In recent years, syphilis notifications have increased dramatically in Japan. We carried out molecular typing and macrolide resistance analyses of Treponema pallidum subsp.
KEYWORDS: macrolide resistance, molecular typing, syphilis, Treponema pallidum
ABSTRACT
In recent years, syphilis notifications have increased dramatically in Japan. We carried out molecular typing and macrolide resistance analyses of Treponema pallidum subsp. pallidum samples collected from patients at four clinics and a hospital in Tokyo and Osaka prefectures in 2017. The macrolide resistant strain type 14d/f (SS14-like clade) was found in significantly more cases of syphilis among heterosexuals than in those among men who have sex with men (MSM); i.e., 79% (31/39) of the strains from heterosexuals were 14d/f compared to 37% (7/19) of those from MSM (odds ratio [OR], 6.6; 95% confidence interval [CI], 1.7 to 26.7; P = 0.002). In addition, 83% (50/60) of the strains were identified as macrolide resistant with an A2058G mutation in the 23S rRNA gene; 90% (35/39) of the strains from heterosexuals were macrolide resistant compared to 58% (11/19) of those from MSM. The odds of having the resistant mutation were considerably higher in the former (OR, 6.4; 95% CI, 1.3 to 33.5; P = 0.02). Heterosexual women and heterosexual men showed similar distributions, and the association remained the same when restricted to men. The strain type distribution and the prevalence of macrolide resistance differed substantially between syphilis strains from heterosexual cases and from MSM cases, suggesting distinct epidemiologic profiles for the two communities and providing important insight into the dynamics of syphilis in Japan.
INTRODUCTION
With the global reemergence of syphilis, the number of reports of syphilis has been increasing rapidly in Japan. Unlike in other industrialized countries (1), the recent increase in syphilis case reports in Japan since 2013 has mostly been associated with transmission among heterosexual men and women (i.e., men who have sex with women [MSW] and women who have sex with men [WSM]), rather than through transmission among men who have sex with men (MSM) (2). This has increased the level of public health concern given the risk of congenital syphilis (2). Large numbers of syphilis cases have been reported in urban areas in Japan, such as Tokyo and Osaka prefectures. The reports of molecular typing and analysis of macrolide resistance of the Treponema pallidum subsp. pallidum strains circulating in Japan have been limited and not comprehensive. In particular, the relative data for strains from the heterosexual and MSM subpopulations remain unknown. Such information may provide useful insights into the epidemiology of syphilis in Japan, including the current state of macrolide resistance and the dynamics of syphilis transmission. Therefore, we conducted this study to determine the prevalence and distribution of T. pallidum subsp. pallidum strains circulating in Japan, taking into consideration the key subpopulations affected by the current syphilis outbreak.
MATERIALS AND METHODS
Sample collection.
Samples were collected from suspected syphilis patients in four clinics and a hospital specializing in sexually transmitted infections and other infectious diseases, located in Tokyo and Osaka prefectures in Japan, between January and December 2017. While there was no defined sampling scheme, the samples were collected from patients clinically suspected of having syphilis during the first visit for each patient. One sample was collected per patient, and these samples were transported to the laboratory of the National Institute of Infectious Diseases in Tokyo. The majority of samples were swabs of genital, anal, or oral lesions, and the rest of the samples were cerebrospinal fluid, blood, or a solid tissue sample, such as from a vegetation or muscle with a suspicious lesion. Information on each patient’s gender and self-reported transmission route (i.e., gender of the sexual partner[s] suspected as the source of infection) was also collected when possible. The results from serological tests for syphilis were also obtained when available.
PCR analyses and molecular typing.
PCR amplification of polA and tpp47 genes was carried out as described previously to detect T. pallidum subsp. pallidum DNA (3, 4). Since the DNA polymerase used in this study could be employed with a variety of biological samples (see below) directly as the templates of PCR, swab samples from genital, anal, and oral lesions suspended in Tris-EDTA (TE) buffer were directly used as the PCR templates. For cerebrospinal fluid, blood, and solid surgical tissue samples, DNA was extracted using a DNeasy Blood & Tissue kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol, and the extracted DNA was used as the template. A sample was considered to be T. pallidum subsp. pallidum DNA positive when PCR amplification was positive for at least one of the two treponemal genes (polA or tpp47).
DNA extracted from the swabs and other samples was used for molecular typing and macrolide resistance analysis. Molecular typing was based on a combination of the number of 60-bp repeats in the arp gene, the restriction fragment length polymorphism (RFLP) pattern of the tpr genes, and the sequence of the tp0548 gene (5). Macrolide resistance was determined by the presence of a mutation in one or both of the T. pallidum subsp. pallidum 23S rRNA genes (A2058G and A2059G) by direct DNA sequencing of the 628-bp PCR amplicons of the two 23S RNA genes containing these residues (6).
These analyses were carried out as described previously (5, 6), except for a modification in the PCR cycling conditions; i.e., the annealing and extension steps were combined in a single step at 68°C, using the PCR enzyme TaKaRa Mighty Amp DNA polymerase (TaKaRa Bio, Inc., Shiga, Japan).
Statistical analyses.
Statistical analyses were performed using Stata version 10. While these were mostly descriptive, a limited number of statistical significance tests were performed to compare the data between the MSM and heterosexual samples, on the basis of the two-tailed Fisher’s exact test; P values of <0.05 were considered to be statistically significant. To indicate the magnitude of an association, the odds ratios (ORs) and associated exact 95% confidence interval (CI) were also determined.
This study was approved by the institutional review board of the National Institute of Infectious Diseases (approval numbers 508 and 705).
Accession number(s).
The sequence for a newly identified variant of tp0548 has been deposited in the GenBank DDBJ (accession number LC380837).
RESULTS
A total of 156 samples were collected for this study from patients clinically suspected of having syphilis. A PCR analysis showed that 82 of the samples were positive for T. pallidum subsp. pallidum DNA (data not shown). Among the remaining 74 DNA-negative samples, there was no information on the results of a serological test for syphilis diagnosis for 19 samples. For the other 55 DNA-negative samples, 33 were serologically negative and 22 were serologically positive for syphilis (data not shown). The 33 DNA-negative serology-negative samples were excluded from further study. Thus, among the remaining 123 samples, 82 were DNA positive (67%), 22 were serology positive (18%), and 19 (15%) had no DNA or serology data.
Of the 82 DNA-positive samples, 25 were from MSM, 36 were from heterosexual men, and 12 were from heterosexual women. The remaining 9 samples were from patients where the gender of the partner(s) was unknown and were excluded, leaving 73 DNA-positive samples for further analysis.
The molecular typing results for the 73 DNA-positive samples in this study, including the partially typed samples, are summarized in Table 1. Fully typed profiles were obtained for 58/73 (79%) samples (Table 1): four arp types (i.e., 7, 10, 12, and 14), ten tpr types (i.e., a, b, d, e, f, j, k, l, o, and p), and six tp0548 types (i.e., a, c, d, f, g, and a new variant type of f). This new type that was identified in this study had a type f single nucleotide polymorphism (SNP) with G at residue 170 in the tp0548 gene reading frame replaced by T: this sequence has been deposited in the GenBank DDBJ (accession number LC380837). We designated this new type “o.” This type was not identical to any other tp0548 sequence from T. pallidum subsp. pallidum, T. pallidum subsp. pertenue, or T. pallidum subsp. endemicum that has been deposited in the GenBank DDBJ as of 20 August 2018. A total of 16 strain types were identified, with 14d/f representing the most common strain type (i.e., 38/58 samples [66%]), followed by 14d/g (i.e., 3/58 samples [5%]), and the other minor types shown in Table 1 and Fig. 1 (Simpson’s diversity index, 0.56).
TABLE 1.
Overall genetic information of 73 T. pallium DNA-positive samples from patients with a known transmission route
| Type | Typing status | arp type | tpr type | tp0548 type | No. of samples | Genetic groupa | 23S rRNA genes (total no. of samples [no. from HW, HM, and MSM])b
|
Transmission route (no. of samples) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistant | Sensitive | NAc | HW | HM | MSM | |||||||
| 7o/c | Complete | 7 | o | c | 1 | Nichols-like | 1 | 1 | ||||
| 10b/a | Complete | 10 | b | a | 1 | Nichols-like | 1 | 1 | ||||
| 10o/c | Complete | 10 | o | c | 1 | Nichols-like | 1 | 1 | ||||
| 12a/c | Complete | 12 | a | c | 1 | Nichols-like | 1 | 1 | ||||
| 14a/o | Complete | 14 | a | o | 1 | SS14-like | 1 | 1 | ||||
| 14b/c | Complete | 14 | b | c | 1 | Nichols-like | 1 | 1 | ||||
| 14b/d | Complete | 14 | b | d | 1 | Nichols-like | 1 | 1 | ||||
| 14d/c | Complete | 14 | d | c | 1 | Nichols-like | 1 | 1 | ||||
| 14d/f | Complete | 14 | d | f | 38 | SS14-like | 34 (6, 24, 4) | 3 (0, 0, 3) | 1 (1, 0, 0) | 7 | 24 | 7 |
| 14d/g | Complete | 14 | d | g | 3 | SS14-like | 3 (0, 0, 3) | 3 | ||||
| 14e/f | Complete | 14 | e | f | 1 | SS14-like | 1 | 1 | ||||
| 14f/f | Complete | 14 | f | f | 1 | SS14-like | 1 | 1 | ||||
| 14j/f | Complete | 14 | j | f | 2 | SS14-like | 1 (0, 1, 0) | 1 (0, 1, 0) | 2 | |||
| 14k/f | Complete | 14 | k | f | 1 | SS14-like | 1 | 1 | ||||
| 14l/f | Complete | 14 | l | f | 2 | SS14-like | 2 (0, 2, 0) | 2 | ||||
| 14p/f | Complete | 14 | p | f | 2 | SS14-like | 1 (1, 0, 0) | 1 (0, 1, 0) | 1 | 1 | ||
| 14/NA/f | Partial | 14 | NA | f | 1 | SS14-like | 1 | 1 | ||||
| 14/NA/NA | Partial | 14 | NA | NA | 1 | unknown | 1 | 1 | ||||
| 14d/NA | Partial | 14 | d | NA | 1 | unknown | 1 | 1 | ||||
| NA/d/f | Partial | NA | d | f | 2 | SS14-like | 2 (1, 1, 0) | 1 | 1 | |||
| NA/e/f | Partial | NA | e | f | 1 | SS14-like | 1 | 1 | ||||
| NA/NA/c | Partial | NA | NA | c | 2 | Nichols-like | 1 (0, 0, 1) | 1 (1, 0, 0) | 1 | 1 | ||
| NA/NA/f | Partial | NA | NA | f | 2 | SS14-like | 1 (0, 1, 0) | 1 (0, 1, 0) | 2 | |||
| NA/NA/g | Partial | NA | NA | g | 1 | SS14-like | 1 | 1 | ||||
| NA/NA/NA | Failed | NA | NA | NA | 4 | unknown | 4 (1, 2, 1) | 1 | 2 | 1 | ||
Determined as described in reference 8.
HW, heterosexual women; HM, heterosexual men; MSM, men who have sex with men.
NA, data not available.
FIG 1.
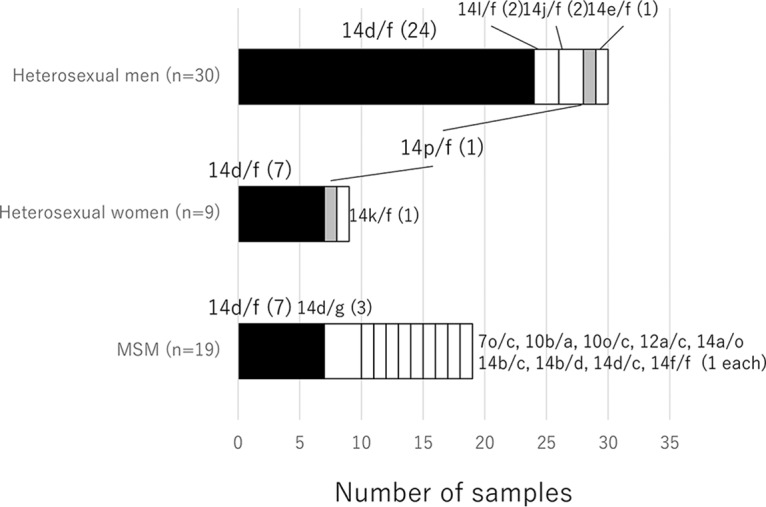
Distribution of the molecular types of 58 Treponema pallidum subsp. pallidum strains in heterosexual men, heterosexual women, and men who have sex with men (MSM). The 58 completely typed Treponema pallidum subsp. pallidum strains in this study were categorized by their self-reported transmission route (heterosexual men, heterosexual women, and MSM). The numbers of strains of each molecular type are shown by the bar size for that type. Molecular types specific to one transmission route are shown in white. Type 14p/f strains, specific to heterosexuals (men and women), are shown in gray. Type 14d/f strains, common in all three groups, are shown in black.
Previous reports have noted that the tp0548 types determined by the molecular typing method used in this study (5) can be divided into either Nichols-like clade or SS14-like clade strains. In this division, tp0548 types a, b, c, d, and h were classified in the Nichols-like clade, and types e, f, g, i, and k were classified in the SS14-like clade (7). The new tp0548 type identified in this study, type “o,” was classified in the SS14-like clade because it had a type f SNP (see above). Therefore, 51 of the 58 fully typed strains (88%) in this study were classified as the SS14-like clade, and 7 of the 58 strains (12%) were classified as the Nichols-like clade. Among the partially typed samples (n = 11), tp0548 was determined for 9 of the samples: 7 strains were the SS14-like clade and 2 strains were the Nichols-like clade (Table 1).
Of the 58 fully typed strains in this study, 39 (67%) were from heterosexuals (30 from men and 9 from women), and 19 strains (33%) were from MSM (Table 1). Six strain types were identified in heterosexuals (5 in men and 3 in women), with type 14d/f being the most common strain type, accounting for 31/39 (79%) of the samples: 24/30 (80%) of the samples from men and 7/9 (78%) of the samples from women (Simpson’s diversity index, 0.36) (Fig. 1). All 39 strains from heterosexuals were tp0548 type f; therefore, they were all classified as the SS14-like clade (Fig. 1) (see above). In contrast, the 19 strains from MSM were 11 types: 7/19 (37%) were type 14d/f, 3/19 (16%) were type 14d/g, and 9/19 (47%) were a wide spectrum of strain types (Simpson’s diversity index, 0.81) (Fig. 1). Nichols-like clade strains were all from MSM in the 58 completely typed strains. Among the partially typed strains (n = 11), tp0548 was determined in 9 samples from 6 heterosexuals and 3 samples from MSM. Only 1 of the 6 samples from heterosexuals may have been a Nichols-like clade strain. Type 14d/f strains were significantly associated with heterosexuals, and the odds of having this strain type were six times greater in heterosexuals than in MSM (OR, 6.6; 95% CI, 1.7 to 26.7; P = 0.002). The type 14d/f association was the same when the heterosexual group was restricted to men (OR, 6.9; 95% CI, 1.6 to 30.6; P = 0.006).
Macrolide resistance was determined in 60/73 (82%) strains in this study at both of the 23S rRNA gene loci. The majority of these (50/60 [83%]) strains had the A2058G mutation in both of the 23S rRNA genes, indicating macrolide resistance in these strains (Table 1). The remaining 10/60 (17%) strains did not have an A2058G or A2059G mutation in either 23S rRNA gene and therefore were identified as the wild type, which indicated that they were macrolide sensitive (Table 1).
The 23S rRNA gene sequences were determined for 52/58 (90%) of the SS14-like clade strains, including both completely and partially typed strains (Tables 1 and 2). The A2058G mutation was detected in 46/52 strains (88%). The other 6 strains had the wild-type sequence, suggesting macrolide sensitivity: 5 of these 6 strains were from MSM. For the 8 Nichols-like clade strains from MSM (Tables 1 and 2), 4/8 (50%) were macrolide resistant and 4/8 (50%) were macrolide-sensitive types.
TABLE 2.
Distribution of clades and macrolide resistance of the 54 fully analyzed samples from patients with a known transmission route
Almost all of the completely typed strains from heterosexuals (35/39 [90%]) were identified as macrolide resistant: 28/30 (93%) of the strains from heterosexual men and 7/9 (78%) of the strains from heterosexual women, compared to 11/19 (58%) of the strains from MSM (Tables 1 and 2). Macrolide resistance was significantly associated with heterosexuals, with 6-fold odds of the mutation being present in heterosexuals compared to that in MSM (OR, 6.4; 95% CI, 1.3 to 33.5; P = 0.02). The association was similar when the heterosexual group was restricted to men (OR, 10.2; 95% CI, 1.6 to 107.2; P = 0.009). Of the 19 strains from MSM, 8 strains (42%) were identified as wild type and macrolide sensitive (Table 2).
We also carried out a further analysis of the distribution of macrolide resistance and of the T. pallidum subsp. pallidum strain type for samples from each transmission route group. All but one type 14d/f strain from heterosexuals were macrolide resistant; i.e., 30/31 (97%). These macrolide-resistant strains were SS14-like clade strains, including types 14e/f, 14j/f, 14l/f, and 14p/f. However, only 4/7 (57%) samples from MSM, which were strain type 14d/f, were identified as macrolide resistant. Among the type 14d/f strains (n = 38), macrolide resistance was significantly associated with samples from heterosexuals (OR, 22.5; 95% CI, 1.3 to 1218; P = 0.030).
Overall, 30/39 (77%) samples from heterosexuals were type 14d/f strains and macrolide resistant, but only 4/19 (21%) samples from MSM were type 14d/f and macrolide resistant. Type 14d/g strains that were macrolide resistant were only found in samples from MSM but were not highly prevalent in MSM; i.e., 3/19 (16%). The other macrolide-resistant strains from MSM were typed as 7o/c, 10o/c, 12a/c, and 14b/d. All of these strains were classified as Nichols-like clade, which were not found in macrolide-resistant strains from heterosexuals. In addition, type 14d/f strains with macrolide sensitivity alleles (wild type) were only found in samples from MSM. This result was based on molecular typing and suggested that strains of various T. pallidum subsp. pallidum types were disseminated in MSM populations.
DISCUSSION
This is the first report on the molecular typing of T. pallidum subsp. pallidum strains in samples from heterosexual and MSM populations in Japan and of the macrolide resistance of these strains. The samples in this study were isolated in 2017 when the number of syphilis cases in Japan, in particular, among heterosexuals, was increasing dramatically and T. pallidum subsp. pallidum type 14d/f (SS14-like clade) strains with macrolide resistance were being detected worldwide (8–12).
The results presented here showed type 14d/f (SS14-like clade) strains with macrolide resistance were the dominant T. pallidum subsp. pallidum strains in Japan. The prevalence of type 14d/f (SS14-like clade) strains was similar to that in previous reports from China (8–10). Although a high prevalence of 14d/f strains was also reported in Peru, Russia, and Argentina, they were not reported to have a high prevalence of macrolide resistance (13–15). The second most common strains in this study were type 14d/g (also SS14-like clade), which were only found in samples from MSM, and were the second most prevalent type found in MSM samples. Type 14d/g strains were also previously reported to be the dominant strain type in the United States, France, Australia, Canada, and Italy (6, 11, 12, 16, 17). A strong link has been reported between type 14d/g strains and macrolide resistance (6, 11, 12, 16, 17).
Novel multilocus sequence typing (MLST) and sequence-based molecular typing (SBMT) methods with greater discrimination have recently been proposed and demonstrated (15, 18, 19). In the T. pallidum clade classifications in these studies, 26.8% of the strains studied in Argentina in 2006 and 2013 were Nichols-like clade strains (15), 5.9% of the strains studied in France in 2010 to 2016 were Nichols-like clade strains (18), and 9.3% of the strains studied in France and Switzerland in 2011 to 2015 were Nichols-like clade strains (19). In this study, 20.7% of the strains studied in Japan in 2017 were Nichols-like clade strains.
The results presented here indicated large differences in the distribution of strain types and macrolide resistance among T. pallidum subsp. pallidum strains in samples from heterosexuals and MSM in Japan. Strains that were both type 14d/f and macrolide resistant were strongly associated with samples from heterosexuals. Strains in samples from heterosexuals were also tp0548 type f. Therefore, all of the completely typed strains from heterosexuals were classified in the SS14-like clade (7), although 1 of the 6 partially typed strains from heterosexuals was a Nichols-like clade (Table 1). In addition, there was a variety of strains in samples from MSM, with no dominant type strain. All 35 strains from heterosexuals were SS14-like clade strains and were macrolide resistant, but 8/19 (42%) of the strains from MSM were SS14-like clade strains and were macrolide resistant.
Macrolide resistance was more prevalent among SS14-like clade strains than among Nichols-like clade strains: 46/52 (89%) among SS14-like clade strains compared to 4/8 (50%) among Nichols-like clade strains (Table 2). These values were comparable to those in previous reports: 81% among SS14-like clade strains and 60% among Nichols-like clade strains (18), and 88.2% among SS14-like strains and 71.4% among the Nichols-like strains (19). In our study, notably, 38/39 (97%) of the strains from heterosexuals were SS14-like clade and macrolide resistant, in contrast to only 8/21 (38%) of the strains among MSM; while pronounced among heterosexuals, macrolide resistance was also more prevalent in SS14-like clade strains among MSM.
The results of this study suggested that recent T. pallidum subsp. pallidum strains circulating in the heterosexual community in Japan appear to be more homogenous than those circulating in the MSM community. Also, Nichols-like clade strains and macrolide-sensitive SS14-like clade strains may continue to circulate in the MSM community.
Although the number of samples in this study was relatively small, the homogeneity observed in strains from heterosexual men and women during 2017, when reports of heterosexual syphilis cases continued to increase considerably, was noteworthy. Although sexual networks can overlap between the heterosexual and MSM communities, the differential molecular profile between these two groups indicates that such bridging may be limited. In addition, there may be localized circulation of T. pallidum subsp. pallidum strains within the heterosexual community that is sustaining the recent increase of syphilis cases in Japan.
There were some limitations in this study regarding the geographical origin of the samples available for this study. Since samples were only collected in Tokyo and Osaka prefectures, our results may not be generalizable to T. pallidum subsp. pallidum strains across Japan. However, these two prefectures warrant particular study because they have the highest syphilis burden in Japan, both in the absolute number of notifications and the notification rate per population (2). In addition, as MSM patients and heterosexual patient samples were both obtained from the same urban areas, the MSM and heterosexual sample strains could be compared without variation in geographic location. Another limitation in this study was the self-reported nature of the transmission route (i.e., heterosexual versus MSM). Given the societal context in Japan, misclassification of MSM as heterosexual men for social desirability reasons is possible; i.e., MSM misreporting themselves as heterosexual. However, such bias would lead to an underestimate of the observed associations.
In conclusion, this study found important differences in the strain types and macrolide resistance of T. pallidum subsp. pallidum strains recently circulating in the heterosexual and MSM communities in Japan. Together with epidemiological information, these molecular findings provide a better understanding of the transmission dynamics of the syphilis epidemic, both within Japan and also within the broader global context, to help inform the public health response for syphilis control.
ACKNOWLEDGMENTS
We thank Igen Hongo, Masayuki Sawamura, Takashi Hamada, and Hiroshi Kameoka who generously and continuously provided us with specimens from clinically suspected syphilis cases. We also thank Kimiko Matsumoto for her technical support in the detection and typing of T. pallidum subsp. pallidum, including PCR procedures.
This study was supported by a grant from the Ministry of Health, Labor and Welfare of Japan (H-28-Shinkou-Gyousei-Ippan-008).
REFERENCES
- 1.Kamb ML, Taylor MM, Ishikawa N. 2018. Rapid increases in syphilis in reproductive-aged women in Japan: a warning for other countries? Sex Transm Dis 45:144–146. doi: 10.1097/OLQ.0000000000000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi T, Arima Y, Yamagishi T, Nishiki S, Kanai M, Ishikane M, Matsui T, Sunagawa T, Ohnishi M, Oishi K. 2018. Rapid increase in reports of syphilis associated with men who have sex with women and women who have sex with men, Japan, 2012 to 2016. Sex Transm Dis 45:139–143. doi: 10.1097/OLQ.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H, Rodes B, Chen CY, Steiner B. 2001. New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J Clin Microbiol 39:1941–1946. doi: 10.1128/JCM.39.5.1941-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orle KA, Gates CA, Martin DH, Body BA, Weiss JB. 1996. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol 34:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marra C, Sahi S, Tantalo L, Godornes C, Reid T, Behets F, Rompalo A, Klausner JD, Yin Y, Mulcahy F, Golden MR, Centurion-Lara A, Lukehart SA. 2010. Enhanced molecular typing of treponema pallidum: geographical distribution of strain types and association with neurosyphilis. J Infect Dis 202:1380–1388. doi: 10.1086/656533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, Engelman J, Mitchell SJ, Rompalo AM, Marra CM, Klausner JD. 2004. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med 351:154–158. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 7.Arora N, Schuenemann VJ, Jäger G, Peltzer A, Seitz A, Herbig A, Strouhal M, Grillová L, Sánchez-Busó L, Kühnert D, Bos KI, Davis LR, Mikalová L, Bruisten S, Komericki P, French P, Grant PR, Pando MA, Vaulet LG, Fermepin MR, Martinez A, Centurion Lara A, Giacani L, Norris SJ, Šmajs D, Bosshard PP, González-Candelas F, Nieselt K, Krause J, Bagheri HC. 2016. Origin of modern syphilis and emergence of a pandemic Treponema pallidum cluster. Nat Microbiol 2:16245. doi: 10.1038/nmicrobiol.2016.245. [DOI] [PubMed] [Google Scholar]
- 8.Dai T, Li K, Lu H, Gu X, Wang Q, Zhou P. 2012. Molecular typing of Treponema pallidum: a 5-year surveillance in Shanghai, China. J Clin Microbiol 50:3674–3677. doi: 10.1128/JCM.01195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen XS, Yin YP, Wei WH, Wang HC, Peng RR, Zheng HP, Zhang JP, Zhu BY, Liu QZ, Huang SJ. 2013. High prevalence of azithromycin resistance to Treponema pallidum in geographically different areas in China. Clin Microbiol Infect 19:975–979. doi: 10.1111/1469-0691.12098. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Liu S, Liu Z, Xie Y, Jiang C, Xu M, Zhao F, Zeng T, Yu J, Wu Y. 2016. Molecular subtyping and surveillance of resistance genes in Treponema pallidum DNA from patients with secondary and latent syphilis in Hunan, China. Sex Transm Dis 43:310–316. doi: 10.1097/OLQ.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 11.Read P, Tagg KA, Jeoffreys N, Guy RJ, Gilbert GL, Donovan B. 2016. Treponema pallidum strain types and association with macrolide resistance in Sydney, Australia: new tp0548 gene types identified. J Clin Microbiol 54:2172–2174. doi: 10.1128/JCM.00959-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuel M, Hayden K, Kadkhoda K, Tsang RSW. 2018. Molecular typing and macrolide resistance of syphilis cases in Manitoba, Canada, from 2012 to 2016. Sex Transm Dis 45:233–236. doi: 10.1097/OLQ.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 13.Flores JA, Vargas SK, Leon SR, Perez DG, Ramos LB, Chow J, Konda KA, Calvo GM, Salvatierra HJ, Klaussner JD, Caceres CF. 2016. Treponema pallidum pallidum genotypes and macrolide resistance status in syphilitic lesions among patients at 2 sexually transmitted infection clinics in Lima, Peru. Sex Transm Dis 43:465–466. doi: 10.1097/OLQ.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 14.Khairullin R, Vorobyev D, Obukhov A, Kuular U-H, Kubanova A, Kubanov A, Unemo M. 2016. Syphilis epidemiology in 1994-2013, molecular epidemiological strain typing and determination of macrolide resistance in Treponema pallidum in 2013–2014 in Tuva Republic, Russia. APMIS 124:595–602. doi: 10.1111/apm.12541. [DOI] [PubMed] [Google Scholar]
- 15.Gallo Vaulet L, Grillová L, Mikalová L, Casco R, Rodríguez Fermepin M, Pando MA, Šmajs D. 2017. Molecular typing of Treponema pallidum isolates from Buenos Aires, Argentina: frequent Nichols-like isolates and low levels of macrolide resistance. PLoS One 12:e0172905. doi: 10.1371/journal.pone.0172905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grange PA, Allix-Beguec C, Chanal J, Benhaddou N, Gerhardt P, Morini JP, Deleuze J, Lassau F, Janier M, Dupin N. 2013. Molecular subtyping of Treponema pallidum in Paris, France. Sex Transm Dis 40:641–644. doi: 10.1097/OLQ.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 17.Giacani L, Ciccarese G, Puga-Salazar C, Dal Conte I, Colli L, Cusini M, Ramoni S, Delmonte S, D’Antuono A, Gaspari V, Drago F. 2018. Enhanced molecular typing of Treponema pallidum subspecies pallidum strains from 4 Italian hospitals shows geographical differences in strain type heterogeneity, widespread resistance to macrolides, and lack of mutations associated with doxycycline resistance. Sex Transm Dis 45:237–242. doi: 10.1097/OLQ.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pospíšilová P, Grange PA, Grillová L, Mikalová L, Martinet P, Janier M, Vermersch A, Benhaddou N, Del Giudice P, Alcaraz I, Truchetet F, Dupin N, Šmajs D. 2018. Multi-locus sequence typing of Treponema pallidum subsp. pallidum present in clinical samples from France: infecting treponemes are genetically diverse and belong to 18 allelic profiles. PLoS One 13:e0201068. doi: 10.1371/journal.pone.0201068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grillová L, Bawa T, Mikalová L, Gayet-Ageron A, Nieselt K, Strouhal M, Sednaoui P, Ferry T, Cavassini M, Lautenschlager S, Dutly F, Pla-Díaz M, Krützen M, González-Candelas F, Bagheri HC, Šmajs D, Arora N, Bosshard PP. 2018. Molecular characterization of Treponema pallidum subspp. pallidum in Switzerland and France with a new multilocus sequence typing scheme. PLoS One 13:e0200773. doi: 10.1371/journal.pone.0200773. [DOI] [PMC free article] [PubMed] [Google Scholar]


