The sustained increase in the incidence of nontuberculous mycobacterial (NTM) infection and the difficulty in distinguishing these infections from tuberculosis constitute an urgent need for NTM species-level identification. The MeltPro Myco assay is the first diagnostic system that identifies 19 clinically relevant mycobacteria in a single reaction based on multicolor melting curve analysis run on a real-time PCR platform.
KEYWORDS: melting curve analysis, nontuberculous mycobacteria, real-time PCR, species identification
ABSTRACT
The sustained increase in the incidence of nontuberculous mycobacterial (NTM) infection and the difficulty in distinguishing these infections from tuberculosis constitute an urgent need for NTM species-level identification. The MeltPro Myco assay is the first diagnostic system that identifies 19 clinically relevant mycobacteria in a single reaction based on multicolor melting curve analysis run on a real-time PCR platform. The assay was comprehensively evaluated regarding its analytical and clinical performances. The MeltPro Myco assay accurately identified 51 reference mycobacterial strains to the species/genus level and showed no cross-reactivity with 16 nonmycobacterial strains. The limit of detection was 300 bacilli/ml, and 1% of the minor species was detected in the case of mixed infections. Clinical studies using 1,163 isolates collected from five geographically distinct health care units showed that the MeltPro Myco assay correctly identified 1,159 (99.7%) samples. Further testing with 94 smear-positive sputum samples showed that all samples were correctly identified. Additionally, the entire assay can be performed within 3 h. The results of this study confirmed the efficacy of this assay in the reliable identification of mycobacteria, suggesting that it might potentially be used as a screening tool in regions endemic for tuberculosis.
INTRODUCTION
Nontuberculous mycobacteria (NTM) are ubiquitous organisms frequently isolated from environmental sources (1). Some NTM species can cause severe diseases in individuals, especially those with reduced or compromised immune function (2–5). The most common clinical manifestation of NTM infections is lung disease that resembles tuberculosis (TB) (6); however, it cannot be treated as TB because NTM harbor natural resistance mechanisms against antituberculosis drugs. In addition, NTM species differ remarkably among each other regarding pathogenicity and drug susceptibility profiles (6–8). In recent years, the mortality caused by NTM infections has increased significantly due to misdiagnosis and inappropriate therapy (9). Identification of NTM to the species and subspecies levels has thus become essential for facilitating early and precise treatment of NTM diseases.
Traditionally, mycobacteria are identified by phenotypic methods based on cell culturing techniques. Testing is laborious, difficult, and time-consuming. In the last decade, advances in molecular methods have enabled rapid identification of many mycobacterial species. Several molecular systems are already commercially available; for example, the line probe assay (LPA) represented by GenoType Mycobacterium CM/AS (Hain Lifescience GmbH, Nehren, Germany) (10) and INNO-LiPA Mycobacteria (Innogenetics, Ghent, Belgium) (11) assays can detect mycobacteria up to 30 and 17 species/complex, respectively. These assays, however, require various post-PCR manipulations and are prone to amplicon contamination. The real-time PCR-based Real Myco-ID assay (Optipharm, Osong, South Korea) is run in a closed-tube format and can thus avoid the above-mentioned problems; nevertheless, multiple PCRs are required for identification of the 17 different species (12). So far, Sanger sequencing is still regarded as the gold standard for NTM identification, yet it is often restricted to research use and may encounter difficulties with mixed-infection samples, which are not uncommon in mycobacterial disease.
The MeltPro Myco assay (Zeesan Biotech, Xiamen, China) is a newly released qualitative diagnostic assay that can identify 19 clinically relevant Mycobacterium species. This assay is based on a unique multicolor melting curve analysis (MMCA) (13) that can detect dozens of targets in a single reaction using a real-time PCR machine. This is achieved by using a two-dimensional (2D) labeling strategy combining fluorescence color and melting temperature (Tm) for target identification (14). One distinct feature of the MeltPro Myco assay is its ability to identify the 19 Mycobacterium species in a single reaction. The closed-tube detection format avoids post-PCR manipulation, making it easy to use, rapid, and less prone to carryover contamination. To assess its suitability for clinical use, we evaluated its analytical performance regarding species identification accuracy, limit of detection (LOD), and ability to detect mixed infections. We further investigated its clinical performance by testing 1,163 clinical mycobacterial isolates and 94 smear-positive sputum specimens.
MATERIALS AND METHODS
MeltPro Myco assay.
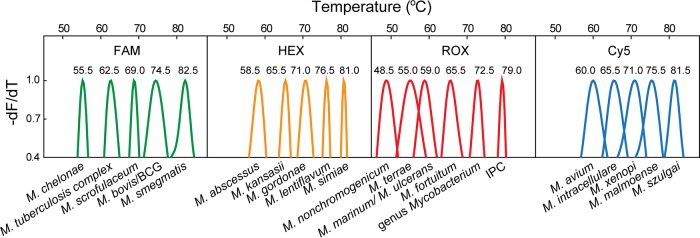
The MeltPro Myco assay targets the intergenic transcribed spacer (ITS) region between the 16S rRNA and 23S rRNA genes of mycobacteria using a panmycobacterial primer set. Eighteen species-specific probes were designed to identify 17 NTM species (Mycobacterium ulcerans and Mycobacterium marinum complex were not differentiated) and the M. tuberculosis complex (MTBC). One additional genus-specific probe was used to identify the Mycobacterium genus. To differentiate Mycobacterium bovis and the bacillus Calmette-Guérin (BCG) vaccine from M. tuberculosis, a set of primers and a probe were designed to target an uninterrupted 229-bp sequence in the M. bovis genome, which is otherwise interrupted by a unique 12.7-kb fragment in M. tuberculosis (15). Moreover, a fragment of the Arabidopsis thaliana sucrose-proton symporter 2 (SUC2) gene was included as an internal positive control (IPC) for an indication of PCR inhibition. In total, 21 labels were assigned to 19 mycobacterial species, the genus Mycobacterium, and IPC (Fig. 1). The result interpretation was as follows: a positive IPC signal indicates successful amplification with no or negligible inhibition, the appearance of a genus Mycobacterium melting peak indicates the existence of mycobacteria, and the presence of any 19 species-specific positive signals indicates the existence of the corresponding species; on the other hand, the absence of all species-specific melting peaks indicates the existence of mycobacterial species beyond the 19 species in the assay and the readout is Mycobacterium species. Finally, absence of the genus Mycobacterium melting peak indicates that no mycobacteria are detected.
FIG 1.
Melting peaks for each species in the MeltPro Myco assay in line with the 2D label strategy. To visually compare the melting temperature values of different Mycobacterium species, the melting curves showing the negative derivative of fluorescence intensity with respect to temperature were first normalized between 0 and 1, and then the data between 0.4 and 1 were plotted.
The MeltPro Myco assay was run in a Slan-96S real-time PCR system (Zeesan Biotech). For sample detection, 5 μl of extracted DNA was added to reaction tubes prefilled with a 20-μl PCR mixture. The running program included decontamination at 50°C for 2 min, denaturation at 95°C for 10 min, 55 cycles of 95°C for 15 s, 57°C for 20 s, and 78°C for 20 s, followed by denaturation at 95°C for 2 min, hybridization at 45°C for 2 min, and temperature increase from 45°C to 90°C at a ramp rate of 0.04°C/s. When completed, results regarding species were automatically provided by a dedicated software (MeltPro Manager version 1.0; Zeesan Biotech) according to the result interpretation guidelines.
Analytical evaluation.
For evaluating analytical specificity, we analyzed the most common microflora found in sputum consisting of 51 mycobacterial species and 16 nonmycobacterial species. The 51 mycobacterial strains included 22 NTM species or subspecies that could be identified by the MeltPro Myco assay and 29 other NTM species (Table 1). They were obtained from the National Center for Medical Culture Collections (CMCC) affiliated with the National Institutes for Food and Drug Control (NIFDC, China). All strains were stored in culture medium at approximately 106 bacilli/ml. Genomic DNA was obtained by heating lysis. Briefly, 0.5 ml of Mycobacterium culture in liquid medium was centrifuged at 13,000 × g for 10 min, and then the precipitate was suspended in 100 μl DNA extracting solution (10 mM Tris-HCl [pH 8.5], 1 mM EDTA, and 1% Triton X-100). The suspension was heated at 99°C for 20 min, followed by centrifugation at 13,000 × g for 10 min. The supernatant was kept at −20°C before use.
TABLE 1.
Results of strains tested with the MeltPro Myco assay
| Strain | Bacterium | Results obtained by the MeltPro Myco assay |
||||
|---|---|---|---|---|---|---|
| FAM (°C)a | HEX (°C)a | ROX (°C)a | Cy5 (°C)a | Identificationb | ||
| 95001 | M. avium | - | - | 72.3, 79.2 | 59.6 | M. avium |
| 95002 | M. intracellulare | - | - | 72.5, 79.0 | 65.0 | M. intracellulare |
| 95003 | M. xenopi | - | - | 72.5, 79.0 | 71.0 | M. xenopi |
| 95004 | M. ulcerans | - | - | 58.5, 72.4, 78.9 | - | M. marinum/M. ulcerans |
| 95005 | M. terrae | - | - | 55.0, 72.5, 79.0 | - | M. terrae |
| 95007 | M. nonchromogenicum | - | - | 48.6, 72.5, 79.0 | - | M. nonchromogenicum |
| 95010 | M. malmoense | - | - | 72.5, 79.0 | 75.8 | M. malmoense |
| 95013 | M. kansasii | - | 65.6 | 72.4, 79.0 | - | M. kansasii |
| 95014 | M. marinum | - | - | 58.6, 72.5, 79.0 | - | M. marinum/M. ulcerans |
| 95015 | M. simiae | - | 80.7 | 72.5, 79.1 | - | M. simiae |
| 95017 | M. scrofulaceum | 68.7 | - | 72.4, 79.0 | - | M. scrofulaceum |
| 95018 | M. gordonae | - | 70.8 | 72.5, 79.0 | - | M. gordonae |
| 95019 | M. szulgai | - | - | 72.5, 79.0 | 81.2 | M. szulgai |
| 95020 | M. chelonae | 55.0 | - | 72.4, 78.8 | - | M. chelonae |
| 95021 | M. abscessus | - | 58.5 | 72.5, 79.0 | - | M. abscessus |
| 95022 | M. fortuitum | - | - | 65.0, 72.5, 79.0 | - | M. fortuitum |
| 95023 | M. smegmatis | 82.4 | - | 72.5, 79.0 | - | M. smegmatis |
| 93009 | M. tuberculosis | 62.3 | - | 72.5, 79.0 | - | MTBC |
| 95048 | BCG | 62.3, 74.6 | - | 72.5, 79.0 | - | M. bovis/BCG |
| 95049 | M. bovis | 62.3, 74.6 | - | 72.4, 79.0 | - | M. bovis/BCG |
| 95054 | M. africanum | 62.3 | - | 72.5, 79.0 | - | MTBC |
| D2PB302 | M. microti | 62.3 | - | 72.5, 79.0 | - | MTBC |
| 95006 | M. gastri | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95008 | M. shimoidei | - | - | 72.3, 78.8 | - | Mycobacterium spp. |
| 95009 | M. triviale | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95012 | M. farcinogenes | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95016 | M. asiaticum | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95024 | M. phlei | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95025 | M. thermoresistibile | - | - | 72.5, 79.1 | - | Mycobacterium spp. |
| 95026 | M. aichiense | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95027 | M. aurum | - | - | 72.4, 79.0 | - | Mycobacterium spp. |
| 95028 | M. chubuense | - | - | 72.4, 79.0 | - | Mycobacterium spp. |
| 95029 | M. duvalii | - | - | 72.6, 79.0 | - | Mycobacterium spp. |
| 95030 | M. flavescens | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95031 | M. gadium | - | - | 72.5, 79.1 | - | Mycobacterium spp. |
| 95032 | M. gilvum | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95033 | M. komossense | - | - | 72.4, 79.0 | - | Mycobacterium spp. |
| 95034 | M. neoaurum | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95035 | M. obuense | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95036 | M. parafortuitum | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95037 | M. rhodesiae | - | - | 72.5, 79.2 | - | Mycobacterium spp. |
| 95038 | M. tokaiense | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95039 | M. porcinum | - | - | 72.4, 79.0 | - | Mycobacterium spp. |
| 95040 | M. pulveris | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95041 | M. senegalense | - | - | 72.3, 79.0 | - | Mycobacterium spp. |
| 95042 | M. fallax | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95043 | M. agri | - | - | 72.5, 79.1 | - | Mycobacterium spp. |
| 95044 | M. austroafricanum | - | - | 72.4, 79.0 | - | Mycobacterium spp. |
| 95045 | M. diernhoferi | - | - | 72.5, 79.0 | - | Mycobacterium spp. |
| 95046 | M. chitae | - | - | 72.5, 79.2 | - | Mycobacterium spp. |
| 93467 | M. vaccae | - | - | 72.4, 79.0 | - | Mycobacterium spp. |
Melting temperature of melting peak. “-” means no melting peak was detected.
The identification “Mycobacterium spp.” represents bacteria that could only be detected at the genus level by the MeltPro Myco assay.
The 16 nonmycobacterial strains were obtained from our laboratory (see Table S1 in the supplemental material). These strains were provided in the form of genomic DNA of approximately 103 copies/μl.
For evaluating analytical sensitivity, four NTM species, i.e., M. tuberculosis, M. kansasii, M. fortuitum, and M. avium, were chosen as representatives of the four fluorescence channels; their exact concentrations were determined by direct microscopic counting using a hemocytometer. Bacterial suspensions were prepared in a serial dilution of 3 × 105, 3 × 104, 3 × 103, 3 × 102, and 3 × 101 bacilli/ml in 0.9% NaCl. Each bacterial suspension was subjected to DNA extraction using the Lab-Aid 824 MTB DNA extraction kit (Zeesan Biotech). The LOD was defined as the lowest concentration that gives no more than one negative result in 20 replicates (i.e., positive rate, ≥95%).
To determine the efficiency of MeltPro Myco assay in detecting mixed infections, we tested two pathogens that occur in the same fluorescence channel. M. chelonae and M. tuberculosis were chosen, as the two occur in the 6-carboxyfluorescein (FAM) channel as neighboring species. Plasmid DNA (103 copies/μl) containing the ITS region of M. chelonae was mixed with plasmid DNA (103 copies/μl) containing the ITS region of M. tuberculosis in different percentages to generate DNA templates containing 0%, 1%, 5%, 25%, 50%, 75%, 95%, 99%, and 100% M. chelonae. They were subjected to the MeltPro Myco assay and tested in triplicate. We also tested two pathogens occurring in different fluorescence channels. To this end, Mycobacterium intracellulare in the Cy5 channel was paired with M. tuberculosis in the FAM channel. Plasmid DNA templates containing different percentages of M. intracellulare were subjected to the MeltPro Myco assay, as mentioned above.
Clinical evaluation.
Two batches of samples were used for clinical evaluation of the MeltPro Myco assay consisting of 1,163 clinical isolates and 94 smear-positive sputum samples.
The clinical isolates used met the following eligibility criteria, were MGIT 960 culture positive, and were consecutively collected. They were collected from five health care units. Among them, 378 isolates were from southern China (Guangzhou Chest Hospital, Guangzhou, Guangdong), 189 isolates were from southeastern China (Xiamen Center for Disease Control and Prevention, Xiamen, Fujian), 217 isolates were from central China (Henan Provincial Center for Disease Control and Prevention, Zhengzhou, Henan), 297 isolates were from northeastern China (Infectious Disease Hospital of Heilongjiang Province, Harbin, Heilongjiang), and 82 isolates were from northwestern China (Chest Hospital of Xinjiang Uyghur Autonomous Region, Wulumuqi, Xinjiang Uyghur Autonomous Region). DNA extraction from clinical isolates was carried out by heating lysis, as described previously.
Patient samples consisted of 94 smear-positive sputum samples that were consecutively collected from Guangzhou Chest Hospital. Each sample was decontaminated by using the N-acetyl-l-cysteine (NALC)-NaOH method, followed by neutralization with sterile phosphate-buffered saline (PBS; pH 6.8). After centrifugation at 3,000 × g for 15 min, the pellet was resuspended in 2 ml PBS buffer. A 1.5-ml sample of the decontaminated specimens was subjected to DNA extraction using the Lab-Aid 824 MTB DNA extraction kit.
To confirm the detection results of the MeltPro Myco assay, a variety of comparison methods were used. First, an IS6110-based MTBC test kit (Zeesan Biotech) was used as a screening tool to confirm MTBC-containing samples. Second, a spoligotyping assay was used to differentiate between M. bovis and M. tuberculosis (16). Third, Sanger sequencing was used to confirm all NTM species by sequencing the 5′ region of the 16S rRNA gene (∼500 bp) and the ITS region between the 16S rRNA and 23S rRNA genes, as previously described (17). The sequences obtained from the two regions were subjected to BLAST analysis against the NCBI GenBank database.
To validate the mixed infections identified by the MeltPro Myco assay, PCR products from the ITS region were separated by agarose gel electrophoresis (AGE) or polyacrylamide gel electrophoresis (PAGE). DNA recovered from the bands was subjected to Sanger sequencing. For amplicons that cannot be separated by AGE or PAGE, PCR detection of species-specific genes was used to confirm the coexisting strains; M. tuberculosis was confirmed by the MTBC test kit that targets IS6110, M. avium was confirmed by the detection of IS901, and M. intracellulare was confirmed by the detection of DT1 (an insertion element harboring transposase gene), as previously described (18, 19).
RESULTS
Analytical evaluation.
Evaluation of analytical specificity showed that the MeltPro Myco assay could correctly detect all 51 mycobacterial strains to the species/genus level and exhibited no cross-reactivity with the 16 nonmycobacterial strains (Tables 1 and S1).
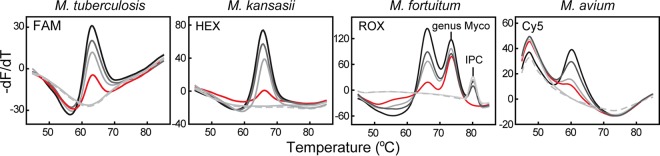
The four studied strains, viz. M. tuberculosis, M. kansasii, M. fortuitum, and M. avium, gave reproducible readouts in 20 replicates when the concentration was equal to or higher than 300 bacilli/ml; therefore, the MeltPro Myco assay LOD was determined to be 300 bacilli/ml (Fig. 2).
FIG 2.
Analytical sensitivity of the MeltPro Myco assay. Melting curves of the four representative species, i.e., M. tuberculosis, M. kansasii, M. fortuitum, and M. avium, ranged from 3 × 105 to 3 × 101 bacilli/ml and are shown (gray lines) together with the melting curves representing the LOD (red lines) and no-template control (NTC) (dashed lines). Myco, Mycobacterium.
We then evaluated the ability of the MeltPro Myco assay to detect mixed infections by using two-species mixed samples as models. Our results showed that minor species could be detected at as low as 1% of the abundant species, regardless of whether the two species were in identical or different fluorescence channels (Fig. 3).
FIG 3.
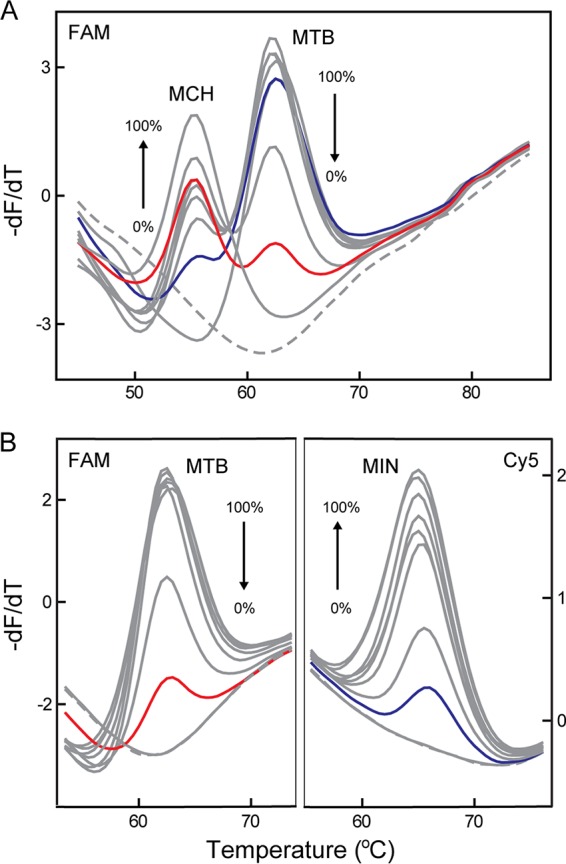
MeltPro Myco assay for mixed-infection detection. (A) Two coexisting species, M. tuberculosis (MTB) and M. chelonae (MCH), were detected in one channel (FAM). (B) Two coexisting species, MTB and M. intracellulare (MIN), were detected in two channels (FAM and Cy5). The overall template concentration was 5 × 103 copies per reaction. Melting curves of artificial plasmid templates containing two species with various ratios (0:100, 1:99, 5:95, 25:75, 50:50, 75:25, 95:5, 99:1 to 100:0) are shown (gray lines) together with melting curves of the mixed template containing 1% MTB (red line), 1% of MCH (A), or 1% of MIN (B) (blue line), and NTC (dashed line).
Clinical evaluation.
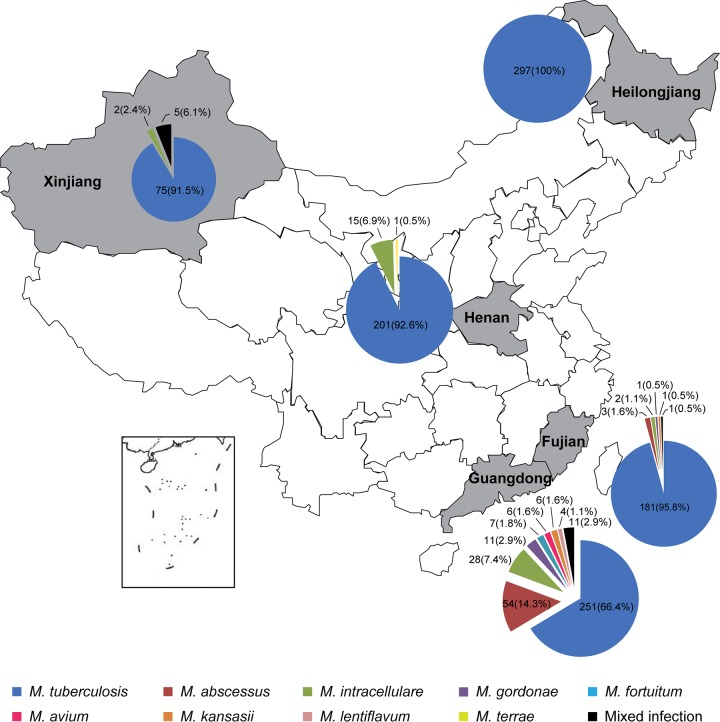
We first assessed the MeltPro Myco assay using 1,163 clinical isolates collected from five geographically distinct health care units in China. Of the 378 isolates from Guangzhou, 251 M. tuberculosis, 116 NTM, and 11 mixed infections were identified. Of the 189 isolates from Xiamen, 181 M. tuberculosis, 7 NTM, and one mixed infection were detected. From Henan, 201 M. tuberculosis and 16 NTM were detected in 217 samples. From the Heilongjiang samples, all 297 isolates were identified as M. tuberculosis. Of the 82 isolates from Xinjiang, 75 M. tuberculosis, 2 NTM, and 5 mixed infections were identified. In total, 1,146 samples of single infections (1,005 samples of M. tuberculosis and 141 samples of NTM) and 17 samples of mixed infections were identified by the MeltPro Myco assay. The species frequencies are shown with their location of origin in Fig. 4.
FIG 4.
Mycobacterium species distribution over five regions in China.
To verify the above-mentioned results, we first screened all samples using the MTBC test kit. The results showed that all 1,005 samples identified as M. tuberculosis gave positive results, whereas none of the 141 NTM and 14 of 17 mixed infections were positive.
To confirm the 141 NTM single-infection results, Sanger sequencing was used to analyze the 5′ region of the 16S rRNA gene and the ITS region between the 16S rRNA and 23S rRNA genes. Concordant results were found for 137 of the 141 NTM samples. Of the 4 discordant samples from Guangzhou Chest Hospital, 3 samples identified as M. intracellulare by the MeltPro Myco assay were determined to be Mycobacterium marseillense, Mycobacterium chimera, and M. avium complex through sequencing. Notably, these three species belong to the M. avium complex, which also contains M. intracellulare, indicating a close relationship between them. The other sample identified as M. fortuitum alone by the MeltPro Myco assay displayed overlapping peaks in the sequencing chromatogram, indicating mixed species. The PCR products were then subjected to PAGE for separation of possible mixed amplicons, and two bands were recovered. Sequencing analysis revealed that they were amplicons of M. fortuitum and Mycobacterium senegalense. As M. senegalense was outside the species identification groups in the MeltPro Myco assay, sequencing analysis confirmed the assay results.
To confirm the 17 mixed infection results, we first used AGE or PAGE to separate and recover their ITS amplicons by taking advantage of the length differences (>10 bp). Otherwise, we used PCR detection of species-specific target genes. Through these approaches, all 17 samples containing mixed species detected by MeltPro Myco assay were confirmed to be correct (Table 2).
TABLE 2.
Validation of 18 mixed infections found in 1,163 clinical isolates
| Sample | Mixed infection bacteria | Discrepant bpa | Validation assayb |
|---|---|---|---|
| GZ k1624623 | M. avium-M. abscessus | 38 (AGE) | ITS |
| GZ k1624078 | M. intracellulare-M. abscessus | 38 (AGE) | ITS |
| GZ k1624914 | M. tuberculosis-M. abscessus | 36 (AGE) | ITS |
| GZ k1621849 | M. tuberculosis-M. abscessus | 36 (AGE) | ITS |
| GZ k1623927 | M. tuberculosis-M. abscessus | 36 (AGE) | ITS |
| GZ k1620589 | M. tuberculosis-M. abscessus | 36 (AGE) | ITS |
| GZ k1621157 | M. tuberculosis-M. abscessus | 36 (AGE) | ITS |
| GZ k1617424 | M. xenopi-M. lentiflavum | 25 (PAGE) | ITS |
| GZ k1623332 | M. fortuitum-M. senegalense | 14 (PAGE) | ITS |
| GZ k1618570 | M. tuberculosis-M. gordonae | 10 (PAGE) | ITS |
| GZ k1621958 | M. tuberculosis-M. avium | 2 | IS6110, IS901 |
| GZ k1616819 | M. tuberculosis-M. intracellulare | 2 | IS6110, DT1 |
| XM TA15018 | M. tuberculosis-M. intracellulare | 2 | IS6110, DT1 |
| XJ 5504 | M. tuberculosis-M. intracellulare | 2 | IS6110, DT1 |
| XJ 5541 | M. tuberculosis-M. intracellulare | 2 | IS6110, DT1 |
| XJ 5551 | M. tuberculosis-M. intracellulare | 2 | IS6110, DT1 |
| XJ 5614 | M. tuberculosis-M. intracellulare | 2 | IS6110, DT1 |
| XJ 5563 | M. tuberculosis-M. intracellulare | 2 | IS6110, DT1 |
Discrepant base pairs between two amplicon fragments amplified from mixed strains using panmycobacterial ITS primers, as previously described (17). Agarose gel electrophoresis (AGE) or polyacrylamide gel electrophoresis (PAGE) was used to separate two different amplicons based on corresponding discrepant base pairs.
For mixed infections with two different amplicons that can be separated by AGE or PAGE, both PCR products were subjected to ITS sequencing. For M. tuberculosis-M. avium and M. tuberculosis-M. intracellulare, M. tuberculosis, M. avium, and M. intracellulare were confirmed by using their respective specific genes, i.e., IS6110, IS901, and DT1.
Taken together, clinical evaluation of the 1,163 clinical isolates showed that the MeltPro Myco assay correctly identified 1,159 (99.7%) samples. Three samples identified as M. intracellulare proved to be species that were closely related and within the M. avium complex, while a mixed-infection sample missed identifying one species, as it is outside the assay coverage.
To explore the suitability of the MeltPro Myco assay in detecting clinical samples, we tested 94 smear-positive sputum samples that were consecutively collected. The MeltPro Myco assay identified 92 species-resolvable samples, 1 Mycobacterium sp. sample, and 1 negative sample (Fig. 5). Of the 92 samples identifiable to the species level, 69 samples contained single MTBC members composed of 68 M. tuberculosis (73.1%) and 1 M. bovis, 21 samples contained single NTM species, and 2 samples contained two mixed species. The MTBC test kit gave concordant results for all M. tuberculosis-containing samples, while M. bovis identification was confirmed by spoligotyping. Sequencing analysis revealed that the Mycobacterium spp. proved to be M. europaeum, an NTM species outside the coverage of the MeltPro Myco assay. The negative sample contained Corynebacterium species, a nonmycobacterial organism that is also beyond the coverage of the MeltPro Myco assay. Consequently, the results of the MeltPro Myco assay for the 94 smear-positive sputum samples were all confirmed to be correct.
FIG 5.
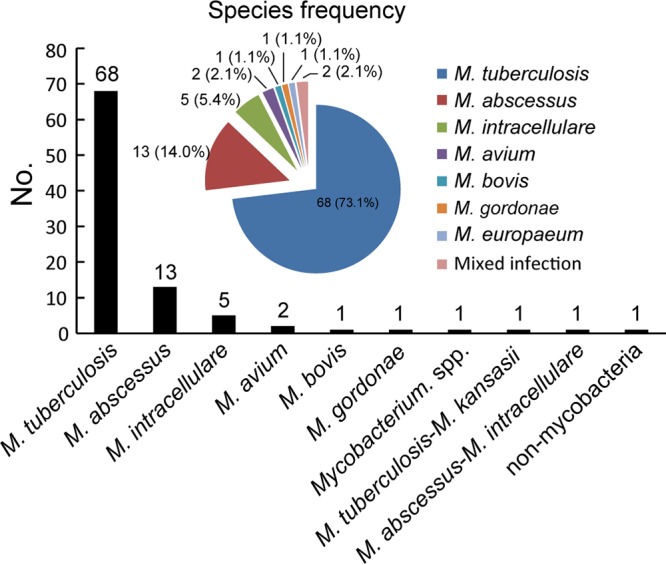
Distribution of the mycobacterial species detected from the 94 smear-positive sputum specimens.
DISCUSSION
Compared to traditional phenotypic methods, molecular identification of mycobacterial species has advantages in rapidness, accuracy, reproducibility, and especially the ability to identify mycobacteria directly from clinical raw sample. Rapid identification of mycobacteria would not only help the treatment of NTM diseases but also benefit TB control. For example, patients infected with NTM can be removed from isolation immediately without any subsequent TB contact investigation after a rapid identification.
In this study, we evaluated the analytical and clinical performances of the MeltPro Myco assay. The MeltPro Myco assay accurately identified 51 reference mycobacterial strains to the species/genus level and showed no cross-reactivity with 16 nonmycobacterial strains. Using representative NTM species of the four fluorescence channels, the MeltPro Myco assay LOD was found to be 300 bacilli/ml. In the case of mixed infections, 1% of the minor species could be detected regardless of whether the two species were in identical or different fluorescence channels. Clinical evaluation using 1,163 isolates collected from five geographically distinct health care units showed that the MeltPro Myco assay could correctly identify M. tuberculosis and NTM species in 1,159 samples. Further testing with direct clinical samples showed that all 94 smear-positive sputum samples were correctly identified.
The MeltPro Myco assay represents the first melting curve analysis-based system designed for the identification of NTM. The unique feature of this assay is its ability to identify 17 clinically important NTM to the species level, as well as to detect the genus Mycobacterium, MTBC, and further M. bovis BCG, all in a single and closed-tube reaction. The MeltPro Myco assay also distinguishes itself from existing systems, such as membrane hybridization assays that require various post-PCR manipulations or real-time PCR assays that need multiple reactions. The turnaround time is within 3 h, and the hands-on time is less than 20 min, as only a single template addition step is required. The assay can detect up to 94 samples with 1 positive- and 1 negative-control sample in a single run on a 96-well real-time PCR machine, facilitating its potential use as a screening tool in clinical settings.
The MeltPro Myco assay utilizes the ITS region as the target sequence in identifying 17 NTM species and detecting the genus Mycobacterium. The ITS region proved to be more specific than other target regions that are currently used in NTM identification. This specificity is reflected in the reduced cross-reactivity among NTM species. Of the 17 NTM resolvable species, only M. marinum and M. ulcerans could not be differentiated. The MeltPro Myco assay correctly identified all 51 common mycobacterial strains to the species/genus level and showed no cross-reactivity with 16 nonmycobacterial species. In contrast, existing systems exhibit extensive cross-reactivity among the target NTM species; for example, a sequencing-based system targeting a hypervariable segment of the 16S rRNA failed to differentiate NTM pairs, such as M. chelonae and M. abscessus, M. kansasii and M. gastri, and M. marinum and M. ulcerans, among others (20). Likewise, a DNA chip assay based on 16S rRNA listed 3 of the 17 NTM species as being cross-reactive with other species (21). A widely used membrane hybridization assay, the GenoType CMdirect version 1.0 (or GenoType Mycobacterium CM version 2.0), utilizes the 23S rRNA target region and had 7 of the 14 species showing cross-reactivity with other target NTM members (10). Even more cross-reactions were observed with the real-time PCR-based system Real Myco-ID, where 6 of 10 NTM species were cross-reactive among the target species (12).
The inclusion of M. bovis BCG in the MeltPro Myco assay could add clinical value, as M. bovis harbors natural resistance to pyrazinamide, a first-line drug used to treat M. tuberculosis. Distinguishing M. bovis from MTBC would prevent the unnecessary usage of pyrazinamide. Moreover, disseminated BCG infections mostly occur in patients with immune deficiencies after vaccination; thus, rapid diagnosis of BCG infections is critical due to its extremely high mortality rate (22).
The low LOD of the MeltPro Myco assay, i.e., 300 bacilli/ml, forms the basis for its application beyond cultured samples to potential smear-positive specimens that contain 5,000 to ∼10,000 bacilli/ml (23). Moreover, its capability in detecting 1% of minor species supports its applicability to mixed-infection samples, which are difficult, if not impossible, for Sanger sequencing.
Clinical evaluation further supported the accuracy of the MeltPro Myco assay in mycobacterial identification. Of all 1,163 isolates, the MeltPro Myco assay correctly identified 1,159 samples when Sanger sequencing was regarded as the gold standard. Three discordant samples detected as M. intracellulare proved to be M. marseillense, M. chimera, and M. avium complex, all of which are rare NTM species that belong to the M. avium complex (MAC) and were not included as identifiable species in the MeltPro Myco assay. These results indicate the cross-reactivity of M. intracellulare-specific probes with its MAC members. According to the American Thoracic Society (ATS) recommendation (6), the differentiation between MAC members is not yet clinically significant. There is no prognostic or treatment advantage for distinguishing MAC isolates into specific species. Thus, these three MAC species could be listed as M. intracellulare equivalents in the MeltPro Myco assay to some extent. However, currently reported multiple outbreaks of M. chimera infections associated with heater cooler units following cardiac surgery (24, 25) indicated that differentiating subspecies within the MAC would be important in the epidemiological survey. The last discordant sample detected as M. fortuitum was confirmed to be a mixed infection of M. fortuitum and M. senegalense; however, M. senegalense is beyond the identifiable species of the MeltPro Myco assay. Thus, the assay detection results were correct according to the interpretation guidelines.
The large number of samples employed for evaluating clinical specificity also provided information on the incidence rate and species distribution of NTM in China. As shown in Fig. 3, South China (Guangzhou and Xiamen) showed a higher incidence rate, with more NTM species (135/567 [23.8%]) than in north-central China (Henan, Heilongjiang, and Xinjiang; 23/596 [3.9%]). These preliminary data support the hypothesis that high humidity and temperature lead to an increased prevalence of NTM (26–28). The 1,163 isolates gave an overall incidence rate of 13.6% for NTM (including mixed infections), which was somewhat lower than that reported in the fifth national tuberculosis epidemiological survey conducted in 2010, where the incidence rate of NTM was 22.9% (29). This discrepancy could probably be attributed to different locations and sample sizes. It is worth noting that M. abscessus, a species of multidrug-resistant NTM, was the most common organism (14.3%) identified in southern China. Previously, M. abscessus was thought to be independently acquired by susceptible individuals from the environment. However, recent studies have shown that the majority of M. abscessus infections were acquired through transmission, potentially via vomit and aerosols (30). The high incidence rate of M. abscessus presents a challenge for prevention and control and highlights the importance of its identification. The MeltPro Myco assay could correctly identify all the M. abscessus samples. However, similar to current commercial assays, it could not differentiate the subspecies of M. abscessus, which are important in the development of treatment regimens (31).
Most existing molecular assays for mycobacterial identification restrict their use to clinical isolates rather than clinical samples (10–12). However, an additional and long culturing step is required to obtain these isolates. Unfortunately, there are no universal culture conditions for all NTM species. For example, NTM species, such as M. chelonae, M. marinum, and M. ulcerans, require low culture temperature (25 to 33°C), while M. ulcerans needs egg yolk for growth and also needs lengthy incubation, which will differentiate it from M. marinum. This drawback can be even more complex in cases where a sample consists of both fast- and slow-growing mycobacteria, as its composition can change along with the culture. These culture-derived problems also impair the performance of matrix-assisted laser desorption ionization–time of fight mass spectrometry (MALDI-TOF MS) systems developed for the identification of mycobacteria. In fact, the accuracy of MALDI-TOF MS systems varied with medium types, incubation periods, sample preparation, and repeat testing (32–35). More seriously, MALDI-TOF MS was unable to identify M. tuberculosis in the presence of polymicrobial culture (36). Apparently, all the above-mentioned problems could be overcome when clinical samples are simply detected without a culture step.
Taking this into account, the success of the MeltPro Myco assay in detecting 94 sputum samples has important implications. Despite the limited number of samples, the clinical samples gave richer information regarding NTM incidence in the real world than in cultured samples. Of the 94 smear-positive sputum specimens consecutively collected in Guangzhou Chest Hospital, 68 M. tuberculosis, 1 M. bovis, 21 NTM, and 2 mixed-infection samples, as well as 1 mycobacterial sample and 1 nonmycobacterial sample, were identified. In comparison, of the 378 clinical isolates consecutively collected in the same hospital, 251 M. tuberculosis, 116 NTM, and 11 mixed-infection samples were identified. The high incidence of NTM infections in this hospital, including mixed infections, detected in sputum (25.5%) was close to the results obtained from clinical isolates (33.6%). Similar incidences of mixed-infection detection were also found between sputum and clinical isolates (2.1% versus 2.9%). As in the clinical samples, M. abscessus was again the predominant NTM species, followed by M. intracellulare in the sputum samples. Despite these similarities, the diverse nature of the sputum samples could be seen with the detection of 1 M. bovis sample as well as 1 M. europaeum and 1 corynebacterium that were additionally detected in such a small sample size.
One limitation of this study was the lack of smear-negative specimens for evaluation. Smear microscopy is a routine and low-cost screening tool in a TB laboratory. A positive specimen can contain 5,000 to ∼10,000 bacilli/ml, which is well above the LOD (300 bacilli/ml) of the MeltPro Myco assay. Therefore, it is promising for the MeltPro assay to identify mycobacteria in the smear-negative specimens. Further studies are needed regarding the outcome and cost-effectiveness for the implementation of this assay into clinical practice.
Another limitation of this study is that some species covered by the MeltPro Myco assay were not detected among the clinical samples. This is mainly due to the limited sample types, which were restricted to respiratory samples. Further study is needed on those nonrespiratory sample types, such as tissues or pus, so that a comprehensive coverage of NTM species might be achieved.
The sustained increase in NTM infection incidence in recent years and the difficulty in distinguishing NTM infection from tuberculosis constitute an urgent need to identify NTM to the species level. In this regard, the MeltPro Myco assay is appropriate due to its rapidness, ease of use, and robustness. As the MeltPro Myco assay can also directly detect smear-positive specimens, it can even be implemented as a screening tool in regions endemic for tuberculosis.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant 81572068) and the Important National Science & Technology Specific project no. 2017ZX10302301.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01096-18.
REFERENCES
- 1.van Ingen J. 2013. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med 34:103–109. doi: 10.1055/s-0033-1333569. [DOI] [PubMed] [Google Scholar]
- 2.Field SK, Cowie RL. 2006. Lung disease due to the more common nontuberculous mycobacteria. Chest 129:1653–1672. doi: 10.1378/chest.129.6.1653. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann P, Tebruegge M, Curtis N, Ritz N. 2015. The management of non-tuberculous cervicofacial lymphadenitis in children: a systematic review and meta-analysis. J Infect 71:9–18. doi: 10.1016/j.jinf.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Wallace RJ Jr, Brown BA, Onyi GO. 1992. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis 166:405–412. doi: 10.1093/infdis/166.2.405. [DOI] [PubMed] [Google Scholar]
- 5.Varley CD, Ku JH, Henkle E, Schafer SD, Winthrop KL. 2017. Disseminated nontuberculous mycobacteria in HIV-infected patients, Oregon, USA, 2007–2012. Emerg Infect Dis 23:533–535. doi: 10.3201/eid2303.161708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 7.Nie W, Duan H, Huang H, Lu Y, Chu N. 2015. Species identification and clarithromycin susceptibility testing of 278 clinical nontuberculosis mycobacteria isolates. Biomed Res Int 2015:506598. doi: 10.1155/2015/506598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Li H, Jiang G, Zhao L, Ma Y, Javid B, Huang H. 2014. Prevalence and drug resistance of nontuberculous mycobacteria, northern China, 2008–2011. Emerg Infect Dis 20:1252–1253. doi: 10.3201/eid2007.131801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, Luo J, Huang H. 2016. The prevalence of non-tuberculous mycobacterial infections in mainland China: systematic review and meta-analysis. J Infect 73:558–567. doi: 10.1016/j.jinf.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Richter E, Rusch-Gerdes S, Hillemann D. 2006. Evaluation of the GenoType Mycobacterium assay for identification of mycobacterial species from cultures. J Clin Microbiol 44:1769–1775. doi: 10.1128/JCM.44.5.1769-1775.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tortoli E, Nanetti A, Piersimoni C, Cichero P, Farina C, Mucignat G, Scarparo C, Bartolini L, Valentini R, Nista D, Gesu G, Tosi CP, Crovatto M, Brusarosco G. 2001. Performance assessment of new multiplex probe assay for identification of mycobacteria. J Clin Microbiol 39:1079–1084. doi: 10.1128/JCM.39.3.1079-1084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HY, Kim H, Kim S, Kim DK, Cho SN, Lee H. 2015. Performance of a real-time PCR assay for the rapid identification of Mycobacterium species. J Microbiol 53:38–46. doi: 10.1007/s12275-015-4495-8. [DOI] [PubMed] [Google Scholar]
- 13.Huang Q, Liu Z, Liao Y, Chen X, Zhang Y, Li Q. 2011. Multiplex fluorescence melting curve analysis for mutation detection with dual-labeled, self-quenched probes. PLoS One 6:e19206. doi: 10.1371/journal.pone.0019206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, Wang X, Sha C, Xia Z, Huang Q, Li Q. 2013. Combination of fluorescence color and melting temperature as a two-dimensional label for homogeneous multiplex PCR detection. Nucleic Acids Res 41:e76. doi: 10.1093/nar/gkt004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakshi CS, Shah DH, Verma R, Singh RK, Malik M. 2005. Rapid differentiation of Mycobacterium bovis and Mycobacterium tuberculosis based on a 12.7-kb fragment by a single tube multiplex-PCR. Vet Microbiol 109:211–216. doi: 10.1016/j.vetmic.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Zeng X, Li H, Zheng R, Kurepina N, Kreiswirth BN, Zhao X, Xu Y, Li Q. 2016. Spoligotyping of Mycobacterium tuberculosis complex isolates by use of ligation-based amplification and melting curve analysis. J Clin Microbiol 54:2384–2387. doi: 10.1128/JCM.00857-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed AM, Kuyper DJ, Iwen PC, Ali HH, Bastola DR, Hinrichs SH. 2005. Computational approach involving use of the internal transcribed spacer 1 region for identification of Mycobacterium species. J Clin Microbiol 43:3811–3817. doi: 10.1128/JCM.43.8.3811-3817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin SJ, Lee BS, Koh WJ, Manning EJ, Anklam K, Sreevatsan S, Lambrecht RS, Collins MT. 2010. Efficient differentiation of Mycobacterium avium complex species and subspecies by use of five-target multiplex PCR. J Clin Microbiol 48:4057–4062. doi: 10.1128/JCM.00904-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae H, Han S, Kim S, Ki C, Huh H, Yong D, Koh W, Shin S. 2017. Development of a one-step multiplex PCR assay for differential detection of major Mycobacterium species. J Clin Microbiol 55:2736–2751. doi: 10.1128/JCM.00549-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cloud JL, Neal H, Rosenberry R, Turenne CY, Jama M, Hillyard DR, Carroll KC. 2002. Identification of Mycobacterium spp. by using a commercial 16S ribosomal DNA sequencing kit and additional sequencing libraries. J Clin Microbiol 40:400–406. doi: 10.1128/JCM.40.2.400-406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Yue J, Yan Z, Han M, Han Z, Jin L, Zhao Y. 2012. Performance assessment of the CapitalBio mycobacterium identification array system for identification of mycobacteria. J Clin Microbiol 50:76–80. doi: 10.1128/JCM.00320-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbot EA, Perkins MD, Silva SF, Frothingham R. 1997. Disseminated bacille Calmette-Guérin disease after vaccination: case report and review. Clin Infect Dis 24:1139–1146. doi: 10.1086/513642. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 24.Scriven J, Scobie A, Verlander N, Houston A, Collyns T, Cajic V, Kon O, Mitchell T, Rahama O, Robinson A, Withama S, Wilson P, Maxwell D, Agranoff D, Davies E, Llewelyn M, Soo S, Sahota A, Cooper M, Hunter M, Tomlins J, Tiberi S, Kendall S, Dedicoat M, Alexander E, Fenech T, Zambon M, Lamagni T, Smith E, Chand M. 2018. Mycobacterium chimaera infection following cardiac surgery in the United Kingdom: clinical features and outcome of the first 30 cases. Clin Microbiol Infect 24:1164–1170. doi: 10.1016/j.cmi.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 25.van Ingen J, Kohl T, Kranzer K, Hasse B, Keller P, Katarzyna Szafrańska A, Hillemann D, Chand M, Schreiber P, Sommerstein R, Berger C, Genoni M, Rüegg C, Troillet N, Widmer A, Becker S, Herrmann M, Eckmanns T, Haller S, Höller C, Debast S, Wolfhagen M, Hopman J, Kluytmans J, Langelaar M, Notermans D, Ten Oever J, van den Barselaar P, Vonk A, Vos M, Ahmed N, Brown T, Crook D, Lamagni T, Phin N, Smith E, Zambon M, Serr A, Götting T, Ebner W, Thürmer A, Utpatel C, Spröer C, Bunk B, Nübel U, Bloemberg G, Böttger E, Niemann S, Wagner D, Sax H. 2017. Global outbreak of severe Mycobacterium chimaera disease after cardiac surgery: a molecular epidemiological study. Lancet Infect Dis 17:1033–1041. doi: 10.1016/S1473-3099(17)30324-9. [DOI] [PubMed] [Google Scholar]
- 26.Falkinham JO., III. 2015. Environmental sources of nontuberculous mycobacteria. Clin Chest Med 36:35–41. doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Falkinham JO., III. 2009. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol 107:356–367. doi: 10.1111/j.1365-2672.2009.04161.x. [DOI] [PubMed] [Google Scholar]
- 28.van Ingen J, Boeree MJ, Dekhuijzen PN, van Soolingen D. 2009. Environmental sources of rapid growing nontuberculous mycobacteria causing disease in humans. Clin Microbiol Infect 15:888–893. doi: 10.1111/j.1469-0691.2009.03013.x. [DOI] [PubMed] [Google Scholar]
- 29.Technical Guidance Group of the Fifth National TB Epidemiological Survey. 2012. The fifth national tuberculosis epidemiological survey in 2010. Chin J Antituber 348:485–508. [Google Scholar]
- 30.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, Jabbour N, Johnson GR, Knibbs LD, Morawska L, Sly PD, Jones A, Bilton D, Laurenson I, Ruddy M, Bourke S, Bowler IC, Chapman SJ, Clayton A, Cullen M, Daniels T, Dempsey O, Denton M, Desai M, Drew RJ, Edenborough F, Evans J, Folb J, Humphrey H, Isalska B, Jensen-Fangel S, Jonsson B, Jones AM, et al. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastian S, Veziris N, Roux A, Brossier F, Gaillard J, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y, Wang L, Ma P, Fan W, Gu B, Ju S. 2018. Accuracy of matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of mycobacteria: a systematic review and meta-analysis. Sci Rep 8:4131. doi: 10.1038/s41598-018-22642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotz A, Ferroni A, Beretti JL, Dauphin B, Carbonnelle E, Guet-Revillet H, Veziris N, Heym B, Jarlier V, Gaillard JL, Pierre-Audigier C, Frapy E, Berche P, Nassif X, Bille E. 2010. Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 48:4481–4486. doi: 10.1128/JCM.01397-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forbes BA, Hall GS, Miller MB, Novak SM, Rowlinson MC, Salfinger M, Somoskovi A, Warshauer DM, Wilson ML. 2018. Practice guidelines for clinical microbiology laboratories: mycobacteria. Clin Microbiol Rev 31:e00038-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Eck K, Faro D, Wattenberg M, de Jong A, Kuipers S, van Ingen J. 2016. Matrix-assisted laser desorption ionization–time of flight mass spectrometry fails to identify nontuberculous mycobacteria from primary cultures of respiratory samples. J Clin Microbiol 54:1915–1917. doi: 10.1128/JCM.00304-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang TS, Lee CC, Tu HZ, Lee SS. 2018. Rapid identification of mycobacteria from positive MGIT broths of primary cultures by MALDI-TOF mass spectrometry. PLoS One 13:e0192291. doi: 10.1371/journal.pone.0192291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.