Over the past ten years, standard diagnostics for cryptococcal meningitis in HIV-infected persons have evolved from culture to India ink to detection of cryptococcal antigen (CrAg), with the recent development and distribution of a point-of-care lateral flow assay. This assay is highly sensitive and specific in cerebrospinal fluid (CSF), but is also sensitive in the blood to detect CrAg prior to meningitis symptoms.
KEYWORDS: Cryptococcus, diagnostics, point-of-care, prevention
ABSTRACT
Over the past ten years, standard diagnostics for cryptococcal meningitis in HIV-infected persons have evolved from culture to India ink to detection of cryptococcal antigen (CrAg), with the recent development and distribution of a point-of-care lateral flow assay. This assay is highly sensitive and specific in cerebrospinal fluid (CSF), but is also sensitive in the blood to detect CrAg prior to meningitis symptoms. CrAg screening of HIV-infected persons in the blood prior to development of fulminant meningitis and preemptive treatment for CrAg-positive persons are recommended by the World Health Organization and many national HIV guidelines. Thus, CrAg testing is occurring more widely, especially in resource-limited laboratory settings. CrAg titer predicts meningitis and death and could be used in the future to customize therapy according to burden of infection.
INTRODUCTION
Globally cryptococcal meningitis causes 15% of AIDS-related deaths, with an estimated 181,100 deaths annually (1). HIV-infected persons with advanced HIV disease are at highest risk of infection. While efforts are under way to increase access to antiretroviral therapy (ART) globally, persons who do not start or who default ART remain at risk of cryptococcal meningitis (2). The majority of cases of cryptococcal meningitis occur in sub-Saharan Africa, where diagnostic facilities, access to optimal antifungal medications, and access to intensive hospital-based treatments are limited (3). Thus, 6-month mortality from cryptococcal meningitis in hospital settings, despite standard-of-care antifungal therapy, ranges from 40% to 60% in resource-limited settings (2, 4). Over the last 10 years, drastic improvements in the development and distribution of a point-of-care lateral flow assay (LFA) for cryptococcal antigen (CrAg) have dramatically improved prevention efforts and diagnosis of this lethal infection.
DIAGNOSTICS FOR CRYPTOCOCCAL MENINGITIS
Diagnosis of cryptococcal meningitis requires cerebrospinal fluid (CSF) culture, India ink, or CrAg testing.
Culture.
CSF culture is considered the gold standard for diagnosis of cryptococcal meningitis. Unfortunately, diagnosis can take days, and up to 1 to 2 weeks for definitive results. Thus, other diagnostic methods have been used to expedite diagnosis and treatment. In research settings, quantitative CSF cultures have been utilized which provide valuable clinical information (5). These CSF quantitative cultures are easily performed in any microbiology laboratory. The simple technique uses a 100-μl input volume of CSF with five 1:10 serial dilutions in water, plating on Sabouraud dextrose agar, and then quantitative culture counting on the plate with the least growth (5). Increasing quantitative culture burden is a risk factor for 2-week mortality, with an ∼40% increase in odds of mortality per log10 CFU/ml CSF increase in Cryptococcus growth (6). The change in quantitative culture growth with subsequent serial lumbar punctures gives clinicians valuable feedback on the rate of CSF Cryptococcus clearance and on when the CSF is likely to be sterile.
India ink.
India ink microscopy has historically been a quick, low-resource method to detect Cryptococcus in the CSF (7). The stain fills the background field, but it is not taken up by the thick Cryptococcus capsule, forming a halo of light by which it can be visualized using a light microscope. While simple and readily accessible in resource-limited settings, where the burden of cryptococcal infection is the greatest, unfortunately, sensitivity is low (86% in expert hands), meaning that 1 in 7 diagnoses are missed by India ink microscopy (8). For persons presenting early in disease process with lower burden of infection, India ink’s sensitivity is only 42% when the CSF Cryptococcus CFU value is <1,000 per ml of CSF (8). CSF centrifugation can likely increase the sensitivity of microscopy; however, microscopy is less sensitive than testing for CrAg (8).
Cryptococcal antigen.
CrAg can be identified using latex agglutination, which historically has a sensitivity and specificity of >99% in blood and CSF (8). More recent comparisons have reported sensitivities of 97% to 98% and specificities of 86% to 100%, dependent on the specific manufacturer (8). Results can be qualitative, or semiquantitative with titers by 1:2 serial dilution. The latex agglutination test detects polysaccharide antigens of the Cryptococcus capsule, but the process of performing the test requires testing in a laboratory environment, and thus skilled laboratory workers, steady electricity, heat inactivation, cold-chain shipping, and refrigeration of reagents (9). While feasible in high-income country laboratories, these requirements are frequently prohibitive in countries where the majority of cryptococcal infection occurs. The major expense of the test in high-income countries is laboratory labor. In low-income countries, the major expense is cold-chain shipping and storage. Thus, clinicians historically have relied on India ink for diagnosis in low-income countries, despite its lower sensitivity. Overall, CrAg latex agglutination is now an archaic test, as latex agglutination is more expensive, more labor-intensive, less sensitive, less specific, and requires cold-chain shipping/storage (8).
The CrAg lateral flow assay (LFA), approved by the U.S. Food and Drug Administration (FDA) in 2011 (Immy, Norman, OK), is an immunochromatographic dipstick assay that also detects antigen with qualitative or semiquantitative results (9). If CrAg is present in the drop of serum, plasma, or CSF sample, it will bind to the gold-conjugated, anticryptococcal antibodies on the test strip to cause a visible line. This FDA-approved point-of-care dipstick test has changed the diagnostic landscape of cryptococcal meningitis, as it does not require laboratory infrastructure. Semiskilled health care workers without laboratory training can perform this test in clinics or at the patients’ bedsides. No refrigeration is required, and results are available after 10 min. Conversely, laboratory-based CrAg testing using latex agglutination takes approximately 5 h from test ordering to availability of results in high-income settings (8).
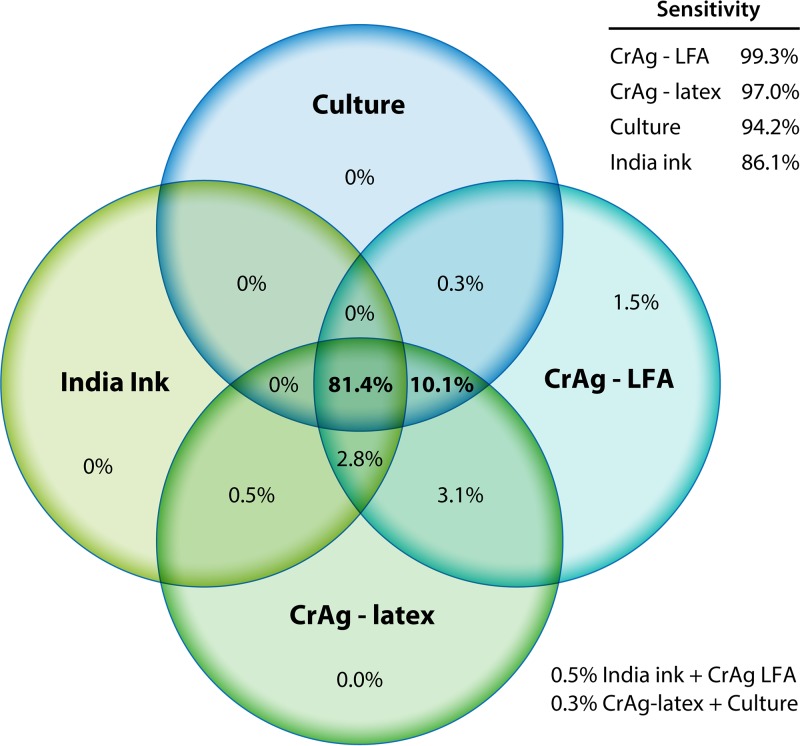
In a large validation study in South Africa and Uganda, 832 HIV-infected persons underwent diagnostic testing for cryptococcal meningitis. The CrAg LFA performed best, with a sensitivity of 99.3% and specificity of 99.1% for CSF (Fig. 1). CrAg testing by either latex agglutination or LFA was more sensitive than CSF culture, which has historically been considered the gold standard for diagnosis. Importantly, the LFA identified 6 additional persons with cryptococcal meningitis that were not detected by any other means (8).
FIG 1.
Venn diagram of distribution of CSF diagnostic testing in Uganda and South Africa during 2006 to 2012 (n = 832) (8).
In 2018, there are five manufacturers of CrAg LFAs. The first CrAg LFA by Immy is FDA approved in the United States and European Conformity (CE)-marked in Europe, and it has been used among hundreds of thousands of persons globally, with large multisite validation studies. Three other CrAg LFAs have more limited validation data, and none are FDA-approved. Second is the Biosynex CryptoPS LFA (Biosynex, Paris, France), which is CE-marked in Europe. This CryptoPS LFA is also marketed by Bio-Rad (Hercules, CA). To date, a single-site validation study has been performed, testing 186 serum/plasma and 23 CSF samples from Cameroon (10). In comparison to the FDA-approved Immy CrAg LFA, the CryptoPS LFA had 78% (11/14) sensitivity in serum, 92% (11/12) in plasma, and 100% (4/4) in CSF (10). Specificity was excellent (100%) in all sample types (10). In the initial validation study, among serum specimens positive by Immy CrAg LFA with Immy titers of <1:100, only 2 of 5 were positive by the Biosynex, missing titers positive at 1:10 and 1:20 dilution using the Immy LFA (10). More validation is needed among serum specimens with low titers and among CSF specimens.
A third test is the StrongStep (Liming Bio, China). In a two-site validation study in Uganda, 143 CSF and 167 plasma samples were tested in comparison to the Immy CrAg LFA and CSF culture (11). The StrongStep test performed well in CSF, with 100% (101/101) sensitivity and 98% (41/42) specificity (11). However, the specificity was only 90% (101/112) in plasma (11), with 98% sensitivity (54/55). The limited specificity of plasma in the setting of CrAg screening and the 9% CrAg prevalence rate equate to a positive predictive value of only 50% (11). This test appears to be highly sensitive; however, there are substantial challenges with specificity (11).
Two other manufactured tests exist. The Dynamiker CrAg LFA and FungiXpert Cryptococcal Capsular Polysaccharide K-Set are manufactured in China. No published validation studies exist for either test, although Dynamiker had clinical validation studies ongoing in 2018. Neither test is approved in Europe or the United States.
CrAg testing in the blood to detect meningitis.
Management of cryptococcal meningitis is complex and resource intensive. Specifically, persons with cryptococcal meningitis frequently have elevated intracranial pressure caused by the large polysaccharide capsule of the cryptococcal organism plugging the arachnoid villa and obstructing CSF outflow (12). This elevated intracranial pressure is associated with increased mortality (13). Therapeutic lumbar punctures are therefore needed to release this pressure and reduce mortality in persons with cryptococcal meningitis (14, 15). In resource-limited settings, where access to lumbar punctures is difficult, combining the diagnostic lumbar puncture with the first therapeutic lumbar puncture would streamline care (16). Thus, using peripheral blood for a rapid point-of-care diagnosis of cryptococcal meningitis allows clinicians to remove large amounts of CSF and reduce intracranial pressure with the first lumbar puncture, as well as to confirm the diagnosis.
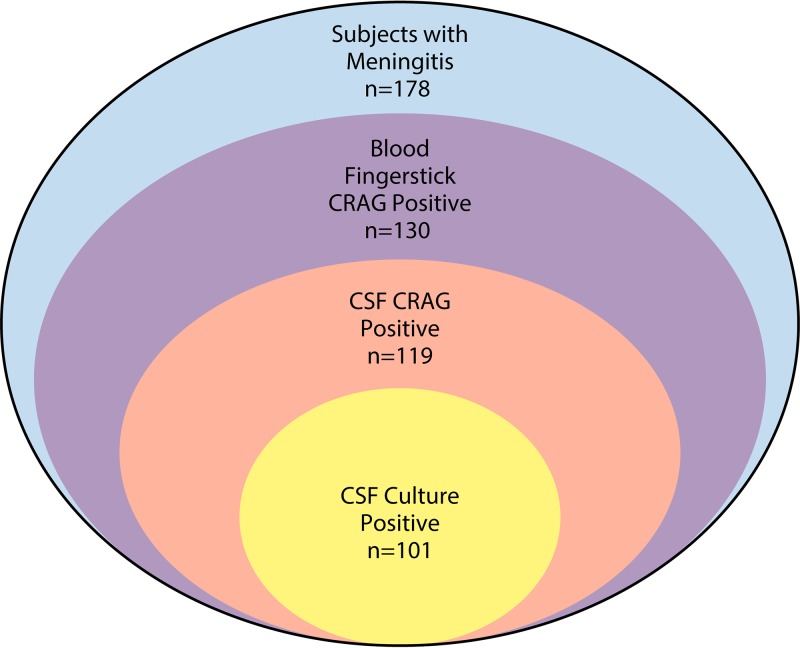
Performance of the CrAg LFA as a fingerstick point-of-care test has been evaluated in this context (16). Specifically, for those with suspected meningitis, a point-of-care fingerstick CrAg LFA was performed prior to lumbar puncture and compared to subsequent plasma and CSF CrAg LFA. The positive predictive value of fingerstick LFA for the detection of CrAg in the blood was 100%, with 93% detection for cryptococcal meningitis (Fig. 2). Those (7%) that had a positive fingerstick but negative CSF CrAg were found to have positive serum/plasma CrAg; thus, these were not false positives but actual cryptococcal infection in the blood. Fingerstick results had 100% concordance with serum or plasma CrAg results, and 100% negative predictive value for excluding cryptococcal meningitis. Fingerstick CrAg testing does have limitations in asymptomatic populations with low fungal burdens, where false negatives can occur in comparison to serum or plasma testing (17). Pipetting fingerstick whole blood onto the LFA improves diagnostic performance over direct application of blood to the CrAg LFA sample pad (17).
FIG 2.
Venn diagram of the distribution of positivity by blood CrAg, CSF CrAg, and CSF culture (16).
Other diagnostics.
Other available diagnostics in high-income country settings include PCR. The FilmArray meningitis/encephalitis panel (Biofire, Salt Lake City, UT) is a multiplex PCR assay that detects 14 meningitis-causing pathogens (bacteria, viruses, and fungi), including Cryptococcus. FilmArray PCR detected Cryptococcus in 96% (95% confidence interval [CI], 83% to 99%) of CSF when there were >100 Cryptococcus CFU per ml of CSF, and specificity was 100% (18). The expense of multiplex PCR does not make this an ideal cryptococcal assay, but the multiplex component does make this very nice as an overall meningitis assay for common causes of community-acquired meningitis. As well, matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF) has also been reported to detect Cryptococcus in clinical specimens (19).
CrAg-BASED SCREENING FOR CRYPTOCOCCAL MENINGITIS
Early cryptococcal meningitis.
The majority of persons with cryptococcal meningitis present with signs and symptoms of meningitis (headache, neck stiffness, and fever), and are found to be positive for CSF CrAg by diagnostic lumbar punctures and peripheral blood CrAg through fingerstick or venipuncture. However, there is a population with symptoms of meningitis who are negative for CSF CrAg and negative by CSF culture but CrAg positive in the blood (20). In one cohort in Uganda, this represented 4.3% of those HIV-infected individuals with suspected meningitis. This population (blood CrAg positive, with symptoms of meningitis, but CSF CrAg negative) was presumed to have early cryptococcal central nervous system (CNS) infection, and in-hospital mortality was 39%, which was similar to the in-hospital mortality of those with fulminant cryptococcal meningitis (32%). Larger studies are needed to further characterize the natural history of this population.
Cryptococcal antigen screening.
CrAg is detectable in blood weeks to months before onset of meningitis symptoms (21). Prevalence of asymptomatic cryptococcal antigenemia varies from 1% to 15% among HIV-infected persons with advanced HIV disease (1, 22). In high-income countries, the average prevalence of asymptomatic CrAg is 2.6% (1). Asymptomatic CrAg positivity is an independent predictor of meningitis and death (21, 23). Preemptively treating those with cryptococcal antigenemia with high-dose fluconazole before symptoms of meningitis develop prevents mortality. This has been evaluated most rigorously in a randomized controlled trial of 2,000 persons with advanced HIV disease in sub-Saharan Africa, which demonstrated a 28% survival benefit with CrAg screening and preemptive treatment, along with adherence support (24). Given this clear survival benefit, the World Health Organization and numerous national HIV guidelines now recommend CrAg screening for those with advanced HIV disease and preemptively treating those who are CrAg positive with high-dose fluconazole (25).
CrAg titer.
Both latex agglutination and CrAg LFA can be semiquantified using titers. CrAg LFA titers are performing by the same serial dilution, and the titer is the last positive test before the dilution turns negative. Titers across LFA manufacturers are not comparable, and even the titer between the Immy latex agglutination test and Immy LFA are not comparable; the median difference was 2.5-fold (interquartile range [IQR], 1.25- to 5-fold) higher with the CrAg LFA (8). This difference in titer confirms the better analytical sensitivity of the CrAg LFA over latex agglutination, but the titer difference is variable.
CrAg titer is predictive of meningitis and death (26–29). Plasma CrAg titers of ≤1:80 by Immy CrAg have an exceedingly low probability of meningitis (26, 27). As serum or plasma CrAg titers rise from 1:160 to 1:320 to 1:640, the probability of CSF involvement increases (27). CrAg titers of ≥1:1,280 have near-universal central nervous system (CNS) involvement (26, 27). The Biosynex CrAg LFA provides a semiquantification with both low- and high-titer test lines. The high CrAg titer band equates to approximately 1:1,024 Immy CrAg LFA titer.
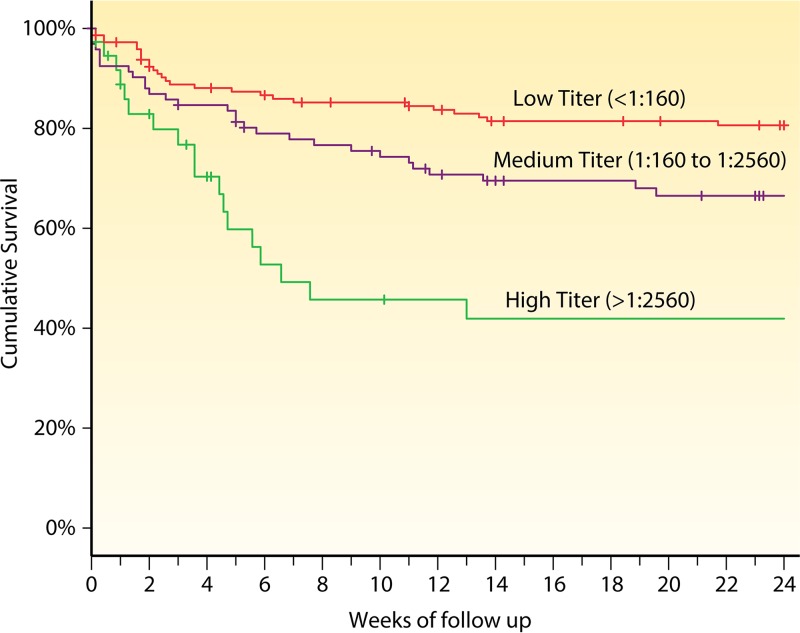
There have been four published cohorts of asymptomatic CrAg-positive persons investigating CrAg titer versus outcome, each relatively small (26–29). We combined these cohorts to summarize the effect of CrAg titer on survival when preemptive fluconazole monotherapy is given to CrAg-positive persons. Of 415 records, 287 had plasma CrAg titers measured at time of starting fluconazole. Survival was measured for those with low CrAg titers (<1:160), medium titers (1:160 to 1:2,560), and high titers (>1:2,560). Survival decreased as the plasma CrAg titer increased (log-rank P < 0.0001) (Fig. 3). Among asymptomatic CrAg-positive persons, CrAg titers of ≥1:160 are associated with increased mortality despite receiving fluconazole preemptive therapy (28, 29).
FIG 3.
Survival by CrAg titer in 287 asymptomatic HIV-infected persons with cryptococcal antigenemia in four cohorts in Ethiopia, South Africa, Tanzania, and Uganda (26, 27, 29, 32).
How to screen for CrAg.
There are two possible methods for implementing CrAg screening into laboratories. The first is reflexive laboratory testing. With this method, those with a CD4 cell count result of ≤100 cells/μl would routinely have a CrAg test performed on the remaining plasma specimen from the CD4 test. Thus, the ordering provider would not be responsible for initiating this test, but testing would occur via a laboratory protocol. The result would be presented with the CD4 lab test result, with a brief explanation of what to do if the CrAg result is positive.
The alternative is to depend on health care providers to order a CrAg test when a CD4 lab value returns as ≤100 cells/μl. While a seemingly simple task, in settings that are already overburdened and understaffed, asking providers to remember to order this lab test has proved challenging. In one South African evaluation, only 27% of eligible persons were CrAg screened using a provider-initiated approach (30). Conversely, with reflex laboratory testing, >95% of eligible persons were screened (30). Additionally, by having a provider order the test, there is a delay in testing. A new CrAg-positive result in an untreated patient is a critical laboratory result, requiring urgent action to prevent progression to meningitis and death.
The difficulties with laboratory-based screening are that there is often a delay between receipt of the lab result and bringing the patient back to the clinic for results and potential treatment. Conversely, with provider-initiated screening, there is less uptake of initial screening, but if done in the clinic room with the patient, the results are potentially available in 10 min, and the patient can initiate therapy immediately, if needed. In the United States, CrAg testing is not a U.S. Clinical Laboratory Improvement Amendments (CLIA)-waived test, so provider point-of-care testing would not be allowable. In most high-prevalence settings where CrAg screening occurs, a reflexive laboratory-based approach has been adopted. South Africa performs reflexive CrAg screening at national laboratories where CD4 testing is performed.
Future implications and conclusions.
Given the importance of CrAg titer in predicting meningitis and/or death, CrAg titer will likely be used in the future to customize therapy both for prevention and treatment of cryptococcal meningitis. For example, if someone is found to be asymptomatic with a low CrAg titer (<1:160), they could be treated with fluconazole preemptive therapy, per current standard of care. However, given the high mortality despite high-dose fluconazole in asymptomatic persons with a high CrAg titer, more intensive therapies should be evaluated to improve survival. Such therapies may include liposomal amphotericin and/or flucytosine. It is also possible that, in those with fulminant meningitis, those with high titers may benefit from longer duration of therapy compared to those with low titers. Thus, titer will likely play a significant role in the management of cryptococcal infection, both in low-income areas and in high-income settings. Infectious Diseases Society of America (IDSA) guidelines currently recommend 4 weeks of amphotericin for those with cryptococcal meningitis without HIV infection. This recommendation is based on no empirical data. Evaluation of how to shorten duration of therapy based on burden of infection (i.e., titer or quantitative culture) would be novel and would spare patients exposure to toxic antifungal therapy.
In the last 20 years, cryptococcal testing has progressed from culture, which takes days for results, and thereby is clinically unhelpful with initial diagnosis, to India ink, which is technically easy and quick, but with poor sensitivity, to a highly sensitive and specific point-of-care lateral flow assay that can be done at the patient bedside for rapid diagnosis. This evolution has greatly improved meningitis diagnosis and expedited initiation of effective treatment. Furthermore, the cost of $2.50 to $3.00 for the Immy CrAg LFA in resource-limited settings has made screening for cryptococcal infection a cost-effective strategy to prevent meningitis and death (9, 31). CrAg titer predicts meningitis and death. Future areas of research include evaluation of customized therapy according to titer in persons with cryptococcal infection. The evolution of cryptococcal diagnostics highlights the enormous impact of point-of-care diagnostics in enhancing medical care and public health programs.
REFERENCES
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhein J, Hullsiek KH, Evans EE, Tugume L, Nuwagira E, Ssebambulidde K, Kiggundu R, Mpoza E, Musubire AK, Bangdiwala AS, Bahr NC, Williams DA, Abassi M, Muzoora C, Meya DB, Boulware DR. 2018. Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis 5:ofy122. doi: 10.1093/ofid/ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oladele RO, Bongomin F, Gago S, Denning DW. 2017. HIV-associated cryptococcal disease in resource-limited settings: a case for “prevention is better than cure”? J Fungi (Basel) 3:E67. doi: 10.3390/jof3040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler EK, Boulware DR, Bohjanen PR, Meya DB. 2012. Long term 5-year survival of persons with cryptococcal meningitis or asymptomatic subclinical antigenemia in Uganda. PLoS One 7:e51291. doi: 10.1371/journal.pone.0051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyal J, Akampurira A, Rhein J, Morawski BM, Kiggundu R, Nabeta HW, Musubire AK, Bahr NC, Williams DA, Bicanic T, Larsen RA, Meya DB, Boulware DR. 2016. Reproducibility of CSF quantitative culture methods for estimating rate of clearance in cryptococcal meningitis. Med Mycol 54:361–369. doi: 10.1093/mmy/myv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, Longley N, Muzoora C, Phulusa J, Taseera K, Kanyembe C, Wilson D, Hosseinipour MC, Brouwer AE, Limmathurotsakul D, White N, van der Horst C, Wood R, Meintjes G, Bradley J, Jaffar S, Harrison T. 2014. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 58:736–745. doi: 10.1093/cid/cit794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kambugu A, Meya DB, Rhein J, O'Brien M, Janoff EN, Ronald AR, Kamya MR, Mayanja-Kizza H, Sande MA, Bohjanen PR, Boulware DR. 2008. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis 46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulware DR, Rolfes MA, Rajasingham R, von Hohenberg M, Qin Z, Taseera K, Schutz C, Kwizera R, Butler EK, Meintjes G, Muzoora C, Bischof JC, Meya DB. 2014. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis 20:45–53. doi: 10.3201/eid2001.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajasingham R, Meya DB, Boulware DR. 2012. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J Acquir Immune Defic Syndr 59:e85–e91. doi: 10.1097/QAI.0b013e31824c837e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Temfack E, Kouanfack C, Mossiang L, Loyse A, Fonkoua MC, Molloy SF, Koulla-Shiro S, Delaporte E, Dromer F, Harrison T, Lortholary O. 2018. Cryptococcal antigen screening in asymptomatic HIV-infected antiretroviral naive patients in Cameroon and evaluation of the new semi-quantitative Biosynex CryptoPS test. Front Microbiol 9:409. doi: 10.3389/fmicb.2018.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mpoza E, Mukaremera L, Kundura DA, Akampurira A, Luggya T, Tadeo KK, Pastick KA, Bridge SC, Tugume L, Kiggundu R, Musubire AK, Williams DA, Muzoora C, Nalintya E, Rajasingham R, Rhein J, Boulware DR, Meya DB, Abassi M. 2018. Evaluation of a point-of-care immunoassay test kit ‘StrongStep’ for cryptococcal antigen detection. PLoS One 13:e0190652. doi: 10.1371/journal.pone.0190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson EJ, Najjuka G, Rolfes MA, Akampurira A, Jain N, Anantharanjit J, von Hohenberg M, Tassieri M, Carlsson A, Meya DB, Harrison TS, Fries BC, Boulware DR, Bicanic T. 2014. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J Infect Dis 209:74–82. doi: 10.1093/infdis/jit435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graybill JR, Sobel J, Saag M, van Der Horst C, Powderly W, Cloud G, Riser L, Hamill R, Dismukes W, The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. 2000. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. Clin Infect Dis 30:47–54. doi: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 14.Rolfes MA, Hullsiek KH, Rhein J, Nabeta HW, Taseera K, Schutz C, Musubire A, Rajasingham R, Williams DA, Thienemann F, Muzoora C, Meintjes G, Meya DB, Boulware DR. 2014. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis 59:1607–1614. doi: 10.1093/cid/ciu596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DA, Kiiza T, Kwizera R, Kiggundu R, Velamakanni S, Meya DB, Rhein J, Boulware DR. 2015. Evaluation of fingerstick cryptococcal antigen lateral flow assay in HIV-infected persons: a diagnostic accuracy study. Clin Infect Dis 61:464–467. doi: 10.1093/cid/civ263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wake RM, Jarvis JN, Harrison TS, Govender NP. 2018. Brief report: Point of care cryptococcal antigen screening: pipetting finger-prick blood improves performance of immunomycologics lateral flow assay. J Acquir Immune Defic Syndr 78:574–578. doi: 10.1097/QAI.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhein J, Bahr NC, Hemmert AC, Cloud JL, Bellamkonda S, Oswald C, Lo E, Nabeta H, Kiggundu R, Akampurira A, Musubire A, Williams DA, Meya DB, Boulware DR. 2016. Diagnostic performance of a multiplex PCR assay for meningitis in an HIV-infected population in Uganda. Diagn. Microbiol Infect Dis 84:268–273. doi: 10.1016/j.diagmicrobio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarumoto N, Sakai J, Kodana M, Kawamura T, Ohno H, Maesaki S. 2016. Identification of disseminated cryptococcosis using MALDI-TOF MS and clinical evaluation. Med Mycol J 57:E41–E46. doi: 10.3314/mmj.16-00002. [DOI] [PubMed] [Google Scholar]
- 20.Kenneth S, Bangdiwala A, Kwizera R, Kandole TK, Tugume L, Kiggundu R, Mpoza E, Nuwagira E, Williams DA, Lofgren SM, Abassi M, Musubire AK, Cresswell FV, Rhein J, Muzoora C, Hullsiek KH, Boulware DR, Meya DB, ASTRO-CM team. 2018. Symptomatic cryptococcal antigenemia presenting as early cryptococcal meningitis. Clin Infect Dis doi: 10.1093/cid/ciy817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, Downing R, Coutinho A, Mermin J. 2007. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health 12:929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 22.Ford N, Shubber Z, Jarvis JN, Chiller T, Greene G, Migone C, Vitoria M, Doherty M, Meintjes G. 2018. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: a systematic review and meta-analysis. Clin Infect Dis 66:S152–s159. doi: 10.1093/cid/cix1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, Kamya MR, Bohjanen PR, Boulware DR. 2010. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count ≤100 cells/μL who start HIV therapy in resource-limited settings. Clin Infect Dis 51:448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, Chijoka C, Masasi A, Kimaro G, Ngowi B, Kahwa A, Mwaba P, Harrison TS, Egwaga S, Jaffar S. 2015. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet doi: 10.1016/s0140-6736(15)60164-7. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2018. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/guidelines/cryptococcal-disease/en/. [PubMed] [Google Scholar]
- 26.Beyene T, Zewde AG, Balcha A, Hirpo B, Yitbarik T, Gebissa T, Rajasingham R, Boulware DR. 2017. Inadequacy of high-dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)-positive human immunodeficiency virus-infected persons in an Ethiopian CrAg screening program. Clin Infect Dis 65:2126–2129. doi: 10.1093/cid/cix613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, Nel JS, Mashamaite S, Adelekan A, Chiller TM, Jarvis JN, Harrison TS, Govender NP. 2018. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among human immunodeficiency virus-infected patients. Clin Infect Dis 66:686–692. doi: 10.1093/cid/cix872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morawski BM, Boulware DR, Nalintya E, Kiragga A, Kakooza F, Rajasingham R, Park BJ, Manabe Y, Kaplan JE, Meya DB. 2016. Pre-ART cryptococcal antigen titer associated with preemptive fluconazole failure, abstr 159. Abstr Conference on Retroviruses and Opportunistic Infections. Boston, Massachusetts, USA. [Google Scholar]
- 29.Letang E, Muller MC, Ntamatungiro AJ, Kimera N, Faini D, Furrer H, Battegay M, Tanner M, Hatz C, Boulware DR, Glass TR. 2015. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis 2:ofv046. doi: 10.1093/ofid/ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallabhaneni S, Longley N, Smith M, Smith R, Osler M, Kelly N, Cross A, Boulle A, Meintjes G, Govender NP. 2016. Implementation and operational research: evaluation of a public-sector, provider-initiated cryptococcal antigen screening and treatment program, Western Cape, South Africa. J Acquir Immune Defic Syndr 72:e37–e42. doi: 10.1097/QAI.0000000000000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassim N, Schnippel K, Coetzee LM, Glencross DK. 2017. Establishing a cost-per-result of laboratory-based, reflex cryptococcal antigenaemia screening (CrAg) in HIV+ patients with CD4 counts less than 100 cells/μl using a lateral flow assay (LFA) at a typical busy CD4 laboratory in South Africa. PLoS One 12:e0171675. doi: 10.1371/journal.pone.0171675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nalintya E, Meya DB, Lofgren S, Huppler Hullsiek K, Boulware DR, Rajasingham R. 2018. A prospective evaluation of a multisite cryptococcal screening and treatment program in HIV clinics in Uganda. J Acquir Immune Defic Syndr 78:231–238. doi: 10.1097/QAI.0000000000001669. [DOI] [PMC free article] [PubMed] [Google Scholar]