Manual treponemal and nontreponemal serologic testing has historically been used for the diagnosis of syphilis. This approach is simple and reproducible but labor intensive.
KEYWORDS: syphilis, BioPlex, RPR, FTA, traditional algorithm
ABSTRACT
Manual treponemal and nontreponemal serologic testing has historically been used for the diagnosis of syphilis. This approach is simple and reproducible but labor intensive. Recently, the FDA cleared the fully automated BioPlex 2200 Syphilis Total & RPR assay for the detection of treponemal and nontreponemal antibodies. We evaluated the clinical performance of this assay at a tertiary medical center with a high syphilis prevalence. Prospective consecutively collected (n = 400) and known RPR-positive (n = 100) specimens were compared using predicate manual rapid plasma reagin (RPR) and fluorescent treponemal antibody absorption (FTA) methods and the BioPlex 2200 Syphilis Total & RPR assay. Positive and negative percent agreements (PPA and NPA, respectively) between the assays were calculated. The PPA and NPA between the manual and BioPlex 2200 RPR results for the prospective population were 85% (17/20; 95% confidence interval [CI], 69% to 100%) and 98% (373/380; 95% CI, 97% to 99%), respectively. The PPA for the manual RPR-positive population was 88% (88/100; 95% CI, 82% to 94%). Overall, the manual and BioPlex 2200 RPR titers demonstrated 78% (99/127) concordance within ±1 dilution and 94% (120/127) within ±2 dilutions. An interpretation of the syphilis serologic profile using the traditional algorithm showed a concordance of 99.5% in the prospective population and 85% in the manual RPR-positive cohort. The performance of the BioPlex 2200 Syphilis Total & RPR assay is comparable to those of manual methods. The high NPA of this assay combined with the ability to automate a historically labor-intensive assay is an appealing attribute for syphilis screening in a high-volume laboratory.
INTRODUCTION
Syphilis, which is caused by the bacterium Treponema pallidum subspecies pallidum, is a major cause of genital ulcerative disease and other manifestations worldwide. The sexually transmitted infection is divided into primary, secondary, or tertiary stages that depend on clinical manifestations (1). Asymptomatic infection is known as latent syphilis. Inadequate treatment of syphilis can lead to severe neurological and cardiovascular sequelae and congenital transmission to an infant from an infected mother. Thus, timely recognition of disease is essential to prevent morbidity and further transmission. The diagnosis of syphilis is typically made via serologic testing (2). However, diagnosis is complicated by the wide array of disease manifestations and the lack of a single optimal diagnostic test.
Serologic testing methods for syphilis include treponemal-specific and nontreponemal tests that detect antibodies directed against host antigens released upon cell damage (3). In the traditional algorithm for syphilis testing, screening is via a nontreponemal test, such as venereal disease research laboratory (VDRL) or rapid plasma reagin (RPR) tests. As these tests lack specificity, positive results are confirmed by a treponemal-specific test, such as a T. pallidum enzyme immunoassay (TP-EIA), the T. pallidum particle agglutination (TPPA), or the fluorescent treponemal antibody absorption (FTA) tests. While nontreponemal tests are useful to screen for active infection and monitor the response to treatment, treponemal tests typically produce positive results for life and do not distinguish between current and past infections.
The traditional screening algorithm is a cost-effective and reliable approach to syphilis diagnosis. However, these methods are labor intensive, and the interpretation of results for nontreponemal tests is often subjective. Due to the availability of high-throughput automated TP-EIAs, some laboratories have instituted a reverse screening algorithm in which initial testing consists of an automated treponemal specific method. Positive results are followed by nontreponemal testing to distinguish active infection. The main advantage of the reverse algorithm is that the first step of the screen is automated. Thus, only positive tests need to be confirmed by manual nontreponemal testing, which is appealing to high-volume laboratories. Studies have demonstrated that this method may be useful for the detection of patients with untreated latent syphilis in whom nontreponemal testing is negative (4, 5). However, discordant results between treponemal and nontreponemal testing can lead to uncertainty in patient management and counseling (6, 7).
The BioPlex 2200 Syphilis Total & RPR assay is a multiplex flow immunoassay intended for the simultaneous detection of nontreponemal reagin antibodies and total (IgG/IgM) treponemal antibodies in human serum or plasma (8). The fully automated assay employs antigen-coated fluoromagnetic beads with unique fluorescent signatures to identify the presence of IgG or IgM antibodies to reagin antigens or T. pallidum. Thus, this is the first FDA-cleared assay that enables fully automated serologic testing for syphilis. While the Syphilis Total & RPR assays are performed simultaneously, the Syphilis Total results can be reported selectively on the basis of a positive RPR result, enabling the use of the traditional forward algorithm for interpretation. The objective of this study was to evaluate the diagnostic performance of this assay at a tertiary medical center with a high rate of syphilis.
(This work was presented in part as a poster presentation at AACC, Chicago, IL, July 2018.)
MATERIALS AND METHODS
Study population.
Two sample populations were utilized in this study: (i) prospective, 400 consecutive residual adult and pediatric serum samples sent to the Barnes-Jewish Hospital clinical laboratory for physician-ordered syphilis screening as part of a standard of care (July to August 2017); (ii) retrospective, 100 known RPR-positive residual serum samples from pediatric and adult patients sent to the Barnes-Jewish Hospital clinical laboratory for physician-ordered syphilis screening as part of a standard of care between November 2016 and September 2017 (excluding the time the prospective specimens were collected). Samples were frozen at −80°C until testing on the BioPlex 2200. No sample was frozen for >10 months. The Barnes-Jewish Hospital is a tertiary academic medical center with >1,250 beds located in an urban environment with a high rate of syphilis (32.4 cases per 100,000 people compared to 9.2 cases per 100,000 people at a national level) (9). This study was approved by the Washington University Human Research Protection Office (HRPO).
Manual testing.
All serum samples were tested by the predicate manual method using a traditional screening approach. The algorithm consisted of nontreponemal testing using the Wampole Impact RPR card test according to the manufacturer’s package insert (Alere North America, Inc., Orlando, FL) with confirmation by the Zeus IFA FTA-ABS Test system according to the manufacturer’s package insert (Zeus Scientific, Branchburg, NJ). The titers were determined for all positive RPR samples using doubling dilutions and were reported from 1:1 to endpoint.
BioPlex 2200 testing.
After thawing at room temperature, the samples were tested with the BioPlex 2200 Syphilis Total & RPR assay (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s package insert (8). The BioPlex 2200 Syphilis Total & RPR assay is a multiplex flow immunoassay for the qualitative detection of IgG and IgM antibodies to Treponema pallidum (Total) and qualitative detection with optional titer determination of nontreponemal regain antibodies (RPR). For this study, the titers were determined for all reactive RPR samples by on-board dilution and were reported as <1:4, 1:4, 1:8, 1:16, 1:32, or >1:64. Because on-board titers reported via the BioPlex 2200 RPR assay have different start and endpoints than the manual method, any titers at or below 1:4 and at or above 1:64 were considered equivalent between the two methods (see Table S1 in the supplemental material).
Discordant testing.
Any discrepant results between the manual and BioPlex 2200 assays (either RPR or treponemal testing) were further evaluated using a Serodia Treponema pallidum particle-agglutination (TPPA) assay according to the manufacturer’s package insert (Fujirebio, Malvern, PA, USA). Discrepant samples were sent (with results blinded) to Bio-Rad for TPPA testing.
Data analysis.
The performance of the BioPlex 2200 RPR assay was evaluated by calculating positive percent agreement (PPA) and negative percent agreement (NPA) against the manual RPR method. Confidence intervals (CIs) were calculated using the Wilson score method. The final comparator categorical results were interpreted using the traditional algorithm, and overall concordance was calculated. Per the traditional algorithm, positive RPR testing was followed by confirmatory FTA testing. RPR nonreactive results or RPR reactive/FTA-negative results were interpreted as negative for syphilis infection. RPR and FTA reactive specimens were considered positive for syphilis infection.
RESULTS
Characteristics of the study populations.
Prospective consecutively collected specimens (n = 400) and manual RPR-positive specimens (n = 100) were used to evaluate the performance of the BioPlex 2200 Syphilis Total & RPR assay in this study. As shown in Table 1, the majority of the prospective population (81% [323/400]) was 18 years or older (median, 29 years; range, 0 to 85 years), and 66% (263/400) were female, with 14% (36/263) known to be pregnant. For the prospective specimens, 7.5% (30/400) were collected from HIV-positive subjects, of whom 87% (26/30) were male. Of the manual RPR-positive specimens, 99 of 100 (99%) were collected from adults of 18 years or older, with a median age of 30 years (range, 18 to 79 years). In this specimen population, 50% (8/16) of the female subjects were pregnant and 47% (47/100) were HIV-positive individuals.
TABLE 1.
Demographics of all populations categorized by sex and subcategorized by age, pregnancy, and HIV status
| Parameter | Prospective population (n = 400) |
Known RPR+ population (n = 100) |
||
|---|---|---|---|---|
| Male (n [%]) | Female (n [%]) | Male (n [%]) | Female (n [%]) | |
| Age (yrs) | ||||
| <1 | 37 (9.2) | 30 (7.5) | 1 (1.0) | 0 (0) |
| 15–17 | 0 (0) | 10 (2.5) | 0 (0) | 0 (0) |
| ≥18 | 100 (25.0) | 223 (55.8) | 83 (83.0) | 16 (16.0) |
| Pregnancy | ||||
| Positive | 36 (13.7) | 8 (50.0) | ||
| Negative | 166 (63.1) | 4 (25.0) | ||
| Unknown | 61 (23.2) | 4 (25.0) | ||
| HIV | ||||
| Positive | 26 (6.5) | 4 (1.0) | 45 (45.0) | 2 (2.0) |
| Negative | 59 (14.8) | 210 (52.5) | 36 (36.0) | 12 (12.0) |
| Unknown | 52 (12.2) | 49 (12.2) | 3 (3.0) | 2 (2.0) |
| Total | 137 (34.0) | 263 (66.0) | 84 (84.0) | 16 (16.0) |
Comparison of the performance of BioPlex 2200 RPR assay with that of the manual RPR method.
The PPA and NPA for the 400 prospectively collected specimens were 85% (17/20; 95% CI, 69% to 100%) and 98% (373/380; 95% CI, 97% to 99%), respectively (Table 2). Of the 3 prospective specimens that were reactive with the manual RPR but negative by the BioPlex 2200 RPR assay, one specimen was confirmed as negative by both FTA and TPPA treponemal testing, suggesting a false-positive manual RPR result. The other two specimens were obtained from adult HIV-positive men with low RPR titers reported by the manual method (≤1:4 in both cases). The overall concordance of the RPR results in the prospective population was 97.5% (390/400). At a subpopulation level, the PPA and NPA for HIV-positive individuals were 82% (9/11; 95% CI, 59% to 100%) and 100% (19/19; 95% CI, 100%), respectively. The PPA and NPA for pregnant women were 0% (0/1) and 100% (35/35), respectively.
TABLE 2.
Agreement between the manual RPR and BioPlex 2200 RPR results from 400 prospectively collected specimens
| Specimen source | RPR result (n) |
PPAa (95% CI) | NPAb (95% CI) | |||
|---|---|---|---|---|---|---|
| BioPlex 2200 | Manualc
|
|||||
| R | NR | Total | ||||
| All | R | 17 | 7d | 24 | 85 (69–100) | 98 (97–99) |
| NR | 3e | 373 | 376 | |||
| Total | 20 | 380 | 400 | |||
| HIV positive | R | 9 | 0 | 9 | 82 (59–100) | 100 |
| NR | 2 | 19 | 21 | |||
| Total | 11 | 19 | 30 | |||
| Pregnant | R | 0 | 0 | 0 | 0 | 100 |
| NR | 1 | 35 | 36 | |||
| Total | 1 | 35 | 36 | |||
| Infant | R | 0 | 1 | 1 | 99 (96–100) | |
| NR | 0 | 66 | 66 | |||
| Total | 0 | 67 | 67 | |||
PPA, positive percent agreement.
NPA, negative percent agreement.
R, reactive; NR, nonreactive.
BioPlex RPR titers were ≤1:4 for 7 discordant specimens. TPPA testing was nonreactive in all samples.
Manual RPR titers were ≤1:4 for 3 discordant samples. TPPA testing was negative for 1/3 samples.
As shown in Table 3, the PPA of the manual RPR-positive population was 88% (88/100; 95% CI, 82% to 94%). Seven of the twelve discordant results demonstrated low positive RPR titers ≤1:4 by the manual RPR method. A subpopulation analysis of HIV-positive individuals and pregnant women showed PPAs of 91% (43/47; 95% CI, 84% to 99%) and 75% (6/8; 95% CI, 45% to 100%), respectively.
TABLE 3.
Agreement between the manual RPR and BioPlex 2200 RPR results from 100 known RPR-positive specimens
| Specimen source | RPR result (n) |
PPAa (95% CI) | |||
|---|---|---|---|---|---|
| BioPlex 2200 | Manualb
|
||||
| R | NR | Total | |||
| Known RPR+ population | R | 88 | 0 | 88 | 88 (82–94) |
| NR | 12c | 0 | 12 | ||
| Total | 100 | 0 | 100 | ||
| HIV positive | R | 43 | 0 | 43 | 91 (84–99) |
| NR | 4 | 0 | 4 | ||
| Total | 47 | 0 | 47 | ||
| Pregnant | R | 6 | 0 | 6 | 75 (45–100) |
| NR | 2 | 0 | 2 | ||
| Total | 8 | 0 | 8 | ||
| Infant | R | 1 | 0 | 1 | 100 |
| NR | 0 | 0 | 0 | ||
| Total | 1 | 0 | 1 | ||
PPA, positive percent agreement.
R, reactive; NR, nonreactive.
For 12 discordant results, 11 had titers ≤1:4 and one had a titer of 1:8. TPPA testing was reactive for 10/12 discordant results.
Concordance of BioPlex 2200 RPR and manual RPR titers.
Positive RPR results are routinely reported with antibody titers. Including both study populations, a total of 127 specimens were RPR positive by one or both assays. Of the titers reported on these specimens, 99 of 127 (78%) results were concordant within ±1 doubling dilution and 120 of 127 (94%) within ±2 doubling dilutions (Table 4). The majority of RPR titers reported from positive specimens using the manual method (53%) or the BioPlex 2200 RPR assay (60%) demonstrated titers ≤1:4 (Fig. 1).
TABLE 4.
Concordance of BioPlex 2200 RPR titer with the manual RPR titer resulta
| Titer variation from manual RPR (no. of dilutions) |
No. of results | % concordance |
|
|---|---|---|---|
| ±1 dilution | ±2 dilutions | ||
| >−2 | 4 | ||
| −2 | 15 | ||
| = or ±1 | 99 | 78 (99/127) | 94 (120/127) |
| +2 | 6 | ||
| >+2 | 3 | ||
Results for 127 specimens with RPR-positive result by either method: 27 from the prospective population, and 100 from the known RPR-positive population.
FIG 1.
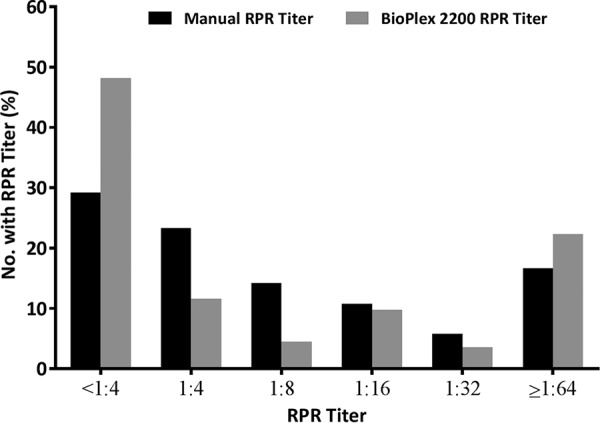
Distribution of RPR titers via manual and BioPlex 2200 methods obtained from RPR-positive specimens.
Concordance of syphilis algorithm interpretation.
Using the traditional algorithm to interpret syphilis serologic testing results, the status of syphilis infection was categorized for each population. In the prospective population, 381 of 400 specimens were interpreted as negative for active syphilis infection, of which 373 were RPR nonreactive by both methods, 7 were manual RPR nonreactive and BioPlex 2200 RPR reactive followed by negative BioPlex 2200 Syphilis Total results, and one specimen that was manual RPR reactive followed by FTA negative that was BioPlex 2200 RPR nonreactive. Seventeen of four hundred specimens were interpreted as positive by both methods (RPR reactive followed by positive FTA and BioPlex 2200 Syphilis Total results) (Table 5). Two specimens characterized as active syphilis by manual methods (RPR and FTA reactive) were interpreted as negative by the BioPlex 2200 RPR assay. Both specimens tested positive by TPPA. The overall concordance of test interpretation between the BioPlex 2200 and manual methods was 99.5% (398/400). An analysis of these data using the reverse algorithm for result interpretation in the prospectively collected specimens revealed that 17 of 400 (4.3%) specimens would have screened positive by the BioPlex 2200 Syphilis Total assay but negative by the BioPlex 2200 RPR assay.
TABLE 5.
Concordance of syphilis interpretation using traditional algorithm for the prospective and known RPR positive populations
| Population | Result (n) |
% concordance | |||
|---|---|---|---|---|---|
| BioPlex 2200 | Manuala
|
||||
| RPR− | RPR+ FTA−b | RPR+ FTA+ | |||
| Prospective (n = 400) | RPR− | 373 | 1 | 2 | 99.5 (398/400) |
| RPR+ ST−c | 7 | 0 | 0 | ||
| RPR+ ST+ | 0 | 0 | 17 | ||
| Known RPR+ (n = 100) | RPR− | 0 | 12 | 85 (85/100) | |
| RPR+ ST− | 0 | 2 | |||
| RPR+ ST+ | 1 | 85 | |||
Shading indicates discordant results when interpreted using forward algorithm, i.e., treponemal assay performed only if RPR positive.
FTA, fluorescent treponemal antibody absorption.
ST, syphilis total.
In the known RPR-positive population, 14 specimens that were interpreted by the traditional algorithm as active syphilis by the manual method (RPR positive, FTA positive) tested negative for syphilis infection by the BioPlex 2200 assay (12 were nonreactive by RPR and 2 were RPR reactive but Syphilis Total negative). Notably, 4 of the 14 positive specimens tested negative by a third TPPA method, indicating that these were likely falsely reactive by the manual method. One specimen that was negative by the manual method (RPR positive but FTA nonreactive) demonstrated positive RPR and Syphilis Total results on the BioPlex 2200 (Table 5). TPPA testing was nonreactive for this specimen, indicating agreement with the manual method. Thus, the overall concordance of test interpretation in the manual RPR-positive population was 85% (85/100). If the results of discrepant analyses are taken into consideration, the concordance of this population was 89% (89/100).
DISCUSSION
Here, we report the clinical performance of the BioPlex 2200 Syphilis Total & RPR assay at an urban tertiary medical center with a high volume of syphilis testing. To our knowledge, this is the first evaluation of the recently FDA-cleared BioPlex 2200 Syphilis Total & RPR assay. Because of the manual nature of RPR assays, which are the first step of the traditional forward algorithm, many laboratories have transitioned to a reverse algorithm, in which the first step consists of testing with a treponemal EIA that is amenable to automation (10–12). This enables an increased throughput in laboratories with a high volume of testing.
With the reverse algorithm, laboratories eliminate the need for manual RPR testing in any specimen with a negative treponemal screen. However, the frequent occurrence of discordant results (positive treponemal screening tests with a negative RPR) may lead to clinical uncertainty. In such cases, the CDC recommends additional treponemal testing by a second assay to help resolve discrepancies (13). However, because this “tiebreaker” test is a treponemal assay, it suffers from similar limitations to the original screening test, making past or present syphilis hard to differentiate. Prior studies have noted significantly more positive screens using the reverse algorithm in a low-prevalence population (4, 14). This effect is amplified in a high-prevalence population such as our cohort. In fact, the use of the reverse algorithm to interpret the data in the prospective cohort would have necessitated a third test method in 4% of specimens that tested positive by the BioPlex 2200 Syphilis Total assay but negative by BioPlex 2200 RPR assay. To reduce the need for additional confirmatory testing in laboratories using the reverse algorithm, several studies have attempted to correlate the signal strength of a positive treponemal test to predict a reactive confirmatory test result (15, 16). The use of the traditional algorithm would preclude the need for establishing signal strength cutoffs or for performing extra confirmatory testing.
The BioPlex 2200 Syphilis Total & RPR assay enables full automation of both the screen and confirmatory tests on a single instrument. This facilitates the interpretation of test results using the traditional algorithm and avoids the confusion of the reverse algorithm by performing the nontreponemal assay first. This is particularly advantageous for laboratories in areas with high rates of syphilis in which background seropositivity is high. A potential pitfall of this assay is the potential for a higher cost than that of the manual method. Although further investigation is required to definitively ascertain the cost benefit, potential additional costs may be offset by decreased hands-on time in the laboratory and through downstream effects in patient care from faster results and less need for follow-up testing. Additional benefits of the BioPlex 2200 Syphilis Total & RPR assay include higher throughput, interface with laboratory information systems, decreased opportunity for manual errors, result standardization, immediate availability of confirmatory testing after a positive RPR, and automated dilutions for titers.
The performance of the BioPlex 2200 RPR assay was comparable to that of the manual method, with overall PPA and NPA of 85% and 98%, respectively, in the prospective population. Follow-up discrepant testing was negative for one specimen, suggesting a false-positive manual RPR result. The other two discrepant specimens were obtained from adult HIV-positive males with low RPR titers (≤1:4), and both specimens were positive by manual FTA and BioPlex 2200 Syphilis Total methods. While no additional clinical information was available to arbitrate these discrepancies, these results may suggest a recent history of treated syphilis or the possibility that an attenuated immune response hampered the detection by the BioPlex 2200 assay in these specimens.
We observed excellent overall concordance of the interpretation of the serologic profile using the traditional algorithm in the prospective population. In the manual RPR-positive population, the concordance of the syphilis serologic interpretation dropped to 85%, mostly due to 12 specimens that tested negative for RPR on the BioPlex 2200 assay and two that were negative on the BioPlex 2200 Syphilis Total but positive by FTA. The decreased concordance in this population is likely an effect of selection bias, as the analyzed population consisted only of manual RPR-positive patients and is not representative of the population intended to be analyzed by this method. While we lack clinical data to aid the interpretation of these results, 4 of the 14 specimens tested negative by the second treponemal TPPA method, suggesting the possibility of false-positive results by the more subjective and less specific manual RPR and FTA methods.
Positive RPR results are typically reported in conjunction with titers, which are used clinically to monitor therapeutic response (17). The BioPlex 2200 RPR assay is capable of performing onboard dilutions for optional titer determination. Due to the inherent variability of titer determination, we considered any BioPlex 2200 RPR result within one dilution of the manual result concordant. Good concordance (78%) of the BioPlex 2200 RPR assay was observed with the predicate manual method. Discordant results were mostly attributed to titer results of <1:4 on the BioPlex 2200 RPR assay in specimens with titers of 1:8 or 1:16 determined by the manual method. Notably, more than 94% of the reported titers were within two dilutions between the manual and BioPlex 2200 RPR methods.
One limitation of this study is that the specimens were obtained with limited clinical data. Thus, we were not able to clinically stage syphilis into primary, secondary, latent, and tertiary infections. Second, due to lack of adequate clinical information, it is difficult to arbitrate discordant results, even after a discrepant analysis was performed by a third treponemal assay (TPPA). Third, this study was performed at a single large tertiary medical center with a high syphilis prevalence. Further investigation may be needed to evaluate the clinical performance of BioPlex 2200 Syphilis Total & RPR assay in areas with low syphilis prevalence. Lastly, BioPlex 2200 RPR titers were reported on the instrument at a maximum dilution of 1:64. Thus, we are unable to assess the accuracy of this method at higher titers. For clinicians following titers as a measure of a response to therapy, onboard determination of higher RPR titers with a prior offline dilution step is now supported on the BioPlex 2200. However, this feature was not assessed in our study. A major advantage of this study is that a large number of well-preserved clinical specimens, including 100 manual RPR-positive specimens, were evaluated, which enabled the assessment of all aspects of syphilis diagnostic testing, from nontreponemal and treponemal assays to serologic interpretation.
This study, for the first time, establishes the clinical performance of a recently FDA-cleared automated BioPlex 2200 Syphilis Total & RPR assay at a tertiary medical center with a high rate of syphilis infection. The performance of the BioPlex 2200 Syphilis Total & RPR assay was comparable to those of the manual RPR and FTA methods. While the sensitivity of the BioPlex 2200 RPR method may be lower than that of traditional manual RPR assays, the ability to interpret the results using the traditional algorithm is a major benefit of this method. The high NPA of this assay, in combination with the ability to automate a historically labor-intensive method, make it well suited for use as a diagnostic testing modality for syphilis in a high-volume laboratory.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rhonda Porche-Sorbet, Karl Hock, James Andrew Krekeler, and Alexander Gronowski for technical assistance with this project.
This study was funded by Bio-Rad Laboratories. A.M.G. has received research funding from Instrumentation Laboratories. M.L.Y. has received personal fees from Bio-Rad and Agena Biosciences.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01487-18.
REFERENCES
- 1.Lafond RE, Lukehart SA. 2006. Biological basis for syphilis. Clin Microbiol Rev 19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 3.Yarbrough ML, Burnham CA. 2016. The ABCs of STIs: an update on sexually transmitted infections. Clin Chem 62:811–823. doi: 10.1373/clinchem.2015.240234. [DOI] [PubMed] [Google Scholar]
- 4.Binnicker MJ, Jespersen DJ, Rollins LO. 2012. Direct comparison of the traditional and reverse syphilis screening algorithms in a population with a low prevalence of syphilis. J Clin Microbiol 50:148–150. doi: 10.1128/JCM.05636-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control Prevention. 2008. Syphilis testing algorithms using treponemal tests for initial screening–four laboratories, New York City, 2005–2006. MMWR Morb Mortal Wkly Rep 57:872–875. [PubMed] [Google Scholar]
- 6.Loeffelholz MJ, Binnicker MJ. 2012. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J Clin Microbiol 50:2–6. doi: 10.1128/JCM.06347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seña AC, White BL, Sparling PF. 2010. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis 51:700–708. doi: 10.1086/655832. [DOI] [PubMed] [Google Scholar]
- 8.Bio-Rad. 2017. BioPlex 2200 Syphilis Total & RPR package insert. Bio-Rad, Hercules, CA. [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2018. Sexually transmitted disease surveillance 2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 10.Binnicker MJ, Jespersen DJ, Rollins LO. 2011. Treponema–specific tests for serodiagnosis of syphilis: comparative evaluation of seven assays. J Clin Microbiol 49:1313–1317. doi: 10.1128/JCM.02555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez E, Jespersen DJ, Harring JA, Binnicker MJ. 2010. Evaluation of the Bio-Rad BioPlex 2200 syphilis multiplex flow immunoassay for the detection of IgM- and IgG-class antitreponemal antibodies. Clin Vaccine Immunol 17:966–968. doi: 10.1128/CVI.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morshed MG. 2014. Current trend on syphilis diagnosis: issues and challenges. Adv Exp Med Biol 808:51–64. doi: 10.1007/978-81-322-1774-9_5. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control Prevention. 2011. Discordant results from reverse sequence syphilis screening–five laboratories, United States, 2006–2010. MMWR Morbid Mortal Wkly Rep 60:133–137. [PubMed] [Google Scholar]
- 14.Dunseth CD, Ford BA, Krasowski MD. 2017. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med 8:52–59. doi: 10.1016/j.plabm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry GJ, Loeffelholz MJ. 2016. Use of treponemal screening assay strength of signal to avoid unnecessary confirmatory testing. Sex Transm Dis 43:737–740. doi: 10.1097/OLQ.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 16.Fakile YF, Jost H, Hoover KW, Gustafson KJ, Novak-Weekley SM, Schapiro JM, Tran A, Chow JM, Park IU. 2018. Correlation of treponemal immunoassay signal strength values with reactivity of confirmatory treponemal testing. J Clin Microbiol 56:e01165-17. doi: 10.1128/JCM.01165-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanowski B, Sutherland R, Fick GH, Mooney D, Love EJ. 1991. Serologic response to treatment of infectious syphilis. Ann Intern Med 114:1005–1009. doi: 10.7326/0003-4819-114-12-1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


