LETTER
Here, we report a significant increase in positivity rate among low-risk health care workers (HCW) undergoing annual tuberculosis (TB) testing in our health system after the fourth-generation QuantiFERON-TB Gold Plus (QFT-Plus) assay replaced the QuantiFERON-TB Gold (QFT) assay. Compared to its predecessor, which stimulates only CD4+ T-lymphocytes in a single TB antigen tube (TB1), the QFT-Plus assay includes a second TB antigen tube (TB2) with a peptide formulation designed to stimulate both CD4+ and CD8+ T-lymphocytes. As recommended by the manufacturer, the QFT-Plus assay is considered positive if either antigen tube (TB1 and/or TB2) result is positive.
The QFT-Plus assay replaced the QFT assay in North America on 30 June 2018. After 11 years of use of the QFT assay for occupational testing, our institution switched to the QFT-Plus assay on 16 July 2018. Within weeks, our occupational health staff noticed increases in the number of health visits for medical evaluation and retesting in HCW with positive test results.
To investigate this, we conducted a retrospective review of HCW tested with the QFT-Plus assay from 16 July 2018 to 4 September 2018 and compared the rates to those in HCW tested with the QFT assay from the exact same time period in 2017. The HCW QFT-Plus positivity rate was significantly increased compared to that of the QFT assay, with 3.5% (95/2,720) positive in 2018 compared to 2.2% (60/2,748) in 2017 (95% confidence interval [CI] of difference, 0.4 to 2.2%, p = 0.003) (Fig. 1). Of the 95 positive tests with the QFT-Plus assay, 19 (20.0%) were positive by TB1 only, and 31 (32.6%) were positive by TB2 only. QFT-Plus testing was repeated in 49 patients; of these, only 12 (24.5%) were positive. Using a previously suggested conservative interpretation (positive only if TB1 and TB2 positive) in low-risk HCW (1), the QFT-Plus positivity rate would have been 1.7% (45/2,720), which is more comparable to 2017 QFT positivity rates.
FIG 1.
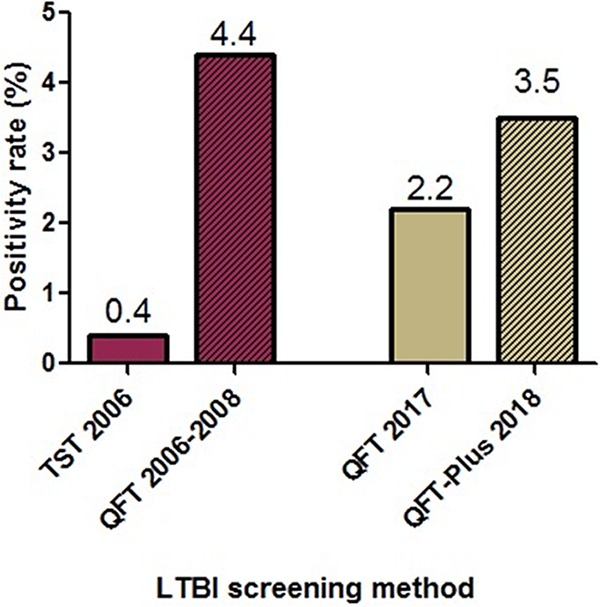
Increase in positivity rate with the QFT-Plus assay at Stanford Health Care occupational health clinic. Red bars represent tuberculin skin test (TST) positivity rate from 2006 compared to QFT positivity rate from 2006 to 2008. Tan bars represent QFT positivity rate from 2017 (16 July to 4 September) compared to QFT-Plus positivity rate from 2018 (16 July to 4 September). Striped pattern represents new QFT assay introduced for each period.
Although concerning, this is not the first time we have experienced higher positivity rates for annual TB screening after adoption of a new gamma interferon (IFN-γ) release assay (IGRA). Shortly after QFT was approved by the U.S. Food and Drug Administration (FDA) in 2005 as a replacement for the tuberculin skin test (TST), many North American health systems, including ours (Fig. 1) (2), started to report unusually high conversion rates with the QFT assay in low-risk HCW, compared with concurrent or historical TST rates (3). Our QFT assay conversion rate has fallen in the recent years (Fig. 1) as a result of preanalytical standardization and manufacturer quality assurance (4).
The QFT-Plus assay was introduced on the IGRA market with the touted advantage of better sensitivity than its predecessor in immunocompromised hosts and after recent exposure. However, studies that have compared the QFT-Plus assay to the QFT assay have shown similar sensitivity in patients with active TB and similar test performance in HCW (1, 5). As reported here, similarly to the implementation of the original QFT (3), we are seeing an increase in nonreproducible positive results in low-risk serially tested HCW (Fig. 1). We anticipate other institutions in the United States reporting higher positivity rates. Higher rates need to be thoughtfully considered because the QFT-Plus assay is more expensive than the QFT assay, due to the increased cost of reagents (approximately $10 increase per test), and lower throughput. The lower specificity of QFT-Plus testing prompts additional HCW visits for repeat testing and counselling and causes HCW anxiety and loss of productivity. Further studies will be required to validate our proposed conservative interpretation of QFT-Plus results and confirm its usefulness in identifying false-positive results in low-risk HCW.
REFERENCES
- 1.Moon HW, Gaur RL, Tien SS, Spangler M, Pai M, Banaei N. 2017. Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 55:1650–1657. doi: 10.1128/JCM.02498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slater ML, Welland G, Pai M, Parsonnet J, Banaei N. 2013. Challenges with QuantiFERON-TB Gold assay for large-scale, routine screening of U.S. healthcare workers. Am J Respir Crit Care Med 188:1005–1010. doi: 10.1164/rccm.201305-0831OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. 2014. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banaei N, Gaur RL, Pai M. 2016. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? J Clin Microbiol 54:845–850. doi: 10.1128/JCM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theel ES, Hilgart H, Breen-Lyles M, McCoy K, Flury R, Breeher LE, Wilson J, Sia IG, Whitaker JA, Clain J, Aksamit TR, Escalante P. 2018. Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold in-tube interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol 56:e00614-18. doi: 10.1128/JCM.00614-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


