Nontyphoidal Salmonella (NTS) bacteremia causes hospitalization and high morbidity and mortality. We linked Gastrointestinal Bacteria Reference Unit (GBRU) data to the Hospital Episode Statistics (HES) data set to study the trends and outcomes of NTS bacteremias in England between 2004 and 2015.
KEYWORDS: antimicrobial resistance, bacteremia, bloodstream infection, hospital admission, human, invasive infection, nontyphoidal Salmonella, salmonellosis
ABSTRACT
Nontyphoidal Salmonella (NTS) bacteremia causes hospitalization and high morbidity and mortality. We linked Gastrointestinal Bacteria Reference Unit (GBRU) data to the Hospital Episode Statistics (HES) data set to study the trends and outcomes of NTS bacteremias in England between 2004 and 2015. All confirmed NTS isolates from blood from England submitted to GBRU between 1 January 2004 and 31 December 2015 were deterministically linked to HES records. Adjusted odds ratios (AOR), proportions, and confidence intervals (CI) were calculated to describe differences in age, sex, antibiotic resistance patterns, and serotypes over time. Males, neonates, and adults above 65 years were more likely to have NTS bacteremia (AOR, 1.54 [95% CI, 1.46 to 1.67]; 2.57 [95% CI, 1.43 to 4.60]; and 3.56 [95% CI, 3.25 to 3.90], respectively). Proportions of bacteremia increased from 1.41% in 2004 to 2.67% in 2015. Thirty-four percent of all blood isolates were resistant to a first-line antibiotic, and 1,397 (56%) blood isolates were linked to an HES record. Of the patients with NTS bacteremia, 969 (69%) had a cardiovascular condition and 155 (12%) patients died, out of which 120 (77%) patients were age 65 years and above. NTS bacteremia mainly affects older people with comorbidities placing them at increased risk of prolonged hospital stay and death. Resistance of invasive NTS to first-line antimicrobial agents appeared to be stable in England, but the emergence of resistance to last-resort antibiotics, such as colistin, requires careful monitoring.
INTRODUCTION
Nontyphoidal Salmonella (NTS) is an important cause of food poisoning, causing gastrointestinal infections that range from asymptomatic to clinically severe illness. Known risk factors for acquiring NTS include consumption of contaminated food items, such as eggs, meat, vegetables, and dairy products, as well as overseas travel (1). Although the majority of nontyphoidal salmonellosis infections are self-limiting, a proportion of cases go on to develop bacteremia or disseminated infection. Invasive NTS infections can be life-threatening and usually affect infants, the elderly, and immunocompromised patients.
Since the late 1990s, there has been a significant decline in the incidence of reported nontyphoidal salmonellosis in the United Kingdom. The number of NTS isolates from humans reported to Public Health England (PHE) from England and Wales decreased by 40% from 14,283 reports in 2004 to 8,630 reports in 2016. This reduction was seen in other developed countries as well and is thought to primarily result from improved standards of hygiene in poultry and egg production (2). PHE’s Gastrointestinal Bacteria Reference Unit (GBRU) has identified more than 2,600 different serovars of NTS to date, with the two most commonly reported serovars being Salmonella enterica subsp. enterica serovars Enteritidis (27%) and Typhimurium (20%) (3). In 2016, there were 12 reported foodborne outbreaks of Salmonella spp. across England and Wales. S. Enteritidis was responsible for eight of the outbreaks. The largest outbreak involving 115 cases was due to S. Enteritidis, with 14 cases ending up hospitalized; it was a multicountry outbreak linked to shell eggs (3).
Salmonella spp. are a significant cause of morbidity and mortality. In the United States, for example, Salmonella spp. cause an estimated 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths annually. Male patients are more likely than females to have bacteremia (odds ratio [OR], 1.23; 95% confidence interval [95% CI], 1.09 to 1.39), and older persons age 65 to 84 years are twice as likely to have bacteremia (OR, 2.04; 95% CI, 1.73 to 2.41) than those age 18 to 64 years (4).
Antibiotics are not routinely used to treat uncomplicated NTS gastroenteritis. Current recommendations are that antibiotics be reserved for patients with severe disease or patients who are at high risk for invasive disease (5). However, antibiotic-resistant NTS is increasingly reported, which complicates the management of those patients for whom antibiotic treatment is indicated. Globally, surveillance data have demonstrated an increase in overall antimicrobial resistance among salmonellae from 20% to 30% in the early 1990s to as high as 70% in some countries at the turn of the century (6). In developed countries, antimicrobial drug resistance in NTS is an almost inevitable consequence of the use of antimicrobial drugs in livestock and meat and milk entering the food chain (7–9).
Serovars of particular importance in the UK since the early 1990s which have been associated with multidrug resistance include S. Typhimurium DT104, Salmonella enterica subsp. enterica serovar Virchow, and Salmonella enterica subsp. enterica serovar Hadar. There has been an increase in the incidence of reduced susceptibility to quinolones in these serovars in late 1990s; specifically, S. Virchow is known to be associated with invasive disease (9). This increase is thought to coincide with licensing of the use of enrofloxacin in veterinary medicine in food animals. Third-generation cephalosporins are considered a first-line treatment for Salmonella bacteremia where reduced susceptibility to quinolones is well known; increasing global resistance in NTS is of particular concern (10, 11). The risk factors for invasive disease in adults and children include immunosuppression of any cause (including HIV-positive status), malaria infection, severe anemia, and malnutrition (12). Other risk factors include diabetes, malignancy, and autoimmune disorders (13).
NTS bacteremia has been linked with an overall in-hospital mortality rate of 12%. The mortality rate for primary bacteremia is 24.8%, and for endovascular infection, it is 14.3%. Predictors of in-hospital death are found to be age, extraintestinal infection, and solid-organ malignancy (13). The development of extraintestinal infections is associated with more severe septic manifestations, longer hospital stays, longer duration of antimicrobial therapy, and a higher rate of mortality. In adults, NTS bacteremia poses significant health threats because affected patients usually have underlying diseases (14, 15).
Here, we report the national rate and trends of NTS bacteremia and invasive serovars, together with the patterns of antimicrobial resistance and comorbidities associated with NTS bacteremia in England between 2004 and 2015.
MATERIALS AND METHODS
Bacterial isolates.
PHE’s Gastrointestinal Bacteria Reference Unit (GBRU) receives isolates of presumptive Salmonella spp. from all hospital microbiology laboratories in England for confirmation of serovar identification. All nontyphoidal Salmonella spp. isolated from stool and sterile sites submitted to the GBRU between January 2004 and December 2015 were included in this study. Quality assurance specimens were excluded. All pure isolates between January 2004 and March 2015 were serotyped with O- and H-antigen-specific antisera according to the Kaufmann and White scheme. Isolates received from April 2014 to December 2015 were identified by a combination of serotyping and whole-genome sequencing (WGS) (16).
Antibiotic susceptibility testing.
Isolates from blood were tested for antibiotic susceptibility by a breakpoint agar dilution method, using Iso-Sensitest or Mueller-Hinton agar. The antimicrobial concentrations used for screening of resistance from 2013 were 8 mg/liter ampicillin, 8 and 16 mg/liter chloramphenicol, 2 mg/liter colistin, 256 mg/liter sulfonamide, 2 mg/liter gentamicin, 2 mg/liter tobramycin, 8 mg/liter amikacin, 16 mg/liter streptomycin, 8 mg/liter tetracycline, 2 mg/liter trimethoprim, 16 mg/liter nalidixic acid, 0.064 and 0.5 mg/liter ciprofloxacin, 1 and 2 mg/liter ceftazidime, 0.5 and 1 mg/liter cefotaxime, 8 mg/liter cefoxitin, 8 mg/liter cefpirome, 0.064 and 0.5 mg/liter ertapenem, and 128 mg/liter temocillin (17, 18). Prior to 2013, the panel included ceftriaxone (1 mg/liter), and the screening concentrations of 0.125 and 1 mg/liter ciprofloxacin and 64 mg/liter sulfonamide were used. The following antimicrobials were not tested for prior to 2013: azithromycin, tobramycin, ertapenem, temocillin, cefoxitin, and cefpirome.
Antimicrobial susceptibility testing was subjected to internal quality assurance (QA) in accordance with the published methods and to external quality assurance in collaboration with laboratories within the European Union Reference Laboratory for Antibiotic Resistance (EURL) (17, 19). NTS isolates were classified as multidrug resistant if they were resistant to three or more antimicrobial agents. Isolates which were resistant to 1 mg/liter cefotaxime were subjected to an in-house PCR assay to detect mechanisms of β-lactam resistance (CTX-M extended-spectrum β-lactamases [ESBLs] and genes encoding AmpC, SHV, TEM, GES, VEB, and PER β-lactamases [10]). Isolates with ertapenem resistance were subject to a PCR for detection of carbapenemases (20).
Data linkage.
Data for all NTS isolates taken between 1 January 2004 and 31 December 2015 were extracted from PHE’s Laboratory Information Management Systems (LIMS). The LIMS contains microbiological information, including species identification, phage type or serotype, antimicrobial susceptibility, and site of isolation, as well as patient demographic details.
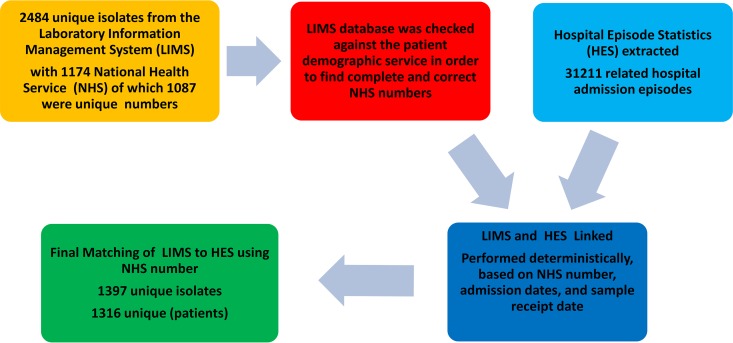
Data for NTS isolates from blood were deterministically linked to the corresponding inpatient records derived from the Hospital Episode Statistics (HES) data set, which contains information about patients admitted to National Health Service (NHS) hospitals in England. The data linkage process is illustrated in more detail in Fig. 1.
FIG 1.
Data linkage between Hospital Episode System (HES) and Laboratory Information Management System (LIMS).
Data were cleaned and analyzed using Stata SE 13.1 for Windows. We calculated proportions and 95% confidence intervals of NTS bacteremia and antimicrobial resistance and estimated the odds ratios of NTS bacteremia, unadjusted and adjusted for age, sex, and international travel, using logistic regression. We examined relative proportions of different strains and strain-specific trends over time. The mean length of hospital stay according to age group and the proportions of primary/secondary ICD-10 codes of those admitted to hospital were examined.
This study is based on an analysis of routinely collected surveillance data and does not require ethics committee review. Public Health England is authorized to process confidential information for communicable disease purposes under section 251 of the NHS Act 2006.
RESULTS
There were 116,362 isolates of NTS from England tested by the GBRU between January 2004 and December 2015, of which 2,484 (2.1%) were blood isolates. The age distribution of all NTS isolates was relatively stable over time, although the proportion increased slightly in infants (6.0% in 2015 versus 4.1% in 2004), decreased in adults age 18 to 64 (55.3% in 2015 versus 61.4% in 2004), and increased in adults age 65+ (13.6% in 2015 versus 9.1% in 2004). The proportion of total NTS isolates in children remained stable (25.0% in 2015 versus 25.2% in 2004).
NTS bacteremia.
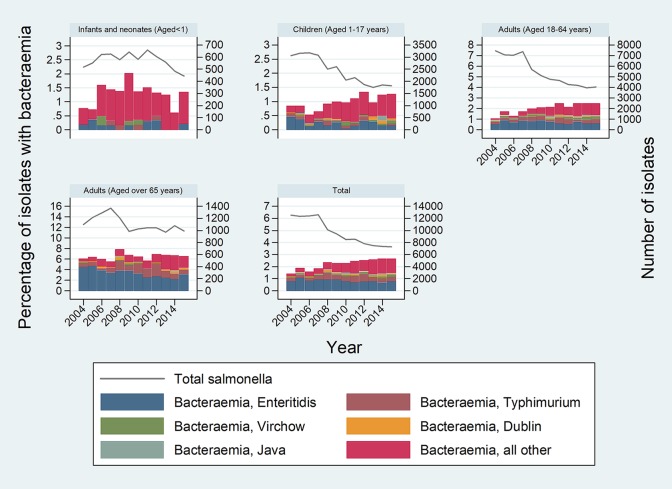
Males were more likely than females to develop NTS bacteremia (unadjusted OR, 1.46; 95% CI, 1.35 to 1.59). The median age of cases was 56 years (range, 0 to 114 years). Neonates and older adults (age 65+ years) were more likely than younger adults (age 18 to 44 years) to develop bacteremia (unadjusted OR, 2.35; 95% CI, 1.32 to 4.21; and OR, 3.53; 95% CI, 3.23 to 3.85, respectively) (Table 1). Overall proportions of bacteremia increased over time, from 1.41% in 2004 to 2.67% in 2015, with a broadly linear trend over time. The multivariable model with age, sex, and linear trend provided results very similar to those of the univariable analysis. Trends were comparable across age groups, although there was no evidence of an increase in bacteremia in those age 65+ years (OR per year increase, 1.01; 95% CI, 0.99 to 1.03), and the trends for neonates and infants were nonsignificant due to small counts. Figure 2 shows total numbers of NTS cases over time according to age group and percentages of NTS bacteremia.
TABLE 1.
Demographic information and travel history of patients with nontyphoidal Salmonella infections from, England, 2004 to 2015a
| Characteristic | No. (%) of blood isolates | No. of all other isolates | Unadjusted OR (95% CI) | Adjusted OR (95% CI)b |
|---|---|---|---|---|
| Group | ||||
| Neonates (0–28 days) | 12 (4.4) | 272 | 2.35 (1.32–4.21) | 2.56 (1.45–4.65) |
| Infants (29 days to 1 yr) | 78 (1.2) | 6,603 | 0.61 (0.48–0.77) | 0.60 (0.47–0.75) |
| Children (1–17 yr) | 271 (0.9) | 29,088 | 0.48 (0.42–0.55) | 0.48 (0.42–0.55) |
| Adults 18–64 yr | 1,261 (1.9) | 65,586 | 1 (ref) | 1 (ref) |
| Adults >65 yr | 861 (6.5) | 13,314 | 3.53 (3.23–3.85) | 3.47 (3.17–3.80) |
| Unknown | 1 (0.1) | 1,497 | ||
| Sex | ||||
| Male | 1,426 (2.5) | 56,459 | 1.46 (1.35–1.59) | 1.54 (1.42–1.67) |
| Female | 976 (1.7) | 56,111 | 1 (ref) | 1 (ref) |
| Unknown | 82 (2.2) | 3,790 | ||
| OR per yr increase | NA | NA | 1.06 (1.04–1.07) | 1.05 (1.03–1.06) |
| International travel | ||||
| Yes | 560 (1.9) | 29,247 | 0.91 (0.80–1.03) | |
| No | 505 (1.7) | 28,974 | 1 (ref) | |
| Unknown (including blank) | 1,419 (2.4) | 58,139 | ||
| Total | 2,484 (2.1) | 116,360 |
NA, not applicable; ref, reference group. All isolates tested include blood and nonblood isolates (e.g., feces and pus).
Adjusted for age, sex, and year.
FIG 2.
Total numbers of nontyphoidal Salmonella (NTS) cases over time according to age group, proportion with bacteremia, and the five most common serovars.
Only 1,065/2,484 (43%) cases of NTS bacteremia had documented travel history, and there was no difference in the likelihood of bacteremia between those isolates from cases with and those without documented travel (Table 1).
NTS serovars causing bacteremia.
A total of 767 NTS serovars were identified across all NTS strains, and 148 serovars were associated with bacteremia. Table 2 shows that S. Enteritidis was the most common serovar identified for all (blood and nonblood) isolates, while Salmonella enterica subsp. enterica serovar Dublin was the serovar most commonly isolated from blood (OR, 26.96; 95% CI, 20.39 to 35.65).
TABLE 2.
Most frequent serovars of nontyphoidal Salmonella from blood isolates from England, 2004 to 2015
| Salmonella enterica subsp. enterica serovar | Total no. of isolates (n = 116,362) |
No. (%) of blood isolates (n = 2,484) |
Odds ratioa | 95% confidence interval |
|---|---|---|---|---|
| Salmonella Enteritidis | 50,992 | 1,009 (2.0) | Reference | |
| Salmonella Dublin | 227 | 80 (35.2) | 26.96 | 20.39–35.65 |
| Salmonella Panama | 273 | 25 (9.2) | 4.99 | 3.29–7.57 |
| Salmonella Colindale | 191 | 15 (7.9) | 4.22 | 2.48–7.18 |
| Salmonella Poona | 455 | 33 (7.3) | 3.87 | 2.70–5.55 |
| Salmonella Brandenburg | 232 | 15 (6.5) | 3.42 | 2.02–5.80 |
| Salmonella Chester | 336 | 19 (5.7) | 2.97 | 1.86–4.74 |
| Salmonella Heidelberg | 449 | 21 (4.7) | 2.43 | 1.56–3.78 |
| Salmonella Oranienburg | 870 | 38 (4.4) | 2.26 | 1.62–3.15 |
| Salmonella Virchow | 3,017 | 129 (4.3) | 2.21 | 1.84–2.67 |
| Salmonella Bovis-Morbificans | 405 | 15 (3.7) | 1.91 | 1.13–3.20 |
| Salmonella Schwarzengrund | 574 | 21 (3.7) | 1.88 | 1.21–2.92 |
| Salmonella Corvallis | 679 | 21 (3.1) | 1.58 | 1.02–2.45 |
| Salmonella Java | 1,480 | 43 (2.9) | 1.48 | 1.09–2.02 |
| Salmonella Saintpaul | 922 | 22 (2.4) | 1.21 | 0.79–1.86 |
| Salmonella unnamedb | 4,122 | 95 (2.3) | 1.17 | 0.94–1.45 |
| Salmonella Typhimurium | 19,655 | 404 (2.1) | 1.04 | 0.93–1.17 |
| Salmonella Montevideo | 981 | 20 (2.0) | 1.03 | 0.66–1.61 |
| Salmonella Stanley | 1,633 | 28 (1.7) | 0.86 | 0.59–1.26 |
| All other serovars | 24,177 | 381 (1.6) | 0.79 | 0.70–0.89 |
| Salmonella Newport | 2,833 | 31 (1.1) | 0.55 | 0.38–0.79 |
| Salmonella Infantis | 1,859 | 19 (1.0) | 0.51 | 0.32–0.81 |
Odds ratio is odds of serotype in blood isolate/odds of S. Enteritidis in blood isolate.
bSalmonella unnamed refers to all isolates that could not be fully typed serologically.
The other top five Salmonella enterica subsp. enterica serovars most commonly isolated from blood were S. Panama (OR, 4.99; 95% CI, 3.29 to 7.25), S. Colindale (OR, 4.22; 95% CI, 2.48 to 7.18), S. Poona (OR, 3.87; 95% CI, 2.70 to 5.55), and S. Brandenburg (OR, 3.42; 95% CI, 2.02 to 5.80).
Using 2009 to 2012 as the baseline category, logistic regression showed no significant change in the proportion of blood isolates from 2009 to 2012 to the years 2013 to 2015 for any of the top 20 strains, although Salmonella serovars Enteritidis, Newport, and Poona had lower odds in the preceding period (2004 to 2008 versus 2009 to 2012).
Antibiotic susceptibility testing.
Out of 2,484 NTS isolates from blood, 2,308 (93%) isolates were tested for antibiotic susceptibility, and 1,366 (59% of all isolates tested) were fully susceptible to all first-line antibiotics (ceftriaxone, ciprofloxacin, azithromycin, and ampicillin), as shown in Table 3. Resistance to the first-line agents ampicillin and ceftriaxone did not show any significant change over the period 2004 to 2015, and any difference in the proportion of resistance in blood isolates from one year to the next was not statistically significant. There was evidence of less resistance to colistin in the 2004 to 2008 period (OR, 0.38; 95% CI, 0.20 to 0.73; P = 0.004).
TABLE 3.
Antibiotic resistance in nontyphoidal Salmonella blood isolates in England, 2004 to 2015
| Antimicrobial resistance type | No. (%) of isolates by yr |
OR (95% CI), P value vs 2009–2012 fora
: |
|||
|---|---|---|---|---|---|
| 2004–2008 | 2009–2012 | 2013–2015 | 2004–2008 | 2013–2015 | |
| Total no.b | 1,010 | 760 | 538 | ||
| Fully sensitive | 595 (58.9) | 447 (58.8) | 324 (60.2) | 1.00 (0.83–1.22), 0.968 | 1.06 (0.85–1.33), 0.611 |
| Agentsc | |||||
| Ampicillin | 172 (17.0) | 133 (17.5) | 91 (16.9) | 0.97 (0.75–1.24), 0.795 | 0.96 (0.72–1.29), 0.783 |
| Ciprofloxacin | 220 (21.8) | 153 (20.1) | 104 (19.3) | 1.10 (0.88–1.39), 0.399 | 0.95 (0.72–1.26), 0.721 |
| Nalidixic acid | 216 (21.4) | 150 (19.7) | 104 (19.3) | 1.11 (0.88–1.40), 0.397 | 0.97 (0.74–1.29), 0.856 |
| Cefoxitin | 4 (0.4) | 2 (0.3) | 0 (0.0) | 1.51 (0.28–8.25), 0.636 | NA |
| Cefotaxime | 14 (1.4) | 6 (0.8) | 6 (1.1) | 1.77 (0.68–4.62), 0.246 | 1.42 (0.45–4.42), 0.548 |
| Ceftriaxone | 13 (1.3) | 6 (0.8) | 4 (0.7) | 1.64 (0.62–4.33), 0.319 | 0.94 (0.26–3.35), 0.926 |
| Sulfonamide | 201 (19.9) | 136 (17.9) | 97 (18.0) | 1.14 (0.90–1.45), 0.287 | 1.01 (0.76–1.35), 0.950 |
| Trimethoprim | 117 (11.6) | 88 (11.6) | 47 (8.7) | 1.00 (0.75–1.34), 0.997 | 0.73 (0.50–1.06), 0.099 |
| Tetracycline | 191 (18.9) | 120 (15.8) | 91 (16.9) | 1.24 (0.97–1.60), 0.088 | 1.09 (0.81–1.46), 0.588 |
| Chloramphenicol | 77 (7.6) | 64 (8.4) | 56 (10.4) | 0.90 (0.64–1.27), 0.540 | 1.26 (0.87–1.84), 0.224 |
| Amikacin | 7 (0.7) | 5 (0.7) | 3 (0.6) | 1.05 (0.33–3.33), 0.929 | 0.85 (0.20–3.56), 0.820 |
| Gentamicin | 21 (2.1) | 10 (1.3) | 9 (1.7) | 1.59 (0.75–3.40), 0.230 | 1.28 (0.51–3.16), 0.599 |
| Streptomycin | 160 (15.8) | 109 (14.3) | 86 (16.0) | 1.12 (0.86–1.46), 0.385 | 1.14 (0.84–1.54), 0.415 |
| Tobramycin | 10 (1.0) | 3 (0.4) | 1 (0.2) | 2.52 (0.69–9.20), 0.161 | 0.47 (0.05–4.53), 0.514 |
| Temocillin | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | NA |
| Colistin | 14 (1.4) | 27 (3.6) | 16 (3.0) | 0.38 (0.20–0.73), 0.004 | 0.83 (0.44–1.56), 0.567 |
| Ertapenem | 1 (0.1) | 1 (0.1) | 1 (0.2) | 0.75 (0.05–12.05), 0.841 | 1.41 (0.09–22.65), 0.807 |
| Azithromycin | 0(0) | NA | NA | ||
| Patternsd | |||||
| First-line agente | 353 (35.0) | 255 (33.6) | 176 (32.7) | 1.06 (0.87–1.30), 0.540 | 0.96 (0.76–1.22), 0.752 |
| First-line agentf | 225 (22.3) | 156 (20.5) | 104 (19.3) | 1.11 (0.88–1.40), 0.375 | 0.93 (0.70–1.22), 0.596 |
| ≥1 agent | 361 (35.7) | 254 (33.4) | 179 (33.3) | 1.11 (0.91–1.35), 0.310 | 0.99 (0.79–1.26), 0.955 |
NA, not applicable.
Isolates not tested were excluded from total n. Percentages are rounded up to 1 decimal place.
For single antimicrobials and resistance to ≥1 agent, resistance is defined as an intermediate or resistant MIC. Multidrug resistance is resistance to >3 antimicrobial agents.
Azithromycin was not routinely tested before 2013.
Resistant to ≥1 of the following agents: ampicillin, ceftriaxone, ciprofloxacin, and/or azithromycin.
Resistant to ≥1 of the following agents: ceftriaxone, ciprofloxacin, and/or azithromycin.
A total of 26 isolates showed resistance to third-generation cephalosporins, 2/26 were also resistant to cefoxitin, and a further 4 were resistant only to cefoxitin. Seven out of 26 isolates underwent further molecular characterization; one demonstrated SHV (S. Enteritidis), one had CMY-2 (Salmonella enterica subsp. enterica serovar Kibusi), and one had CTX-M-15 (Salmonella enterica subsp. enterica serovar Ajiobo). No molecular markers of carbapenem resistance were found in the three ertapenem-resistant isolates. A total of 57 isolates from 22 different serovars showed resistance to colistin over 2004 to 2015, ranging from 2 to 10 isolates per year; the majority (n = 18) were S. Enteritidis. The most invasive top 5 serovars did not show significantly higher drug resistance to first-line antibiotics compared to S. Enteritidis.
Hospital Episode Statistics and NTS bacteremia.
Out of 2,484 NTS blood isolates, a total of 1,397 (56%) isolates were linked to a hospital admission record. Table 4 shows the length of hospital stay for each age group.
TABLE 4.
Length of in-hospital stay for patients with nontyphoidal Salmonella bacteremia in each age group
| Age of patient with NTS bacteremia | No. of patients with NTS bacteremia | Hospital stay (mean [SD; range]) (days) |
|---|---|---|
| Adult 18–64 yr | 664 | 10.4 (17.1; 0–204) |
| Adult >65 yr | 549 | 16.6 (18.9; 0–183) |
| Child (1–17 yr) | 138 | 7.1 (10.5; 0–99) |
| Infant (29 days–1 yr) | 39 | 5.2 (5.4; 0–21) |
| Neonate (0–28 days) | 7 | 13.8 (15.4; 0–43) |
| Age unknown | 1,087 | Unlinked to hospital stay records |
| Total | 2,484 |
Out of the matched data, there were 1,316 unique admissions with a known discharge method; 155 (12%) patients with a bacteremia died on discharge, and 120 (77%) of them were adults age 65 years or older.
Cardiovascular disease was the most common comorbidity recorded for a hospital admission associated with NTS bacteremia. A total of 969 (69%) admissions out of all 1,397 blood isolates matched with a hospital admission episode had a primary or secondary diagnosis of cardiovascular disease recorded (Table 5). Fifty-six (4%) cases had mycotic aneurysms recorded as a part of the clinical illness. There were no cases matched with sickle cell disease.
TABLE 5.
Comorbidities, clinical details, or complications recorded for hospital inpatients (n = 1,397) with a nontyhoidal Salmonella bacteremia in England, 2004 to 2015
| ICD-10 group of conditions | No. (%) of primary or secondary ICD-10 diagnosis |
|---|---|
| Cardiovascular conditions | 969 (69) |
| NTS infectionsa | 818 (59) |
| Pulmonary | 499 (36) |
| Renal genitourinary | 485 (35) |
| Gastrointestinal | 460 (33) |
| Musculoskeletal | 405 (29) |
| Other conditions | 389 (28) |
| Metabolic syndrome | 311 (22) |
| Malignancy (solid-organ and hematological) | 309 (22) |
| Malignancy (others) | 304 (22) |
| Endocrine, including diabetes | 280 (20) |
| Hematological (nonmalignant) | 207 (15) |
| Complications of NTSb | 206 (15) |
| Other Salmonella infections | 155 (11) |
| Other systemic bacterial infection | 150 (11) |
| Neuropsychiatric disorder | 149 (11) |
Includes Salmonella enteritis, Salmonella sepsis, localized Salmonella infections.
Includes Salmonella meningitis, Salmonella pneumonia, Salmonella arthritis, Salmonella osteomyelitis, Salmonella pyelonephritis, Salmonella with other localized infection, other specified Salmonella infections, Salmonella infections unspecified.
DISCUSSION
We confirmed findings from previous studies that males and older adults are more likely to develop NTS blood infections (4, 21–23). We did not find that international travel was associated with an increased risk of hospitalization for NTS bacteremia; however, we did not have travel history information in 42% of cases. This contrasts with studies reporting that international travel is a notable risk factor for being hospitalized with NTS bacteremia (24). A recent study from the United States found quinolone resistance in S. Enteritidis to be strongly associated with international travel (25).
S. Enteritidis was the most common serovar isolated from all specimens and from blood isolates in England during the study, but the risk of developing a bacteremia was highest for patients infected with S. Dublin, followed by S. Panama, S. Colindale, and S. Poona. The most invasive serovars from England (S. Poona, S. Panama, and S. Dublin) have also been noted in other developed countries, like the United States and Australia (4, 26). We found that S. Typhimurium was not the most invasive serovar in England, which is very different from other countries and regions, such as Australia, South East Asia, and sub-Saharan Africa, where invasive S. Typhimurium predominates (26–28).
In this study, the rates of resistance to ampicillin and ciprofloxacin were 17.3% and 20.7%, respectively. More than one-third (34%) of all blood isolates showed resistance to a first-line agent, including ampicillin, ceftriaxone, and ciprofloxacin. No azithromycin resistance was found, but this may be partly because it was not routinely tested before 2013.
Ciprofloxacin is a first-line treatment which is particularly active against Gram-negative bacteria, such as Salmonella spp. (29). We found that the levels of ciprofloxacin resistance have fallen from around 27% of NTS blood isolates in 2004 to 17% in 2014, even though the change was not statistically significant. A previous study conducted in England and Wales showed increasing resistance to ciprofloxacin (MICs, 0.25 to 1.0 mg/liter) among NTS from humans between 1994 and 1997 (9). Resistance was associated with the 4 most common serotypes of S. Enterica, comprising 89% of the isolates identified in the study. Increased ciprofloxacin resistance has also been reported in the United States between 2003 and 2013, but the trend for blood isolates was not statistically significant (4). Resistance to ceftriaxone remained at very low levels between 2004 and 2015. Based on these findings, we recommend that clinicians in the UK use third-generation cephalosporins for empirical treatment of NTS bacteremia and rationalize treatment according to antimicrobial susceptibility results. Quinolones should be avoided as empirical treatment due to the high prevalence of resistance. Azithromycin can be a suitable oral follow-up option after the initial intravenous therapy, as it has excellent intracellular penetration and is very useful for the control of deep-seated metastatic foci in complicated infections. The duration of intravenous treatment ranges from 7 to 14 days for bacteremia and can be extended to 6 to 12 weeks for complicated infections, such as endocarditis or bone and joint infections. One of the drawbacks of the study was that the HES data did not capture the details of treatment received by the cases.
We found 57 isolates with resistance to colistin during the study period, 18 of which were S. Enteritidis. Colistin is being used as a last-resort drug to treat infections caused by multidrug-resistant (MDR) Gram-negative bacteria. Colistin resistance is low in Salmonella spp., with some serovars having inherent chromosomal resistance to colistin; however, ongoing surveillance for transmissible resistance is vital (14).
NTS bacteremia in admitted hospital patients was closely associated with cardiovascular, pulmonary, and genitourinary comorbidities and specific conditions, such as diabetes, primary hypertension, and acute kidney failure. This is consistent with reports that NTS bacteremia may follow more commonly in patients who are immunocompromised or have comorbid diseases, and mortality generally rises with increasing age (14). A higher rate of mortality in the elderly is thought to be due to the presence of several comorbidities (21).
These analyses indicate an increase in bacteremia from 2004 to 2015. This was partly explained by a change in the age distribution of isolates, with an increase in isolates in those age 65+ years, although the linear trend persisted after accounting for demographic variables (adjusted OR, 1.05 per year increase).
This study has some limitations. LIMS stores information based on isolates submitted rather than on patients. This means that the analysis is also based on isolates rather than individual patients, with the exception of the mortality analysis.
The results of data linkage provided reasonable results, with the majority of the data being linked on the basis of the NHS number. However, some records did not have an NHS number or there were errors in the number preventing the record from being linked without doing further work. Where NHS numbers were missing or incorrect, the name, sex, and date of birth allowed for an NHS number to be found from the patient demographic survey; therefore, these data were reasonably accurate. There is a risk of bias being introduced into the hospital admissions analysis because not all LIMS data were matched to a hospital admission.
It was not possible to calculate the odds ratio for antimicrobial resistance for invasive NTS, as the study did not look at resistance for all NTS isolates. Due to the absence of testing for a number of antimicrobials before 2013, it was not possible to analyze trends for the entire period of the study for certain antibiotics, such as azithromycin.
The hospital admissions data can contain up to 21 fields of diagnostic ICD-10 data for each admitted person. This means that a detailed analysis of comorbidities was not feasible and beyond the scope for inclusion in this study. Further studies are needed on hospital admissions data to provide more precise estimates on the prevalence of comorbidities associated with NTS bacteremia, including subgroup analysis. Further enhanced surveillance of NTS bacteremias with a focus on the details of recent travel, comorbidities, treatment, and outcome will be useful to improve the understanding of this disease.
NTS bacteremia tends to develop in older people with comorbidities. The outcomes for older people can lead to prolonged hospital stays and can result in death. Resistance of NTS to some first-line antimicrobial agents appears to have been stable in England over the last decade, but the emergence of resistance to last-choice antibiotics requires careful monitoring, as it could limit future treatment options.
ACKNOWLEDGMENTS
We acknowledge the NHS hospitals and PHE microbiology specialist laboratories for contributing isolates and hospital data and health protection teams for their help in the national Salmonella surveillance program.
We received no specific funding for this work.
REFERENCES
- 1.O’Brien SJ. 2013. The “decline and fall” of nontyphoidal Salmonella in the United Kingdom. Clin Infect Dis 56:705–710. doi: 10.1093/cid/cis967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick ME, Adcock PM, Gomez TM, Altekruse SF, Holland BH, Tauxe RV, Swerdlow DL. 2004. Salmonella Enteritidis infections, United States, 1985–1999. Emerg Infect Dis 10:1–7. doi: 10.3201/eid1001.020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England. 2017. Salmonella data 2006 to 2015: national laboratory data for residents of England and Wales, Public Health England, London, United Kingdom: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/598401/Salmonella_2016_Data.pdf. [Google Scholar]
- 4.Angelo KM, Reynolds J, Karp BE, Hoekstra RM, Scheel CM, Friedman C. 2016. Antimicrobial resistance among nontyphoidal Salmonella isolated from blood in the United States, 2003–2013. J Infect Dis 214:1565–1570. doi: 10.1093/infdis/jiw415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galanakis E, Bitsori M, Maraki S, Giannakopoulou C, Samonis G, Tselentis Y. 2007. Invasive non-typhoidal salmonellosis in immunocompetent infants and children. Int J Infect Dis 11:36–39. doi: 10.1016/j.ijid.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Su L-H, Chiu C-H, Chu C, Ou JT. 2004. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin Infect Dis 39:546–551. doi: 10.1086/422726. [DOI] [PubMed] [Google Scholar]
- 7.Prestinaci F, Pezzotti P, Pantosti A. 2015. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Threlfall EJ. 2002. Antimicrobial drug resistance in Salmonella: problems and perspectives in food- and water-borne infections. FEMS Microbiol Rev 26:141–148. doi: 10.1111/j.1574-6976.2002.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 9.Threlfall EJ, Ward LR, Rowe B. 1999. Resistance to ciprofioxacin in non-typhoidal salmonellas from humans in England and Wales— the current situation. Clin Microbiol Infect 5:130–134. doi: 10.1111/j.1469-0691.1999.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 10.Burke L, Hopkins KL, Meunier D, de Pinna E, Fitzgerald-Hughes D, Humphreys H, Woodford N. 2014. Resistance to third-generation cephalosporins in human non-typhoidal Salmonella enterica isolates from England and Wales, 2010–12. J Antimicrob Chemother 69:977–981. doi: 10.1093/jac/dkt469. [DOI] [PubMed] [Google Scholar]
- 11.Liang Z, Ke B, Deng X, Liang J, Ran L, Lu L, He D, Huang Q, Ke C, Li Z, Yu H, Klena JD, Wu S. 2015. Serotypes, seasonal trends, and antibiotic resistance of non-typhoidal Salmonella from human patients in Guangdong Province, China, 2009–2012. BMC Infect Dis 15:53. doi: 10.1186/s12879-015-0784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morpeth SC, Ramadhani HO, Crump JA. 2009. Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis 49:606–611. doi: 10.1086/603553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu R-B, Tsay Y-G, Chen J, Chu S-H. 2003. Risk factors for primary bacteremia and endovascular infection in patients without acquired immunodeficiency syndrome who have nontyphoid salmonellosis. Clin Infect Dis 36:829. doi: 10.1086/367932. [DOI] [PubMed] [Google Scholar]
- 14.Gradel O, Dethlefsen C, Schønheyder C, Nielsen H. 2008. Magnitude of bacteraemia is associated with increased mortality in non-typhoid salmonellosis: a one-year follow-up study. APMIS 116:147. doi: 10.1111/j.1600-0463.2008.00886.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen PL, Chang CM, Wu CJ, Ko NY, Lee NY, Lee HC, Shih HI, Lee CC, Wang RR, Ko WC. 2007. Extraintestinal focal infections in adults with nontyphoid Salmonella bacteraemia: predisposing factors and clinical outcome. J Intern Med 261:91–100. doi: 10.1111/j.1365-2796.2006.01748.x. [DOI] [PubMed] [Google Scholar]
- 16.Ashton PM, Nair S, Peters TM, Bale JA, Powell DG, Painset A, Tewolde R, Schaefer U, Jenkins C, Dallman TJ, de Pinna EM, Grant KA, Salmonella Whole Genome Sequencing Implementation Group. 2016. Identification of Salmonella for public health surveillance using whole genome sequencing. PeerJ 4:e1752. doi: 10.7717/peerj.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Threlfall EJ, Fisher IS, Ward LR, Tschape H, Gerner-Smidt P. 1999. Harmonization of antibiotic susceptibility testing for Salmonella: results of a study by 18 national reference laboratories within the European Union-funded Enter-net group. Microb Drug Resist 5:195–200. doi: 10.1089/mdr.1999.5.195. [DOI] [PubMed] [Google Scholar]
- 18.European Committee on Antimicrobial Susceptibility Testing. 2014. Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0. European Committee on Antimicrobial Susceptibility Testing, Vaxjo, Sweden. [Google Scholar]
- 19.Meakins S, Fisher IST, Berghold C, Gerner-Smidt P, Tschäpe H, Cormican M, Luzzi I, Schneider F, Wannett W, Coia J, Echeita A, Threlfall EJ. 2008. Antimicrobial drug resistance in human nontyphoidal Salmonella isolates in Europe 2000–2004: a report from the Enter-net International Surveillance Network. Microb Drug Resist 14:31–36. doi: 10.1089/mdr.2008.0777. [DOI] [PubMed] [Google Scholar]
- 20.Ellington MJ, Findlay J, Hopkins KL, Meunier D, Alvarez-Buylla A, Horner C, McEwan A, Guiver M, McCrae L-X, Woodford N, Hawkey P. 2016. Multicentre evaluation of a real-time PCR assay to detect genes encoding clinically relevant carbapenemases in cultured bacteria. Int J Antimicrob Agents 47:151–154. doi: 10.1016/j.ijantimicag.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Chen PL, Lee HC, Lee NY, Wu CJ, Lin SH, Shih HI, Lee CC, Ko WC, Chang CM. 2012. Non-typhoidal Salmonella bacteraemia in elderly patients: an increased risk for endovascular infections, osteomyelitis and mortality. Epidemiol Infect 140:2037–2044. doi: 10.1017/S0950268811002901. [DOI] [PubMed] [Google Scholar]
- 22.Kiratisin P. 2008. Bacteraemia due to non-typhoidal Salmonella in Thailand: clinical and microbiological analysis. Trans R Soc Trop Med Hyg 102:384. doi: 10.1016/j.trstmh.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. 2006. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol 6:101. doi: 10.1186/1471-2180-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch K, Kristensen B, Holt HM, Ethelberg S, Molbak K, Schonheyder HC. 2011. International travel and the risk of hospitalization with non-typhoidal Salmonella bacteremia. A Danish population-based cohort study 1999–2008 BMC Infect Dis 11:277. doi: 10.1186/1471-2334-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell AT, Vieira AR, Huang JY, Whichard J, Cole D, Karp BE. 2014. Quinolone-resistant Salmonella enterica serotype Enteritidis infections associated with international travel. Clin Infect Dis 59:e139–e141. doi: 10.1093/cid/ciu505. [DOI] [PubMed] [Google Scholar]
- 26.Williamson DA, Lane CR, Easton M, Valcanis M, Strachan J, Veitch MG, Kirk MD, Howden BP. 2018. Increasing antimicrobial resistance in nontyphoidal Salmonella isolates in Australia from 1979 to 2015. Antimicrob Agents Chemother 62:e02012-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dekker D, Krumkamp R, Eibach D, Sarpong N, Boahen KG, Frimpong M, Fechtner E, Poppert S, Hagen RM, Schwarz NG, Adu-Sarkodie Y, Owusu-Dabo E, Im J, Marks F, Frickmann H, May J. 2018. Characterization of Salmonella enterica from invasive bloodstream infections and water sources in rural Ghana. BMC Infect Dis 18:47. doi: 10.1186/s12879-018-2957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mather AE, Phuong TLT, Gao Y, Clare S, Mukhopadhyay S, Goulding DA, Hoang NTD, Tuyen HT, Lan NPH, Thompson CN, Trang NHT, Carrique-Mas J, Tue NT, Campbell JI, Rabaa MA, Thanh DP, Harcourt K, Hoa NT, Trung NV, Schultsz C, Perron GG, Coia JE, Brown DJ, Okoro C, Parkhill J, Thomson NR, Chau NVV, Thwaites GE, Maskell DJ, Dougan G, Kenney LJ, Baker S. 2018. New variant of multidrug-resistant Salmonella enterica serovar Typhimurium associated with invasive disease in immunocompromised patients in Vietnam. mBio 9:e01056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joint Formulary Committee. 2017. British National Formulary. 5.1.12. Quinolones, on BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com/#/content/bnf/_333860278?hspl=ciprofloxacin.