Abstract
Background:
Nighttime food intake has rarely been studied in inpatient settings and only one study observed a relation between self-reported nighttime eating and weight gain.
Objective:
We investigated the prevalence of nighttime eating and its effect on weight change.
Design:
Healthy nondiabetic Pima Indians (n = 117; 67 M, 50 F) and whites (n = 43; 29 M, 13 F) were admitted to a clinical research unit. After consuming a standardized diet for 3 d, participants ate ad libitum from a computer-operated vending machine that recorded the time of food selection. Energy intake was calculated as mean kcal/d. Follow-up weight was available for 94 volunteers.
Results:
Fifty-five subjects (36%) were nighttime eaters (NEs; persons who ate between 2300 and 0500 on ≥1 of the 3 d). Prevalence was similar among whites and Pima Indians (37% and 35%, respectively). There were no significant differences in body mass index or percentage body fat between NEs and non-NEs. NEs consumed more calories per day (4758) than did non-NEs (4244; P = 0.02), but the percentage of calories from macronutrients did not differ. NEs consumed ≈15% (690 kcal) of their daily energy during nighttime episodes. After control for baseline weight and follow-up time (), NEs (n = 29) gained more weight (6.2 kg) than did non-NEs (n = 65; 1.7 kg; P = 0.03).
Conclusions:
Nighttime eating was common, and it predicted weight gain. It remains to be determined whether this behavior indicates abnormal sleep patterns leading to nighttime wakefulness and food intake in those prone to weight gain. Am J Clin Nutr 2008;88:900–5.
INTRODUCTION
In 1955, Stunkard described what was termed the night eating syndrome (NES) as an eating disorder that encompasses morning anorexia, evening hyperphagia, and sleep disturbances (1). Despite the syndrome name, eating at night was not specifically considered one of the cardinal features of the syndrome, but it has now been proposed as such (2–4). In fact, evening hyperphagia (now accepted as the consumption of ≥25% of daily calories after the evening meal) and nocturnal ingestions (defined as awakening from sleep ≥3 times/wk to eat) often co-exist (5). The prevalence of NES is reportedly low in the general population[0.4–1.6% (6–8)], and the relation between NES and obesity varies. Some studies showed no association (7, 9), but others showed a rising prevalence of NES in specific obese populations (10, 11)—prevalences of 6–16% in persons presenting to weight-loss clinics (12–15) and 8–42% in bariatric surgery candidates (16–18). In a study in psychiatric clinics, obese patients were 5 times more likely to be diagnosed with NES than were normal-weight patients (19). Obese persons with NES have less successful weight-loss outcomes than do those without NES (1, 15, 20).
NES is associated with significant psychological distress (4, 21, 22) and is more common in selected obese populations, but whether its cardinal feature (nighttime food consumption) is related to greater calorie intake and hence greater weight gain is unclear. Only one study to date has prospectively assessed the relation between nighttime eating and subsequent weight gain, and it found an association only in obese women (23).
The purposes of the current study, therefore, were to retrospectively assess the prevalence of nighttime eating in a population of Pima Indian and white adults who had previously participated in a carefully controlled inpatient study of food intake and to relate the effect of nighttime eating to overall calorie consumption and subsequent weight gain.
SUBJECTS AND METHODS
Subjects
One hundred seventeen Pima Indians (67 M, 50 F) and 43 whites (29 M, 14 F) were recruited between December 1999 and November 2005 from the Gila River Indian Community 40 miles southeast of Phoenix (Pima Indians) and by advertisement throughout the greater Phoenix area (whites).
All subjects were found to be free of disease according to physical examination, medical history, and laboratory testing. On admission to the metabolic ward, subjects were fed a standard weight-maintaining diet (20%, 30%, and 50% of daily calories provided as protein, fat and carbohydrate, respectively) for 3 d before testing. Weight-maintenance energy needs (WMEN) on the metabolic ward were calculated for each subject according to weight and sex [men: 9.5 × weight (kg) + 1973; women: 9.5 × weight (kg) + 1745 (24)]. Body composition was measured by using dual-energy X-ray absorptiometry (DPX-L; Lunar Corp, Madison, WI) as described previously (25, 26). Glucose tolerance was assessed by using a 75-g oral-glucose-tolerance test (OGTT) according to the criteria of the World Health Organization. Only nondiabetic subjects participated in this study.
Before participation, all volunteers were fully informed of the nature and purpose of the study, and written informed consent was obtained. The experimental protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
Assessment of food preferences
After admission to the metabolic ward, subjects completed an 80-item food preferences questionnaire containing typical breakfast, lunch, dinner, and snack items as described previously (27). Briefly, foods were categorized as being high (>45% kcal) or low (<20% kcal) in fat and, within each of these categories, as being high in simple sugar (>30% kcal), complex carbohydrate (>30% kcal), or protein (>13% kcal) (28). Subjects were asked to assign each food a hedonic rating by using a 9-point Likert scale with the following anchors: 1 = dislike extremely; 5 = neutral; and 9 = like extremely. An option to indicate that the food item had never been tasted was also included.
Ad libitum food intake using a computerized vending machine system
During the final 3 d on the metabolic ward, subjects were asked to self-select all of their food by using a computer-operated vending machine system, which was previously described, validated, and tested for reproducibility [intraclass correlation for energy intake = 0.90; P < 0.0001 (27, 29, 30)]. Briefly, an automated food-selection system is made up of a refrigerated vending machine (model 3007; U-Select-It, Des Moines, IA) that contains 40 trays.
The 40 food items made available to the subjects on each of the 3 d consisted of those foods to which the subject had assigned an intermediate high (between 4 and 8) hedonic rating on the food preferences questionnaire. In addition, a core group of condiments, beverages, breads, and spreads was provided to each subject each day. The same selection was offered each day, and it accommodated the appropriateness of certain foods for breakfast, lunch, dinner, or evening snacks. Subjects had free access to the vending machines ad libitum for 23.5 h/d. The refrigerated machines were housed in a separate eating area, and subjects were instructed to eat only in the vending room and to eat whatever they wanted whenever they wished. They were instructed to return all uneaten portions and wrappers. Television viewing during food consumption was prohibited.
The time of day and the foods eaten were recorded during each visit to the vending machine. With the use of the CBORD Professional Diet Analyzer Program (CBORD Inc, Ithaca, NY), daily energy, protein, fat, and carbohydrate intakes were calculated from the actual weights of food and condiments consumed; the database was modified to reflect the nutrient content of specific food items as indicated by the food manufacturer. Results are presented as the mean ± SD of the 3 d; the percentage WMEN was calculated as [(mean daily energy consumed/WMEN) × 100].
Nighttime eating status
The Clinical Research Unit requires that all subjects be in their rooms with the lights out at 2300. During the present study, volunteers were required to be in bed with the lights out at that time, but they were permitted to get up and use the vending machines during the night if so desired. Subjects are routinely awakened at 0500 so that morning weight can be measured. Therefore, subjects who had energy intakes recorded between 2300 and 0500 on any of the 3 d during which they used the vending machines were considered nighttime eaters (NEs), and those who never consumed any food after 2300 were categorized as non-nighttime eaters (non-NEs).
Eating behavior and body image
Eating behavior was assessed by using the Three-Factor Eating Questionnaire (31), a 51-item instrument composed of 3 subscales measuring dietary restraint (ie, the cognitive control of eating), disinhibition (ie, the tendency to have an uninhibited response to food), and perceived hunger (ie, the susceptibility of eating in response to subjective feelings of hunger). The Gormally Binge Eating Scale (32), which discriminates among persons having no, moderate, or severe binge-eating problems, was used to assess binge-eating behavior. Body image perception was assessed by using the Figure Rating Scale (33), which consists of 9 male and female silhouettes ranging from very thin to overweight. Participants indicated by number (from 10 to 90) their current body size and their ideal figure. The questionnaires were self-administered by each subject before the ad libitum use of the vending machines.
Follow-up study
Subjects with follow-up weights obtained ≥6 mo after their baseline weight and who did not have diabetes at the follow-up visit were included. Follow-up weights were collected from repeat visits to the Clinical Research Unit or from a longitudinal study of health in the Gila River Indian Community, in which persons are invited to participate every 2 y and to undergo an OGTT and a measurement of height and weight. In the case of multiple follow-up visits, the weight obtained at the last visit in which a subject was not diabetic was used. Weight change was expressed as absolute change (final–baseline weight), weight change per year (final weight–baseline)/follow-up time, percentage weight change (final–baseline/baseline) × 100, and percentage weight change per year (% weight change/follow-up time).
Statistical analysis
All procedures were carried out with SAS software (version8.2; SAS Inst, Cary, NC). Pearson’s correlation coefficients were used to describe relations between continuous variables, and a chi-square test was used for categorical data. Linear regression models were used to calculate adjusted least-squares means and 95% CIs for differences in anthropometric, metabolic, and behavioral characteristics and energy intake and weight change adjusted for covariates by night eating status. Statistical significance was set at P < 0.05.
RESULTS
Baseline characteristics
Fifty-five (36%) subjects (16 whites, 39 Pima Indians) were categorized as NEs. and 99 (27 whites, 72 Pima Indians) were categorized as non-NEs. Frequency of nighttime eating did not differ significantly between whites and Pima Indians (37% and 35%, respectively; P = 0.77) and between men and women (22% and 14%, respectively; P = 0.79).
The physical and behavioral characteristics of the study participants are shown in Table 1. These variables did not differ by race (data not shown), but females had significantly higher body mass index (BMI; in kg/m2) and percentage body fat than did males (P = 0.002 and P < 0.0001, respectively). There were no significant differences between NEs and non-NEs in body weight, percentage body fat, or BMI, although NEs were slightly younger (31 ± 8 y) than non-NEs (34 ± 8 y; P = 0.05).
TABLE 1.
Metabolic characteristics and intake data for nighttime eaters (NEs) and non-nighttime eaters (non-NEs)1
| Non-NEs | NEs | P | |
|---|---|---|---|
| Age (y)2 | 34 ± 8 | 31 ± 8 | 0.05 |
| Weight (kg) | 95 (89, 100)3 | 98 (91, 105) | 0.47 |
| Body fat (%) | 40 (38, 41) | 40 (38, 43) | 0.43 |
| BMI (kg/m2) | 34 (32, 35) | 33 (32, 36) | 0.63 |
| Mean energy intake (kcal) | 4244 (3971, 4517) | 4758 (4409, 5107) | 0.02 |
| Energy intake (% of WMEN) | 151 (141, 160) | 170 (158, 182) | 0.01 |
| Cholesterol intake (% of kcal) | 49 (48, 51) | 50 (49, 52) | 0.4 |
| Protein intake (% of kcal) | 14 (14, 15) | 14 (13, 15) | 0.5 |
| Fat intake (% of kcal) | 40 (38, 41) | 39 (37, 40) | 0.4 |
WMEN, weight-maintenance energy needs. n = 99 (59 M, 40 F) and 55 (34 M, 21 F) for non-NEs and NEs, respectively. General linear regression models were adjusted for sex, age, and race.
(all such values); Student’s t test.
Adjusted ; 95% CIs in parentheses (all such values).
Energy intake
Table 1 lists the energy intake data for the NEs and non-NEs. NEs consumed significantly (P = 0.02) more calories (: 4758; 95% CI: 4409, 5107) than did non-NEs (4244; 3971, 4517). On average, NEs consumed significantly (P = 0.01) more of their estimated percentage WMEN than did non-NEs (170%; 158, 182 and 151%; 141, 160, respectively). The difference in energy intake between the groups after the calories consumed during nighttime ingestions were subtracted was not significant (P = 0.43). NEs consumed significantly more grams of protein (P = 0 0.04) and carbohydrate (P = 0.01) and had a trend toward consuming more fat (P = 0.07) than did non-NEs; however, the percentages of calories from protein, carbohydrate, and fat did not differ significantly between the groups.
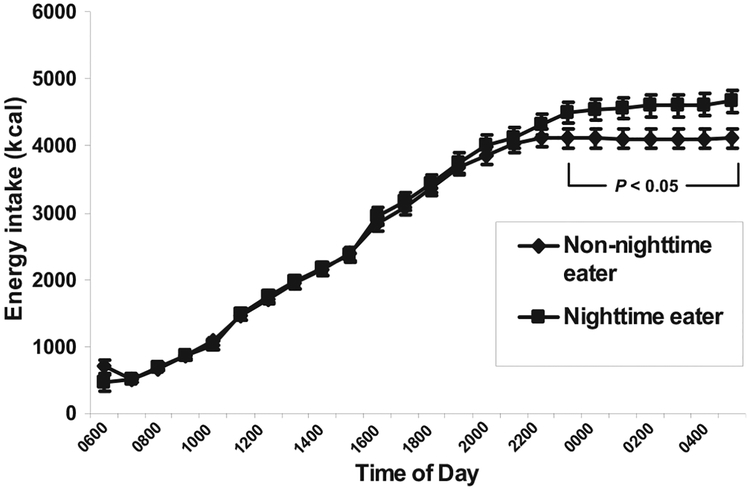
As illustrated in Figure 1, the differences in energy intake between NEs and non-NEs began appearing at 2200 (P = 0.07); at that time, non-NEs ceased consuming food, whereas NEs, by definition, continued to eat between 2300 and 0500 (P < 0.05 for all time-points). Examination of the distribution of 24-h energy intakes assessed by time periods during the day (morning: 1600–1059; afternoon: 1100–1659; evening: 1700–2259; and night: 2300–0559) showed that NEs did not differ significantly from non-NEs during any time period except the night (P < 0.0001).
FIGURE 1.
24-h Cumulative energy intake. n = 154 [99 non-nighttime eaters (non-NEs), 55 nighttime eaters (NEs)]. Measured as kcal/d = mean kilocalories over a 3-d period while subjects selected all food ad libitum from a computer-operated vending machine system. NEs were subjects who had energy intake recorded between 2300 and 0500 on any of the 3 d on which they used the vending machines; non-NEs were subjects who never consumed any food after 2300. All comparisons of mean kcal between NEs and non-NEs at each hour from 2300 through 0500m differed significantly (P < 0.05).
Nighttime ingestions
NEs consumed an average of 690 ± 436 kcal between 2300 and 0500, and this food represented ≈15 ± 3% of their daily caloric intake. Carbohydrates constituted the bulk of the calories consumed during these nighttime episodes (61.5%); fat (30.9%) and protein intakes (8.7%) were much lower.
Eating behavior psychopathology and body image
Women had significantly (P = 0.02) higher binge-eating scores than did men, but there were no significant differences between whites and Pima Indians or between NEs and non-NEs. There was a trend toward greater restrained eating in non-NEs than in the NEs [F = 3.6; P = 0.06], but there were no significant differences by eating groups, race, or sex for disinhibition or cognitive hunger ratings. On the Figure Rating Scale, there were no significant differences between NEs and non-NEs in the figures chosen to represent the subjects’ current or ideal appearance (Table 2).
TABLE 2.
Eating behavior and body image scores for nighttime eaters (NEs) and non-nighttime eaters (non-NEs)1
| Non-NEs | NEs | P | |
|---|---|---|---|
| Restrained eating (TFEQ) | 7.3 (6.4, 8.2)2 | 5.9 (4.8, 7.1) | 0.06 |
| Cognitive hunger (TFEQ) | 5.2 (4.5, 5.9) | 5.8 (4.8, 6.7) | 0.32 |
| Disinhibition (TFEQ) | 6.1 (38, 41) | 6.2 (38, 43) | 0.86 |
| Binge eating (BES) | 10.6 (8.9, 12.3) | 11.4 (9.2, 13.5) | 0.54 |
| Current appearance (FRS) | 5.4 (5.0, 5.8) | 5.8 (5.3, 6.3) | 0.17 |
| Ideal appearance (FRS) | 3.4 (3.3, 3.7) | 3.7 (3.5, 4.0) | 0.13 |
TFEQ, Three-Factor Eating Questionnaire; BES, Gormally Binge Eating Scale; FRS, Figure Rating Scale. Body image scores were based on general linear regression models. All means were adjusted for sex, age, and race.
Adjusted ; 95% CIs in parentheses (all such values).
Follow-up
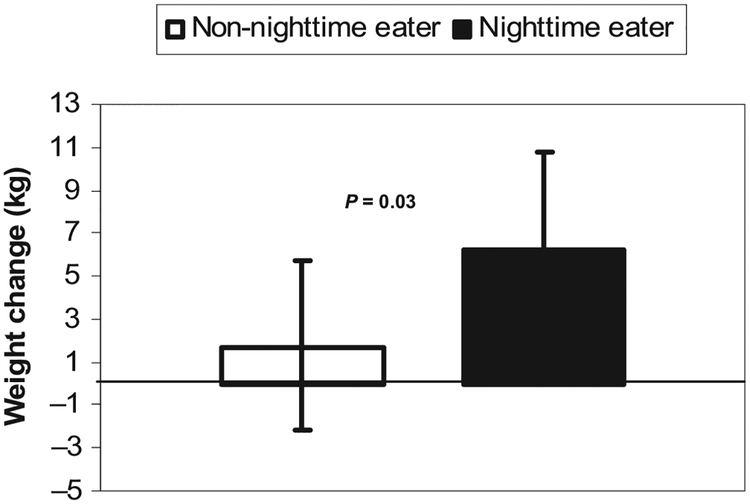
Ninety-four subjects (29 NEs, 65 non-NEs; 55 M, 39 F; 7 whites, 87 Pima Indians) had follow-up weights available ( follow-up time: 3.4 ± 1.8 y; range: 0.5–7.4 y). There was no significant difference in follow-up time between NEs and non-NEs. After control for sex, age, race, baseline weight, and follow-up time, NEs gained significantly (P = 0.03) more weight(6.2 kg) than did non-NEs (1.7 kg) (Figure 2). Additional analyses showed similar significant differences for percentage weight change (6.9% and 2.3%, respectively; P = 0.04), weight change per year (2.7 and 1.1 kg/y, respectively; P = 0.02), and percentage weight change per year (3.1% and 1.5%, respectively; P = 0.04).
FIGURE 2.
Weight gain in nighttime eaters (NEs) was greater at follow-up than that in non-nighttime eaters (non-NEs). n = 94 (29 NEs, 65 non-NEs). Weight change was measured as the change in weight from admission to last identified follow-up weight, adjusted for sex, age, race, baseline weight, and follow-up time. Error bars epresent 95% CIs.
DISCUSSION
Nighttime eating was common in this carefully controlled inpatient study of food intake, and it did not vary in prevalence between Pima Indian and whites. NEs consumed more total calories than did non-NEs, and the difference in intake resulted from the calories ingested at night. Although the groups did not differ by either BMI or percentage body fat at baseline, NEs gained significantly more weight during the follow-up period than did non-NEs.
This study design is the first to investigate nighttime eating in an inpatient setting by using computerized vending machines to accurately record food consumption over multiple days. Several previous studies have relied on 24-h food diaries to assess nighttime ingestions related to NES (5, 34, 35), and only 2 overnight laboratory studies of NES have been conducted (35, 36). In the first inpatient study of NES, Birketvedt et al (35) collected 24-h blood measurements while subjects were fed 4 meals at 0800, 1200, 1600, and 2000. However, food was not provided at night, and thus the food intake was not representative of the subjects’ typical pattern. Allison et al (36) improved on this design by observing ad libitum nighttime food intake in a study in which subjects were served 3 meals/d, and ad libitum snacks were available at bedside for access during nighttime awakenings. Obvious strengths of the current study are that subjects had ad libitum access to food for nearly 24 h/d on 3 consecutive days, and follow-up data were available (unlike either of the abovementioned inpatient studies).
It is interesting that the eating pattern we observed was not consistent with that previously reported in studies of classic NES (35, 36). For example, our nighttime eaters did not consume less during the morning or afternoon hours. However, the importance of this pattern has been challenged, because recent studies indicated that morning anorexia was not significantly related to total score on the night eating diagnostic questionnaire (22) and thus may not be an important distinguishing diagnostic symptom of NES (37). Furthermore, a report comparing waking at night to eat with simply eating late at night (without having gone to bed first) did not find a pattern of lower morning intake (13). We did not use subjective appetite ratings to assess morning anorexia.
We failed to observe differences between the groups in measures of eating psychopathology (ie, binge eating, restraint, hunger, and disinhibition) or body image. These findings are consistent with a recent study in which no relations were observed between nocturnal snacking and hunger, disinhibition, or cognitive restraint scores on the Three-Factor Eating Questionnaire (11). Although greater eating-related psychopathologic behavior was observed previously (4, 21, 22), our ability to observe such differences may have been limited because the present study population was not previously defined as having NES, as was done in the earlier reports. We also did not collect data on psychological measures at follow-up, and it is possible that the pathologic eating behavior of the NEs worsened over time as their weight increased.
A limitation of the present study is that we could not assess whether the NEs would have met the full criteria for NES. Although we selected the time period from 2300 to 0500 to represent the time when patients were presumably asleep with the lights out, sleep onset was not documented. Thus, it is possible that these subjects were not waking up to eat but were simply eating late at night. Although we have accurate recordings of the timing of all ingestions, we cannot identify which ingestions were considered by the subjects to be meals. Thus, evening hyperphagia, defined by the percentage of calories consumed after the evening meal and considered a feature of NES, could not be classified. The NEs also did not appear to eat more than the non-NEs until after 2300. However, because nocturnal ingestions appear to explain the highest amount of variance in a diagnosis of NES (22; R Gupta, A Geliekter, unpublished observations, 2007), and because the vast majority of persons with nocturnal ingestions also reported consuming ≥25% of their daily calories after the evening meal (5), we suspect that a subset of our subjects may also have met this criterion and thus the full criteria for NES. It must be acknowledged that, on average, all persons overate throughout the 24-h period, which made comparisons with previous NES studies difficult. Nevertheless, NEs still clearly ate more and, at least as important, gained more weight over time than did non-NEs.
In a previous study of circadian eating and sleeping patters in NES, O’Reardon et al (34) observed a phase delay in energy consumption and more nocturnal awakenings in those with NES than in controls but no differences in the timing of the sleep-wake cycle. Given that the relation between sleep deprivation and hyperphagia has been well documented (38), it remains unclear as to whether the primary disturbance observed in the present study is due to altered circadian rhythms or whether it indicates the presence of true NES.
In summary, on the basis of the isolated behavior described here of recorded food intake during the night and its predisposition to subsequent weight gain, we have identified nighttime eating as a maladaptive behavior. Our findings confirm those of Andersen et al (23), who observed a greater weight gain in obese women who reported getting up at night to eat, without identification of any additional NES criteria. Whether the nighttime eating behavior observed in the present study identified persons with NES or only those with altered circadian rhythms is unknown. Regardless, these findings show an association between altered sleep patterns and greater adiposity, which suggests that the identification and avoidance of nighttime eating could be a strategy for preventing weight gain in a substantial number of persons.
We thank John Graves and the dietary staff and Carol Massengill and the nursing staff of the National Institutes of Health Clinical Unit and the staff of the Diabetes Epidemiology and Clinical Research Section, National Institute of Diabetes and Digestive and Kidney Diseases. Most of all, we thank the volunteers for their participation in the study.
The authors’ responsibilities were as follows—MEG: analysis of the data and writing the manuscript; CV: design of the study, collection of the data, data entry, and review and revision of the manuscript; AS: design of the study, collection and analysis of the data, and review and revision of the manuscript; and JK: significant advice and consultation regarding data analysis and review and revision of the manuscript. None of the authors had a personal or financial conflict of interest.
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
REFERENCES
- 1.Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome; a pattern of food intake among certain obese patients Am J Med 1955;19:78–86. [DOI] [PubMed] [Google Scholar]
- 2.O’Reardon JP, Stunkard AJ, Allison KC. Clinical trial of sertraline in the treatment of night eating syndrome. Int J Eat Disord 2004;35:16–26. [DOI] [PubMed] [Google Scholar]
- 3.Stunkard AJ, Allison KC, O’Reardon JP. The night eating syndrome: a progress report. Appetite 2005;45:182–6. [DOI] [PubMed] [Google Scholar]
- 4.Allison KC, Grilo CM, Masheb RM, Stunkard AJ. Binge eating disorder and night eating syndrome: a comparative study of disordered eating. J Consult Clin Psychol 2005;73:1107–15. [DOI] [PubMed] [Google Scholar]
- 5.Allison KC, Lundgren JD, O’Reardon JP, Moore R, Stunkard AJ. Comparing the clinical significance of the different typologies of night eating syndrome. Obesity (Silver Spring) 2007;15:A9–10 (abstr). [Google Scholar]
- 6.Rand CS, Kuldau JM. Eating patterns in normal weight individuals: bulimia, restrained eating and the night eating syndrome. Int J Eat Disord 1986;5:75–84. [Google Scholar]
- 7.Rand CS, Macgregor AM, Stunkard AJ. The night eating syndrome in the general population and among postoperative obesity surgery patients. Int J Eat Disord 1997;22:65–9. [DOI] [PubMed] [Google Scholar]
- 8.Striegel-Moore RH, Dohm FA, Hook JM, Schreiber GB, Crawford PB, Daniels SR. Night eating syndrome in young adult women: prevalence and correlates. Int J Eat Disord 2005;37:200–6. [DOI] [PubMed] [Google Scholar]
- 9.Striegel-Moore RH, Franko DL, May A, Ach E, Thompson D, Hook JM. Should night eating syndrome be included in the DSM? Int J Eat Disord 2006;39:544–9. [DOI] [PubMed] [Google Scholar]
- 10.Aronoff NJ, Geliebter A, Zammit G. Gender and body mass index as related to the night-eating syndrome in obese outpatients. J Am Diet Assoc 2001;101:102–4. [DOI] [PubMed] [Google Scholar]
- 11.Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 2007;31:1722–30. [DOI] [PubMed] [Google Scholar]
- 12.Adami GF, Campostano A, Marinari GM, Ravera G, Scopinaro N. Night eating in obesity: a descriptive study. Nutrition 2002;18:587–9. [DOI] [PubMed] [Google Scholar]
- 13.Ceru-Bjork C, Andersson I, Rossner S. Night eating and nocturnal eating—two different or similar syndromes among obese patients? Int J Obes Relat Metab Disord 2001;25:365–72. [DOI] [PubMed] [Google Scholar]
- 14.Stunkard A, Berkowitz R, Wadden T, Tanrikut C, Reiss E, Young L. Binge eating disorder and the night-eating syndrome. Int J Obes Relat Metab Disord 1996;20:1–6. [PubMed] [Google Scholar]
- 15.Gluck ME, Geliebter A, Satov T. Night eating syndrome is associated with depression, low self-esteem, reduced daytime hunger, and less weight loss in obese outpatients. Obes Res 2001;9:264–7. [DOI] [PubMed] [Google Scholar]
- 16.Adami GF, Meneghelli A, Scopinaro N. Night eating and binge eating disorder in obese patients. Int J Eat Disord 1999;25:335–8. [DOI] [PubMed] [Google Scholar]
- 17.Allison KC, Wadden TA, Sarwer DB, et al. Night eating syndrome and binge eating disorder among persons seeking bariatric surgery: prevalence and related features. Surg Obes Relat Dis 2006;2:153–8. [DOI] [PubMed] [Google Scholar]
- 18.Hsu LK, Betancourt S, Sullivan SP. Eating disturbances before and after vertical banded gastroplasty: a pilot study. Int J Eat Disord 1996;19:23–34. [DOI] [PubMed] [Google Scholar]
- 19.Lundgren JD, Allison KC, Crow S, et al. Prevalence of the night eating syndrome in a psychiatric population. Am J Psychiatry 2006;163:156–8. [DOI] [PubMed] [Google Scholar]
- 20.Powers PS, Perez A, Boyd F, Rosemurgy A. Eating pathology before and after bariatric surgery: a prospective study. Int J Eat Disord 1999;25: 293–300. [DOI] [PubMed] [Google Scholar]
- 21.Allison KC, Crow SJ, Reeves RR, et al. Binge eating disorder and night eating syndrome in adults with type 2 diabetes. Obesity (Silver Spring) 2007;15:1287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison KC, Lundgren JD, O’Reardon JP, et al. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the night eating syndrome. Eat Behav 2008;9:62–72. [DOI] [PubMed] [Google Scholar]
- 23.Andersen GS, Stunkard AJ, Sorensen TI, Petersen L, Heitmann BL. Night eating and weight change in middle-aged men and women. Int J Obes Relat Metab Disord 2004;28:1338–43. [DOI] [PubMed] [Google Scholar]
- 24.Ferraro R, Boyce VL, Swinburn B, De GM, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 1991;53:1368–71. [DOI] [PubMed] [Google Scholar]
- 25.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990;51:1106–12. [DOI] [PubMed] [Google Scholar]
- 26.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 1995;62:730–4. [DOI] [PubMed] [Google Scholar]
- 27.Salbe AD, Tschop MH, DelParigi A, Venti CA, Tataranni PA. Negative relationship between fasting plasma ghrelin concentrations and ad libitum food intake. J Clin Endocrinol Metab 2004;89:2951–6. [DOI] [PubMed] [Google Scholar]
- 28.Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav 1998;63: 919–28. [DOI] [PubMed] [Google Scholar]
- 29.Rising R, Alger S, Boyce V, et al. Food intake measured by an automated food-selection system: relationship to energy expenditure. Am J Clin Nutr 1992;55:343–9. [DOI] [PubMed] [Google Scholar]
- 30.Salbe AD, Venti CA, Franks P, Krakoff J, Tataranni PA. Reproducibility of ad libitum energy intake in humans. Obes Res 2006;13:A174–5 (abstr). [Google Scholar]
- 31.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29:71–83. [DOI] [PubMed] [Google Scholar]
- 32.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav 1982;7:47–55. [DOI] [PubMed] [Google Scholar]
- 33.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983;60:115–20. [PubMed] [Google Scholar]
- 34.O’Reardon JP, Ringel BL, Dinges DF, et al. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res 2004;12:1789–96. [DOI] [PubMed] [Google Scholar]
- 35.Birketvedt GS, Florholmen J, Sundsfjord J, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA 1999; 282:657–63. [DOI] [PubMed] [Google Scholar]
- 36.Allison KC, Ahima RS, O’Reardon JP, et al. Neuroendocrine profiles associated with energy intake, sleep, and stress in the night eating syndrome. J Clin Endocrinol Metab 2005;90:6214–7. [DOI] [PubMed] [Google Scholar]
- 37.Kelly C, Allison KC, Scott G, et al. Evaluation of diagnostic criteria for night eating syndrome using item response theory analysis. Eating Behav (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep 1989;12:13–21. [DOI] [PubMed] [Google Scholar]