Abstract
BACKGROUND AND OBJECTIVE:
Children with neurologic impairment (NI) are commonly hospitalized for different types of pneumonia, including aspiration pneumonia. We sought to compare hospital management and outcomes of children with NI diagnosed with aspiration versus nonaspiration pneumonia.
METHODS:
A retrospective study of 27 455 hospitalized children aged 1 to 18 years with NI diagnosed with pneumonia from 2007 to 2012 at 40 children’s hospitals in the Pediatric Health Information System database. The primary exposure was pneumonia type, classified as aspiration or nonaspiration. Outcomes were complications (eg, acute respiratory failure) and hospital utilization (eg, length of stay, 30-day readmission). Multivariable regression was used to assess the association between pneumonia type and outcomes, adjusting for NI type, comorbid conditions, and other characteristics.
RESULTS:
In multivariable analysis, the 9.7% of children diagnosed with aspiration pneumonia experienced more complications than children with nonaspiration pneumonia (34.0% vs 15.2%, adjusted odds ratio [aOR] 1.2 (95% confidence interval [CI] 1.1–1.3). Children with aspiration pneumonia had significantly longer length of stay (median 5 vs 3 days; ratio of means 1.2; 95% CI 1.2–1.3); more ICU transfers (4.3% vs 1.5%; aOR 1.4; 95% CI 1.1–1.9); greater hospitalization costs (median $11 594 vs $5162; ratio of means 1.2; 95% CI 1.2–1.3); and more 30-day readmissions (17.4% vs 6.8%; aOR 1.3; 95% CI 1.2–1.5).
CONCLUSIONS:
Hospitalized children with NI diagnosed with aspiration pneumonia have more complications and use more hospital resources than when diagnosed with nonaspiration pneumonia. Additional investigation is needed to understand the reasons for these differences.
What’s Known on This Subject:
Children with neurologic impairment are frequently hospitalized with pneumonia. They are at high risk for aspiration from a variety of factors including underlying muscle weakness, gastroesophageal reflux, and dysphagia.
What This Study Adds:
Hospitalized children with neurologic impairment diagnosed with aspiration pneumonia experience more diagnostic testing, more systemic complications such as acute respiratory failure, longer hospitalizations, and more readmissions than when diagnosed with nonaspiration pneumonia.
Children with neurologic impairment (NI) account for an increasing and disproportionate amount of inpatient hospital resources.1 A variety of neurologic diseases are responsible for NI (eg, hypoxic-ischemic encephalopathy, lissencephaly). NI can lead to respiratory insufficiency from central hypoventilation, gastroesophageal reflux, impaired cough, oromotor dysfunction and dysphagia, and respiratory muscle weakness.2,3 This insufficiency predisposes children with NI to respiratory infection from exogenous sources (eg, community acquired viral or bacterial pneumonia) and endogenous sources (eg, aspiration of saliva or gastric contents). In children with NI, pneumonia is one of the most common reasons for hospitalization, admission to an ICU, and death.1,4–6
In our clinical experience, pneumonia in children with NI, compared with otherwise healthy children, can be challenging to diagnose and treat because no validated clinical practice guidelines exist for pneumonia in children with NI. Little is known about the evaluation, management, and outcomes of pneumonia in children with NI. Moreover, there is a paucity of information on differences in these attributes between aspiration and nonaspiration pneumonia. In adults, aspiration pneumonia is associated with different treatment approaches (eg, higher use of anaerobic antimicrobial agents), increased hospital resource use, and worse health outcomes than nonaspiration pneumonia.7–11
Therefore, the objective of this study was to compare evaluation, management, and outcomes associated with a diagnosis of aspiration versus nonaspiration pneumonia in a multiinstitutional cohort of hospitalized children with NI.
Methods
Study Design and Data Source
This multicenter, retrospective, cohort study included data from the Pediatric Health Information System (PHIS), an administrative database of 43 not-for-profit, tertiary care, US pediatric hospitals affiliated with Children’s Hospital Association (Overland Park, KS). PHIS contains data regarding patient demographics, diagnoses and procedures (with International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes), and daily billed resource utilization (eg, laboratory studies, radiologic imaging, and pharmaceuticals). Encrypted medical record numbers permit identification of patients across multiple visits to the same hospital. Data quality and reliability are ensured through the Children’s Hospital Association and participating hospitals. Three hospitals were excluded because of data quality issues.
Study Population
Inclusion Criteria
Hospitalizations of children 1 to 18 years of age who were discharged between July 1, 2007, and June 30, 2012, were included if they had a NI ICD-9-CM diagnosis code1 and a principal diagnosis of either aspiration pneumonia or nonaspiration pneumonia. NI was defined as functional and/or intellectual impairment that resulted from a neurologic disease using a previously defined set of 606 ICD-9-CM diagnosis codes.1 Infants <1 year were excluded because many NI diagnoses (eg, cerebral palsy) are not assigned until an older age. Hospitalizations with a principal diagnosis of pneumonia (aspiration or nonaspiration) were identified with ICD-9-CM diagnosis codes based on previously used methods.7,8,10–13 Principal diagnosis is the condition established as chiefly responsible for hospital admission.14 Aspiration pneumonia codes were 507.× (eg, “pneumonitis due to solids and liquids”).7,8,10,11,13 Nonaspiration pneumonia codes included those for pneumonia (480.0-2, 480.8-9, 481, 482.0, 482.30-2, 482.41-2, 482.83, 482.89-90, 483.8, 484.3, 485, 486, 487.0) or pulmonary effusion/empyema (510.0, 510.9, 511.0-1, 511.8-9, 513).12
For children with multiple hospitalizations (13% of sample), 1 admission was randomly selected for inclusion. This method helped prevent findings from being heavily influenced by a small group of children experiencing a large number of admission or by 1 type of admission (eg, readmissions).
Exclusion Criteria
We excluded children who did not receive an antibiotic in the first 2 calendar days of admission to minimize the likelihood of including children with (1) nonbacterial pneumonia and (2) who were admitted for nonpneumonia diagnosis but treated for hospital-acquired pneumonia. Children transferred in from another hospital were excluded because records from their initial presentation including testing, treatment, and outcomes were not available in PHIS. Finally, children with a diagnosis of HIV or tuberculosis and children who received antiretroviral or antituberculosis therapy during hospitalization were excluded given expected differences in presentation, management, treatment, and outcomes15 (Supplemental Figure 2).
Outcome Measures
The primary outcome was the presence of a pneumonia-associated complication during hospitalization. Local (eg, effusion), systemic (eg, acute respiratory failure), and metastatic (eg, meningitis) complications of pneumonia were identified using previously described ICD-9-CM codes.16 Secondary outcomes were hospital resource utilization and mortality. Hospital resource utilization included length of stay (LOS) measured in billed hospital days, ICU transfer, all-cause readmission within 30 days of discharge,17 and standardized total hospitalization costs.18
Patient Demographics and Clinical Characteristics
Demographic and clinical characteristics that might differ in prevalence between children diagnosed with aspiration versus nonaspiration pneumonia were assessed, including age, gender, race/ethnicity, insurance, discharge disposition, NI category, presence of a complex chronic condition, assistance with medical technology, and presence of additional comorbidities.
Nine NI categories were assessed: (1) static neurologic disease, (2) progressive neurologic disease, (3) anatomic abnormality, (4) epilepsy, (5) genetic or metabolic condition, (6) cerebrovascular disease, (7) peripheral neurologic disease, (8) behavioral, and (9) not otherwise specified/other. These NI categories are not mutually exclusive (ie, patients may have diagnoses in multiple categories).
Complex chronic conditions (CCC)19 are “any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.”20 We assessed type and number of CCCs endured by each child. The neurologic and neuromuscular CCC category was not examined because all children in our cohort had NI diagnoses. Medical technology included devices (eg, gastrostomy, tracheostomy) used to optimize children’s health and functional status.21 Additional comorbidities known to correlate with severity of NI in children (eg, gastroesophageal reflux, scoliosis) were also assessed.
Diagnostic Testing
As our focus on initial evaluation, we assessed testing that occurred in first 2 calendar days of admission. Laboratory testing, assessed with billing data, included complete blood count, C-reactive protein, erythrocyte sedimentation rate, blood gas, blood chemistry, coagulation studies, liver function studies, and microbiologic studies (ie, viral testing, blood cultures, respiratory cultures, and urine cultures). Imaging studies included chest radiography, ultrasonography, and computed tomography.
Treatment of Pneumonia
Empirical antibiotic therapy, β agonist therapy (eg, albuterol), systemic corticosteroids (eg, prednisolone), and inhaled corticosteroids (eg, fluticasone) were examined as treatments received in the first 2 calendar days of hospitalization. Empirical antibiotic therapy included oral and intravenous antibiotics aside from oral vancomycin and oral aminoglycosides, which are not adequately absorbed to effectively treat pneumonia. Antibiotics were classified and examined by antimicrobial spectra of activity against the most commonly recognized pathogens of aspiration and nonaspiration pneumonia: (1) Streptococcus pneumoniae (eg, aminopenicillins), (2) anaerobes (eg, fluoroquinolones), and (3) Pseudomonas aeruginosa (eg, fourth-generation cephalosporins).22
Statistical Analysis
Continuous data were described with median and interquartile ranges (IQR) given nonnormal distributions. Categorical data were described with counts and frequencies. Demographics, clinical characteristics, diagnostic testing, treatment, and outcomes were stratified by pneumonia diagnosis (aspiration and nonaspiration) and compared using χ2 and Wilcoxon rank sum tests for categorical and continuous variables, respectively.
Generalized estimating equations, clustered on hospital, were derived to assess the independent effect of pneumonia diagnosis on outcomes of pneumonia-associated systemic complications, ICU transfer, and 30-day all-cause readmission while adjusting for important differences in demographic and clinical characteristics. Because LOS and costs had a nonnormal, exponential distribution, they were assessed with exponential regression that included a log link function and a random intercept for each hospital (to account for patient clustering).
Covariates were chosen a priori because of their clinical and biological relevance to exposure and outcomes: age, use of gastrointestinal and respiratory technology, nonneurologic CCC count, NI category, presence of additional comorbidities, and antimicrobial treatment. Each NI category and additional medical comorbidity was included in the models, thereby controlling for patients with multiple diagnoses (eg, 4 types of NI and 3 comorbidities). Moreover, presence of any pneumonia-associated complication was an additional covariate in models for utilization outcomes.
Generalized estimating equations results are presented with adjusted odds ratios (aOR) with 95% confidence interval (CI). LOS and cost results are presented as ratios of means (RoM) with 95% CI to depict relative differences in LOS or cost between all children within a hospital with aspiration versus nonaspiration pneumonia (eg, a RoM of 1.3 depicts a 30% longer LOS for children with aspiration pneumonia compared with those with nonaspiration pneumonia).
All analyses were performed with SAS v9.3 (SAS Institute, Cary, NC). P < .05 was considered statistically significant. Patients with missing data (<1% of all data) were removed from analysis. Cincinnati Children’s Hospital Medical Center Institutional Review Board considered this study exempt as it used a de-identified, existing data set.
Results
Study Cohort
Of the 27 455 children included, median age was 4 years (IQR 2–8), and 52.5% were male. Forty-seven percent were non-Hispanic white, 16.2% were non-Hispanic black, and 24.2% were Hispanic. Most children (54.6%) used public insurance (Table 1). Aspiration pneumonia was diagnosed in 9.7% (n = 2659) of the cohort.
TABLE 1.
Demographic and Clinical Characteristics of Children With Neurologic Impairment Admitted for Pneumonia to 40 Freestanding Children’s Hospitals
| Characteristic | Overall Cohort (n = 27455) | Aspiration Pneumonia (n =2659) | Nonaspiration Pneumonia (n = 24796) | P a | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Male | 14 414 | 52.5 | 1445 | 54.3 | 12 969 | 52.3 | .050 |
| Age | <.001 | ||||||
| 1–4 y | 15 375 | 56.0 | 1252 | 47.1 | 14 123 | 57.0 | |
| 5–9 y | 6701 | 24.4 | 606 | 22.8 | 6095 | 24.6 | |
| 10–18 y | 5379 | 19.6 | 801 | 30.1 | 4578 | 18.5 | |
| Race/ethnicity | .320 | ||||||
| Non-Hispanic white | 13 020 | 47.4 | 1287 | 48.4 | 11 733 | 47.3 | |
| Non-Hispanic black | 4440 | 16.2 | 405 | 15.2 | 4035 | 16.3 | |
| Hispanic | 6647 | 24.2 | 621 | 23.4 | 6026 | 24.3 | |
| Primary source of payment | <.001 | ||||||
| Government | 14 999 | 54.6 | 1649 | 62.0 | 13 350 | 53.8 | |
| Private | 9705 | 35.3 | 834 | 31.4 | 8871 | 35.8 | |
| Other | 2751 | 10.0 | 176 | 6.6 | 2575 | 10.4 | |
| NI category | |||||||
| Static neurologic disease | 21 717 | 79.1 | 1742 | 65.5 | 19 975 | 80.6 | <.001 |
| Epilepsy | 5073 | 18.5 | 1503 | 56.5 | 3570 | 14.4 | <.001 |
| Genetic or metabolic condition | 4045 | 14.7 | 635 | 23.9 | 3410 | 13.8 | <.001 |
| Anatomic abnormality | 3361 | 12.2 | 926 | 34.8 | 2435 | 9.8 | <.001 |
| Not otherwise specified/other | 2205 | 8.0 | 716 | 26.9 | 1489 | 6.0 | <.001 |
| Peripheral neurologic disease | 1373 | 5.0 | 251 | 9.4 | 1122 | 4.5 | <.001 |
| Behavioral | 669 | 2.4 | 54 | 2.0 | 615 | 2.5 | .200 |
| Cerebrovascular disease | 299 | 1.1 | 78 | 2.9 | 221 | 0.9 | <.001 |
| Progressive neurologic disease | 252 | 0.9 | 88 | 3.3 | 164 | 0.7 | <.001 |
| CCC | |||||||
| Gastrointestinal | 6092 | 22.2 | 1699 | 63.9 | 4393 | 17.7 | <.001 |
| Congenital/genetic defect | 5187 | 18.9 | 974 | 36.6 | 4213 | 17.0 | <.001 |
| Respiratory | 2780 | 10.1 | 481 | 18.1 | 2299 | 9.3 | <.001 |
| Cardiovascular | 2260 | 8.2 | 348 | 13.1 | 1912 | 7.7 | <.001 |
| Metabolic | 1239 | 4.5 | 232 | 8.7 | 1007 | 4.1 | <.001 |
| Neonatal | 1182 | 4.3 | 222 | 8.3 | 960 | 3.9 | <.001 |
| Hematology/immunodeficiency | 870 | 3.2 | 58 | 2.2 | 812 | 3.3 | .002 |
| Renal | 741 | 2.7 | 123 | 4.6 | 618 | 2.5 | <.001 |
| Malignancy | 670 | 2.4 | 79 | 3.0 | 591 | 2.4 | .060 |
| Transplant | 320 | 1.2 | 20 | 0.8 | 300 | 1.2 | .040 |
| Nonneurologic CCC count | <.001 | ||||||
| 0 | 15 138 | 55.1 | 372 | 14.0 | 14 766 | 59.5 | |
| 1 | 4131 | 15.0 | 358 | 13.5 | 3773 | 15.2 | |
| 2+ | 8186 | 29.8 | 1929 | 72.5 | 6257 | 25.2 | |
| Assistance with medical technology | 7261 | 26.4 | 1856 | 69.8 | 5405 | 21.8 | <.001 |
| Gastrointestinal | 5929 | 21.6 | 1692 | 63.6 | 4237 | 17.1 | <.001 |
| Respiratory | 1898 | 6.9 | 311 | 11.7 | 1587 | 6.4 | <.001 |
| Neurologic/neuromuscular | 1179 | 4.3 | 285 | 10.7 | 894 | 3.6 | <.001 |
| Cardiovascular | 204 | 0.7 | 29 | 1.1 | 175 | 0.7 | .030 |
| Renal | 143 | 0.5 | 23 | 0.9 | 120 | 0.5 | .009 |
| Other | 872 | 3.2 | 160 | 6.4 | 703 | 2.8 | <.001 |
| Noncomplex medical comorbidities | |||||||
| Reflux | 3629 | 13.2 | 1142 | 42.9 | 2487 | 10.0 | <.001 |
| Constipation | 1685 | 6.1 | 365 | 13.7 | 1320 | 5.3 | <.001 |
| Scoliosis | 1578 | 5.7 | 427 | 16.4 | 1151 | 4.6 | <.001 |
| Osteopenia/osteoporosis | 328 | 1.2 | 90 | 3.4 | 238 | 1.0 | <.001 |
| Diabetes insipidus | 166 | 0.6 | 38 | 1.4 | 128 | 0.5 | <.001 |
P value for χ2 test for categorical variables and for Wilcoxon rank sum test for continuous variables.
Demographics and Clinical Characteristics
Compared with children diagnosed with nonaspiration pneumonia, children with aspiration pneumonia were older (median age 5 years [IQR 2–11] vs 4 years [IQR 2–8], P < .001] and used public insurance more often (62.0% vs 53.8%, P < .001; Table 1).
Children diagnosed with aspiration pneumonia had a different profile of NI, with higher prevalence of epilepsy (56.4% vs 14.4%), genetic or metabolic conditions (23.9% vs 13.8%), and progressive neurologic disease (3.3% vs 0.7%), P < .001 for all.
Children diagnosed with aspiration pneumonia had a higher prevalence of CCCs (eg, gastrointestinal: 63.9% vs 17.7%, P < .001) and technology dependence (69.8% vs 21.8%, P < .001; Table 1). The presence of additional medical comorbidities was also more frequent in children diagnosed with aspiration pneumonia (eg, gastroesophageal reflux [42.9% vs 10.0%], P < .001; Table 1).
Diagnostic Testing
Children diagnosed with aspiration pneumonia were more likely than children with nonaspiration pneumonia to receive diagnostic laboratory tests (eg, complete blood count: 82.7% vs 68.8%, P < .001), with the exception of erythrocyte sedimentation rate (5.4% vs 5.6%, P = .6; Table 2). Children diagnosed with aspiration pneumonia were more likely to receive a chest radiograph (91.8% vs 86.1%) and less likely to receive chest computed tomography (1.2% vs 2.5%) or ultrasound (0.6% vs 1.8%), P < .001 for all (Table 2).
TABLE 2.
Diagnostic Testing and Treatment Within 48 Hours of Admission in Children With NI Hospitalized for Pneumonia
| Characteristic | Overall Cohort (n = 27455) | Aspiration Pneumonia (n = 2659) | Nonaspiration Pneumonia (n = 24796) | P a | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Laboratory Testing | |||||||
| Complete blood count | 19 262 | 70.2 | 2198 | 82.7 | 17 064 | 68.8 | <.001 |
| Blood culture | 16 041 | 58.4 | 1779 | 66.9 | 14 262 | 57.5 | <.001 |
| Chemistry profile | 11 967 | 43.6 | 1522 | 57.2 | 10 445 | 42.1 | <.001 |
| Viral study | 10 481 | 38.2 | 1091 | 41.0 | 9390 | 37.9 | .001 |
| C-reactive protein | 7277 | 26.5 | 780 | 29.3 | 6497 | 26.2 | .001 |
| Blood gas | 7166 | 26.1 | 1220 | 45.9 | 5946 | 24.0 | <.001 |
| Urine culture | 4136 | 15.1 | 732 | 27.5 | 3404 | 13.7 | <.001 |
| Respiratory culture | 2157 | 7.9 | 414 | 15.6 | 1743 | 7.0 | <.001 |
| Erythrocyte sedimentation rate | 1544 | 5.6 | 144 | 5.4 | 1400 | 5.6 | .620 |
| Coagulation study | 1492 | 5.4 | 234 | 8.8 | 1258 | 5.1 | <.001 |
| Liver function test | 645 | 2.3 | 126 | 4.7 | 519 | 2.1 | <.001 |
| Radiographic imaging | |||||||
| Chest radiograph | 23 793 | 86.7 | 2440 | 91.8 | 21 353 | 86.1 | <.001 |
| Chest computed tomography | 650 | 2.4 | 33 | 1.2 | 617 | 2.5 | <.001 |
| Chest ultrasound | 453 | 1.6 | 15 | 0.6 | 438 | 1.8 | <.001 |
| Treatment | |||||||
| Empirical antimicrobial coverage | <.001 | ||||||
| Pneumococcal | 16 777 | 61.1 | 303 | 11.4 | 16 474 | 66.4 | |
| Pneumococcal and anaerobic | 6107 | 22.2 | 1820 | 68.4 | 4287 | 17.3 | |
| Other combinations | 2509 | 9.1 | 99 | 3.7 | 2410 | 9.7 | |
| Pneumococcal, anaerobic, and antipseudomonal | 2062 | 7.5 | 437 | 16.4 | 1625 | 6.6 | |
| Adjunct therapy | |||||||
| Inhaled β agonist | 18 784 | 68.4 | 1830 | 68.8 | 16954 | 68.4 | .640 |
| Inhaled corticosteroids | 7737 | 28.2 | 1182 | 44.5 | 6555 | 26.4 | <.001 |
| Systemic steroids | 78 | 0.3 | 8 | 0.3 | 70 | 0.3 | .860 |
P value for χ2 test for categorical variables and for Wilcoxon rank sum test for continuous variables.
Treatment
Children diagnosed with aspiration pneumonia received a different profile of antibiotics than children with nonaspiration pneumonia (Table 2). The most common antimicrobial coverage for aspiration pneumonia were pneumococcal and anaerobic combined (68.4%) and then pneumococcal, anaerobic, and antipseudomonal combined (16.4%). Children with nonaspiration pneumonia most commonly received pneumococcal coverage alone (66.4%).
Children diagnosed with aspiration and nonaspiration pneumonia received inhaled β agonists (68.8% vs 68.4%, P = .6) and systemic steroids (0.3% vs 0.3%, P = .9) at similar rates. Children diagnosed with aspiration pneumonia were more likely to receive inhaled corticosteroids (44.5% vs 26.4%, P < .001; Table 2).
Clinical Outcomes and Utilization
Pneumonia-Associated Complications
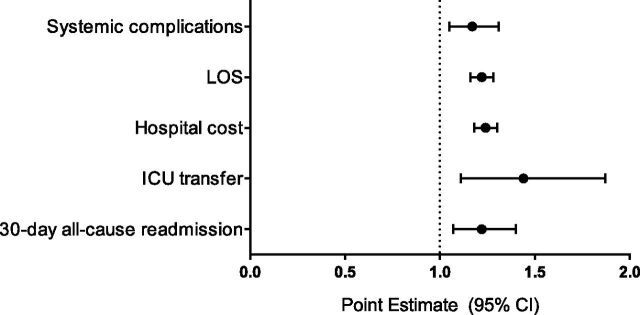
Twenty-four percent of children experienced a pneumonia-associated complication. Systemic complications were most common (17.0%), followed by local (9.1%) and metastatic (0.1%) (Table 3). In multivariable analysis, children diagnosed with aspiration pneumonia were more likely to have systemic complications (aOR 1.2, 95% CI 1.1–1.3; Fig 1).
TABLE 3.
Bivariable Analysis of Pneumonia-Associated Complications and Hospital Resource Use in Children with NI Admitted for Aspiration and Nonaspiration Pneumonia
| Outcome | Overall Cohort (n = 27 455) | Aspiration Pneumonia (n = 2659) | Nonaspiration Pneumonia (n = 24 796) | P a | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Pneumonia-associated complications | 6594 | 24.0 | 5621 | 22.7 | 973 | 36.3 | <.001 |
| Any local | 2490 | 9.1 | 137 | 5.2 | 2353 | 9.5 | <.001 |
| Empyema | 2432 | 8.9 | 132 | 5.0 | 2300 | 9.3 | <.001 |
| Lung abscess | 240 | 0.9 | 9 | 0.3 | 231 | 0.9 | .002 |
| Bronchopleural fistula | 36 | 0.1 | 1 | 0.0 | 35 | 0.1 | .160 |
| Necrotizing pneumonia | 15 | 0.1 | 0 | 0.0 | 15 | 0.1 | .210 |
| Any systemic | 4667 | 17.0 | 903 | 34.0 | 3764 | 15.2 | <.001 |
| Acute respiratory failure | 4554 | 16.6 | 884 | 33.2 | 3670 | 14.8 | <.001 |
| SIRS/sepsis | 341 | 1.2 | 74 | 72.8 | 267 | 1.0 | <.001 |
| ECMO | 32 | 0.1 | 4 | 0.2 | 28 | 0.1 | .590 |
| Hemolytic uremic syndrome | 16 | 0.1 | 0 | 0.0 | 16 | 0.1 | .190 |
| Any metastatic | 37 | 0.1 | 7 | 0.3 | 30 | 0.1 | .060 |
| Meningitis | 7 | 0.0 | 0 | 0.0 | 7 | 0.0 | .390 |
| CNS abscess | 4 | 0.0 | 1 | 0.0 | 3 | 0.0 | .300 |
| Mastoiditis | 1 | 0.0 | 1 | 0.0 | 0 | 0.0 | .002 |
| Pericarditis | 8 | 0.0 | 1 | 0.0 | 7 | 0.0 | .790 |
| Endocarditis | 3 | 0.0 | 0 | 0.0 | 3 | 0.0 | .570 |
| Osteomyelitis | 13 | 0.0 | 5 | 0.2 | 8 | 0.0 | <.001 |
| Septic arthritis | 1 | 0.0 | 0 | 0.0 | 4 | 0.0 | .510 |
| LOS, median d, IQR | 3 | 2–5 | 5 | 3–10 | 3 | 2–5 | <.001 |
| 30-d all-cause readmission rate | 2141 | 7.8 | 463 | 17.4 | 1678 | 6.8 | <.001 |
| Intensive care | |||||||
| Admission | 4265 | 15.5 | 761 | 28.6 | 3504 | 14.1 | <.001 |
| Transfer | 479 | 1.7 | 115 | 4.3 | 364 | 1.5 | <.001 |
| Cost, $, median, IQR | 5509 | 3213–11 522 | 11 594 | 5841–25562 | 5162 | 3080–10249 | <.001 |
| Death | 201 | 0.7 | 46 | 1.7 | 155 | 0.6 | <.001 |
ECMO, extracorporeal membrane oxygenation; SIRS, systemic inflammatory response syndrome.
P value for χ2 test for categorical variables and for Wilcoxon rank sum test for continuous variables
FIGURE 1.
Multivariable analysis of pneumonia-associated complications and hospital resource use in children with NI admitted for aspiration and nonaspiration pneumonia. Generalized estimating equations used to calculated aOR for systemic complications, ICU transfer, and 30-day all-cause readmission; exponential regression used to calculate adjusted RoM for outcomes of LOS and hospital cost. Models adjusted for age, NI category, nonneuromuscular CCC count, gastrointestinal and respiratory technology, noncomplex medical comorbidity antimicrobial treatment, and presence of any pneumonia-associated complication (for utilization outcomes).
LOS
Median hospital LOS for the total cohort was 3 days (IQR 2–5; Table 3). In multivariable analysis, children diagnosed with aspiration pneumonia had a 20% longer LOS than children with nonaspiration pneumonia (RoM 1.2, 95% CI 1.2–1.3; Fig 1).
ICU Care
Fifteen percent of children required ICU admission with an additional 1.7% requiring ICU transfer after admission (Table 3). In multivariable analysis, children diagnosed with aspiration pneumonia had 40% greater odds of ICU transfer than children with nonaspiration pneumonia (aOR 1.4, 95% CI 1.1–1.9; Fig 1).
Readmission
The 30-day readmission rate was 7.8% (Table 3). The most common readmission diagnoses were pneumonia (10.3%), aspiration pneumonia (5.9%), and asthma (2.3%). In multivariable analysis, children diagnosed with aspiration pneumonia had a 20% greater odds of readmission within 30 days than children with nonaspiration pneumonia (aOR 1.2, 95% CI 1.1–1.4; Fig 1).
Hospital Costs
Median hospitalization cost for the total cohort was $5509 IQR $3213–$11 522; Table 3). In multivariable analysis, hospitalization cost was 20% higher for children diagnosed with aspiration pneumonia compared with children with nonaspiration pneumonia (RoM 1.2, 95% CI 1.2–1.3; Fig 1).
Mortality.
Less than 1% of the cohort died during hospitalization. Children with aspiration pneumonia were more likely to die during hospitalization than children with nonaspiration pneumonia (1.7% vs 0.6%, P < .001). Due to small numbers, mortality was not assessed in multivariable analysis.
Discussion
In this retrospective cohort of children with NI hospitalized for pneumonia, we observed differences in the characteristics, evaluation, treatment, and outcomes between diagnoses of aspiration and nonaspiration pneumonia. Children diagnosed with aspiration pneumonia were older, used public insurance more often, had a different NI profile (eg, a higher prevalence of epilepsy, progressive NI), and had more complex chronic conditions, more additional comorbidities, and more use of medical technology. These findings suggest that children with NI diagnosed with aspiration pneumonia had a higher severity of NI, medical complexity, and fragility. However, their higher complication rates, longer and costlier hospitalizations, and higher readmission rates were not fully explained by those attributes in multivariable analysis. These findings are important to consider as hospital clinicians move forward with subsequent investigations to optimize care and outcomes for children with NI hospitalized with pneumonia.
This retrospective study of administrative claims data are not positioned to verify with complete accuracy the type of pneumonia diagnosed by hospital clinicians. Although we used principal diagnosis to distinguish pneumonia type, validated clinical criteria for diagnosing aspiration pneumonia in children do not exist. The value of clinical signs and symptoms (eg, a history that includes a choking episode followed by fever and respiratory distress) or certain test results (eg, right upper lobe infiltration on chest radiograph) to diagnose aspiration pneumonia remain unknown. Aspiration can be silent, sans signs and symptoms, and it can affect different parts and sides of the lung depending on patient position during aspiration. Tests that might verify aspiration of saliva or gastric contents into the airway (eg, bronchoscopy or transtracheal mucous aspirate) are not commonly used in the setting of acute pneumonia. As a result, when caring for children with a higher severity of NI, some clinicians may be more likely to apply a diagnosis of and treat for aspiration pneumonia by subjective suspicion rather than affirmation from hard evidence. Prospective studies are needed to assess these issues and to determine how to best distinguish aspiration pneumonia in children with NI.
Our findings complement a growing body of literature suggesting that health outcomes are worse and hospital resource use is higher in patients diagnosed with aspiration versus nonaspiration pneumonia.7–11,13 Further investigation is needed to explain how much these findings are a manifestation of aspiration pathophysiology versus an artifact of making the diagnosis. For children truly experiencing aspiration, the penetration of oropharyngeal saliva or acidic gastric contents, along with enteric bacteria, into the airway can result in a higher severity of epithelial disruption, inflammation, impaired gas exchange, and prolonged healing than when the airway is exposed to exogenous bacteria alone.23–25 For children not experiencing aspiration but diagnosed with it, the diagnosis might actually reflect a high-severity, nonaspiration bacterial pneumonia (eg, with sepsis or respiratory failure) that led to worse outcomes and more hospital resource use. We controlled for pneumonia severity in our multivariable analyses; it did not fully explain why children with NI diagnosed with aspiration experienced worse health outcomes. Nonetheless, applying the diagnosis of aspiration pneumonia might indicate a higher severity of illness that is unmeasurable with administrative data used in this study.
Little is known about how much airway injury, inflammation, and bronchospasm from aspiration or nonaspiration pneumonia in children with NI may be responsive to treatments such as inhaled β-agonists and inhaled or systemic corticosteroids. Use of β-agonists was common for children with both aspiration and nonaspiration pneumonia. Treatment with systemic corticosteroids was uncommon. Children with aspiration pneumonia were more frequently prescribed inhaled corticosteroids, which could reflect presence of underlying chronic lung disease and/or persistent asthma. The high rates of respiratory complications, including respiratory failure, experienced by children with aspiration pneumonia suggest that heightened attention and subsequent investigation to respiratory treatments and therapies intended to maximize airway patency, oxygenation, and ventilation may be warranted.
We observed substantial variation in antimicrobial coverage for children with aspiration pneumonia, which may reflect the paucity of information on which enteric bacteria are causative. Although aspiration pneumonia has traditionally been attributed to anaerobic bacteria, a study of 74 hospitalized children with aspiration pneumonia isolated 5 bacteria (a mixture of anaerobes and aerobes), on average from transtracheal aspirates.26 In adult patients, although anaerobes occur in one-third of aspiration pneumonias, aerobic Gram-negative bacteria, including enteric bacteria (eg, Escherichia coli and Pseudomonas spp) and Gram-positive bacteria (eg, methicillin-sensitive and methicillin-resistant Staphylococcus aureus), are recovered more often (ie, in 50% of cultures).27–30 We are aware of only 1 randomized controlled trial that compared effectiveness of antibiotic treatment in hospitalized children with aspiration pneumonia; no difference in effectiveness between penicillin G and clindamycin was found.31
Our study has several limitations in addition to those described earlier. Clinical data may be preferable to administrative data to identify children with NI and the types of pneumonia they encounter. Misclassification of pneumonia type using ICD-9-CM codes is possible because there is no “gold standard” for diagnosis of aspiration pneumonia.3,27 Aside from use of technology assistance, PHIS administrative data are not equipped to distinguish granular grades of functional status (eg, ability to cough, chest wall control and strength) that might influence health outcomes with pneumonia in children with NI. Interpretation of ICU admission may be limited by variability of admission practices and policies between hospitals. PHIS does not include readmissions to a different hospital, which could have led to undercounting. Although children with NI predominately use children’s hospitals,1 results may not generalize to children with NI hospitalized with pneumonia at non–children’s hospitals. We randomly selected for analysis hospitalizations in children with multiple admissions; using alternative methods could have led to different findings. In a post hoc analysis excluding children with multiple admissions, findings did not change. We limited our study to initial evaluation and treatment of pneumonia in children with NI; subsequent studies are needed to assess the clinical course throughout hospitalization including further tests and treatments.
Despite these limitations, we believe these findings highlight the serious health consequences of pneumonia that occur in children with NI. It is important that these consequences are discussed with families of children with NI, especially the high rates of complications (34%) and 30-day hospital readmission (18%) in children diagnosed with aspiration pneumonia. Yet there remains a need to understand (1) how to accurately distinguish pneumonia type; (2) which antibiotics are most effective; and (3) how bronchodilators, steroids, and other respiratory treatments can be leveraged to maximize pulmonary function. We hope our findings will prompt subsequent investigations to optimize treatment and outcomes in hospitalized children with NI diagnosed with pneumonia.
Supplementary Material
Glossary
- aOR
adjusted odds ratio
- CCC
complex chronic condition
- CRP
C-reactive protein
- CI
confidence interval
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
interquartile range
- LOS
length of stay
- NI
neurologic impairment
- PHIS
Pediatric Health Information System
- RoM
ratio of means
Footnotes
Dr Thomson conceptualized and designed the study, interpreted the data, and drafted the initial manuscript; Dr Hall aided in study design, conducted the statistical analyses data analysis, supervised interpretation of the data, and reviewed and revised the manuscript; Drs Ambroggio, Stone, and Srivastava aided study design, participated in the interpretation of the data, and reviewed and revised the manuscript; Drs Shah and Berry supervised the conceptualization and design of the study, supervised interpretation of the data, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FUNDING: Dr Thomson was supported by funds from the Academic Pediatric Association Young Investigator Award and from National Research Service Award T32HP10027-14. Jay Berry was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant K23 HD058092) and the Agency for Healthcare Research and Quality (grant R21 HS023092).
References
- 1.Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012;9(1):e1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava R, Jackson WD, Barnhart DC. Dysphagia and gastroesophageal reflux disease: dilemmas in diagnosis and management in children with neurological impairment. Pediatr Ann. 2010;39(4):225–231 [DOI] [PubMed] [Google Scholar]
- 3.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671 [DOI] [PubMed] [Google Scholar]
- 4.Graham RJ, Dumas HM, O’Brien JE, Burns JP. Congenital neurodevelopmental diagnoses and an intensive care unit: defining a population. Pediatr Crit Care Med. 2004;5(4):321–328 [DOI] [PubMed]
- 5.Plioplys AV. Survival rates of children with severe neurologic disabilities: a review. Semin Pediatr Neurol. 2003;10(2):120–129 [DOI] [PubMed] [Google Scholar]
- 6.Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88(1):75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbar U, Dham B, He Y, et al. Incidence and mortality trends of aspiration pneumonia in Parkinson’s disease in the United States, 1979–2010. Parkinsonism Relat Disord. 2015;21(9):1082–1086 [DOI] [PubMed] [Google Scholar]
- 8.Baine WB, Yu W, Summe JP. Epidemiologic trends in the hospitalization of elderly Medicare patients for pneumonia, 1991–1998. Am J Public Health. 2001;91(7):1121–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komiya K, Ishii H, Kadota J. Healthcare-associated pneumonia and aspiration pneumonia. Aging Dis. 2015;6(1):27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90 [DOI] [PMC free article] [PubMed]
- 11.Siddique R, Neslusan CA, Crown WH, Crystal-Peters J, Sloan S, Farup C. A national inpatient cost estimate of percutaneous endoscopic gastrostomy (PEG)-associated aspiration pneumonia. Am J Manag Care. 2000;6(4):490–496 [PubMed] [Google Scholar]
- 12.Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanspa MJ, Peyrani P, Wiemken T, Wilson EL, Ramirez JA, Dean NC. Characteristics associated with clinician diagnosis of aspiration pneumonia: a descriptive study of afflicted patients and their outcomes. J Hosp Med. 2015;10(2):90–96 [DOI] [PMC free article] [PubMed]
- 14.ICD-9-CM Official Guidelines for Coding and Reporting . http://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf. Accessed July 7, 2015
- 15.Pickering LK, Baker CJ, Kimberline DW; AAP Committee on Infectious Diseases . Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012 [Google Scholar]
- 16.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126(2):204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Quality Measures Clearinghouse . Plan all-cause readmissions: the number of acute inpatient stays during the measurement year that were followed by an acute readmission for any diagnosis within 30 days and the predicted probability of an acute readmission, for members 18 years of age and older. http://www.qualitymeasures.ahrq.gov/content.aspx?id=47277. Accessed April 30, 2015
- 18.Keren R, Luan X, Localio R, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network . Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164 [DOI] [PubMed] [Google Scholar]
- 19.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. [DOI] [PubMed] [Google Scholar]
- 21.Berry JGHD, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, eds. The Sanford Guide to Antimicrobial Therapy 2014. 44th ed. Sperryville, VA: Antimicorbial Therapy, Inc [Google Scholar]
- 23.Pawlik MT, Schreyer AG, Ittner KP, et al. Early treatment with pentoxifylline reduces lung injury induced by acid aspiration in rats. Chest. 2005;127(2):613–621 [DOI] [PubMed] [Google Scholar]
- 24.Amigoni M, Bellani G, Scanziani M, et al. Lung injury and recovery in a murine model of unilateral acid aspiration: functional, biochemical, and morphologic characterization. Anesthesiology. 2008;108(6):1037–1046 [DOI] [PubMed] [Google Scholar]
- 25.Folkesson HG, Matthay MA, Hébert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest. 1995;96(1):107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brook I, Finegold SM. Bacteriology of aspiration pneumonia in children. Pediatrics. 1980;65(6):1115–1120 [PubMed] [Google Scholar]
- 27.DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care. 2015;30(1):40–48 [DOI] [PubMed] [Google Scholar]
- 28.Allewelt M, Schuler P, Bolcskei PL, Mauch H, Lode H. Ampicillin + sulbactam vs clindamycin +/− cephalosporin for the treatment of aspiration pneumonia and primary lung abscess. Clin Microbiol Infect. 2004;10(2):163–170 [DOI] [PubMed]
- 29.Ito I, Kadowaki S, Tanabe N, et al. Tazobactam/piperacillin for moderate-to-severe pneumonia in patients with risk for aspiration: comparison with imipenem/cilastatin. Pulm Pharmacol Ther. 2010;23(5):403–410 [DOI] [PubMed] [Google Scholar]
- 30.Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276–1282 [DOI] [PubMed] [Google Scholar]
- 31.Jacobson SJ, Griffiths K, Diamond S, et al. A randomized controlled trial of penicillin vs clindamycin for the treatment of aspiration pneumonia in children. Arch Pediatr Adolesc Med. 1997;151(7):701–704 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.