Abstract
Background:
In patients with mild ischemic stroke, small but eloquent infarcts may have devastating effects, particularly on health-related quality of life.
Aim:
This study investigates the association between acute infarct location and three-month health-related quality of life in patients with mild ischemic stroke.
Methods:
We evaluated consecutively enrolled patients from a single center between August 2012 and July 2013. Our primary outcome at three months was impairment in any health-related quality of life domain (upper extremity, lower extremity, executive function, and general concerns) defined by a T-score <45. We analyzed the association between acute infarct locations and impaired health-related quality of life at three months in univariate and multivariable analysis.
Results:
Among 229 patients (mean age 64.9 years, 55% male, 29.7% black, and median initial NIHSS score 1), impaired health-related quality of life was noted in 84 (36.2%) patients at three months. In univariate analysis, patients with subcortical infarcts (56.0% vs. 39.3%, p = 0.02) and brainstem infarcts (21.4% vs. 10.3%, p = 0.02) were more likely to have impaired health-related quality of life. In multivariable analysis, patients with subcortical and/or brainstem infarcts had increased odds of impaired health-related quality of life (adjusted OR 2.54, 95% CI 1.29–5.01, p = 0.01).
Conclusions:
After mild ischemic stroke, subcortical and brainstem infarct locations predict impairment in health-related quality of life.
Keywords: Quality of life, stroke outcome, ischemic stroke, magnetic resonance imaging
Introduction
Mild ischemic strokes (MISs) account for the majority of strokes in the United States, with a median National Institute of Health Stroke Scale (NIHSS) score of 3.1 Despite mild deficits on presentation, disability and impairment in health-related quality of life (HRQOL) are reported in 25% and 36% of MIS patients, respectively, at long-term follow-up.2 A critical gap, however, exists in understanding the biologic mechanism of poor outcome following MIS, thereby limiting the ability to predict which patients with MIS will develop impaired HRQOL and disability.
Recently, we reported that acute infarct volume, a valuable biomarker in predicting disability, was a poor predictor of HRQOL after acute ischemic stroke.3
Besides infarct volume, acute infarct location has been associated with disability after stroke as measured by the modified Rankin Scale (mRS).4 Only one prior study has evaluated infarct location as it relates to HRQOL, but did not specifically target patients with MIS.5 In patients with MIS, small but eloquent infarcts may have devastating effects. Therefore, we sought to evaluate whether acute infarct location predicts three-month HRQOL in patients with MIS. We hypothesized that specific infarct locations predict impaired HRQOL at three months.
Subjects/materials and methods
Participants
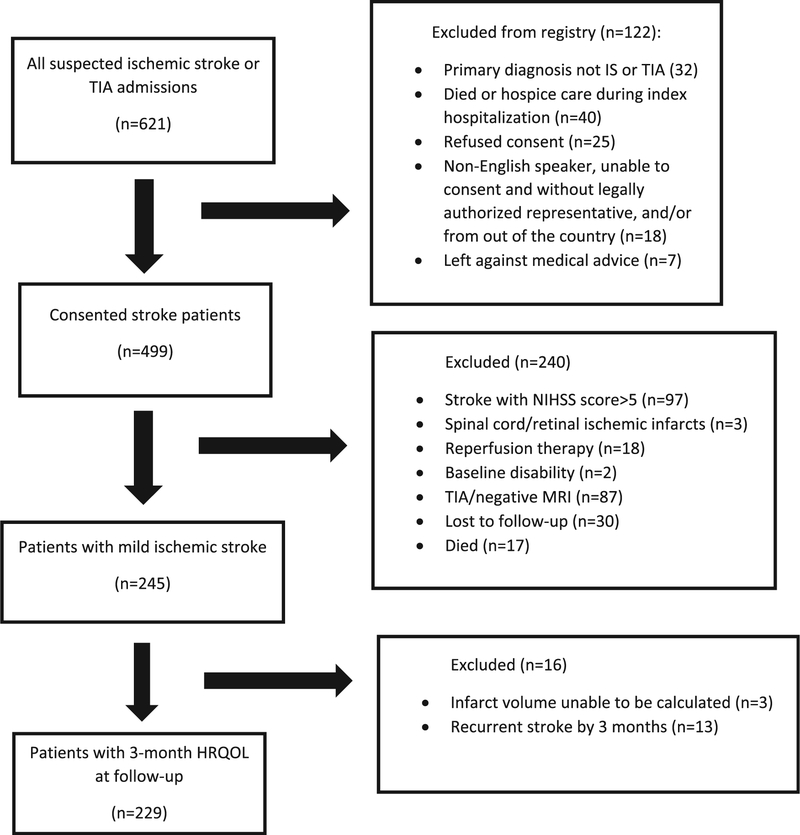
The local Institutional Review Board approved the study. Consecutive patients ≥ 18 years with a confirmed acute ischemic stroke over a period of 12 months (1 August 2012 through 31 July 2013) were enrolled in a prospective registry. Written informed consent was obtained from the patient or their legal representative. Patients with MIS were defined as those with an initial NIHSS score ≤5 and MRI evidence of acute infarct as identified on diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) images.6 Diagnosis was made by a board-certified vascular neurologist in each case. Consecutive patients with the following criteria were included: (1) MIS as previously defined; (2) absence of acute reperfusion treatment; (3) ability to walk independently at baseline; (4) absence of recurrent stroke at three months; and (5) complete follow-up data at three months (Figure 1). Reperfusion therapy was defined as receipt of intravenous or intra-arterial tissue plasminogen activator and/or mechanical thrombectomy. Recurrent stroke during or after hospitalization was recorded based on deterioration in NIHSS score with imaging confirmation during hospitalization or based on a validated questionnaire of reported symptoms of stroke and review of medical records for confirmation after hospitalization. Patients were also excluded if they died, were lost to follow-up, or had missing or incomplete HRQOL scores at three months in more than two domains.
Figure 1.
Flowchart for study inclusion.
Patient data
Patient demographics, premorbid characteristics, insurance status, initial NIHSS, and risk factors and comorbidities were collected prospectively. Board-certified vascular neurologists prospectively reviewed clinical and radiographic data to determine Trial of Org 10172 in Acute Stroke Treatment (TOAST) subtype by consensus adjudication to avoid inter-rater reliability concerns.7
Image acquisition and acute infarct volume measurement
Images were acquired on MRI (Siemens Medical Systems, Erlangen, Germany) during index hospitalization as standard of care. We used previous methodology to semi-automatically determine acute infarct volume.3 In summary, pre-contrast images were preferentially used if available, otherwise post-contrast T1 images were used (typical sequence parameters: TR/TE 1500/45, 13–15 slices, in-plane resolution1.15 × 1.15 mm, slice thickness 5 mm, FOV 22 cm, matrix 192 × 192, flip angle 30). DWI sequences (TR/TE 4000/88 ms, in-plane resolution 1.39 × 1.39 mm, slice thickness 5 mm, matrix 192 × 192) were acquired at b-values of 0 s/mm2 and 1000 s/mm2 and ADC maps were generated in-line on the scanner. After masking out all non-brain tissue using anatomic reference images, we used a threshold approach to delineate the ischemic core.8 To reduce the likelihood of false positive infarcts, a minimum cluster size threshold of 0.145 mL was applied to arrive at the infarct volumes.
Infarct location
Neuroimaging was reviewed by four trained investigators (SP, AJ, RS, and CL) for presence of acute infarct(s) along with its location(s) and vascular territories, blinded to outcome data. Infarct locations were determined using a validated neuroanatomical atlas, the IMAIOS online anatomy atlas (http://www.imaios.com/es/e-Anatomy/Cabeza-ycuello/Cerebro-IRM-de-cortes-axiales), and visually inspected by our trained investigators using consensus adjudication.9
We grouped the infarct locations based on region of involvement: cortex (surface gray matter), subcortex, thalamus, cerebellum, and brainstem (midbrain, pons, and medulla). Subcortical structures were defined as all supratentorial, non-cortical gray matter structures excluding the thalamus but including the basal ganglia and corona radiata. Infarcts were coded for lateralization (left, right, or both).
Outcomes
Three-month outcomes were assessed using functional outcome scales and domain-specific HRQOL scores using Neuro-QOL. Noting proxy answers when indicated, we obtained follow-up mRS data by telephone interview, using a validated method for assessment.10 We defined poor functional outcome as mRS ≥2. The following four domains of Neuro-QOL were used: upper extremity function, lower extremity function, executive function, and general concerns. Instrument results are expressed as T-scores normalized to general US population demographics with means of 50 and standard deviations (SDs) of 10 (additional information available at www.neuroqol.org).11 Patients were determined to have impaired HRQOL if any of the domains had T scores <45, which is >0.5 SD from the normalized population mean score, reported as a conservative minimal clinical important difference estimate.6,12
Statistical analysis
Data are expressed as number (percent), mean (SD), or median (range) as appropriate. We calculated the proportions of patients with impaired HRQOL in any of the four Neuro-QOL domains (primary outcome) and disability (defined by mRS ≥2; secondary outcome) by infarct location groups. We assessed differences in baseline demographic, clinical, and imaging variables among those with and without impaired HRQOL at three months using Pearson’s Chi-square tests for categorical variables (Fisher’s exact test when appropriate) and t-tests for normally distributed continuous variables.
We used logistic regression models to evaluate the association between infarct locations groups and HRQOL. In the univariate analyses, we evaluated each infarct location for association with HRQOL. In the multivariable logistic regression models, we included infarct locations that were associated with impaired HRQOL in the univariate analyses (p < 0.05) adjusting for other covariates. Based on the univariate results, we re-categorized infarct locations as having or not having involvement of subcortical and/or brainstem regions. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for the association between baseline variables and impaired HRQOL at three months. We performed analyses for each domain of HRQOL, with impairment defined as T-score <45. Finally, we repeated the analyses for the secondary outcome, disability at three months defined as mRS >1. All statistical analyses were performed using SAS 9.4 (Cary, NC). Statistical significance was considered p-value < 0.05 in final models.
Results
Among 499 acute ischemic stroke patients, 229 (45.9%) patients with MIS were included for analysis (Figure 1). Among these patients, the mean age was 64.9 years, 55% were male, and 29.7% were black. Median initial NIHSS score was 1 and median infarct volume was 0.74 cc. There were 84 (36.7%) patients with impaired HRQOL at three months. The overall mean (SD) of the Neuro-QOL domains was: upper extremity 51.24 (±5.82), lower extremity 49.18 (±8.20), executive function 53.27 (±6.96), and general concerns 55.19 (±5.90). There was no difference in the baseline and clinical characteristics of patients with MIS excluded from those included in the study (Supplemental Table 1).
On univariate analysis (Table 1), patients with impaired HRQOL at three months had a lower percentage of private insurance (21.4% vs. 46.2%, p < 0.001) and current smoking (11.9% vs. 23.4%, p = 0.03) and higher initial NIHSS score (2 vs. 1, p < 0.001). Age, sex, race, baseline mRS, medical comorbidities, and acute infarct volumes were similar between those with and without impaired HRQOL. Those with impaired HRQOL were more likely to have subcortical (56.0% vs. 39.3%, p = 0.02) and brainstem (21.4% vs. 10.3%, p = 0.02) infarct locations and less likely to have cortical infarcts (53.6% vs. 66.7%, p = 0.05). The impaired HRQOL group also had more disability at three months (28.6% vs. 0.7%, p < 0.001), proxy reporting (20.2% vs. 4.2%, p < 0.001), and received more rehabilitation after discharge (65.5% vs. 24.1%, p < 0.001).
Table 1.
Baseline demographics, clinical characteristics, acute infarct location, and disability among those with normal versus impaired HRQOL at three months after mild ischemic stroke (n = 229)a
| Normal HRQOL (n= 145) | Impaired HRQOL (n = 84) | p | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Age (SD), y | 63.0 (14.9) | 68.3 (14.8) | 0.47 | ||
| Male, n (%) | 84 (57.9%) | 42 (50.0%) | 0.25 | ||
| Black, n (%) | 39 (26.9%) | 29 (34.5%) | 0.22 | ||
| Private insurance, n (%) | 67 (46.2%) | 18 (21.4%) | <0.01 | ||
| mRS score at baseline, median (range) | 0 (0–3) | 0 (0–3) | 0.38 | ||
| Comorbid medical conditions, n (%) | |||||
| Diabetes mellitus | 29 (20%) | 24 (28.6%) | 0.14 | ||
| Hypercholesterolemia | 107 (74.4%) | 55 (65.5%) | 0.18 | ||
| Atrial fibrillation/flutter | 10 (6.9%) | 10 (11.9%) | 0.20 | ||
| Cardiac disease | 29 (20%) | 23 (27.4%) | 0.20 | ||
| Prior stroke | 22 (14.9%) | 18 (21.4%) | 0.23 | ||
| Chronic kidney disease | 10 (6.9%) | 9 (10.7%) | 0.31 | ||
| Hypertension | 106 (73.1%) | 66 (78.6%) | 0.36 | ||
| Peripheral artery disease | 6 (4.1%) | 8 (9.5%) | 0.10 | ||
| Smoking within past six months | 34 (23.4%) | 10 (11.9%) | 0.03 | ||
| Clinical characteristics | |||||
| Initial NIHSS score, median (range) | 1 (0–5) | 2 (0–5) | <0.01 | ||
| TOAST subtype, n (%) | 0.35 | ||||
| Cardioembolic | 26 (17.6%) | 18 (21.4%) | |||
| Large artery atherosclerosis | 23 (15.5%) | 18 (21.4%) | |||
| Small artery disease | 21 (14.2%) | 16 (19.0%) | |||
| Other | 21 (14.5%) | 7 (8.3%) | |||
| Cryptogenic | 54 (37.2%) | 25 (29.8%) | |||
| Infarct characteristics, n (%) | |||||
| Median infarct volume (range), cc | 0.73 (0.01–31.6) | 0.74 (0.01–37) | 0.54 | ||
| Cerebellum | 24 (16.6%) | 16 (19.0%) | 0.63 | ||
| Thalamus | 9 (6.2%) | 7 (8.3%) | 0.54 | ||
| Cortical | 97 (66.7%) | 45 (53.6%) | 0.05 | ||
| Subcortical | 57 (39.3%) | 47 (56.0%) | 0.02 | ||
| Brainstem | 15 (10.3%) | 18 (21.4%) | 0.02 | ||
| Brainstem and/or subcortical | 66 (45.5%) | 59 (70.2%) | <0.01 | ||
| Side of infarct, n (%) | 0.28 | ||||
| Left | 52 (35.9%) | 39 (46.4%) | |||
| Right | 66 (45.5%) | 31 (36.9%) | |||
| Both | 27 (18.6%) | 14 (16.7%) | |||
| Supratentorial, n (%) | 71 (49.0%) | 48 (57.1%) | 0.23 | ||
| Left supratentorial, n (%) | 63 (43.4%) | 44 (52.4%) | 0.19 | ||
| Three-month data, n (%) | |||||
| Proxy (vs. patient) report | 7 (4.8%) | 17 (20.2%) | <0.01 | ||
| Received any rehabilitation after discharge | 35 (24.1%) | 55 (65.5%) | <0.01 | ||
| mRS score ≥2 | 1 (0.7%) | 24 (28.6%) | <0.01 | ||
Impaired group demonstrated T-score <45 in any QOL domain.
HRQOL: health-related quality of life; SD: standard deviation; IQR: interquartile range; mRS: modified Rankin Score; NIHSS: National Institutes of Health Stroke Scale; TOAST: trial of org 10172 in acute stroke treatment; cc: cubic centimeters.
Table 2 describes the multivariable models for impaired HRQOL. Patients with subcortical and/or brainstem infarcts had increased odds of impaired HRQOL (adjusted OR 2.54, 95% CI 1.29–5.01, p = 0.01). Other factors that predicted impaired HRQOL included initial NIHSS score (adjusted OR 1.49, 95% CI 1.04–2.14, p = 0.03), private insurance (adjusted OR 0.31, 95% CI 0.15–0.64, p < 0.001), and any rehabilitation post-hospitalization (adjusted OR 4.21, 95% CI 2.15–8.21, p < 0.001). Patients with sub-cortical and/or brainstem infarcts had impaired upper extremity HRQOL (adjusted OR 3.46, 95% CI 1.48–8.09, p = 0.004) and lower extremity HRQOL (adjusted OR 2.52, 95% CI 1.07–5.95, p = 0.04). Those with cortical infarcts only were more likely to have impaired general concerns in HRQOL (OR 6.25, CI 1.19–33.33, p = 0.03). Infarct location was not associated with executive function domain of HRQOL in multivariable analysis.
Table 2.
The effect of brainstem and/or subcortical infarct locations on impaired HRQOL at three month from the adjusted models (n = 229)
| Predictors | Impaired HRQOLa | Disability by mRSb | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Subcortical and/or brainstem infarct location | 2.54 | 1.29–5.01 | <0.01 | 2.46 | 0.37–16.42 | 0.35 | |
| Any rehabilitation after hospital | 4.21 | 2.15–8.21 | <0.01 | 19.71 | 3.60–108.01 | <0.01 | |
| Baseline mRS 2–3 vs. 0–1 | 1.14 | 0.40–3.23 | 0.81 | 1.03 | 0.21–5.01 | 0.97 | |
| Current smoking (<6 months) | 0.62 | 0.26–1.49 | 0.29 | 0.44 | 0.08–2.53 | 0.36 | |
| Private insurance | 0.31 | 0.15–0.64 | <0.01 | 0.41 | 0.11—1.50 | 0.18 | |
| Initial NIHSS score (per point) | 1.17 | 0.94–1.47 | 0.17 | 1.67 | 1.14–2.43 | <0.01 | |
| Proxy follow-up reporting | 2.53 | 0.84–7.59 | 0.10 | 8.87 | 2.42–32.56 | <0.01 | |
| Volume Groups | Q2 (0.02–0.72 cc) | 1.13 | 0.44–2.89 | 0.80 | 0.82 | 0.25–2.64 | 0.73 |
| Q3 (0.73–3.34 cc) | 0.95 | 0.40–2.31 | 0.92 | 0.88 | 0.30–2.54 | 0.81 | |
| Q4 (>3.35 cc) | 1.26 | 0.50–3.17 | 0.63 | 0.84 | 0.28–2.55 | 0.76 | |
| Ql (0.01 cc) | 1.00 | 1.00 | |||||
Impaired HRQOL is defined as 5 points below the population mean of 50 for any of the domain (i.e. T-score < 45). Bold values represent p-values ≤0.05.
Disability is defined by mRS ≥2.
HRQOL: health-related quality of life; mRS: modified Rankin Score; NIHSS: National Institutes of Health Stroke Scale; Q: quartile; MIS: mild ischemic stroke; cc: cubic centimeters.
For the secondary outcome (disability by mRS; Table 2), those with brainstem and/or subcortical infarcts did not have a significantly increased likelihood of disability in univariate (OR 1.69, 0.35–8.18 95% CI, p = 0.52) or multivariable analysis (adjusted OR 1.46, 0.13–2.04 95% CI, p = 0.70). Significant predictors of disability at three months in the multivariable analysis included: initial NIHSS (adjusted OR 1.67, 1.14–2.43 95% CI, p < 0.01), any rehabilitation (adjusted OR 19.71, 3.60–108.01 95% CI, p < 0.01), and proxy-reporting (adjusted OR 8.87, 2.42–32.56 95% CI, p < 0.01).
Discussion
In a prospective single-center urban cohort study of MIS patients, we observed that subcortical and brainstem infarct locations were independently associated with impaired HRQOL at three months. By domain of HRQOL, subcortical and brainstem infarct location predicted worse motor, but not cognitive patient reported outcomes. Infarct location should be considered in rehabilitation evaluation at time of index stroke despite mild deficits and could be used to identify patients for targeted rehabilitation.
To our knowledge, this study is the first to evaluate the impact of acute infarct locations on HRQOL in patients with MIS. A recent study of acute ischemic stroke patients, not limited to MIS alone, found that infarcts in subcortical regions influence functional outcomes as measured by the mRS after one month.4 Others have also noted that acute infarct size and location predict functional dependency at 90 days in patients with hemiplegic stroke.9 Predictors of HRQOL are different than the predictors of function because these are different constructs. HRQOL is likely more correlated with socioeconomic status. Our results showed that patients with private insurance were significantly less likely to have impaired HRQOL but had similar mRS scores compared to patients with other insurance types. Socioeconomic13 and insurance14 status have both been shown to impact stroke mortality and functional outcomes. These factors may impact pre-stroke care including delays in presentation and treatment adherence prior to the stroke. Patients without insurance may also have limited access to follow-up visits for post-stroke care and be more likely to discontinue secondary prevention medications.15
There are several potential mechanisms, whereby patients with MIS develop disability and impaired HRQOL despite initial mild deficits. After initial presentation, patients may experience worsening through infarct growth, hemorrhage or edema formation, or recurrent stroke. We excluded patients with recurrent strokes during or after hospitalization. Alternatively, patients with MIS may present with unrecognized or underappreciated deficits, which become apparent upon returning home. We hypothesize that subtle deficits, due to subcortical and/or brainstem infarct locations evident on index imaging, worsen long-term HRQOL after MIS. Patients with infratentorial and eloquent (e.g. corticospinal tracts) subcortical infarcts can present with subtle findings including weakness, incoordination, and swallowing difficulties that can significantly impact HRQOL but may not be detected using functional measures such as the mRS, especially in patients with MIS. Further discrimination of specific subcortical and brainstem structures such as the corticospinal tract through diffusion tensor imaging may be helpful to define which specific locations affect domain- specific outcomes using quantitative, objective measures of neurologic function such as gait analysis.
We were unable to demonstrate an effect on infarct location on three-month disability using the mRS. Functional measures and objective examination findings can fail to correlate with neuroanatomical findings.16 The National Institute of Neurologic Disorders and Stroke created the Neuro-QOL to address the need to understand HRQOL after neurologic injury.11 While HRQOL and mRS are correlated measures, there are some important differences. We have previously demonstrated HRQOL impairments in patients without significant disability on mRS.6 Floor and ceiling effects, especially in mild stroke patients, may make HRQOL more sensitive than the mRS to identify small but clinically meaningful effects.17 HRQOL can provide more context as to the reasoning for a patient’s impairments that are not expressly captured in a disability measurement like the mRS such as inability to physically walk versus difficulty running errands. Alternatively, we may have been underpowered with our sample size to detect a significant effect on mRS. Our study could therefore be underpowered to demonstrate differences in dichotomous mRS outcomes in a mild stroke population. In addition, we excluded patients who died (mRS 6) after index hospitalization since our primary outcome was HRQOL; though death within three months is not common in patients with MIS, this may have biased our study against finding an effect of infarct location on mRS.
We did not find an HRQOL difference by sex. Prior studies have shown that women have worse functional outcomes after stroke than men.18 These differences have been attributed to women being older at the time of initial stroke and increased pre-stroke disability.18 While prior studies on sex and outcomes after stroke have focused on disability and functional outcomes, our group has not observed differences between men and women in HRQOL after stroke.3,6 Further work should be done to elucidate why sex impacts on functional outcomes but HRQOL after stroke.
Our study strengths include its prospective design, longitudinal follow-up at three months, and adjudicated imaging analysis by trained investigators. There are, however, several limitations. First, as a study from single urban academic medical center, our results may not be generalizable to other settings and populations. Replication of our findings in multicenter cohorts and different settings is needed. Second, HRQOL measures were collected only post-stroke; we do not have pre-stroke or baseline HRQOL assessments to measure change over time. Third, HRQOL assessments require participation from patients. If patients lose interest, assessments are prone to inaccuracy. Fourth, we did not assess other HRQOL domains such as fatigue or depression though these are less likely to have specific infarct location-outcome relationships. Fifth, HRQOL likely impacted by a multitude of factors that were not directly assessed including objective neurocognitive and psychosocial measures.
Conclusions
Acute infarct location predicts impairment in HRQOL outcomes in patients with MIS. Subcortical and brainstem infarcts were independently associated with impaired HRQOL. Future studies should consider the potential of imaging techniques such as diffusion tensor imaging to further elucidate which locations impact HRQOL and disability after MIS.
Supplementary Material
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Reeves M, Khoury J, Alwell K, et al. Distribution of National Institutes of Health stroke scale in the Cincinnati/Northern Kentucky Stroke Study. Stroke 2013; 44: 3211–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EE, Fonarow GC, Reeves MJ, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from get with the guidelines. Stroke 2011; 42: 3110–3115. [DOI] [PubMed] [Google Scholar]
- 3.Lin C, Lee J, Chatterjee N, et al. Predicting domain-specific health-related quality of life using acute infarct volume. Stroke 2017; 48: 1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng B, Forkert ND, Zavaglia M, et al. Influence of stroke infarct location on functional outcome measured by the modified Rankin scale. Stroke 2014; 45: 1695–1702. [DOI] [PubMed] [Google Scholar]
- 5.de Haan RJ, Limburg M, Van der Meulen JH, Jacobs HM and Aaronson NK. Quality of life after stroke. Impact of stroke type and lesion location. Stroke 1995; 26: 402–408. [DOI] [PubMed] [Google Scholar]
- 6.Sangha RS, Caprio FZ, Askew R, et al. Quality of life in patients with TIA and minor ischemic stroke. Neurology 2015; 85: 1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein LB, Jones MR, Matchar DB, et al. Improving the reliability of stroke subgroup classification using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. Stroke 2001; 32: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 8.Purushotham A, Campbell BCV, Straka M, et al. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke 2015; 10: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C-L, Tang F-T, Chen H-C, Chung C-Y and Wong M-K. Brain lesion size and location: effects on motor recovery and functional outcome in stroke patients. Arch PMR 2000; 81: 447–452. [DOI] [PubMed] [Google Scholar]
- 10.Bruno A, Akinwuntan AE, Lin C, et al. Simplified modified rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke 2011; 42: 2276–2279. [DOI] [PubMed] [Google Scholar]
- 11.Gershon RC, Lai JS, Bode R, et al. Neuro-QOL: quality of life item banks for adults with neurological disorders: item development and calibrations based upon clinical and general population testing. Qol Res 2012; 21: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloan JA, Cella D and Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol 2005; 58: 1217–1219. [DOI] [PubMed] [Google Scholar]
- 13.Addo J, Ayerbe L, Mohan KM, et al. Socioeconomic status and stroke: an updated review. Stroke 2012; 43: 1186–1191. [DOI] [PubMed] [Google Scholar]
- 14.McManus M, Ovbiagele B, Markovic D and Towfighi A. Association of insurance status with stroke-related mortality and long-term survival after stroke. J Stroke Cerebrovasc Dis 2015; 24: 1924–1930. [DOI] [PubMed] [Google Scholar]
- 15.Skolarus LE, Meurer WJ, Burke JF, Prvu Bettger J and Lisabeth LD. Effect of insurance status on postacute care among working age stroke survivors. Neurology 2012; 78: 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin C, Loochtan AI, Dresser B, et al. Is carpal tunnel syndrome present in acute stroke patients? An investigative study using clinical and imaging screening tools. J Clin Neurosci 2017; 39: 111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fries J, Rose M and Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol 2011; 38: 1759–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS and Wolf PA. Gender Differences in Stroke Incidence and Poststroke Disability in the Framingham Heart Study. Stroke 2009; 40: 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.