Abstract
Ischemic stroke is a leading cause of death and disability worldwide, with few available treatment options. The pathophysiology of cerebral ischemia involves both early phase tissue damage, characterized by neuronal death, inflammation, and blood-brain barrier breakdown, followed by late phase neurovascular recovery. It is becoming clear that any promising treatment strategy must target multiple points in the evolution of ischemic injury to provide substantial therapeutic benefit. Histone deacetylase (HDAC) inhibitors are a class of drugs that increase the acetylation of histone and non-histone proteins to activate transcription, enhance gene expression, and modify the function of target proteins. Acetylation homeostasis is often disrupted in neurological conditions, and accumulating evidence suggests that HDAC inhibitors have robust protective properties in many preclinical models of these disorders, including ischemic stroke. Specifically, HDAC inhibitors such as trichostatin A, valproic acid, sodium butyrate, sodium 4-phenylbutyrate, and suberoylanilide hydroxamic acid have been shown to provide robust protection against excitotoxicity, oxidative stress, ER stress, apoptosis, inflammation, and blood-brain barrier breakdown. Concurrently, these agents can also promote angiogenesis, neurogenesis and stem cell migration to dramatically reduce infarct volume and improve functional recovery after experimental cerebral ischemia. In the following review, we discuss the mechanisms by which HDAC inhibitors exert these protective effects and provide evidence for their strong potential to ultimately improve stroke outcome in patients.
Keywords: Histone deacetylase inhibitors, ischemia, stroke, neuroprotection, anti-inflammation, angiogenesis, neurogenesis, functional recovery
INTRODUCTION
Stroke is a devastating neurological condition responsible for widespread mortality, serious long-term disability, and enormous financial burden. In the United States alone approximately 795,000 people experience a new or recurrent stroke each year, meaning that, on average, someone suffers an attack every 40 seconds [1]. Stroke is also the fourth leading cause of death, accounting for 1 out of every 18 mortalities [1–2]. The significant majority of cases, 87%, are the result of impaired blood flow to the brain due to cerebral artery occlusion [1]. These strokes are termed ischemic and are the focus of this review. Methods to minimize the negative effects of cerebral ischemia are clearly of critical importance to public health. Unfortunately, progress in this search has thus far been inadequate. Currently the only Food and Drug Administration (FDA)-approved treatment for ischemic stroke is recombinant tissue plasminogen activator (rtPA), which acts by breaking down clots to restore blood flow and preserve brain tissue. rtPA is only effective when given within 4.5 hours of symptom onset, and otherwise carries a risk of intracranial hemorrhage that outweighs the potential benefit [3]. As a result of these limitations, it is estimated that rtPA is only used for 1.8–2.1% of ischemic stroke patients in the United States [4].
One major complication in the search for an effective stroke treatment is that the evolution of ischemic brain injury takes place from hours to weeks after the initial event. With a high basal metabolic rate, a reliance on precisely-maintained ionic gradients, and large concentrations of excitotoxic neurotransmitters such as glutamate and aspartate, brain tissue is particularly susceptible to the immediate effects of glucose and oxygen deprivation [5–6]. In the most severely affected region, known as the ischemic core, limited blood supply rapidly prevents the generation of adenosine triphosphate (ATP), and in turn leads to the failure of ATP-dependent ion pumps such as Na+/K+ ATPase. The subsequent Na+ influx, glutamate accumulation, and Ca2+ overload results in cytotoxic edema, acidosis, organelle and membrane failure, and finally necrotic cell death [5–7]. The area immediately adjacent to the core infarct zone, the ischemic penumbra, is subjected to more moderate reductions in blood flow, and thus more delayed injury mechanisms [8]. Of particular importance, ischemic stroke is associated with a robust immune response involving microglial activation, infiltration of peripheral leukocytes, and amplification of inflammatory signaling cascades [9–10]. These inflammatory reactions exacerbate cell death and disrupt the blood-brain barrier (BBB) to induce brain edema. In the delayed phase after cerebral ischemia the penumbra is the site of endogenous neurovascular repair, including angiogenesis and neurogenesis, which takes place from days to weeks after stroke onset and determines the ultimate extent of tissue regeneration and functional recovery [8, 11]. Because tissue in the ischemic penumbra is damaged but not destroyed, it holds the greatest promise for therapeutic intervention [8].
Given the complex pathophysiological processes involved in stroke, it is evident that an effective treatment must have a broad spectrum of actions, from inhibiting excitotoxicity and inflammation to augmenting tissue repair. Excitingly, recent preclinical advances have identified histone deacetylase (HDAC) inhibitors as promising therapeutic agents in multiple neurological conditions, including stroke [12–16]. By reversing the histone hypoacetylation observed after ischemic brain damage, compounds such as trichostatin A (TSA), valproic acid (VPA), sodium butyrate (SB), and suberoylanilide hydroxamic acid (SAHA, or vorinostat) can induce global changes in gene transcription and protein expression. As a result, HDAC inhibitors regulate processes ranging from cell survival to regeneration, and dramatically reduce infarct volume while promoting neurobehavioral recovery in multiple animal models of cerebral ischemia [17–20]. In this review, we first briefly describe the dynamic regulation of histone acetylation, and then discuss the accumulating evidence supporting the diverse mechanisms and therapeutic promise of HDAC inhibitors in ischemic stroke.
HISTONE ACETYLATION: HOMEOSTASIS AND REGULATION
1. HATs and HDACs
Chromatin is organized into structural units called nucleosomes, which consist of DNA wrapped around an octamer of four core histone proteins (two each of H2A, H2B, H3, and H4). Histone amino-terminal tails are subject to multiple post-translational modifications, including acetylation at specific lysines. By neutralizing the positive charge of these residues, acetylation reduces histone-DNA interaction to promote a loosely packed, open chromatin conformation that is more accessible to transcription factors and machinery. As a result, histone acetylation is generally associated with transcriptional activation and increased gene expression [reviewed in 21, 22]. Endogenously, histone acetylation is tightly regulated by histone acetyltransferases (HATs) and HDACs. These evolutionarily conserved enzymes catalyze acetylation and deacetylation of histones, respectively. Many transcriptional coactivators, such as cAMP response element-binding (CREB) binding protein (CBP)/p300, have been characterized as HATs [23], whereas many corepressor complexes, such as coREST, NuRD and mSin3A, interact with HDACs [24–26]. HATs and HDACs can also regulate the acetylation of non-histone proteins, including α-tubulin, molecular chaperone heat shock protein (HSP) 90, and transcription factors p53, E2F1, nuclear transcription factor kappa B (NF-κB), Smad7, and STAT-3 [reviewed in 27].
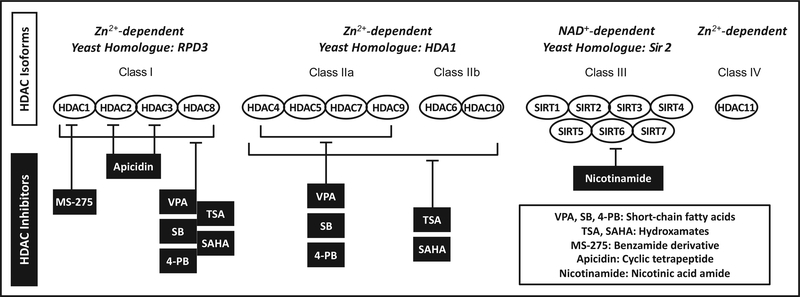
Eighteen human HDACs have been identified which can be divided into four major classes [reviewed in 16, 28]. Class I HDACs (HDAC1, 2, 3 and 8) are ubiquitously expressed, Zn2+-dependent enzymes related to the yeast protein RPD3. HDAC1 and 2 are found in the nucleus, whereas HDAC3 can shuttle between the nucleus and the cytoplasm [16] and HDAC8 is predominantly in the cytosol of smooth muscle cells [29]. Class II HDACs are further subdivided into class IIa (HDAC4, 5, 7, and 9) and class IIb (HDAC6 and 10). Both subclasses are Zn2+-dependent and homologous to the yeast protein HDA1. Unlike class I, class II HDACs show a tissue-specific distribution with the highest expression in heart, skeletal muscle, and brain [16, 28]. Class IIa HDACs are shuttled between the cytoplasm and nucleus, where they act as transcriptional corepressors via interaction with other proteins such as the myocyte enhancer factor 2 family of transcription factors [30]. Of the class IIb HDACs, only HDAC6 is well-characterized. It is located exclusively in the cytoplasm where it deacetylates α-tubulin to regulate microtubule stability [31] and is crucial to the misfolded protein stress response [32]. Notably, in neurons HDAC6 seems to regulate transport of the key neurotrophin brain-derived neurotrophic factor (BDNF), as its inhibition augments BDNF transport and release via increased α-tubulin acetylation [33]. Class III HDACs (SIRT1–7), are related to the yeast protein Sir2 and distinct from other HDACs in both structure and function. Also known as sirtuins, class III HDACs are dependent on nicotinamide adenine dinucleotide (NAD+) and act on diverse substrates in both the nucleus and cytoplasm [16]. The sole class IV HDAC, HDAC11, is Zn2+-dependent and shares characteristics with both class I and class II HDACs. It is found primarily in the cell nucleus of kidney, heart, skeletal muscle, testis, and brain [34–35]. (Fig. 1) provides an overview of HDAC classification.
Fig. (1).
HDAC isoforms and their pharmacological inhibitors. Eighteen human HDACs are divided into 4 major classes: I, IIa/b, III, and IV. The HDAC inhibitors most commonly utilized in models of cerebral ischemia are class I and II inhibitors trichostatin A (TSA) and suberoylanilide hydroxamic acid (SAHA), class I and IIa inhibitors valproic acid (VPA), sodium butyrate (SB), and 4-phenylbutyrate (4-PB), and class I isoform-specific inhibitors MS-275 and apicidin. Nicotinamide is a non-specific inhibitor of class III HDACs.
2. Dysregulation in Neurological Disease
Multiple lines of evidence have established that acetylation homeostasis is crucial for healthy neuronal function and is impaired during neurodegeneration [36]. Diverse models of neurological disorders, including stroke, are associated with a dramatic reduction of histone H3 and H4 acetylation [reviewed in 14]. For example, both our laboratory and others have observed significantly decreased histone acetylation levels after transient or permanent cerebral ischemia in rat and mouse [17–18, 37]. This is presumably due to a deficit in HAT activity, particularly CBP: the onset of neuronal apoptosis in cerebellar granule cells (CGCs) was associated with a specific reduction in CBP/p300 HAT activity due to degradation of CBP by caspases and calpains [38]. Furthermore, loss of CBP has been linked to cortical neuron death induced by activation of amyloid precursor protein-dependent signaling [38], motor neuron degeneration in a mouse model of amyotrophic lateral sclerosis [38], mutant huntingtin-triggered cell death [39], and irreversible cortical culture damage by hypoxia [40]. Under pro-apoptotic conditions, restoring histone acetylation levels by overexpressing CBP or with HDAC inhibitors can significantly reduce neuronal loss in vitro [38, 41]. Importantly, over the past decade HDAC inhibitors have been successfully applied to animal models of neurodegeneration and brain injury including Huntington’s disease [41–42], amyotrophic lateral sclerosis [43], Parkinson’s disease [44], Alzheimer’s disease [45], traumatic brain injury [46], and stroke [19, 47–48].
The most commonly used HDAC inhibitors in preclinical ischemic stroke studies are TSA, SAHA, VPA, SB, and sodium 4-phenylbutyrate (4-PB). These compounds are readily permeable to the BBB and provide relatively nonspecific inhibition of multiple HDAC isoforms. SAHA and TSA are hydroxamates that inhibit class I and II HDACs, although with less efficacy against HDAC8 [49]. VPA, SB, and 4-PB are short chain fatty acid derivatives that inhibit class I and class IIa HDACs, but not IIb [14, 50–51]. Several studies have also made use of the isoform-specific compounds MS275, a benzamide derivative that preferentially inhibits HDAC1, and apicidin, a cyclic tetrapeptide that primarily targets HDAC2 and 3 [49]. Finally, nicotinamide is a nicotinic acid amide that inhibits the NAD+-dependent deacetylase activity of class III HDACs [52]. Since nicotinamide is not specific to sirtuins and also inhibits other NAD+-dependent targets such as poly(ADP-ribose) polymerase (PARP), its coverage in this review is limited [53]. Stroke-relevant HDAC inhibitors are summarized in (Fig. 1).
In the following sections, we discuss in detail the beneficial mechanisms of HDAC inhibitors in both alleviating tissue damage and promoting recovery in cellular and animal models of ischemic brain injury.
HDAC INHIBITION IN CEREBRAL ISCHEMIA I: CELLULAR PROTECTION AND TISSUE PRESERVATION
1. Infarct Volume Reduction
Pre- or post-injury treatment with HDAC inhibitors can dramatically reduce infarct volume after cerebral ischemia. However, published studies vary considerably in injury model, dosing strategy, and the time point at which infarction was measured, making it difficult to directly compare the efficacy of each treatment paradigm. One of the most common models of focal cerebral ischemia in rodents is middle cerebral artery occlusion (MCAO). The MCA can either be permanently occluded (pMCAO), or transiently occluded (tMCAO) by temporarily blocking the origin of the MCA.
Many groups have observed a substantial reduction in infarct volume when animals were treated with HDAC inhibitors prior to the onset of experimental stroke. For example, in one study mice were pretreated with 5 mg/kg TSA by intraperitoneal (i.p.) injection once per day for 14 days and then subjected to 1 hour MCAO. At 24 hours post-injury, infarct volume was reduced by approximately 24% compared to vehicle-treated MCAO animals [54]. Rats given 1200 mg/kg SB by i.p. injection 24 and 4 hours prior to pMCAO had significantly smaller infarct volumes than vehicle-treated controls [55]. In both tMCAO and pMCAO mouse models, i.p. injection of 300 mg/kg VPA 30 minutes prior to ischemia also significantly reduced the infarct volume [56]. The protective effects of 4-PB have been investigated in a mouse model of hypoxia-ischemia (H/I) in which right carotid artery ligation was followed by 30 minutes of hypoxia at 6% O2 [47]. Under these conditions, pretreatment with 40 or 120 mg/kg 4-PB i.p. 30 minutes before H/I and then once per day for 3 days reduced infarct volumes by 40% and 70%, respectively.
Post-stroke treatment with HDAC inhibitors has also proved effective. For instance, in a mouse pMCAO model, 1 mg/kg TSA given by i.p. injection at the onset of occlusion and again 6 hours later diminished infarction by 57% at 48 hours [57]. 25 mg/kg or 50 mg/kg SAHA administered using the same stroke model and injection timing reduced infarction by approximately 30% at 24 hours [18]. Histone H3 acetylation was significantly reduced in the ischemic brains, although interestingly, no changes in HAT and HDAC activities were observed. It is suggested that ischemia-induced histone hypoacetylation may be due to limited HAT activity caused by ischemia-decreased acetyl-CoA contents [18]. SAHA treatment restored histone acetylation levels. However, SAHA’s effect on HDAC or HAT activity after ischemia is unclear and remains to be elucidated. In rats, 300 mg/kg SB injected subcutaneously (s.c.) at the start and 12 hours after pMCAO [17] and 300 mg/kg VPA delivered i.p. at the same time points after 1 hour tMCAO both significantly reduced infarct volume as well [19]. VPA treatment (300 mg/kg, i.p.) immediately after reperfusion was also found to significantly reduce the infarct size in a 2-hour tMCAO mouse model [56].
Of particular importance to their potential therapeutic use in the clinic, HDAC inhibitors remain efficacious when initially administered with a significant time delay after ischemic onset. Notably, when 300 mg/kg VPA was first injected s.c. 3 hours after rat pMCAO, a 32.7% decrease in infarct volume at 24 hours was still observed [17]. Similarly, 300 mg/kg SB first injected s.c. 6 hours after pMCAO resulted in a 33.1% decrease in infarction measured 24 hours post-injury [17]. Finally, when an initial i.p. injection of 4-PB was given 1 hour after H/I, infarct volume was again markedly diminished by both 40 and 120 mg/kg doses [47]. Notably, treatment with HDAC inhibitors increased histone acetylation in rodent brains [17–19, 54–56], suggesting the involvement of HDAC inhibition in brain infarct volume reduction. The consistent protective effects of structurally diverse HDAC inhibitors, most importantly when administration was delayed 1–6 hours after ischemic onset, give this class of drugs great clinical potential for the treatment of acute stroke (Table 1).
Table 1.
Summary of current literature demonstrating infarct volume reduction by HDAC inhibitors (HDACi) in rodent models of cerebral ischemia.
2. Neuroprotection
As alluded to previously, ischemic stroke involves multiple interdependent mechanisms of neuronal death including excitotoxicity, oxidative stress, endoplasmic reticulum (ER) stress, and apoptosis. Evidence suggests that HDAC inhibitors can mitigate many of these pathways to reduce neuronal injury after both in vitro and in vivo insult.
2.1. Excitotoxicity
Excessive glutamate release and inhibition of reuptake mechanisms after ischemia leads to overstimulation of N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxanole propionate (AMPA) receptors. The subsequent Ca2+ influx activates damaging lipases, nucleases, and proteases and can also stimulate pathways such as arachidonic acid metabolism and superoxide production to trigger additional cell death [6, 58–59]. Many studies have demonstrated the striking ability of HDAC inhibitors to protect against excitotoxicity in neurons. For example, pioneering work by Kanai et al used a model of excitotoxicity induced by SYM 2081 ((2S, 4R)-4-methylglutamate), an inhibitor of excitatory amino acid transporters and agonist of low-affinity kainate receptors, in mature CGCs [60]. SYM toxicity was blocked by NMDA receptor antagonist MK-801 and resulted in cell shrinkage, chromatin condensation, and DNA fragmentation, suggesting that it caused glutamate-induced apoptosis by blocking excitatory amino acid reuptake. The study found that 6 days pretreatment with VPA, SB, or TSA resulted in concentration-dependent protection against SYM-induced cell death, with nearly complete protection at 1.6 mM, 1.0 mM, and 100 nM, respectively. VPA or TSA treatment was associated with a selective increase in acetylated histone H3, and VPA suppressed the SYM-induced nuclear translocation of pro-apoptotic glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Soon afterwards, Leng and Chuang demonstrated that VPA, TSA, and 4-PB showed robust protection against glutamate-induced, NMDA receptor-mediated excitotoxicity in CGCs. Treatment with HDAC inhibitors markedly upregulated, while glutamate challenge downregulated, levels of acetylated H3 and induction of α-synuclein [61]. Knockdown of this presynaptic protein completely blocked the neuroprotective effects of VPA against glutamate-induced cell death, suggesting that α-synuclein, previously thought to be neurotoxic, is critical to VPA’s ability to preserve cell viability. This may be due to the α-synuclein-dependent upregulation of anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) and down-regulation of pro-apoptotic ubiquitin-conjugating enzyme E2N. In support of these findings, another study showed that VPA protected CGCs from 6-hydroxydopamine-induced insult by increasing α-synuclein expression and preventing its nuclear translocation [62]. Furthermore, VPA also exhibited neuroprotective effects in a rat rotenone model of Parkinson’s disease, and this neuroprotection may be mediated through its HDAC inhibition, thereby preventing rotenone-induced nuclear accumulation of monoubiquitinated α-synuclein [63]. It is of interest to note that VPA-increased histone H3 acetylation was closely correlated with inhibition of HDAC activity in the rat brains. In addition, in a cortical culture model VPA, TSA, SB, and class I HDAC-specific inhibitors MS-275 and apicidin all robustly increased mRNA, protein, and promoter histone acetylation of HSP70, a stress-inducible molecular chaperone with anti-apoptotic properties [64]. HSP70 upregulation by HDAC inhibitors seemed to be mediated by activation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway and acetylation of the downstream transcription factor Sp1, which binds to the HSP70 promoter. Importantly, transcriptional inhibition of HSP70 suppressed VPA-induced neuroprotection against glutamate excitotoxicity, suggesting that HSP70 may be critical for the anti-excitotoxic effects of HDAC inhibitors. Supporting this notion, superinduction of HSP70 and/or preservation of phospho-Akt have also been observed after protective treatment with VPA, TSA, SB, and SAHA in rodent models of cerebral ischemia [17–19].
The anti-excitotoxic effects of HDAC inhibitors may also involve regulation of the actin-severing protein gelsolin. By modulating the actin cytoskeleton, gelsolin can regulate the damaging Ca2+ influx after NMDA receptor activation: Ca2+ levels and cell death were enhanced in gelsolin-null neurons after oxygen-glucose deprivation (OGD), while infarct volume was significantly increased in gelsolin-null mice after MCAO [65]. Notably, TSA pretreatment was protective against OGD in wild-type, but not gelsolin-deficient, cortical neurons, and was associated with increased gelsolin protein expression and promoter histone acetylation as well as reduced intracellular Ca2+ influx [54, 66]. In vivo, 14 days treatment with TSA (1 or 5 mg/kg i.p.) in mice enhanced histone acetylation, increased gelsolin expression, and conferred actin remodeling. Pretreatment with the higher dose was protective against 60 minutes of MCAO in wild-type but not gelsolin knockout animals, further establishing the key role of this protein in the anti-excitotoxic and neuroprotective properties of TSA [54].
Finally, a study by Baltan et al demonstrated the striking ability of SAHA and MS-275 to reduce excitotoxic white matter damage, an often-overlooked component of ischemic injury [67]. Pretreatment with either drug promoted axon functional recovery and preserved morphology after OGD in isolated mouse optic nerves, a pure white matter tract model. Protection was associated with upregulation of the glutamate transporter GLT-1 in astrocytes, which functions to clear extracellular glutamate. As a result, MS-275 pretreatment reduced glutamate accumulation after OGD, and both drugs preserved axonal mitochondria and ATP levels. It is of interest that this study observed extensive localization of class I HDACs on glial cells in optic nerve preparations, suggesting that the actions of HDAC inhibitors on glia require additional consideration in in vivo stroke models.
2.2. Oxidative Stress
Brain damage following ischemia/reperfusion and NMDA receptor activation is also closely linked to the rapid and prolonged generation of reactive oxygen species (ROS) [6]. An initial burst of ROS occurs due to failure of the mitochondrial respiratory chain, followed by ATP depletion and ROS production by xanthine oxidase [68]. After reoxygenation, activation of Ca2+-dependent NAPDH-oxidase causes a robust increase in superoxide [68–69]. Excitotoxic or ischemic conditions also activate neuronal nitric oxide synthase (nNOS), resulting in a high concentration of nitric oxide (NO) which both facilitates NAPDH oxidase activity and reacts with superoxide to form the damaging oxidant peroxynitrite [59, 70]. The downstream effects of these processes include lipid peroxidation, protein denaturation, PARP activation, and initiation of mitochondria-dependent caspase cascades [71].
HDAC inhibitors provide strong protection against oxidative stress in cell culture. An early study by Ryu et al found that TSA, SAHA, and SB were all protective against an established model of oxidative stress induced by glutathione depletion in cortical neurons [72]. Augmentation of Sp1 acetylation and activation, an adaptive response to glutathione depletion in untreated cells, was necessary for the protective effects of these HDAC inhibitors. Additionally, chronic VPA treatment in cortical cultures increased the expression of glutathione S-transferase, an enzyme that converts oxidized products to non-toxic ones via conjugation with glutathione [73]. Further confirming the antioxidative properties of VPA, rat cortical neurons pretreated for 7 days at a therapeutically relevant dose were protected against the lipid peroxidation and protein oxidation induced by both glutamate [74] and the potent oxidant ferric chloride [75].
The neuroprotective and anti-oxidative properties of HDAC inhibitors might also be related to modulation of transcription factor NF-E2-related factor 2 (Nrf2), which regulates antioxidant-responsive genes. It was recently shown that TSA, which increased neuronal survival after OGD (30 or 60 ng/mL) and reduced infarct volume after murine pMCAO (1 mg/kg i.p.), also suppressed the Nrf2 inhibitor Keap1 and enhanced Nrf2 activation in vitro. TSA induced antioxidative proteins downstream of Nrf2, such as heme oxygenase-1, in neuronal cultures, and was no longer protective against cerebral ischemia in Nrf2-deficient mice [57]. The importance of Nrf2 regulation is further supported by the independent finding that VPA or TSA treatment could enhance histone acetylation and restore Nrf2 levels to protect against oxidative death in astrocyte-enriched cultures [76].
Finally, one problem inherent to studying the protective pathways modulated by HDAC inhibitors is their modest baseline toxicity after prolonged exposure. Langley et al circumvented this issue by pulse-treating cortical cultures with TSA for 2 hours, which was sufficient to prevent oxidative death due to glutathione depletion without toxic effects [55]. In this study, HDAC inhibitor exposure was associated with an upregulation of the cyclin-dependent kinase inhibitor p21waf1/cip1, which was also upregulated after protective SB treatment in a rat model of focal ischemia. p21waf1/cip1 seemed to be acting via a non-canonical protective role in the cytoplasm, and while its overexpression was sufficient to prevent oxidative stress-induced neuronal death, it was not always necessary for the protective effects of HDAC inhibitors.
2.3. ER Stress
The ER is a subcellular organelle crucial for protein folding, processing, and secretion. These functions require a high level of stored Ca2+, which is maintained in the ER lumen by ATP-dependent ion pumps. After cerebral ischemia, reduced ATP and elevated cytosolic Ca2+ lead to ER Ca2+ depletion. This initiates a conserved stress response involving upregulation of a specific set of stress genes and suppressed protein synthesis through phosphorylation of eukaryotic initiation factor 2α (eIF-2α) [77–78]. Severe ER stress can result in apoptosis via activation of the transcription factor C/EBP homologous protein/growth arrest- and DNA damage-inducible gene 153 (CHOP/GADD153) [78] as well as cleavage of procaspase-12 and downstream activation of caspases-9 and –3 [79].
Evidence suggests that HDAC inhibitors can regulate ER stress pathways to mitigate damage after cerebral ischemia. Chronic VPA treatment induced the expression of ER stress proteins glucose-regulated protein (GRP)78, GRP94, and calreticulin in the rat cerebral cortex and hippocampus [80] as well as in rat C6 glioma cells [81]. Importantly, overexpression of these molecular chaperones has been shown to preserve Ca2+ homeostasis, prevent oxidative stress, and reduce cell death [82]. Moreover, treatment with 4-PB (40 or 120 mg/kg i.p.) for three days either pre- or post-H/I significantly reduced infarct volume and hemispheric swelling in mice. These protective effects were attributed to reductions in ER stress-mediated apoptosis via suppression of eIF-2α, CHOP/GADD153, and caspase-12 after injury [47]. In mouse neuroblastoma cells, 4-PB inhibited cell death and caspase-12 expression after hypoxia/reoxygenation or treatment with an ER stress inducer [47]. Reinforcing these findings, the protective effects of 4-PB in a rat model of cerebral ischemia with comorbid type 2 diabetes were also associated with reduced ER stress and DNA fragmentation [83]. It is worth noting that these studies did not directly attribute the protective effects of 4-PB to inhibition of HDACs. Furthermore, in contrast to the effects of 4-PB, the structurally dissimilar HDAC inhibitor TSA has been shown to enhance CHOP stabilization and accumulation [84]. To clarify this discrepancy, the specific effects of HDAC inhibition on ER stress after ischemic insult require further investigation.
2.4. Apoptosis
After cerebral ischemia cellular stressors such as ionic imbalance, protease activation, excess free radical formation, DNA damage, and disruption of mitochondrial membrane integrity can trigger apoptotic cascades, particularly in the penumbra region [85]. The initiation of apoptosis depends on a delicate balance between anti-and pro-apoptotic factors. Prominent anti-apoptotic proteins include Bcl-2 and closely related Bcl-xl, which inhibit mitochondrial transition pore formation [85], as well as HSP70, which, among other actions, reduces stress signal transmission by preventing c-Jun N-terminal kinase (JNK) pathway activation [86]. On the other side of this equilibrium are apoptosis inducers such as p53, p53-upregulated modulator of apoptosis (PUMA), Bcl-2–associated X protein (BAX), as well as activated executioner caspase-3, which is considered a hallmark of apoptosis [85].
Treatment with HDAC inhibitors can both induce the expression of anti-apoptotic factors and attenuate the upregulation of pro-apoptotic proteins to reduce cellular apoptosis after ischemic insult. In rodent models of cerebral ischemia, HDAC inhibitor treatment robustly increased levels of Bcl-2 [18], phospho-Akt [17], and HSP70 [17–19, 87], but prevented the ischemia-induced upregulation of p53 [17] and activation of caspase-3 [19]. These effects were also observed after protective VPA treatment in a rat model of intracerebral hemorrhage [88]. In this study, twice-daily post-injury treatment with 300 mg/kg VPA increased histone acetylation and upregulated pro-survival proteins phospho-extracellular signal-regulated kinase (ERK), phospho-Akt, phospho-CREB, HSP70, Bcl-2, and Bcl-xl while simultaneously downregulating pro-apoptotic BAX, reducing caspase activity, and diminishing the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive apoptotic cells. Similar results have been observed in cortical neuronal cultures undergoing hypoxia, where VPA treatment markedly reduced apoptosis as measured by caspase-3 activation in association with inhibition of JNK. Furthermore, an elegant study by Uo et al demonstrated that structurally distinct HDAC inhibitors could significantly protect neurons from BAX-mediated apoptosis induced by both p53-dependent and independent cell death pathways [89]. Together, these results suggest that the neuroprotective actions of HDAC inhibitors are at least partially due to their potent anti-apoptotic effects in response to the complex convergence of cellular stressors after cerebral ischemia.
3. Anti-inflammation
Although much of early stroke research focused predominantly on neuronal injury, inflammation is now recognized as an integral component of post-ischemic progression and pathology [9]. Cerebral ischemia-reperfusion activates a dynamic and long-lasting inflammatory cascade involving interactions between blood vessels, brain tissue, and peripheral immune cells. Occlusion rapidly upregulates adhesion molecules such as P-selectin and intercellular adhesion molecule 1 (ICAM-1) on endothelial cell walls [90–91]. Within hours, this stimulates the recruitment and infiltration of circulating leukocytes to the injury site, where they amplify inflammatory cytokine/chemokine signaling, produce inducible NO synthase (iNOS) and ROS, promote BBB breakdown, and exacerbate neuronal death [9–10, 92]. Additionally, activation of microglia, the brain’s resident immune cells, is mediated by ATP released from astrocytes and observed within the first 24 hours after stroke [93–94]. Activated microglia act as macrophages and propagate inflammation-mediated neurotoxicity via NF-κB-activation and cytokine release [95]. Together, these inflammatory processes aggravate BBB breakdown, edema, and tissue injury, making them a crucial target for effective stroke therapy.
An early study demonstrating the anti-inflammatory effects of HDAC inhibition showed that pretreatment with VPA could inhibit lipopolysaccharide (LPS)-induced NF-κB nuclear translocation in human monocytic leukemia and glioma cell lines [96]. Under basal conditions, NF-κB is inactive and complexed to the inhibitory protein IB in the cytoplasm. Upon stimulation with a stressor, IκB is rapidly degraded and NF-κB is released for translocation to the nucleus where it activates the transcription of a number of downstream mediators [97]. In line with this mechanism, VPA pretreatment in monocytic cells also suppressed the production of NF-κB-dependent pro-inflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) [96]. The strong anti-inflammatory effects of HDAC inhibition against LPS stimulation have also been established in neuron-glia co-cultures from rat midbrain. In this model, pretreatment with VPA, TSA, or SB protected neurons, notably dopaminergic neurons, from LPS-stimulated neurotoxicity; these effects were in part due to HDAC inhibition-mediated production and release of glial cell line-derived neurotrophic factor (GDNF) from astrocytes [98–99]. Further, pretreatment of these co-cultures with VPA suppressed LPS-induced microglial activation and reduced microglia number, while completely blocking the increase in TNF-α and moderately inhibiting production of NO and intracellular ROS [100]. A subsequent study demonstrated that the anti-inflammatory effects of HDAC inhibition were facilitated by the induction of apoptosis in LPS-activated microglia [101]. The structurally distinct HDAC inhibitor SAHA and its analog ITF2357 were similarly effective in suppressing the LPS-stimulated inflammatory response in mouse primary glial cultures [102].
The robust anti-inflammatory properties of HDAC inhibitors have also been investigated directly in the context of stroke. For example, Kim et al demonstrated that post-insult VPA administration in a rat pMCAO model reduced the activation and number of microglia and attenuated the infiltration of monocytes/macrophages to the ischemic cortex 24 and 48 hours after injury. In addition, VPA blocked the pMCAO-induced increase in iNOS immunoreactivity in the ischemic striatum and frontal cortex. These anti-inflammatory effects were replicated by post-insult treatment with SB, which also suppressed microglia activation and number, and prevented the pMCAO-induced upregulation of iNOS and cyclooxygenase-2 (COX-2) [17]. COX-2, the enzyme responsible for prostaglandin synthesis, interacts with iNOS to propagate inflammation and ROS production after stroke [103]; inhibition of either significantly attenuated ischemia-induced brain damage [104–105]. Therefore, suppression of these enzymes likely contributes significantly to the protective effects of HDAC inhibitors. Moreover, the anti-inflammatory properties of HDAC inhibition in cerebral ischemia are consistent with findings from a rat intracerebral hemorraghic model of stroke. At 24 hours after injury, post-insult treatment with VPA reduced microglial activation and neutrophil infiltration as well as downregulated mRNA levels of inflammatory mediators IL-6 and TNF family member Fas ligand [88]. In both cerebral ischemic and hemorraghic models, VPA treatment led to upregulation of histone acetylation in rat brains, suggesting a link between HDAC inhibition and VPA’s anti-inflammatory effects.
As addressed previously, HDAC inhibitors VPA, TSA, SB, MS-275 and apicidin all superinduced endogenous HSP70 in neurons, likely through acetylation of the transcription factor Sp1, enhanced association between Sp1 and the HAT p300, and recruitment of p300 to the HSP70 promoter [64]. In addition, VPA and MS-275 increased histone 3 lysine 4 dimethylation, which is associated with transcriptional activation, at the HSP70 promoter in neurons and astrocytes [106]. Importantly, overexpression of HSP70 in a mouse tMCAO model decreased infarct volume and reduced the number of activated microglia/macrophages in the ischemic cortex. Moreover, it inhibited activation and nuclear translocation of NF-κB and suppressed the tMCAO-induced upregulation of NF-κB-dependent proinflammatory genes TNF-α, IL-1β, ICAM-1, and iNOS [107]. Since NF-κB was also inhibited by VPA and SB in a rat tMCAO model [37] as well as by TSA in an astrocytic OGD model [108], evidence suggests that superinduction of HSP70 and subsequent inhibition of NF-κB and its downstream mediators constitutes a prominent anti-inflammatory mechanism of HDAC inhibitors after cerebral ischemia.
4. BBB Preservation
The BBB is a highly selective physical barrier between the central nervous system and peripheral blood stream. It is formed by a layer of cerebral capillary endothelial cells as well as surrounding basal lamina, pericytes, astrocytic end-feet, and perivascular neurons. Together, these components are termed the neurovascular unit [109–110]. The low permeability of the BBB is maintained by two types of interendothelial junctions: adherens junctions and tight junctions. Adherens junctions are made up of transmembrane cadherin family proteins and mediate cell-cell adhesion [109]. Tight junctions are comprised of the three transmembrane proteins claudins, occludin, and junctional adhesion molecule, as well as cytoplasmic accessory proteins such as zonula occludens (ZO-1, ZO-2, ZO-3). These junctions form the primary barrier to paracellular permeability across the BBB by sealing adjacent endothelial cells together, maintaining electrical resistance, and regulating leukocyte infiltration [111]. Disruption of tight junctions and increased BBB permeability play a major role in the pathogenesis of stroke [112–113]. BBB breakdown allows circulating blood constituents to leak into the brain parenchyma, leading to vasogenic edema, hemorrhage, and peripheral immune cell infiltration. This occurs in two phases - the first within hours of reperfusion and the second 24–72 hours later - which correlate with the biphasic activation of matrix metalloproteinases (MMPs), zinc-dependent endopeptidases that degrade tight junctions and basal lamina proteins [114]. Evidence suggests that both MMP-2 and MMP-9 increase BBB permeability in the early phase after stroke, whereas MMP-9 is primarily responsible for later phase damage [114–116]. MMP-9 is largely produced by infilitrating leukocytes and upregulated by activation of transcription factor NF-κB [113, 117–118], although immunoreactivity has also been observed in vessels and microglia after MCAO [119].
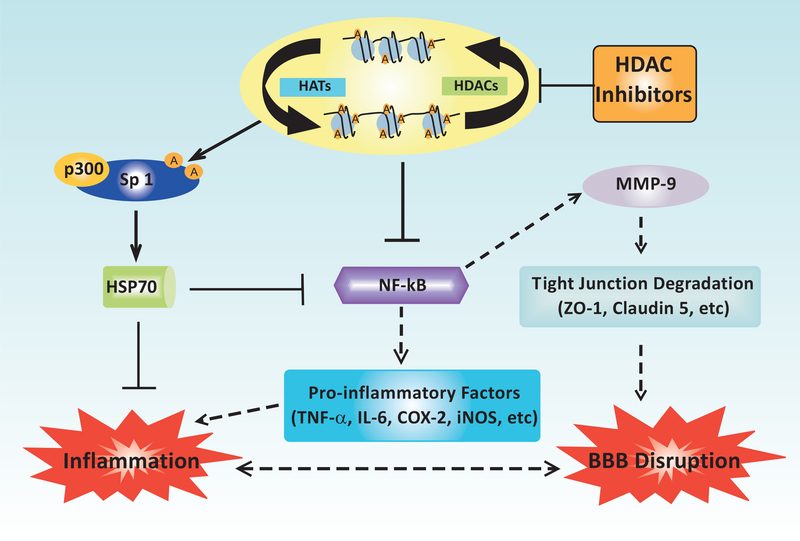
In a rat model of tMCAO, post-insult treatment with VPA (200 or 300 mg/kg i.p.) reduced BBB disruption and brain edema for up to three days after reperfusion [37]. By inhibiting HDACs, VPA suppressed the nuclear translocation of NF-κB subunit p65 and attenuated the activation of MMP-9 in the ischemic cortex and striatum 24 hours post-injury. Furthermore, VPA administration restored the brain levels of tight junction proteins claudin-5 and ZO-1 which were downregulated by ischemia. Treatment with the HDAC inhibitor SB (300 mg/kg i.p.) similarly blocked BBB breakdown, NF-κB nuclear translocation, and MMP-9 activation in this model [37]. These results are supported by recent findings from a rat spinal cord contusion model, which demonstrated that postinjury VPA treatment attenuated blood-spinal cord barrier permeability, inhibited MMP-9 activity, and suppressed degradation of occludin and ZO-1 [120]. In addition, administration of VPA after traumatic brain injury in rats, another acute CNS insult that leads to BBB damage, significantly improved BBB integrity at 48 hours post-insult and enhanced motor and cognitive recovery [46]. The effects of HDAC inhibition on NF-κB–mediated BBB breakdown and inflammation after ischemic stroke are summarized in (Fig. 2).
Fig. (2).
Schematic diagram depicting the effects of HDAC inhibition on inflammation and BBB integrity in ischemic stroke models. Inhibition of HDAC activity results in multiple mechanisms that promote BBB integrity and anti-inflammation. HDAC inhibitors enhance the expression of the neuroprotective molecule HSP70, likely through increasing Sp1 acetylation, enhancing the association between Sp1 and the HAT p300, and promoting the recruitment of p300 to the HSP70 promoter. HSP70 in turn inhibits the activation of NF-κB which reduces the expression of pro-inflammatory factors such as TNF-α, IL-6β, COX-2, and iNOS, thereby suppressing inflammatory damage to both brain tissue and the BBB. NF-κB inhibition induced by HDAC inhibitors also contributes to BBB integrity via tight junction preservation. Reduced NF-κB activation leads to the downregulation of MMP-9, a matrix-degrading enzyme induced after ischemia, ultimately preventing the degradation of tight junction proteins such as ZO-1 and claudin 5. BBB preservation reduces the number of infiltrating immune cells, thereby concurrently suppressing neuroinflammatory damage. Lines with arrows represent stimulatory connections; lines with flattened ends represent inhibitory connections. Dashed lines represent pathways with reduced activity as a result HDAC inhibition. A: acetylation.
HDAC INHIBITION IN CEREBRAL ISCHEMIA II: REGENERATION AND RECOVERY
During the chronic phase (days to weeks) after stroke, there is a shift in the ischemic penumbra from processes that exacerbate tissue damage to those that promote neurovascular regeneration and repair [8]. Accumulating evidence suggests that HDAC inhibitors not only suppress post-stroke injury, but also augment this recovery phase to generate significant functional and behavioral improvement.
1. Pro-angiogenesis
Angiogenesis, the formation of new capillaries through directed migration and proliferation of endothelial progenitor cells from preexisting blood vessels, is a critical component of neurovascular remodeling after stroke. Angiogenesis increases collateral circulation, restoring oxygen and nutrient supply to damaged tissue and promoting concurrent neurogenesis and synaptogenesis [121]. In humans, stroke activates endogenous blood vessel formation in the penumbra, with higher capillary density correlating with increased patient survival time [122]. Revascularization has also been characterized in rodent models of cerebral ischemia. After tMCAO in mice, proliferating endothelial cells are observed as early as 1 day after injury and increased vessel density by 3 days [123]; these growing blood vessels ultimately form a pattern similar to normal brain tissue [124]. Two key molecular players in post-stroke angiogenesis are vascular endothelial growth factor (VEGF) and MMPs. Both are biphasic factors that initially contribute to BBB breakdown but have later regenerative functions. VEGF is a potent proangiogenic factor that stimulates endothelial cell proliferation and interacts with other molecules to direct vessel formation. It is robustly upregulated by multiple cell types after stroke, and remains elevated for several weeks [reviewed in 125]. Notably, delayed administration of recombinant VEGF at 48 hours post-insult enhances angiogenesis and promotes neurological recovery in ischemic rats [126]. There is also a prolonged elevation of MMPs, particularly MMP-2/9, after cerebral ischemia [127]. MMPs degrade extracellular matrix to set the stage for endothelial cell migration, and can cleave matrix-bound VEGF to increase its bioavailability [128]. Delayed administration of MMP inhibitors suppresses neurovascular remodeling and worsens stroke outcome in rats, supporting the role of these proteases in neurovascular recovery [129].
Recent work from our laboratory demonstrated that chronic post-stroke treatment with VPA robustly enhanced histone acetylation, angiogenesis, and functional recovery in a rat tMCAO model [130]. Once daily administration of VPA (200 mg/kg i.p.) initiated at the onset of ischemia significantly reduced infarct volume on day 14 and concurrently increased microvessel density and endothelial cell proliferation in the ipsilateral cortex. Furthermore, VPA treatment markedly enhanced relative cerebral blood flow in the ipsilateral cortex as measured by perfusion-weighted imaging at day 14. These effects were associated with an upregulation of VEGF protein levels and MMP-2/9 activities after VPA treatment. Both VEGF and MMPs are downstream of transcription factor hypoxia-inducible factor (HIF)-1, a highly conserved complex that is activated under hypoxia via stabilization of the α subunit [131]. Notably, post-MCAO VPA treatment was also associated with an increase in HIF-1α, and inhibition of this transcription factor suppressed the VPA-induced augmentation of VEGF protein levels, MMP-2/9 activities, microvessel density, and functional recovery [130]. VPA has additionally been shown to promote endothelial functional preservation through regulation of transforming growth factor-β and VEGF pathways in a swine hemorrhagic shock model [132]. VPA treatment therefore appears to have biphasic effects on post-ischemic endothelial cell function that correlate with endogenous damage/repair processes after stroke. Specifically, it can inhibit NF-κB activation to suppress MMP-9 activity and reduce BBB breakdown at 24 hours after MCAO [37], but then enhance HIF-1 activation to upregulate MMP-2/9 and VEGF and promote angiogenesis during the recovery period.
Supporting these in vivo findings, a recent study demonstrated that both VPA and SAHA improved VEGF-induced endothelial cell spheroid sprout formation [133]. In addition, microvascular pericytes are adjacent to endothelial cells and play a major role in vessel formation, stabilization, and maintenance. An angiogenesis mRNA array demonstrated that chronic VPA treatment in human microvascular pericytes upregulated multiple angiogenesis-related genes involved in vessel stabilization and maturation. This included genes related to endothelial survival, tube formation, maintenance of cell-cell contact between pericytes and endothelial cells, and basement membrane formation [134]. Together, these results indicate that under certain conditions such as ischemic stroke, HDAC inhibitors can promote angiogenesis both in vivo and in vitro, likely contributing to their beneficial effects on long-term recovery after experimental brain ischemia.
2. Pro-neurogenesis
Neurogenesis, the process by which neural progenitor cells proliferate, migrate, and differentiate into functionally integrated neurons, occurs in both adult rodents and primates. Under normal physiological conditions, the two brain regions that typically produce neural progenitor cells are the subventricular zone and the hippocampal dentate gyrus [135–136]. Cerebral ischemia can stimulate neurogenesis in both of these areas [137–138], as well as in non-neurogenic injury regions such as the striatum and cortex [139–140]. Attenuating neural regeneration after global ischemia exacerbated behavioral deficits in gerbils [141], supporting the hypothesis that these new neurons contribute to endogenous functional recovery after stroke.
There is a significant body of evidence supporting the proneurogenic actions of HDAC inhibitors, both in vitro and in vivo. For instance, an early study demonstrated that VPA treatment promoted neurite growth and neurogenesis in primary cortical cultures. This depended on activation of the ERK1/2 pathway, which is also downstream of many canonical neurotrophic factors [142]. In line with these findings, chronic VPA treatment increased neurogenesis in the dentate gyrus of adult mice, possibly through a similar ERK-dependent mechanism [142]. Additionally, VPA, TSA, and SB have all been shown to increase neuronal differentiation of hippocampal neural progenitor cells isolated from adult rats. VPA simultaneously suppressed glial differentiation, and these actions were associated with maintenance of histone acetylation and upregulation of the neuron-specific basic helix-loop-helix transcription factor NeuroD [143]. The pro-neurogenic effects of HDAC inhibition may also be due to induction of BDNF and activation of its receptor TrkB, which have well-established roles in neuronal growth and survival, synaptic plasticity, brain development, and neurogenesis [144]. Notably, treatment with VPA, TSA, SB, or HDAC1-specific siRNA significantly upregulated BDNF promoter IV activity in cortical neurons [145]. Interestingly, VPA has been shown to reduce cell proliferation in the subgranular zone of the dentate gyrus and cause a reduction in BDNF levels in the hippocampus of adult rats [146]. Another study showed that chronic VPA treatment did not affect cell proliferation, cell survival or neurogenesis in the developing rat hippocampus [147]. This discrepancy may be due to differing VPA treatment conditions, and thus further research is required to address this issue.
A key study by Kim et al demonstrated the pro-neurogenic effects of HDAC inhibition in the specific context of ischemic stroke [20]. In a rat model of pMCAO, post-insult treatment with SB or TSA robustly increased cell proliferation in the subventricular zone, dentate gyrus, striatum, and frontal cortex of the ischemic brain. SB concurrently induced multiple neuroblast markers such as nestin and polysialylated neuronal cell adhesion molecule, suggesting that treatment stimulated the migration of neural precursors to areas of injury. In addition, SB treatment upregulated levels of BDNF and downstream pro-neurogenic transcription factor phospho-CREB in multiple brain regions after ischemia. When K252a, a TrkB antagonist, was injected into the lateral ventricle prior to MCAO, SB-induced cell proliferation, neuronal differentiation and CREB activation were inhibited, as were the long-term behavioral benefits of SB in this model. Together, these findings indicate the significance of BDNF-TrkB-dependent neurogenesis on the restorative effects of HDAC inhibition after ischemic injury. Recently, VPA has been shown to promote neurogenesis after brain ischemia [148]. It is worth noting that 100 mg/kg VPA was administered daily to rats for 7 days starting 24 hours after MCAO. The delayed VPA treatment increased the number of neuroblasts in the ischemic boundary zone 28 days after MCAO. Meanwhile, VPA enhanced histone H4 acetylation in neuroblasts and neural progenitor cells, suggesting that HDAC inhibition may be involved in VPA’s proneurogenic effects.
Finally, it is important to note that post-stroke neurogenesis is closely tied to angiogenesis, with neuroblasts migrating along blood vessels for several months after ischemic injury [149]. Therefore, the HDAC inhibitor-induced microvessel proliferation in the ischemic penumbra, described in the previous section, may also promote neurogenesis indirectly.
3. Stem Cell Migration
Research from the past decade has generated great interest in using stem cells to treat stroke. Excitingly, intravenous infusion of bone marrow-derived mesenchymal stem cells (MSCs) successfully improved functional recovery after cerebral ischemia in both rats [150] and human patients [151]. One major obstacle to the widespread application of this technique, however, is the poor baseline homing and migratory capacity of MSCs to a specific region of interest [152]. Interaction between the chemoattractant stromal cell-derived factor-1α (SDF-1α), which is upregulated in the ischemic penumbra, and its cellular receptor CXC chemokine receptor type 4 (CXCR4) plays an essential role in mediating MSC migration to the ischemic brain [153]. Therefore, modulation of this signaling pathway in MSCs has the potential to increase their homing ability and overall therapeutic efficacy.
Groundbreaking work by Tsai et al demonstrated that pulse-treating MSCs with VPA for 3 hours resulted in a dramatic, dose-dependent upregulation of CXCR4 without detrimental effects on proliferation or morphology [154]. This increase correlated with an overall augmentation of acetylated histone H3 as well as histone hyperacetylation at the CXCR4 promoter. Furthermore, CXCR4 upregulation was mimicked by treatment with TSA, SB, or the HDAC1-specific inhibitor MS-275, strongly implicating HDAC inhibition in this phenomenon. When SDF-1α was used as a chemoattractant, 3 hour VPA pretreatment robustly increased the migratory capacity of MSCs, an effect that was abolished by cotreatment with a CXCR4 antagonist. This is consistent with an independent study demonstrating that VPA could similarly enhance CXCR4 transcript expression, promoter histone acetylation, and migration towards a SDF-1α gradient in hematopoietic stem cells [155]. Notably, Tsai and colleagues also showed that combined pretreatment with both VPA and lithium, a glycogen synthase kinase (GSK)-3β inhibitor and upregulator of MMP-9, further increased MSC migration [154]. In a follow-up in vivo study, MSCs primed with VPA were transplanted into ischemic rats 24 hours after 60 minutes of tMCAO [156]. Priming significantly increased MSC homing to the ischemic frontal cortex and striatum. VPA priming also enhanced functional recovery, reduced infarct volume, and increased microvessel density on day 15 after MCAO, with even greater improvements observed in animals that received MSCs co-primed with lithium. Again, this was most likely due to upregulation of CXCR4 and MMP-9, respectively, and suggests that HDAC inhibitor priming may be a promising strategy to increase the clinical utility of MSCs in stroke, particularly when used in combination with a GSK-3β inhibitor such as lithium.
4. Neurobehavioral Recovery
Stroke patients are often severely debilitated as a result of their injury. In a 6 month follow-up study of patients over age 65, 43% suffered moderate to severe neurological deficits, most frequently including hemiparesis (50%) and cognitive decline (46%). Moreover, 26% were institutionalized in nursing homes by 6 months after their initial stroke, reflecting a drastic decline in basic functional abilities [157]. Due to their protective and regenerative actions, treatment with HDAC inhibitors can markedly improve neurological function after ischemic stroke in rodents. In a rat model of pMCAO, post-insult treatment with VPA, SB, or TSA all improved neurological impairment based on motor, sensory, and reflex performance at 24 hours [17]. Of even greater importance to their therapeutic potential in the clinic, delayed administration of VPA or SB 3 hours after injury still significantly improved neurological deficit score and rotarod performance, a measure of motor coordination, at 24 hours. When SB was first given as late as 6 hours after stroke onset, there was a trend toward improvement in both of these tests [17]. Likewise, 120 mg/kg 4-PB delayed by 1 or 3 hours after H/I in mice improved neurological status 1–3 days later [47]. The beneficial effects of HDAC inhibitors are also relatively long-lasting. In the rat pMCAO model described above, immediate post-insult treatment with SB followed by once-daily injections improved neurological scoring and rotarod performance at both days 7 and 14 after injury [20]; post-insult VPA treatment after tMCAO yielded similar rotarod performance improvement [130]. Notably, VPA, injected daily for 7 days starting 24 hours after MCAO, has recently been shown to improve neurological outcomes in foot fault test, adhesive test and neurological severity score as determined 28 days after MCAO [148]. It has been suggested that HDAC inhibition contributes to the neurobehavioral benefits of HDAC inhibitors [17, 130, 148]. The long-term (several weeks to months) effects of HDAC inhibitors on neurological function after stroke are currently unknown and require follow-up.
Cognitive impairment, particularly deficits in memory, language, orientation, and attention, is a frequent outcome for patients who have suffered ischemic stroke. This has significant functional consequences independent from physical disability, including higher rates of dependent living either at home or in nursing facilities [158]. Indirect evidence suggests that HDAC inhibition could contribute to cognitive recovery after cerebral ischemia [reviewed in 15]. An imbalance in chromatin acetylation has been linked to defects in synaptogenesis, plasticity, memory, and cognition which could be restored by treatment with HDAC inhibitors [159–161]. Specifically, Guan et al demonstrated in non-ischemic mice that neuronal overexpression of HDAC2 decreased dendritic spine density, synaptic plasticity, and memory formation whereas HDAC2 knockout mice showed enhanced memory and synapse formation [162]. Chronic treatment with SAHA restored associative learning, spine density, and synapse number in HDAC2 overexpression mice but had no effect in the knockout animals, suggesting that HDAC2 is a primary target for the HDAC inhibitor-mediated enhancement of learning and memory [162]. Since this isoform is categorized as a class I HDAC, it is targeted by all of the pan-HDAC inhibitors commonly evaluated in experimental stroke. Notably, in a rat model of transient global ischemia VPA ameliorated post-injury deficiencies in the Morris water maze task, an established measure of hippocampal-dependent learning and memory [87]. Meanwhile, VPA treatment significantly increased the levels of acetylated histones H3 and H4 in the hippocampus. This raises the exciting possibility that HDAC inhibitors could facilitate cognitive recovery after human stroke, thereby allowing patients to regain functional independence.
CONCLUSION AND PROPOSED NOVEL DIRECTIONS
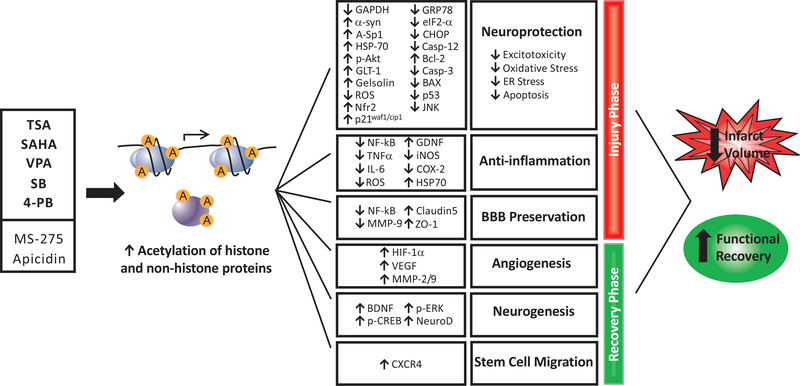
There is strong evidence to suggest that pharmacological inhibition of HDACs can increase the acetylation of histone and non-histone proteins to beneficially modulate a number of endogenous responses to cerebral ischemia. Specifically, HDAC inhibitors can: block excitotoxicity by suppressing GAPDH nuclear accumulation, increasing α-synuclein, HSP70, and gelsolin expression, and preserving white matter; prevent oxidative stress through increasing Sp1 acetylation, activating Nrf2, and upregulating p21waf1/cip1; reduce the expression of ER stress proteins; and upregulate anti-apoptotic Bcl-2, phospho-Akt, and HSP70 while downregulating pro-apoptotic p53 and caspase-3. Concurrently, inhibition of HDACs preserves the BBB and reduces inflammatory processes including microglial activation, immune cell infiltration, NF-κB nuclear translocation, and expression of iNOS, COX-2, and pro-inflammatory cytokines. In the chronic phase after stroke, this class of drugs can promote angiogenesis via delayed upregulation of HIF-1α, MMP-2/9 and VEGF, increase BDNF-TrkB-dependent neurogenesis, and promote stem cell migration to the ischemic brain. As a result, cerebral ischemic animals treated with HDAC inhibitors have reduced infarct volumes and dramatically improved motor, sensory, and reflex responses after injury, as well as potentially enhanced cognitive function. (Fig. 3) illustrates an overview of the beneficial effects and underlying mechanisms of HDAC inhibitors after ischemic stroke.
Fig. (3).
Proposed effects of HDAC inhibition in models of ischemic stroke. Pan-HDAC inhibitors TSA, VPA, SAHA, SB, and 4-PB as well as class I-specific inhibitors MS-275 and apicidin can increase the acetylation of both histone and non-histone proteins to induce gene expression and cause a plethora of downstream effects after cerebral ischemia. To reduce damage in the acute injury phase, this class of drugs inhibits excitotoxicity, prevents oxidative stress, downregulates ER stress proteins, and blocks apoptosis by increasing protective proteins while suppressing pro-apoptotic factors. Furthermore, HDAC inhibitors demonstrate robust anti-inflammatory effects, including inhibition of pleiotropic transcription factor NF-κB and suppression of pro-inflammatory cytokines and enzymes. HDAC inhibitors also preserve the BBB by suppressing NF-κB and matrix-degrading enzyme MMP-9 leading to the upregulation of tight junction proteins. During the chronic recovery phase, lasting days to weeks after stroke, HDAC inhibition promotes important endogenous repair processes. These include angiogenesis, via delayed upregulation of HIF-1α, VEGF, and MMPs, and neurogenesis, via induction of BDNF, p-ERK, and pro-neurogenic transcription factors. Finally, priming with HDAC inhibitors can increase stem cell migration to the site of ischemic injury. Together, these actions both mitigate tissue damage and promote regeneration after cerebral ischemia. As a result, rodents treated with HDAC inhibitors before or after ischemic onset have dramatically reduced infarct volumes and enhanced functional recovery. A: acetylation; p: phosphorylation; casp: caspase; α-syn: α-synuclein.
Despite substantial data supporting the therapeutic efficacy of HDAC inhibition in ischemic stroke, several important unknowns require further investigation. For example, our current understanding of how inhibition of class III HDACs affects ischemic stroke outcome is limited. Nicotinamide, a pan-inhibitor of SIRT1–7, had robust beneficial effects against focal cerebral ischemia in rodents, with a therapeutic window of up to 6 hours [163–165]. However, several important caveats hinder our ability to draw a conclusion about class III HDAC inhibition from these results. First, SIRT1, the most well-characterized of the class III HDAC isoforms thus far and a key regulator of cellular survival, metabolism, and stress response, was protective in multiple neurodegenerative conditions such as Alzheimer’s, Huntington’s, and amyotrophic lateral sclerosis [166–168]. In hippocampal slice cultures exposed to OGD, the neuroprotective effects of resverotrol, a pharmacological SIRT1 inducer, were abolished by SIRT1 inhibition [169]. Together, these results suggest that the beneficial actions of nicotinamide in experimental stroke may be despite its inhibition of SIRT1, rather than because of it. Moreover, this drug has a number of additional targets that may account for its positive effects on stroke outcome. For example, high concentrations of nicotinamide inhibited PARP [170], a NAD+-dependent DNA repair enzyme that is overactivated in ischemia and exacerbates energy depletion, inflammation, and cell death [171–172]. Nicotinamide has also been shown to inhibit iNOS and pro-inflammatory cytokine signaling through additional PARP-independent pathways [173–174]. It is therefore reasonable to hypothesize, for example, that the robust attenuation of immune cell infiltration, NF-κB signaling, and iNOS synthesis by nicotinamide in rat MCAO [165] were due to mechanisms other than suppression of SIRT1, which itself is characterized as an NF-κB inhibitor [175]. The function of SIRT2–7 inhibition in the protective effects of nicotinamide against cerebral ischemic injury cannot be deduced from the current literature.
To directly address the role of class III HDAC inhibition in stroke, antagonists specific for sirtuins, particularly SIRT2–7, must be developed and evaluated. Intriguingly, the SIRT2-specific inhibitor AGK2 and SIRT2 siRNA protected dopaminergic neurons from α-synuclein toxicity in vitro and in a Drosophila model of Parkinson’s disease [176]. Moreover, a study utilizing both healthy CGCs and cultures subjected to potassium depletion-induced cell death found that exogenous administration of SIRT1–7 had opposing effects on cell viability: while SIRT1 and cytoplasmic SIRT5 were neuroprotective, SIRT2, 3, 6, and mitochondrial SIRT5 induced apoptosis in healthy CGCs and SIRT4 and 7 had no effect [177]. It is of particular interest that in this study the neuroprotective effects of SIRT1 were not inhibited by nicotinamide or the SIRT1-specific inhibitor sirtinol, and were reproduced by two SIRT1 mutants lacking deacetylase activity [177]. It is therefore possible that SIRT1 has protective actions independent of its enzymatic activity, which may account for the robust beneficial effects of nicotinamide in experimental stroke despite the putative neuroprotective role of this target. This hypothesis is challenged, however, by the contrasting finding that SIRT1 deacetylase activity was required for SIRT1-mediated neuroprotection in cellular models of Huntington’s disease [168]. Clearly, the effects of sirtuin isoforms on neuronal survival, the mechanisms of their inhibitors, and the relevance of these pathways to cerebral ischemia are highly complex and require further investigation.
In addition, even among class I and class II HDACs the roles of specific isoforms in ischemic pathology and treatment response are unclear [15]. Evidence from Drosophila suggests that each HDAC has a unique expression pattern and regulates a distinct set of genes [178], which has been observed in rodents as well. For instance, as detected by immunohistochemistry in the mouse brain, HDAC1, 2, and 3 have been associated with a higher expression in cortical and hippocampal neurons [179], while HDAC6 has been found to be enriched in serotonin neurons and to be dominantly localized in the dorsal and median raphe nuclei [180–181]. The Allen Mouse Brain Atlas, by RNA in situ hybridization, revealed high transcription of HDAC2 and 3, mild transcription of HDAC6 but trace transcription of HDAC1 in various brain regions (http://mouse.brain-map.org/, accessed on August 7th, 2012). This discrepancy could be owing to different detection methods (protein versus RNA) and the specificity of the brain regions studied. In support of HDAC isoforms regulating distinct sets of gens, Guan et al reported that the detrimental effects of neuronal HDAC2 overexpression on dendritic spine density, synaptic plasticity, and memory formation were not reproduced by overexpression of HDAC1 [162]. Therefore, HDAC2 may be a unique target for facilitating cognitive recovery after stroke. In contrast, inhibition of HDAC1 in cortical neurons led to DNA damage, aberrant cell cycle activity, and neurotoxicity, whereas HDAC1 overexpression in the striatum of ischemic rats conferred significant neuroprotection [182]. Evidence suggests that in some contexts HDAC4 might also have neuroprotective properties, as forced expression provided near-complete protection against both potassium-induced apoptosis in CGCs and oxidative stress in neuroblastoma cells [183]. However, knockdown of HDAC4 was found to reduce infarct size following myocardial ischemia-induced reperfusion injury [184]. Further investigation is necessary to define the role of HDAC4 in stroke. A recent study demonstrated a substantial early induction of HDAC3 and 6 in the mouse cortex after cerebral ischemia and knockdown of HDAC3 or 6 by small hairpin RNAs protected cortical neurons from OGD insult [185]. Poststroke depression and anxiety are common complications following brain ischemia. HDAC6 knockout or loss of its deacetylase activity has been shown to induce antidepressant-like and anxiolytic-like behavior in mice [181]. Therefore, HDAC6 may be a promising therapeutic target for post-stroke depression and anxiety. Given the apparent opposing effects of different HDAC isoforms, it is likely that strategic isoform-specific inhibition could provide improved protection compared to the relatively broad-spectrum effects of drugs such as VPA. Furthermore, a more nuanced understanding of HDAC function throughout the pathophysiology of stroke will be important to guide dose and timing decisions in future trials, both preclinical and clinical.
Another novel strategy to improve the efficacy of HDAC inhibitors in stroke may be combination treatment with other pharmacological agents. Our lab has demonstrated that aging CGCs are insensitive to monotherapy with either HDAC inhibitors or lithium, both of which have robust protective effects against glutamate excitotoxicity in young cultures. However, co-treatment with lithium and VPA, SB, 4-PB, or TSA prior to glutamate challenge resulted in near-complete neuroprotection in this aging model, likely due to potentiation of GSK-3 inhibition [186]. Lithium and VPA co-treatment also caused a more robust upregulation of brain BDNF and HSP70 as well as greater behavioral improvements in transgenic Huntington’s disease mice [187], and delayed disease onset, prolonged lifespan, and decreased neurological deficit scores in a mouse model of amyotrophic lateral sclerosis [188]. Furthermore, as discussed previously, co-priming MSCs with lithium and VPA increased their homing efficiency to the ischemic brain and resulted in superior functional improvement [156]. Taken together, these in vitro and in vivo results suggest that combined treatment with lithium may increase the therapeutic potency of HDAC inhibitors in models of cerebral ischemia. Future studies are necessary to test this hypothesis.
Finally, accumulating evidence suggests that microRNAs (miRNAs), small non-coding RNAs that function in translation repression, play a role in stroke pathogenesis [189]. We recently observed significant miRNA regulation in rats subjected to 60 minutes tMCAO as well as those treated with a therapeutic post-insult dose of VPA [190]. miRNA-331 and miRNA-885–3p were two promising VPA-regulated candidates following brain ischemia and their predicted mRNA targets are suggested to be associated with diverse neurological diseases. Chronic VPA treatment has been shown by others to modulate brain miRNA expression in rats [191], and HDAC inhibition rapidly altered miRNA levels in multiple cancer models [192–193]. Therefore, further evaluation of HDAC inhibitor-regulated miRNAs in the context of cerebral ischemia may offer new insights to the protective mechanisms of these drugs.
In addition to their remarkable therapeutic benefits in the laboratory, the safety profiles of many HDAC inhibitors are already well-established in the clinic. For example, VPA has long been used as a mood stabilizer and anti-epileptic drug [194], and SAHA was FDA-approved in 2006 for the treatment of advanced cutaneous T-cell lymphoma [195]. Given the huge public health burden of stroke and the alarmingly limited treatment options currently available, clinical trials to evaluate HDAC inhibitors in cerebral ischemia are certainly warranted.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH), and the Hsu family gift fund. The authors would like to thank members of the Molecular Neurobiology Section, NIMH, NIH for their helpful discussions and assistance.
ABBREVIATIONS
- 4-PB
4-phenylbutyrate
- 4-PB
Alpha-amino-3-hydroxy-5-methyl-4-Isoxanole propionate
- ATP
Adenosine triphosphate
- BAX
Bcl-2-associated X protein
- BBB
Blood brain barrier
- Bcl-2
B-cell lymphoma 2
- BDNF
Brain-derived neurotrophic factor
- CGCs
Cerebellar granule cells
- CNS
Central nervous system
- COX-2
Cyclooxygenase-2
- CREB
cAMP response element-binding
- CXCR4
CXC chemokine receptor type 4
- eIF-2α
Eukaryotic initiation factor 2α
- ER
Endoplasmic reticulum
- ERK
Extracellular signal-regulated kinase
- GAPDH
Glyceraldehydes-3-phosphate dehydrogenase
- GRP
Glucose-related protein
- GSK
Glycogen synthase kinase
- H/I
Hypoxia/ischemia
- HAT
Histone acetyltransferase
- HDAC
Histone deacetylase
- HIF-1
Hypoxia-inducible factor 1
- HSP
Heat shock protein
- ICAM-1
Intercellular adhesion molecule 1
- iNOS
Inducible NO synthase
- JNK
C-Jun N-terminal Kinase
- LPS
Lipopolysaccharide
- MEF2
Myocyte enhancer factor 2
- MMPs
Matrix metalloproteinases
- MSCs
Mesenchymal stem cells
- NAD+
Nicotinamide adenine dinucleotide
- NF-κB
Nuclear transcription factor kappa B
- NMDA
N-methyl-D-aspartate
- nNOS
Neuronal nitric oxide synthase
- NO
Nitric oxide
- Nrf2
NF-E2-related factor 2
- OGD
Oxygen-glucose deprivation
- p/tMCAO
Permanent/transient middle cerebral artery occlusion
- P13K
Phosphatidylinositol-3-kinase
- PARP
Poly (ADP-ribose) polymerase
- PUMA
p53-upregulated modulator of apoptosis
- ROS
Reactive oxygen species
- rtPA
Recombinant tissue plasminogen activator
- SAHA
Suberoylanilide hydroxamic acid
- SB
Sodium butyrate
- SYM 2081
(2S, 4R)-4-methylglutamate
- TNF-α
Tumor necrosis factor α
- TSA
Trichostatin A
- TUNEL
Terminal deoxynucleotidyl transferase dUPT nick end labeling
- VEGF
Vascular endothelial growth factor
- VPA
Valproic acid
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- [1].Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics––2012 update: a report from the American Heart Association. Circulation 2012; 125: e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Towfighi A, Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke 2011; 42: 2351–5. [DOI] [PubMed] [Google Scholar]
- [3].Hajjar K, Kerr DM, Lees KR. Thrombolysis for acute ischemic stroke. J Vasc Surg 2011; 54: 901–7. [DOI] [PubMed] [Google Scholar]
- [4].Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 2008; 39: 924–8. [DOI] [PubMed] [Google Scholar]
- [5].Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 2010; 17: 197–218. [DOI] [PubMed] [Google Scholar]
- [6].Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 2010; 67: 181–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lipton P Ischemic cell death in brain neurons. Physiol Rev 1999; 79: 1431–568. [DOI] [PubMed] [Google Scholar]
- [8].Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med 2008; 14: 497–500. [DOI] [PubMed] [Google Scholar]
- [9].Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat). Am J Pathol 1994; 144: 188–99. [PMC free article] [PubMed] [Google Scholar]
- [11].Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006; 26: 13007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang ZF, Fessler EB, Chuang DM. Beneficial effects of mood stabilizers lithium, valproate and lamotrigine in experimental stroke models. Acta Pharmacol Sin 2011; 32: 1433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shein NA, Shohami E. Histone deacetylase inhibitors as therapeutic agents for acute central nervous system injuries. Mol Med 2011; 17: 448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci 2009; 32: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Langley B, Brochier C, Rivieccio MA. Targeting histone deacetylases as a multifaceted approach to treat the diverse outcomes of stroke. Stroke 2009; 40: 2899–905. [DOI] [PubMed] [Google Scholar]
- [16].Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 2008; 7: 854–68. [DOI] [PubMed] [Google Scholar]
- [17].Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 2007; 321: 892–901. [DOI] [PubMed] [Google Scholar]
- [18].Faraco G, Pancani T, Formentini L, et al. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol 2006; 70: 1876–84. [DOI] [PubMed] [Google Scholar]
- [19].Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem 2004; 89: 1358–67. [DOI] [PubMed] [Google Scholar]
- [20].Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem 2009; 110: 1226–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grunstein M Histone acetylation in chromatin structure and transcription. Nature 1997; 389: 349–52. [DOI] [PubMed] [Google Scholar]
- [22].Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol 2007; 14: 1008–16. [DOI] [PubMed] [Google Scholar]
- [23].Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 1996; 87: 953–9. [DOI] [PubMed] [Google Scholar]
- [24].You A, Tong JK, Grozinger CM, Schreiber SL. CoREST is an integral component of the CoREST- human histone deacetylase complex. Proc Natl Acad Sci USA 2001; 98: 1454–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev 1999; 13: 1924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nagy L, Kao HY, Chakravarti D, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 1997; 89: 373–80. [DOI] [PubMed] [Google Scholar]
- [27].Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene 2005; 363: 15–23. [DOI] [PubMed] [Google Scholar]
- [28].Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci 2003; 983: 84–100. [DOI] [PubMed] [Google Scholar]
- [29].Waltregny D, De Leval L, Glenisson W, et al. Expression of histone deacetylase 8, a class I histone deacetylase, is restricted to cells showing smooth muscle differentiation in normal human tissues. Am J Pathol 2004; 165: 553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang XJ, Gregoire S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol Cell Biol 2005; 25: 2873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature 2002; 417: 455–8. [DOI] [PubMed] [Google Scholar]
- [32].Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 2003; 115: 727–38. [DOI] [PubMed] [Google Scholar]
- [33].Dompierre JP, Godin JD, Charrin BC, et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci 2007; 27: 3571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol 2004; 338: 17–31. [DOI] [PubMed] [Google Scholar]
- [35].Gao L, Cueto MA, Asselbergs F, Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J Biol Chem 2002; 277: 25748–55. [DOI] [PubMed] [Google Scholar]
- [36].Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ 2006; 13: 539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 2011; 31: 52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rouaux C, Jokic N, Mbebi C, Boutillier S, Loeffler JP, Boutillier AL. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J 2003; 22: 6537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jiang H, Nucifora FC Jr., Ross CA, DeFranco DB. Cell death triggered by polyglutamine-expanded huntingtin in a neuronal cell line is associated with degradation of CREB-binding protein. Hum Mol Genet 2003; 12: 1–12. [DOI] [PubMed] [Google Scholar]
- [40].Jin K, Mao XO, Simon RP, Greenberg DA. Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global cerebral ischemia. J Mol Neurosci 2001; 16: 49–56. [DOI] [PubMed] [Google Scholar]
- [41].McCampbell A, Taye AA, Whitty L, Penney E, Steffan JS, Fischbeck KH. Histone deacetylase inhibitors reduce polyglutamine toxicity. Proc Natl Acad Sci USA 2001; 98: 15179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hockly E, Richon VM, Woodman B, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci USA 2003; 100: 2041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ryu H, Smith K, Camelo SI, et al. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J Neurochem 2005; 93: 1087–98. [DOI] [PubMed] [Google Scholar]
- [44].Gardian G, Yang L, Cleren C, Calingasan NY, Klivenyi P, Beal MF. Neuroprotective effects of phenylbutyrate against MPTP neurotoxicity. Neuromolecular Med 2004; 5: 235–41. [DOI] [PubMed] [Google Scholar]
- [45].Ricobaraza A, Cuadrado-Tejedor M, Perez-Mediavilla A, et al. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology 2009; 34: 1721–32. [DOI] [PubMed] [Google Scholar]
- [46].Dash PK, Orsi SA, Zhang M, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One 2010; 5: e11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol 2004; 66: 899–908. [DOI] [PubMed] [Google Scholar]
- [48].Endres M, Meisel A, Biniszkiewicz D, et al. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci 2000; 20: 3175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Khan N, Jeffers M, Kumar S, et al. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J 2008; 409: 581–9. [DOI] [PubMed] [Google Scholar]
- [50].Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 2006; 5: 769–84. [DOI] [PubMed] [Google Scholar]
- [51].Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 2001; 276: 36734–41. [DOI] [PubMed] [Google Scholar]
- [52].Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell 2005; 17: 855–68. [DOI] [PubMed] [Google Scholar]
- [53].Szabo C Nicotinamide: a jack of all trades (but master of none?). Intensive Care Med 2003; 29: 863–6. [DOI] [PubMed] [Google Scholar]
- [54].Yildirim F, Gertz K, Kronenberg G, et al. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol 2008; 210: 531–42. [DOI] [PubMed] [Google Scholar]
- [55].Langley B, D’Annibale MA, Suh K, et al. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J Neurosci 2008; 28: 163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Qian YR, Lee MJ, Hwang S, Kook JH, Kim JK, Bae CS. Neuroprotection by valproic Acid in mouse models of permanent and transient focal cerebral ischemia. Korean J Physiol Pharmacol 2010; 14: 435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang B, Zhu X, Kim Y, et al. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med 2012; 52: 928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ankarcrona M, Dypbukt JM, Bonfoco E, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995; 15: 961–73. [DOI] [PubMed] [Google Scholar]
- [59].Eliasson MJ, Huang Z, Ferrante RJ, et al. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci 1999; 19: 5910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kanai H, Sawa A, Chen RW, Leeds P, Chuang DM. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J 2004; 4: 336–44. [DOI] [PubMed] [Google Scholar]
- [61].Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci 2006; 26: 7502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Monti B, Polazzi E, Batti L, Crochemore C, Virgili M, Contestabile A. Alpha-synuclein protects cerebellar granule neurons against 6-hydroxydopamine-induced death. J Neurochem 2007; 103: 518–30. [DOI] [PubMed] [Google Scholar]
- [63].Monti B, Gatta V, Piretti F, Raffaelli SS, Virgili M, Contestabile A. Valproic acid is neuroprotective in the rotenone rat model of Parkinson’s disease: involvement of alpha-synuclein. Neurotox Res 2010; 17: 130–41. [DOI] [PubMed] [Google Scholar]
- [64].Marinova Z, Ren M, Wendland JR, et al. Valproic acid induces functional heat-shock protein 70 via Class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J Neurochem 2009; 111: 976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Endres M, Fink K, Zhu J, et al. Neuroprotective effects of gelsolin during murine stroke. J Clin Invest 1999; 103: 347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Meisel A, Harms C, Yildirim F, et al. Inhibition of histone deacetylation protects wild-type but not gelsolin-deficient neurons from oxygen/glucose deprivation. J Neurochem 2006; 98: 1019–31. [DOI] [PubMed] [Google Scholar]
- [67].Baltan S, Murphy SP, Danilov CA, Bachleda A, Morrison RS. Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. J Neurosci 2011; 31: 3990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 2007; 27: 1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Brennan AM, Suh SW, Won SJ, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci 2009; 12: 857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Girouard H, Wang G, Gallo EF, et al. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci 2009; 29: 2545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 2009; 4: 461–70. [DOI] [PubMed] [Google Scholar]
- [72].Ryu H, Lee J, Olofsson BA, et al. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci USA 2003; 100: 4281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang JF, Shao L, Sun X, Young LT. Glutathione S-transferase is a novel target for mood stabilizing drugs in primary cultured neurons. J Neurochem 2004; 88: 1477–84. [DOI] [PubMed] [Google Scholar]
- [74].Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry 2005; 58: 879–84. [DOI] [PubMed] [Google Scholar]
- [75].Wang JF, Azzam JE, Young LT. Valproate inhibits oxidative damage to lipid and protein in primary cultured rat cerebrocortical cells. Neuroscience 2003; 116: 485–9. [DOI] [PubMed] [Google Scholar]
- [76].Correa F, Mallard C, Nilsson M, Sandberg M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3beta. Neurobiol Dis 2011; 44: 142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Owen CR, Kumar R, Zhang P, McGrath BC, Cavener DR, Krause GS. PERK is responsible for the increased phosphorylation of eIF2alpha and the severe inhibition of protein synthesis after transient global brain ischemia. J Neurochem 2005; 94: 1235–42. [DOI] [PubMed] [Google Scholar]
- [78].Tajiri S, Oyadomari S, Yano S, et al. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ 2004; 11: 403–15. [DOI] [PubMed] [Google Scholar]
- [79].Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 2002; 277: 34287–94. [DOI] [PubMed] [Google Scholar]
- [80].Chen B, Wang JF, Young LT. Chronic valproate treatment increases expression of endoplasmic reticulum stress proteins in the rat cerebral cortex and hippocampus. Biol Psychiatry 2000; 48: 658–64. [DOI] [PubMed] [Google Scholar]
- [81].Bown CD, Wang JF, Young LT. Increased expression of endoplasmic reticulum stress proteins following chronic valproate treatment of rat C6 glioma cells. Neuropharmacology 2000; 39: 2162–9. [DOI] [PubMed] [Google Scholar]
- [82].Liu H, Bowes RC 3rd, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem 1997; 272: 21751–9. [DOI] [PubMed] [Google Scholar]
- [83].Srinivasan K, Sharma SS. Sodium phenylbutyrate ameliorates focal cerebral ischemic/reperfusion injury associated with comorbid type 2 diabetes by reducing endoplasmic reticulum stress and DNA fragmentation. Behav Brain Res 2011; 225: 110–6. [DOI] [PubMed] [Google Scholar]
- [84].Ohoka N, Hattori T, Kitagawa M, Onozaki K, Hayashi H. Critical and functional regulation of CHOP (C/EBP homologous protein) through the N-terminal portion. J Biol Chem 2007; 282: 35687–94. [DOI] [PubMed] [Google Scholar]
- [85].Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke 2009; 40: e331–9. [DOI] [PubMed] [Google Scholar]
- [86].Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene 2003; 22: 9041–7. [DOI] [PubMed] [Google Scholar]
- [87].Xuan A, Long D, Li J, Ji W, Hong L, Zhang M, Zhang W. Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sci 2012; 90: 463–8. [DOI] [PubMed] [Google Scholar]
- [88].Sinn DI, Kim SJ, Chu K, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis 2007; 26: 464–72. [DOI] [PubMed] [Google Scholar]
- [89].Uo T, Veenstra TD, Morrison RS. Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms. J Neurosci 2009; 29: 2824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Connolly ES Jr., Winfree CJ, Prestigiacomo CJ, et al. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res 1997; 81: 304–10. [DOI] [PubMed] [Google Scholar]
- [91].Zhang RL, Chopp M, Zaloga C, et al. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res 1995; 682: 182–8. [DOI] [PubMed] [Google Scholar]
- [92].del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol 2000; 10: 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8: 752–8. [DOI] [PubMed] [Google Scholar]
- [94].Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 2003; 183: 25–33. [DOI] [PubMed] [Google Scholar]
- [95].Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci 2008; 28: 2221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ichiyama T, Okada K, Lipton JM, Matsubara T, Hayashi T, Furukawa S. Sodium valproate inhibits production of TNF-alpha and IL-6 and activation of NF-kappaB. Brain Res 2000; 857: 246–51. [DOI] [PubMed] [Google Scholar]
- [97].Lee JI, Burckart GJ. Nuclear factor kappa B: important transcription factor and therapeutic target. J Clin Pharmacol 1998; 38: 981–93. [DOI] [PubMed] [Google Scholar]
- [98].Chen PS, Peng GS, Li G, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry 2006; 11: 1116–25. [DOI] [PubMed] [Google Scholar]
- [99].Wu X, Chen PS, Dallas S, et al. Histone deacetylase inhibitors upregulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol 2008; 11: 1123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Peng GS, Li G, Tzeng NS, et al. Valproate pretreatment protects dopaminergic neurons from LPS-induced neurotoxicity in rat primary midbrain cultures: role of microglia. Brain Res Mol Brain Res 2005; 134: 162–9. [DOI] [PubMed] [Google Scholar]
- [101].Chen PS, Wang CC, Bortner CD, et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 2007; 149: 203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Faraco G, Pittelli M, Cavone L, et al. Histone deacetylase (HDAC) inhibitors reduce the glial inflammatory response in vitro and in vivo. Neurobiol Dis 2009; 36: 269–79. [DOI] [PubMed] [Google Scholar]
- [103].Nogawa S, Forster C, Zhang F, Nagayama M, Ross ME, Iadecola C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc Natl Acad Sci USA 1998; 95: 10966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sugimoto K, Iadecola C. Delayed effect of administration of COX-2 inhibitor in mice with acute cerebral ischemia. Brain Res 2003; 960: 273–6. [DOI] [PubMed] [Google Scholar]
- [105].Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci 1997; 17: 9157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Marinova Z, Leng Y, Leeds P, Chuang DM. Histone deacetylase inhibition alters histone methylation associated with heat shock protein 70 promoter modifications in astrocytes and neurons. Neuropharmacology 2011; 60: 1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab 2008; 28: 53–63. [DOI] [PubMed] [Google Scholar]
- [108].Niu F, Zhang X, Chang L, et al. Trichostatin A enhances OGD-astrocyte viability by inhibiting inflammatory reaction mediated by NF-kappaB. Brain Res Bull 2009; 78: 342–6. [DOI] [PubMed] [Google Scholar]
- [109].Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57: 173–85. [DOI] [PubMed] [Google Scholar]
- [110].Cecchelli R, Berezowski V, Lundquist S, et al. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov 2007; 6: 650–61. [DOI] [PubMed] [Google Scholar]
- [111].Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci 2001; 24: 719–25. [DOI] [PubMed] [Google Scholar]
- [112].Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 2002; 282: H1485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011; 42: 3323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 1998; 29: 2189–95. [DOI] [PubMed] [Google Scholar]
- [115].Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab 1999; 19: 624–33. [DOI] [PubMed] [Google Scholar]
- [116].Barr TL, Latour LL, Lee KY, et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 2010; 41: e123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008; 39: 1121–6. [DOI] [PubMed] [Google Scholar]
- [118].Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 2005; 289: H558–68. [DOI] [PubMed] [Google Scholar]
- [119].Maier CM, Hsieh L, Yu F, Bracci P, Chan PH. Matrix metalloproteinase-9 and myeloperoxidase expression: quantitative analysis by antigen immunohistochemistry in a model of transient focal cerebral ischemia. Stroke 2004; 35: 1169–74. [DOI] [PubMed] [Google Scholar]
- [120].Lee JY, Kim HS, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid attenuates blood-spinal cord barrier disruption by inhibiting matrix metalloprotease-9 activity and improves functional recovery after spinal cord injury. J Neurochem 2012; 121: 818–29. [DOI] [PubMed] [Google Scholar]
- [121].Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci (Lond) 2006; 111: 171–83. [DOI] [PubMed] [Google Scholar]
- [122].Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994; 25: 1794–8. [DOI] [PubMed] [Google Scholar]
- [123].Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 2003; 23: 166–80. [DOI] [PubMed] [Google Scholar]
- [124].Krupinski J, Stroemer P, Slevin M, Marti E, Kumar P, Rubio F. Three-dimensional structure and survival of newly formed blood vessels after focal cerebral ischemia. Neuroreport 2003; 14: 117–16. [DOI] [PubMed] [Google Scholar]
- [125].Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab 2009; 29: 1620–43. [DOI] [PubMed] [Google Scholar]
- [126].Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000; 106: 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol 2008; 8: 82–9. [DOI] [PubMed] [Google Scholar]
- [128].Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 2005; 169: 681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 2006; 12: 441–5. [DOI] [PubMed] [Google Scholar]
- [130].Wang Z, Tsai LK, Munasinghe J, et al. Chronic Valproate Treatment Enhances Post-ischemic Angiogenesis and Promotes Functional Recovery in a Rat Model of Ischemic Stroke. Stroke 2012; 43: 2430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol 2006; 70: 1469–80. [DOI] [PubMed] [Google Scholar]
- [132].Causey MW, Salgar S, Singh N, Martin M, Stallings JD. Valproic acid reversed pathologic endothelial cell gene expression profile associated with ischemia-reperfusion injury in a swine hemorrhagic shock model. J Vasc Surg 2012; 55: 1096–1103 e51. [DOI] [PubMed] [Google Scholar]
- [133].Jin G, Bausch D, Knightly T, et al. Histone deacetylase inhibitors enhance endothelial cell sprouting angiogenesis in vitro. Surgery 2011; 150: 429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Karen J, Rodriguez A, Friman T, Dencker L, Sundberg C, Scholz B. Effects of the histone deacetylase inhibitor valproic acid on human pericytes in vitro. PLoS One 2011; 6: e24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998; 4: 1313–7. [DOI] [PubMed] [Google Scholar]
- [136].Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA 2001; 98: 4752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist 2003; 9: 261–72. [DOI] [PubMed] [Google Scholar]
- [138].Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci 1998; 18: 7768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sun X, Zhang QW, Xu M, et al. New striatal neurons form projections to substantia nigra in adult rat brain after stroke. Neurobiol Dis 2012; 45: 601–9. [DOI] [PubMed] [Google Scholar]
- [140].Kreuzberg M, Kanov E, Timofeev O, Schwaninger M, Monyer H, Khodosevich K. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp Neurol 2010; 226: 90–9. [DOI] [PubMed] [Google Scholar]
- [141].Raber J, Fan Y, Matsumori Y, et al. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol 2004; 55: 381–9. [DOI] [PubMed] [Google Scholar]
- [142].Hao Y, Creson T, Zhang L, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci 2004; 24: 6590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA 2004; 101: 16659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors 2004; 22: 123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 2009; 14: 51–9. [DOI] [PubMed] [Google Scholar]
- [146].Umka J, Mustafa S, ElBeltagy M, et al. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience 2010; 166: 15–22. [DOI] [PubMed] [Google Scholar]
- [147].Chen J, Cai F, Cao J, Zhang X, Li S. Long-term antiepileptic drug administration during early life inhibits hippocampal neurogenesis in the developing brain. J Neurosci Res 2009; 87: 2898–907. [DOI] [PubMed] [Google Scholar]
- [148].Liu XS, Chopp M, Kassis H, et al. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience 2012; 220: 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 2007; 38: 3032–9. [DOI] [PubMed] [Google Scholar]
- [150].Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 2001; 32: 1005–11. [DOI] [PubMed] [Google Scholar]
- [151].Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005; 57: 874–82. [DOI] [PubMed] [Google Scholar]
- [152].Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009; 4: 206–16. [DOI] [PubMed] [Google Scholar]
- [153].Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res 2008; 1195: 104–12. [DOI] [PubMed] [Google Scholar]
- [154].Tsai LK, Leng Y, Wang Z, Leeds P, Chuang DM. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology 2010; 35: 2225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gul H, Marquez-Curtis LA, Jahroudi N, Lo J, Turner AR, Janowska-Wieczorek A. Valproic acid increases CXCR4 expression in hematopoietic stem/progenitor cells by chromatin remodeling. Stem Cells Dev 2009; 18: 831–8. [DOI] [PubMed] [Google Scholar]
- [156].Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke 2011; 42: 2932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis 2003; 12: 119–26. [DOI] [PubMed] [Google Scholar]
- [158].Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry 1994; 57: 202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Alarcon JM, Malleret G, Touzani K, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 2004; 42: 947–59. [DOI] [PubMed] [Google Scholar]
- [160].Peleg S, Sananbenesi F, Zovoilis A, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 2010; 328: 753–6. [DOI] [PubMed] [Google Scholar]
- [161].Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature 2007; 447: 178–82. [DOI] [PubMed] [Google Scholar]
- [162].Guan JS, Haggarty SJ, Giacometti E, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 2009; 459: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Mokudai T, Ayoub IA, Sakakibara Y, Lee EJ, Ogilvy CS, Maynard KI. Delayed treatment with nicotinamide (Vitamin B(3)) improves neurological outcome and reduces infarct volume after transient focal cerebral ischemia in Wistar rats. Stroke 2000; 31: 1679–85. [DOI] [PubMed] [Google Scholar]
- [164].Ayoub IA, Maynard KI. Therapeutic window for nicotinamide following transient focal cerebral ischemia. Neuroreport 2002; 13: 213–6. [DOI] [PubMed] [Google Scholar]
- [165].Chen TY, Lin MH, Lee WT, et al. Nicotinamide inhibits nuclear factor-kappa B translocation after transient focal cerebral ischemia. Crit Care Med 2012; 40: 532–7. [DOI] [PubMed] [Google Scholar]
- [166].Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 2007; 26: 316979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Outeiro TF, Marques O, Kazantsev A. Therapeutic role of sirtuins in neurodegenerative disease. Biochim Biophys Acta 2008; 1782: 363–9. [DOI] [PubMed] [Google Scholar]
- [168].Jiang M, Wang J, Fu J, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med 2012; 18: 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab 2006; 26: 1141–7. [DOI] [PubMed] [Google Scholar]
- [170].Banasik M, Komura H, Shimoyama M, Ueda K. Specific inhibitors of poly(ADP-ribose) synthetase and mono(ADPribosyl)transferase. J Biol Chem 1992; 267: 1569–75. [PubMed] [Google Scholar]
- [171].Eliasson MJ, Sampei K, Mandir AS, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med 1997; 3: 1089–95. [DOI] [PubMed] [Google Scholar]
- [172].Hamby AM, Suh SW, Kauppinen TM, Swanson RA. Use of a poly(ADP-ribose) polymerase inhibitor to suppress inflammation and neuronal death after cerebral ischemia-reperfusion. Stroke 2007; 38: 632–6. [DOI] [PubMed] [Google Scholar]
- [173].Ungerstedt JS, Blomback M, Soderstrom T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin Exp Immunol 2003; 131: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Andrade J, Conde M, Ramirez R, et al. Protection from nicotinamide inhibition of interleukin-1 beta-induced RIN cell nitric oxide formation is associated with induction of MnSOD enzyme activity. Endocrinology 1996; 137: 4806–10. [DOI] [PubMed] [Google Scholar]
- [175].Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004; 23: 2369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Outeiro TF, Kontopoulos E, Altmann SM, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 2007; 317: 516–9. [DOI] [PubMed] [Google Scholar]
- [177].Pfister JA, Ma C, Morrison BE, D’Mello SR. Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS One 2008; 3: e4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Cho Y, Griswold A, Campbell C, Min KT. Individual histone deacetylases in Drosophila modulate transcription of distinct genes. Genomics 2005; 86: 606–17. [DOI] [PubMed] [Google Scholar]
- [179].Baltan S, Bachleda A, Morrison RS, Murphy SP. Expression of Histone Deacetylases in Cellular Compartments of the Mouse Brain and the Effects of Ischemia. Transl Stroke Res 2011; 2: 411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Espallergues J, Teegarden SL, Veerakumar A, et al. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. J Neurosci 2012; 32: 4400–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Fukada M, Hanai A, Nakayama A, et al. Loss of deacetylation activity of Hdac6 affects emotional behavior in mice. PLoS One 2012; 7: e30924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Kim D, Frank CL, Dobbin MM, et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 2008; 60: 803–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Majdzadeh N, Wang L, Morrison BE, Bassel-Duby R, Olson EN, D’Mello SR. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev Neurobiol 2008; 68: 1076–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Granger A, Abdullah I, Huebner F, et al. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J 2008; 22: 3549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Chen YT, Zang XF, Pan J, et al. Expression patterns of histone deacetylases in experimental stroke and potential targets for neuroprotection. Clin Exp Pharmacol Physiol 2012; 39: 751–8. [DOI] [PubMed] [Google Scholar]
- [186].Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci 2008; 28: 2576–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Chiu CT, Liu G, Leeds P, Chuang DM. Combined treatment with the mood stabilizers lithium and valproate produces multiple beneficial effects in transgenic mouse models of Huntington’s disease. Neuropsychopharmacology 2011; 36: 2406–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience 2008; 155: 567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008; 39: 959–66. [DOI] [PubMed] [Google Scholar]
- [190].Hunsberger JG, Fessler EB, Wang Z, Elkahloun AG, Chuang DM. Post-insult valproic acid-regulated microRNAs: potential targets for cerebral ischemia. Am J Transl Res 2012; 4: 316–32. [PMC free article] [PubMed] [Google Scholar]
- [191].Zhou R, Yuan P, Wang Y, et al. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology 2009; 34: 1395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res 2006; 66: 1277–81. [DOI] [PubMed] [Google Scholar]
- [193].Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 2006; 9: 435–43. [DOI] [PubMed] [Google Scholar]
- [194].Brambilla P, Barale F, Soares JC. Perspectives on the use of anticonvulsants in the treatment of bipolar disorder. Int J Neuropsychopharmacol 2001; 4: 421–46. [DOI] [PubMed] [Google Scholar]
- [195].Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 2007; 12: 1247–52. [DOI] [PubMed] [Google Scholar]