Abstract
Nicotine plus flavorings in a propylene glycol (PG) vehicle are the components of electronic cigarette liquids (e-liquids), which are vaporized and inhaled by the user. Dermal exposure to nicotine and e-liquids may occur among workers in mixing and filling of e-cigarettes in the manufacturing process. Inadvertent skin contact among consumers is also a concern. In vitro nicotine permeation studies using heat-separated human epidermis were performed with surrogate and two commercial e-liquids, neat and aqueous nicotine donor formulations. Steady-state fluxes (Jss), and lag times (tlag) were measured for each formulation. In addition, transient (4 h) exposure and finite dose (1–10 μl/cm2) experiments were undertaken using one commercial e-liquid. Average Jss (μg/cm2/h) from formulations were: nicotine in PG (24 mg/ml): 3.97; commercial e-liquid containing menthol (25 mg/ml nicotine): 10.2; commercial e-liquid containing limonene (25 mg/ml nicotine): 23.7; neat nicotine: 175. E-liquid lag times ranged from 5 to 10 h. Absorbed fraction of nicotine from finite doses was ≈0.3 at 48 h. The data were applied to transient exposure and finite dose dermal exposure assessment models and to a simple pharmacokinetic model. Three illustrative exposure scenarios demonstrate use of the data to predict systemic uptake and plasma concentrations from dermal exposure. The data demonstrate the potential for significant nicotine absorption through skin contact with e-cigarette refill solutions and the neat nicotine used to mix them.
Keywords: consumer exposure, Electronic nicotine delivery systems, ejuice, nicotine poisoning, occupational exposure
INTRODUCTION
The use of electronic nicotine delivery systems such as electronic cigarettes (e-cigarettes) has grown substantially over the past several years.1 They offer a means of self-regulated nicotine delivery that is free of many of the toxic components associated with the incineration of tobacco from traditional cigarettes, yet retain the familiar “feel” and ritualistic aspects of smoking. E-cigarettes deliver the potential benefit of weaning nicotine addicts from the known dangers of cigarette smoking, however there are several grounds for concern. These include the growing use of these products by youth and the potential for e-cigarettes to become a gateway to cigarette smoking;2 the possibility of toxic contaminants in e-cigarette liquids (e-liquids) that are vaporized and then inhaled by the user;3,4 ingestion of e-liquids by children;5 and the potential for absorption of toxic levels of nicotine through dermal contact with e-liquids.6,7
With regards to the latter, anecdotal reports in the lay press have raised concern for incidental contact by e-cigarette users. The New York Times6 reported that “In Kentucky….one woman was admitted to the hospital with cardiac problems after her e-cigarette broke in her bed, spilling the e-liquid, which was then absorbed through her skin”. Forbes magazine7 claimed that “Ingestion or skin exposure to small amounts of such solutions, ranging from one teaspoon to a tablespoon based on body weight and skin morphology, carries with it the potential for serious toxicity or even death”.
In addition to concerns related to consumer use, the potential for adverse occupational health effects of dermal contact with e-cigarette liquids and their constituents, including neat nicotine, has been recognized within manufacturing facilities that formulate e-liquids. The US Department of Labor’s Occupational Safety and Health Administration recently cited an e-cigarette manufacturer with workplace safety and health violations.8 Two willful violations included not providing and enforcing the use of protective gloves when workers handled products containing nicotine, and eye protection when handling corrosive chemicals and concentrated nicotine.
E-liquids contain nicotine dissolved in propylene glycol (PG) and/or glycerol plus flavorings. Some common flavoring consti-tuents, for example, limonene and menthol, are terpenes which are known to enhance dermal permeation.9 Workers mix these e-liquids and fill e-cigarette cartridges in the manufacturing process, creating the potential for dermal exposure. Nicotine is a recognized dermal permeant. Numerous studies have documen-ted nicotine absorption and kinetics from transdermal nicotine patches and from farm workers harvesting tobacco leaves (green tobacco sickness). Case studies have demonstrated severe toxicity from dermal contact with nicotine-containing substances10–12). Quantitative measurements of the in vitro human dermal permeation of neat and aqueous solutions of nicotine have been reported.13–16 Although Aungst13 described nicotine absorption from a 1% solution in propylene glycol, no information currently exists on the dermal absorption of nicotine from e-liquids.
The present study was designed to address the need for information on the potential for transdermal nicotine absorption from e-liquid formulations. Infinite dose in vitro human epidermal permeation of nicotine from surrogate and two commercial e-liquids was measured. In addition, donor formulations of neat nicotine and nicotine in water were tested. One e-liquid was selected for further studies using transient and finite dosing regimens. The data were analyzed within a dermal exposure assessment framework to investigate the potential for systemic uptake and toxicity of nicotine through skin contact in illustrative occupational and consumer settings.
MATERIALS AND METHODS
Infinite, transient and finite dose permeation experiments were carried out on heat-separated human epidermal membranes (HEM) mounted on Franz-type diffusion cells (Figure 1), with HPLC quantification of nicotine in receptor compartments.
Figure 1.
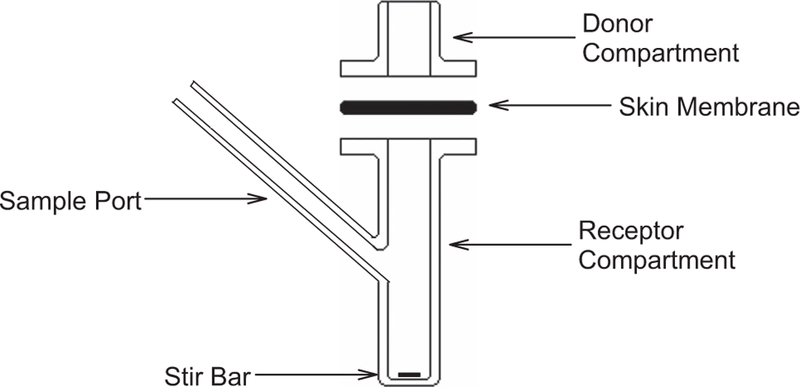
Franz-type diffusion cell. Epidermal membrane is clamped (not shown) between borosilicate glass donor and receptor compartments. Nicotine from the donor formulation applied to skin surface migrates into receptor fluid that is sampled through a port for quantification by HPLC. Stir bar assures mixing of receptor fluid. Not shown is recirculating water jacket that surrounds receptor compartment, maintaining desired temperature.
Chemicals and Solutions
Nicotine ((−)-nicotine, CAS: 54-11-5) was obtained from Sigma-Aldrich (product # N3876, lot # BCBK5694V) with a reported purity of 99.8%. It was stored in a refrigerator (4 °C) in its original container. After opening to remove working quantities, air was evacuated by flowing a stream of nitrogen gas into the container prior to closure. Working dilutions were prepared on the day of the experiment. Propylene glycol (PG; 1,2-propane diol, CAS: 57–55-6) was obtained from Sigma-Aldrich (product # P4347, lot # MKBK7925V) with a reported purity of 99.9% and was stored at room temperature. Lemon-Lime eJuice (product # GJLIQLMNLME) and ICE COLD Menthol eJuice (GJLIQICM) were purchased from Goodejuice.com (Minooka, IL, USA). Both had reported nicotine concentrations of 24 mg/ml and were stored at 4 °C. The manufacturer reported that nicotine plus flavorings were contained in 100% propylene glycol. We detected limonene and menthol respectively in these products using gas chromatography with mass spectral detection; however quantification was not performed.
Buffer was Hanks Balanced Salt Solution (Gibco, Invitrogen, Carlsbad, CA, USA), with 50 mg/l of gentamicin sulfate, 0.32 g/l of sodium bicarbonate and 5.96 g/l of HEPES (Sigma, Saint Louis, MO, USA). The pH of the buffer was adjusted to 7.4 at 37 °C. The buffer was filtered (pore sizes: 0.2 μm, SFCA, Nalgene, ThermoFisher Scientific, Rochester, NY, USA) and degassed prior to use by warming to 40 °C and stirring under laboratory vacuum.
Heat Separation of Human Epidermis
Human skin was received fresh from the West Virginia University Tissue Bank and was processed within 1 day of receipt. Human Subjects Review Boards from both WVU and NIOSH determined that our use of this tissue is not human research. The surgical specimens were taken with consent from six white females, ages 22–53 years, who had undergone breast reduction surgery. HEM were prepared by submersing the skin in buffer at 60 °C for 60 s. Epidermis was separated from remaining dermis using cotton swabs. Epidermal discs were cut using a stainless steel punch (1.59 cm diameter), floated onto a pool of buffer with 10% glycerol on aluminum foil, covered with gauze and stored at − 85 °C. Skin was stored from 49 to 294 days before use. We have demonstrated retention of barrier properties under these conditions.17
In vitro Permeation Studies
HEM were thawed at room temperature then floated on a pool of buffer and mounted on Franz-type (static) diffusion cells (0.64 cm2 diffusion area and 5 ml receptor volume, PermeGear, Hellertown, PA, USA) (Figure 1) with dialysis tubing (MWCO 12–14,000) used as a membrane support. HEM surfaces were rinsed 3 × with water and equilibrated overnight with buffer in receptor compartments. The skin surface was open to air and maintained at 32 °C by recirculating water at 37 °C through the jacketed cells. Relative humidity in the lab was not monitored. All studies were performed on nine skin discs for each formulation, with three taken from each of three individual human donors.
On the day of the experiment, before dosing, HEM barrier function was assessed by measuring transepidermal water loss (TEWL) with an AquaFlux AF200 (Biox Systems, UK). All TEWL’s averaged 12.2 g/m2/h, with a range from 7.4 to 20.2.
Donor formulations.
Donor formulations were: neat nicotine, 24 mg/ml nicotine in water, 24 mg/ml nicotine in PG, Lemon-Lime eJuice, and ICE COLD Menthol eJuice. The nicotine/propylene glycol solution served as a simple surrogate e-liquid with known, well-defined constituents. Nicotine solutions in water and PG were mixed on the day of dosing; the commercial eJuices were used straight from their containers.
Infinite dose studies.
Five hundred microliters of the donor formulation was applied to skin surface and donor cells were capped. The exposure duration for neat and aqueous nicotine formulations was 8 h, and receptor fluid was sampled at 0, 0.5, 1, 2, 3, 4, 5, 6 and 8 h. Exposure duration for other formulations, which exhibited longer lag times, was 26 h with additional sampling at 22, 24 and 26 h.
Transient exposure studies.
Five hundred microliters of Lemon-Lime eJuice was applied to the skin surface and donor cells were capped. At 4 h exposure, it was suctioned from the surface and skin was rinsed for 30 s with a continuous stream of water and suction (~80 ml total), then dried with cotton swabs. Receptor samples were taken hourly from 0 to 8 h, then at 10, 12 and 24 h. The amount of nicotine washed off the skin surface was not quantified.
Finite dose studies.
Finite dose dosing formulations consisted of 1, 5 or 10 μl/cm2 of Lemon-Lime eJuice dissolved in acetone such that the total dosing volume was 25 μl/cm2. Donor cells were not capped. The acetone evaporates within minutes, leaving a film of the eJuice distributed over the skin surface. Receptor samples were taken hourly from 0 to 8 h, then at 10, 12, 22, 24, 28, 32 and 48 h. Unpublished studies in our lab confirmed that 25 μl/cm2 of acetone did not diminish HEM barrier function as measured by transepidermal water loss. Measurements of the evaporation rate of nicotine (unpublished) suggest that as much as 15% of the applied nicotine could have evaporated at 48 h.
HPLC Analysis
Aliquots of 40 μl from each sample were analyzed with an Agilent 1260 HPLC system. A Gemini 5μ C18 110A column, 100 × 4.6 mm (Phenomenex, Torrance, CA, USA) was used and maintained at 22 °C. The isocratic mobile phase consisted of acetonitrile–diethylamine 10 mM solution pH 10.9 (35:65) at a flow rate of 0.6 ml/min. Diethylamine (99.5%) was purchased from Sigma Aldrich and acetonitrile (99.9%) from Fisher Scientific. Nicotine was detected at a wavelength of 261 nm with a retention time of 3.7 min. Calibrations of nicotine in buffer were performed over the range 0.01– 500 μg/ml before each experiment. They were linear (r2 = 0.999) and spanned the entire range of sample concentrations. The limit of detection (LOD) was 0.005 μg/ml and limit of quantification (LOQ) was 0.01 μg/ml.
Nicotine concentrations in Lemon-Lime and ICE COLD Menthol eJuices were quantified by serial dilution of the liquids in water. The average of three determinations was 25 mg/ml for both eJuices, compared with 24 mg/ml reported by the supplier.
Calculation of Permeation Parameters
Steady-state fluxes (JSS), permeability coefficients (kp) and lag times (tlag) were calculated from infinite dose experimental results. The total amount of chemical that penetrated the skin and was absorbed into the receptor fluid, normalized by the area of exposed skin (m(t)) was calculated from the measured receptor concentrations at each sample time point, taking into account the amount that had been removed with each prior sample. Jss and tlag were calculated by nonlinear regression of the experimental data to the diffusion equation, as described previously.18 Briefly, for each diffusion cell, the best fit of the data to the first seven terms of the diffusion equation for a pseudohomogeneous membrane19 was deter-mined using SigmaPlot 12.5 (Systat Software):
| (1) |
The analysis yields estimates for the two variables Jss and tlag. The quality of the regressions was excellent, with an average r2 of 0.997 and a range from 0.988 to 1.000. The permeability coefficient, kp, was calculated as
| (2) |
where Cd is the (constant) concentration of nicotine in the donor formulation. For neat nicotine, the concentration was taken as its density (1010 mg/ml at 20 °C). Note that kp is donor-dependent so that kp’s from aqueous donor are not comparable to kp’s from propylene glycol. kp’s are presented here for convention, but are not discussed further.
Fractional absorption of nicotine from finite dose exposures was calculated as the total mass recovered in receptor cells at 48 h per area of exposure, divided by the applied nicotine dose.
Statistical Tests
The number of replicates for these studies was chosen to exceed OECD guidelines.20 Pre-exposure assessment of barrier integrity was undertaken by measuring TEWL, as described above. No replicates were rejected and subsequent data analyses included all dosed skin samples. Samples were not allocated randomly, but replicates from each human donor were chosen haphazardly for treatment. Statistical tests were performed using SigmaPlot 12.5. Differences among groups were probed for significance using one-way analysis of variance (ANOVA). If non-normality was detected (Shapiro–Wilk test), Kruskal–Wallis ANOVA on ranks was performed. If significance (P < 0.05) was detected, pairwise comparisons were made using the Tukey test. Data in tables and on graphs are presented as means and standard deviations.
RESULTS
Figures 2 and 3 display results from representative infinite dose experiments. Symbols represent mean and standard deviations of three replicates from one human donor for each formulation, and solid lines are regressions of the mean data with Eq. (1). Resulting parameters Jss and tlag are listed in the figure legends. In Figure 2, mass accumulation in receptor fluid over time is displayed for neat and aqueous nicotine donors. Figure 3 displays results for Lemon-Lime and ICE COLD Menthol eJuices and for the surrogate e-liquid of 24 mg/ml nicotine in propylene glycol.
Figure 2.
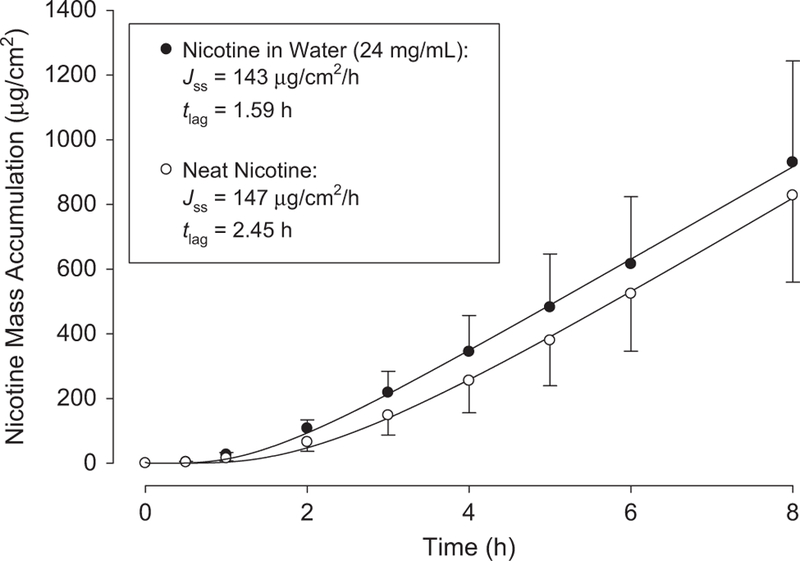
Representative infinite dose mass accumulations for neat and aqueous nicotine. Symbols represent means and SDs of three skin discs from one human donor. One sided error bars are for clarity. Solid lines are regressions with Eq. (1); resulting parameter values for Jss and tlag are listed.
Figure 3.
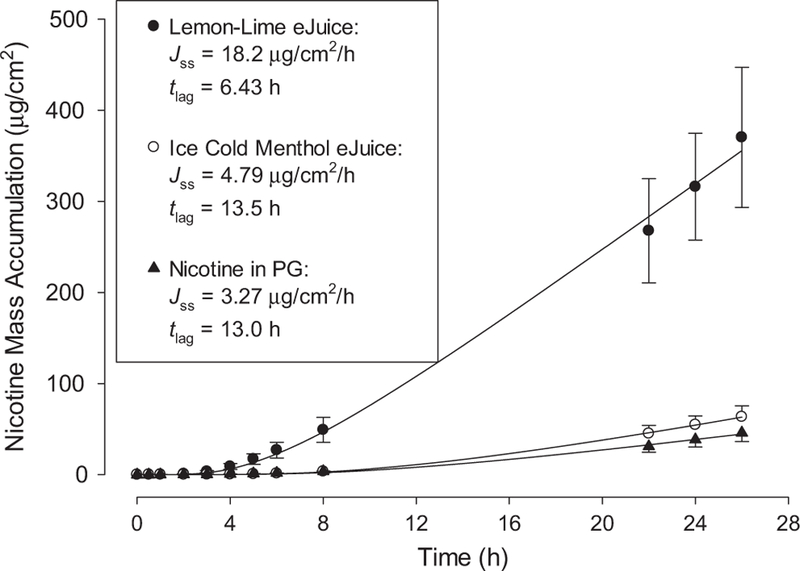
Representative infinite dose mass accumulations for surrogate and commercial e-liquids. Symbols represent means and SDs of three skin discs from one human donor. One sided error bars on lower plots are for clarity. Solid lines are regressions with Eq. (1); resulting parameter values for Jss and tlag are listed.
Table 1 lists infinite dose permeation parameters for all the data, derived from nine replicates for each of the five donor formulations. Kruskal–Wallis one-way ANOVA revealed significant (P < 0.05) differences among eJuices and surrogate e-liquid JSS and tlag, as indicated in Table 1. Large variances were observed in the infinite dose JSS for the surrogate e-liquid and for ICE COLD Menthol eJuice. The latter is driven by one measurement with JSS of 36.2. Removal of that potential outlier results in JSS of 6.96 ± 2.3 μg/cm2/h. The variance observed for the surrogate eliquid appears to reflect a true variance not driven by 1 or 2 measured values.
Table 1.
Infinite dose permeation results for tested nicotine donor formulations
| Nicotine formulation | Jss (μg/cm2/h) | kp (10−4cm/h) | tlag (h) |
|---|---|---|---|
| Neat | 175 ± 57 | 1.73 ± 0.56 | 1.94 ± 0.72 |
| 24 mg/ml in water | 130 ± 60 | 54.2 ± 25.0 | 1.61 ± 0.50 |
| 24 mg/ml in PG | 3.97 ± 2.28a | 1.65 ± 0.95a | 10.1 ± 3.27a |
| Lemon-Lime eJuice (25 mg/ml) | 23.7 ± 7.0a,b | 9.34 ± 2.76a,b | 5.00 ± 1.42a |
| ICE COLD Menthol | 10.2 ± 10.0 | 4.09 ± 4.01 | 8.29 ± 4.08 |
| eJuice (25 mg/ml) | (6.96 ± 2.3)b | (2.78 ± 0.92)b | (8.78 ± 4.07) |
Mean ± SD, n = 9 for each formulation. Jss, steady-state flux; kp, permeability coefficient; tlag, lag time. Numbers in parentheses are values obtained after elimination of one outlier.
Indicate pairs of variables for eJuice and e-liquid surrogate formulations that differ significantly (P < 0.05; ANOVA on ranks).
Figure 4 shows pooled (n = 9 total replicates from three human donors) results for the 4 h transient dose exposures to Lemon-Lime eJuice. The line connects the data points. Note that there is substantial mass accumulation in receptor well after the 4 h exposure: accumulation at 4 h is 16.6 ± 13.7 μg/cm2; at 24 h it is 48.3 ± 14.8 μg/cm2. For comparison, the pooled infinite dose permeation data are also shown in Figure 4. Only the mean values are shown for clarity. Thorough rinsing of the skin surface quite substantially decreases the overall nicotine absorption from these transient dose exposures.
Figure 4.
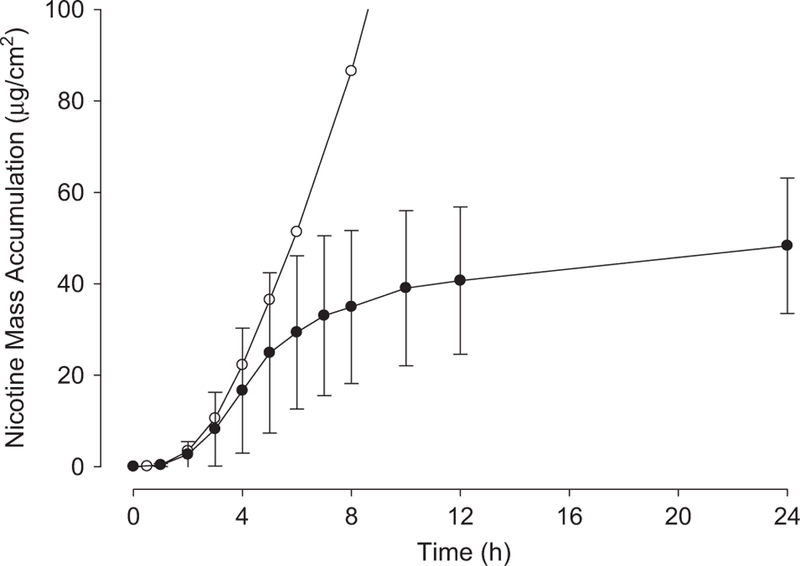
Pooled mass accumulation data from 4 h transient exposure to Lemon-Lime eJuice. Closed circles represent means and SDs for nine total skin discs with three taken from each of three human donors. For comparison, open circles represent mean values of infinite dose experiments without washing at 4 h. As expected, accumulation up to 4 h is similar for both cases.
Figure 5 displays pooled (n = 9 total replicates from three human donors for each load) finite dose mass accumulation data for three loads of Lemon-Lime eJuice. The 1, 5 and 10 μl/cm2 loads represent nicotine loads of 25, 125 and 250 μg/cm2, respectively. The figure displays both mass absorption over time (top) and mass absorption divided by applied mass, or fractional absorption (fabs) (bottom). For clarity, only mean values are shown in the bottom plot. No significant differences in fabs at 48 h were detected (one-way ANOVA).
Figure 5.
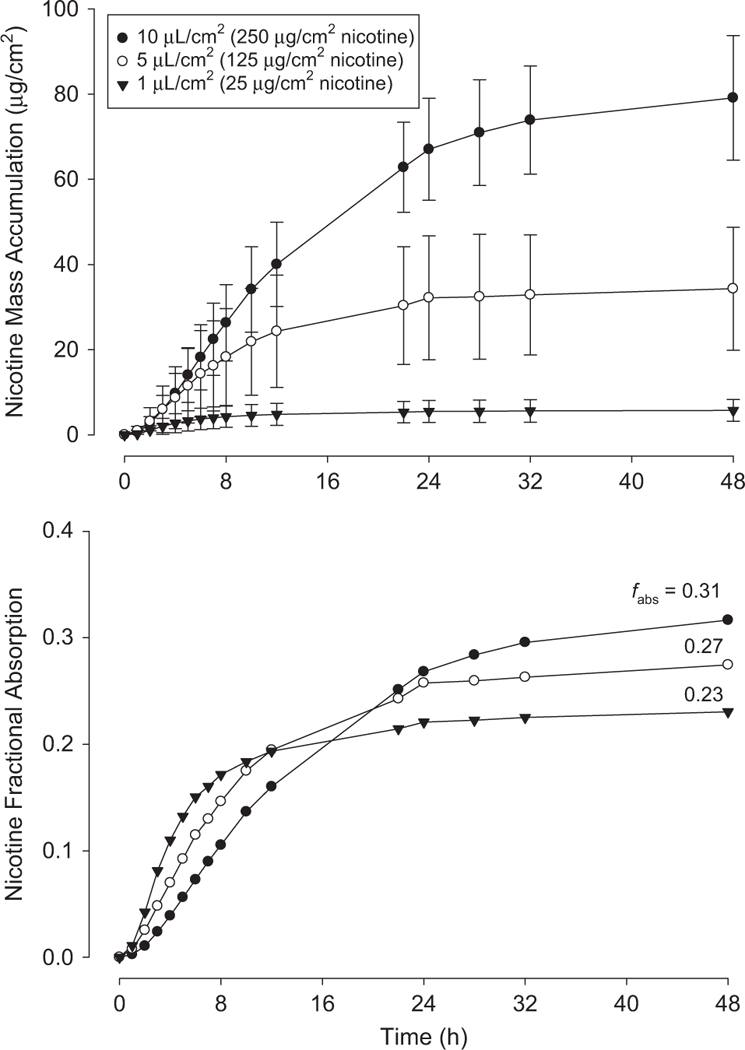
Pooled mass accumulation (top) and fractional absorption (bottom) data for finite dose exposures to specified loads of Lemon-Lime eJuice. Fractions of total dose absorbed (fabs) at 48 h are listed. Each curve represents mean and SD from nine total skin discs with three taken from each of three human donors. Bottom curve shows means only for clarity.
DISCUSSION
Infinite dose permeation results for neat and aqueous nicotine (Table 1) can be compared with previous literature reports. Zorin et al.14 measured JSS of 82 μg/cm2/h, for neat nicotine through dermatomed (~400 μm) human skin, about half our value of 175. However, Zorin et al. derived their measurements from an 80 min exposure. Our measured lag time of ~ 2 h strongly suggests that Zorin et al. did not observe a steady-state flux. Kuswahyuning and Roberts16 report steady-state fluxes of (60.9 and 67.9) μg/cm2/h for neat and 20 mg/ml aqueous solutions, respectively, through HEM of one human donor; exposure duration not specified. Thus, our measured fluxes are higher than previous measurements but may possibly be explained by differences in methodology.
Comparisons among surrogate and commercial e-liquids confirm that terpene flavoring components menthol and limo-nene enhance absorption rate. Limonene increased JSS nearly six-fold over the surrogate with similar nicotine content but no flavorings. Limonene decreased lag time by half, which also is not unprecedented.21,22
We measured transient exposure permeation for a single exposure duration of 4 h (Figure 4). The tested e-liquid (Lemon-Lime eJuice) was selected for its higher permeation rate compared with other formulations. In retrospect, the 24 h sampling duration was insufficient to capture the entire accumulation process. Based on model results,23 we estimate that roughly 90% of the total eventual mass absorption occurred at 24 h, which leads to a value used in subsequent calculations of 55 μg/cm2 systemic absorption for a 4-h exposure to Lemon-Lime eJuice.
Our finite dose results (Figure 5) show fractional absorption trending upward with dose, from 0.23 at 1 μl/cm2 to 0.31 at 10 μl/cm2. Although these differences are not significantly different, the upward trend may explained by higher fractional losses from evaporation at the lower initial loads. For subsequent calculations, fabs = 0.3 is used. As expected, the time it takes to reach final absorbed dose increases with load. The reason that the remaining ~ 70% of the applied load was not detected in the receptor solution is unknown. Potential causes are metabolism (unlikely in previously frozen skin); evaporation (as mentioned above, some evaporative losses are anticipated but not to this extent); binding of nicotine to SC proteins (70% binding is reasonable24 but would have to be irreversible or exhibit very slow reverse kinetics to explain our observations); and binding of nicotine to glass or other components of the diffusion cell system (unknown). Further studies employing a mass balance approach would provide additional information.
Assessment of Risk from Dermal Exposures
The risk from dermal exposures to neat nicotine and eliquids can be assessed by estimating both total nicotine uptake and resulting plasma concentrations from skin contact. Several dermal exposure scenarios are presented here to demonstrate this approach.
The nicotine absorption from dermal exposures may be compared in magnitude with circulatory uptake from cigarette smoking. Although this uptake varies broadly with nicotine content and individual behavior, averages of 1 mg per cigarette have been reported25,26 and this value is used here. Thus, each milligram of nicotine taken up by the dermal route is considered to be equivalent to that of one smoked cigarette. However, dermal uptake kinetics are quite slow compared with pulmonary absorption, which increases blood nicotine levels within seconds of inhalation.
Because of the large differences in the nicotine uptake kinetics of smoking compared with dermal absorption, mT may not be the best metric to assess the risk from skin contact with nicotine and e-liquid. Instead, comparisons of plasma concentrations would be more meaningful. A simple single compartment pharmacokinetic model is used here to obtain rough estimates:
| (3) |
where C is nicotine plasma concentration, Vdis is the volume of distribution for the chemical, kel is the elimination rate constant (related to elimination half time as kel = ln(2)/t1/2) and is the rate of entry of the chemical into the systemic circulation. Values for Vdis (347 l) and kel (0.2/h) used here are from Molander et al.27
For the cigarette, a bolus is assumed with and initial concentration C0 = m/Vdis, where m is the mass of nicotine absorbed for one cigarette (~1 mg). For a single cigarette,
| (4) |
For multiple cigarettes, the average plasma concentration is:
| (5) |
where τ is the constant time interval between cigarettes. For smokers of one cigarette per hour,
| (6) |
For dermal exposure, C0 = 0 and , steady-state flux times area of exposure, and
| (7) |
Steady-state concentration is given by:
| (8) |
Historically the lethal dose of nicotine in adults has been cited as ~ 60 mg. However, a recent review of the literature28 revealed poor documentation and questionable anecdotal evidence surrounding the broad acceptance of this value. Mayer concludes that at least 500 mg of orally ingested nicotine is required to kill an adult. With an oral bioavailability range of 20–45%,29 perhaps 100–225 mg of dermally absorbed nicotine could be lethal. Assuming rapid oral uptake, an initial plasma concentration (C0) of 288–648 ng/ml is predicted as the bioavailable oral dose divided by Vdis.
Acute toxicological effects of nicotine uptake by dermal contact with e-liquids may be insinuated by comparison with case studies reporting nicotine overdoses from transdermal patch abuse. The nicotine patch is designed to deliver a constant transdermal nicotine rate. Nine case studies of nicotine overdose were reported by Woolf et al.,30 some with fairly complete histories of patch exposure. Nicotine patches had a targeted uptake rate 21 mg/24 h (875 μg/h) delivery. These patients all recovered within hospital stays of 1–5 days.
Patient 1:
A 72-year-old female placed two patches on her skin and replaced them every 12 h for 60 h, for an estimated nicotine uptake of 2 × 875 μg/h × 60 h = 105 mg. She exhibited vomiting, weakness and dizziness leading to ataxia and coma. A steady-state plasma concentration may be estimated as:
| (9) |
Patient 2:
A 41-year-old female applied 3 patches and smoked 20 cigarettes over a 12 h period, for an estimated nicotine uptake of 3 × 875 μg/h × 12 h+20 mg = 52 mg; symptoms were vomiting and coma, likely complicated by co-intoxicants. Plasma nicotine concentration is estimated as the sum of mean from smoking plus an assumed steady-state from the patches:
| (10) |
Patient 3:
A 37-year-old male applied 12 patches for 2 h (estimated nicotine absorption = 12 × 875 μg/h × 2 h = 21 mg) and presented with nausea, pale and sweaty skin, dizziness and stupor. If a steady-state uptake was achieved, then the plasma nicotine level is estimated as:
| (11) |
Note that among these three patients, estimated total nicotine absorption varied inversely with plasma concentration, as there were substantial differences in the durations of exposure.
E-liquid dermal exposure scenarios.
Estimates of the systemic uptake and toxicity of nicotine from occupational and consumer dermal exposures may be made using these data along with model-based estimates. Three exposure scenarios are presented to demonstrate application of the data.
Scenario 1:
A worker mixing e-liquids is exposed to e-liquid or neat nicotine continuously over a 4 h shift on the volar surfaces of all their fingertips, or to just the thumb and index fingertips. After the shift, surface contamination is thoroughly washed. This scenario may be applicable to workers who mix e-liquids from neat nicotine or fill cartridges from tanks of e-liquid using a metered pump system—both manual processes in smaller manufacturing facilities. For these assumptions, the transient exposure data are used to calculate the total systemic mass uptake of nicotine, mT. Volar fingertip surface areas were taken from human cadavers measurements.31
Estimates of mT and C, plasma nicotine concentration, are presented in Table 2. For mT estimates, the experimental value of 55 μg/cm2 is used for Lemon-Lime eJuice. For neat nicotine, in absence of experimental data, the model-based approach described in detail in Frasch and Bunge23 was used to estimate mT with the assumption of no nicotine evaporation. Two estimates have been made. First, Eq. (24) from that reference was used directly, and second, that result was modified assuming 30% availability of applied nicotine, based on results from the finite dose studies. These model-based estimates are listed as mT* in Table 2. The estimates for C were obtained from Eq. (8) above with the assumption that steady-state fluxes (from Table 1) are approached. This assumption will overestimate C.
Table 2.
Results for exposure scenarios described in text.
| Exposure scenario | Nicotine formulation | Exposure | Area (cm2) | mT(mg) | mT* (mg) | C (ng/ml) |
|---|---|---|---|---|---|---|
| 1 | Neat nicotine | All fingers, immersion | 73 | — | 99.7 (29.9) | 184 |
| Thumb and index, immersion | 35 | — | 47.8 (14.3) | 88.3 | ||
| Lemon-Lime eJuice | All fingers, immersion | 73 | 4.02 | 21.4 (6.42) | 24.9 | |
| Thumb and index, immersion | 35 | 1.93 | 10.3 (3.09) | 12.0 | ||
| 2 | Lemon-Lime eJuice | 1 ml spread over skin | 154 | 7.51 | — | 52.6 |
| 3 | Lemon-Lime eJuice | 5 ml spread over skin | 769 | 37.5 | — | 263 |
| 15 ml spread over skin | 2307 | 112 | — | 789 |
mT, total mass of nicotine systemically absorbed from exposures to the specified formulation. C is plasma nicotine concentration, calculated from Eq. (8) assuming steady-state fluxes listed in Table 1. Scenario 1 assumes 4 h exposure followed by washing; others assume 48 h finite dose exposures. mT* are model-based transient exposure estimates, as described in the text. Surface areas for Scenario 1 are the volar surface areas of the specified digits of both hands.
Scenario 2:
An individual spills 1 ml of e-liquid onto the skin. This scenario addresses what might have happened in the anecdotal case described in the New York Times,6 in which an individual suffered “…cardiac problems after her e-cigarette broke in her bed, spilling the e-liquid, which was then absorbed through her skin”. A typical e-cigarette cartridge holds about 1 ml of e-liquid.
As the liquid spilled from the woman’s e-cigarette, it presumably formed a film on her skin. Research on the thickness of liquid films on skin is sparse, but the EPA published a study32 on the surface retention of three oils following hand immersion. For cooking oil, with a reported kinematic viscosity of 59.2 cSt, the EPA calculated a film thickness was 65 μm. The kinematic viscosity of propylene glycol is similar at 52 cSt at 21.1 °C33 so a 65 μm film is used here, which for propylene glycol density of 1.01 g/cm2, translates to a load ~ 6.5 μl/cm2 (nicotine load = 162.5 μg/cm2). The finite dose experimental loads reported here (Figure 5) span this load and so the fractional absorptions measured here are applicable to this exposure scenario. One milliliter of a 65 μm film would spread over a skin surface of 154 cm2. Depletion of nicotine from the film is expected and a finite dose model is applicable. This would represent a “worst-case” scenario in terms of maximum contaminated surface area.
Thus, for this scenario, the finite dose regime is applicable with fabs equal to 0.3:
| (12) |
Note that the finite dose model does not account for possible surface decontamination, which would reduce mT. C is estimated using Eq. (8), again with the assumption that JSS (Table 1) is approached.
Scenario 3:
One teaspoon (~5 ml) or one tablespoon (~15 ml) of e-liquid is spread over the skin in a thin film that is not washed off. This scenario addresses the claim in Forbes7 that “…skin exposure to small amounts of such solutions, ranging from one teaspoon to a tablespoon based on body weight and skin morphology, carries with it the potential for serious toxicity or even death.” For the thin film assumption, depletion of nicotine from the film is expected and the finite dose data is used to predict mT (Eq. (12)). A 65 μm film of 5 ml volume would cover an area of 769 cm2; 15 ml would cover 2307 cm2. For perspective, 5 ml is more than enough to cover an entire hand of an average adult male (535 cm2), and 15 ml would nearly cover both arms of an adult female (2370 cm2).34 These represent worst-case scenarios in which the entire dose is exposed to a maximum surface area that is not decontaminated until mass absorption is complete. For these e-liquids, a 65 μm film translates to a nicotine load of 162.5 μg/cm2. Calculations for mT and C proceed as above for Scenario 2.
Exposure scenario results and discussion.
Table 2 shows calculated nicotine dermal absorption amounts and plasma concentrations for these scenarios. Scenario 1 is applicable to a potential occupational dermal exposure while mixing e-liquids with no gloves. These estimates of total nicotine uptake suggest that substantial quantities can be absorbed in this occupational scenario. For Lemon-Lime eJuice, 2–4 mg of nicotine might be absorbed from a 4 h exposure. For neat nicotine, up to 100 mg nicotine uptake is calculated based on the model equations of Frasch and Bunge.23 Based on a 30% free nicotine availability, the amount is reduced to 30 mg which still represents a substantial total nicotine uptake, equivalent to 30 smoked cigarettes. The same equations were used to predict uptake from the Lemon-Lime eJuice, and the results are comparable (~1.5-fold greater) to the measured uptake, as shown in Table 2. Thus, the 30% availability assumption seems appropriate, even if the exact reasons are unknown at this time.
Estimates of plasma nicotine concentration range from 12 to 184 ng/ml for this exposure scenario. Twelve ng/ml is approximately equivalent to the concentration of one who smokes one cigarette an hour (Eq. (6)), while 184 ng/ml is comparable to the nicotine patch overdose patient with the highest plasma level. The use of steady-state dermal flux values will overestimate C for short-term exposures however with repeated twice-daily exposures of this duration, the skin depot would saturate and present a steady source for elevated levels of nicotine absorption. These results point to the possibility of substantial nicotine uptake and the potential for toxic effects from exposures to mixing eliquids.
Scenario 2 results are also presented in Table 2. Actual results would depend on the disposition of e-liquid once it had spilled onto the individual, and these calculations assume that the exposed surface in Table 2 represent the area for 1 ml of e-liquid to form a 65 μm film spread evenly on the skin.
The calculation suggests total nicotine uptake of ~ 7.5 mg. Recall however that for the experimental transient dose that only about 30% of the total was absorbed at t = 4 h. For the 5 μl/cm2 finite dose experiments, about 25% of the total was absorbed at 4 h. Thus, if we assume the individual presented to the hospital 4 h after the initial exposure, perhaps < 2 mg would have been systemically absorbed at that time. It therefore appears uncertain whether the cardiac problems experienced by this woman can be attributed to dermal absorption of nicotine from the spilled e-liquid. Again, the calculated plasma concentration assumes a steady-state level of flux had been achieved, which is clearly not the case for a 4 h exposure. The calculated value of 52.6 ng/ml would lead to acute toxicity, if compared with the nicotine patch overdose patients.
Table 2 also presents results from Scenario 3, in which 5 ml (~1 teaspoon) or 15 ml (~1 tablespoon) are spread evenly over skin in a film of 65 μm thickness. Substantial systemic nicotine uptake, potentially over 100 mg, is calculated by extrapolation from the experimental data. Furthermore, the calculated plasma concentrations could approach the lethal doses estimated (above) at 288–646 ng/ml. It can be concluded that the uptake of nicotine from this exposure scenario could lead to toxic effects of acute nicotine poisoning as suggested in Forbes magazine.7 The possibility of death from one tablespoon of e-liquid cannot be dismissed. However, one may consider the likelihood of such exposures which, as described above, could cover both arms and remain there indefinitely.
CONCLUSIONS
The potential for substantial nicotine uptake from dermal contact with e-cigarette refill liquids and neat nicotine has been demonstrated. This hazard is particularly acute in occupational settings in which workers mix e-liquids from neat nicotine and other constituents, and fill cartridges from tanks of e-liquid. The use of personal protective equipment, including adequate gloves, is essential. The same applies to individuals who mix their own e-liquids for personal consumption. Neat nicotine is particularly hazardous, with a steady-state flux over seven times greater than that of the higher permeating e-liquid, Lemon-Lime eJuice.
Casual contact by end users, on the other hand, appears to be less hazardous. A worst-case scenario, in which the entire contents of one e-cigarette cartridge are spread over the body in a thin film, suggests less than 8 mg of nicotine absorption, over a time course exceeding 24 h. However, intentional misuse of e-liquids could lead to serious toxic effects.
The dermal uptake estimations presented here are not intended to be definitive. Many different factors could contribute to different outcomes, and e-liquid mixtures with different nicotine contents should be evaluated accordingly. The simple one-compartment kinetic model for nicotine plasma concentration (Eq. (3)) could be expanded and solved numerically using actual time-dependent fluxes measured herein. The estimates are presented here to demonstrate the application of the measured permeation data within dermal exposure assessments. Carefully designed in vitro experiments, including infinite dose, transient and finite doses, can generate the types of data that are necessary to make reasonable estimates of dermal uptake from various exposure scenarios. Informed decisions also make judicious use of auxiliary information from a variety of sources combined with reasonable assumptions, as exemplified in the analyses presented here.
ACKNOWLEDGEMENTS
Intramural funding for this research was provided by the National Institute for Occupational Safety and Health, which is part of the Centers for Disease Control. We thank Professor Annette L. Bunge of the Colorado School of Mines for helpful comments and suggestions to incorporate estimates of plasma nicotine concentra-tions for the assessment of risk from dermal contact. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation 2014; 129: 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. E-cigarette use triples among middle and high school students in just one year Centers for Disease Control Press Release; (2015). Available at http://www.cdc.gov/media/releases/2015/p0416-e-cigarette-use.html. Accessed 9 February 2015. [Google Scholar]
- 3.FDA. FDA and Public Health Experts Warn About Electronic Cigarettes Food and Drug Administration Press Release; Available at 2009: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm173222.htm. Accessed 21 November 2016. [Google Scholar]
- 4.Konstantinos EF, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf 2014; 5(2): 67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatham-Stephens K, Law R, Taylor E, Melstrom P, Bunnell R, Wang B et al. Notes from the field: calls to Poison centers for exposures to electronic cigarettes — United States, September 2010–February 2014. MMWR 2014; 63: 292–293. [PMC free article] [PubMed] [Google Scholar]
- 6.Richtel M, Selling a poison by the barrel: liquid nicotine for e-cigarettes New York Times, 23 March 2014. Available at http://www.nytimes.com/2014/03/24/business/selling-a-poison-by-the-barrel-liquid-nicotine-for-e-cigarettes.html?_r=0. Accessed 21 November 2016. [Google Scholar]
- 7.Glatter R, The real dangers of liquid nicotine Forbes, 24 March 2014. Available at http://www.forbes.com/sites/robertglatter/2014/03/24/the-real-danger-of-liquid-nicotine/. Accessed 21 November 2016. [Google Scholar]
- 8.OSHA. Occupational Safety and Health Administration Regional News Release, 2013. Available at https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=NEWS_RELEASES&p_id=23844. Accessed 21 November 2016.
- 9.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliver Rev 2004; 56: 603–618. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner JM. Nicotine poisoning by absorption through the skin. JAMA 1933; 100: 1664–1665. [Google Scholar]
- 11.Benowitz NL, Lake T, Keller KH, Lee BL. Prolonged absorption with development of tolerance to toxic effects after cutaneous exposure to nicotine. Clin Pharmacol Ther 1987; 42: 119–120. [DOI] [PubMed] [Google Scholar]
- 12.Davis P, Levy S, Pahari A, Martinez D. Case report: acute nicotine poisoning associated with a traditional remedy for eczema. Arch Dis Child 2001; 85: 500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aungst BJ. Nicotine skin penetration characteristics using aqueous and non-aqueous vehicles, anionic polymers, and silicone matrices. Drug Dev Ind Pharm 1988; 14: 1481–1494. [Google Scholar]
- 14.Zorin S, Kuylenstierna F, Thulin H. In vitro test of nicotine’s permeability through human skin. Risk evaluation and safety aspects. Ann Occup Hyg 1999; 43: 405–413. [PubMed] [Google Scholar]
- 15.Qvist MH, Hoeck U, Kreilgaard B, Madsen F, Frokjaer S. Evaluation of the Göttingen minipig skin for transdermal in vitro permeation studies. Eur J Pharm Sci 2000; 11: 59–68. [DOI] [PubMed] [Google Scholar]
- 16.Kuswahyuning R, Roberts MS. Concentration dependency in nicotine skin penetration flux from aqueous solutions reflects vehicle induced changes in nicotine stratum corneum retention. Pharm Res 2014; 31: 1501–1511. [DOI] [PubMed] [Google Scholar]
- 17.Barbero AM, Frasch HF. Effect of frozen human epidermis storage duration and cryoprotectant on barrier function using two model compounds. Skin Pharmacol Physiol 2016; 29: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasch HF, Barbero AM. Application of solid-phase microextraction to in vitro skin permeation experiments: example using diethyl phthalate. Toxicol In vitro 2005; 19: 253–259. [DOI] [PubMed] [Google Scholar]
- 19.Crank J The Mathematics of Diffusion, 2nd edn. Clarendon Press: Oxford, 1975); p 51. [Google Scholar]
- 20.OECD. OECD Guideline for the Testing of Chemicals Organization for Economic Co-Operation and Development: Paris, 2004. [Google Scholar]
- 21.Lim PFC, Liu XY, Kang L, Ho PCL, Chan YW, Chan SY. Limonene GP1/PG organogel as a vehicle in transdermal delivery of haloperidol. Int J Pharm 2006; 311: 157–164. [DOI] [PubMed] [Google Scholar]
- 22.Candrashekar NS, Hiremath SR. In vivo immunomodulatory, cumulative skin irritation, sensitization and effect of d-limonene on permeation of 6-mercaptopurine through transdermal drug delivery. Biol Pharm Bull 2008; 31: 656–661. [DOI] [PubMed] [Google Scholar]
- 23.Frasch HF, Bunge AL. The transient dermal exposure II: post-exposure absorption and evaporation of volatile compounds. J Pharm Sci 2015; 104: 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen S, Selzer D, Schaefer UF, Kasting GB. An extended database of keratin binding. J Pharm Sci 2011; 100: 1712–1726. [DOI] [PubMed] [Google Scholar]
- 25.Benowitz NL, Jacob P 3rd. Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther 1984; 35: 499–504. [DOI] [PubMed] [Google Scholar]
- 26.Gori GB, Lynch CJ. Analytical cigarette yields as predictors of smoke bioavailability. Regul Toxicol Pharmacol 1985; 5: 314–326. [DOI] [PubMed] [Google Scholar]
- 27.Molander L, Hansson A, Lunell E. Pharmacokinetics of nicotine in healthy elderly people. Clin Pharmacol Ther 2001; 69: 57–65. [DOI] [PubMed] [Google Scholar]
- 28.Mayer B How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol 2014; 88: 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005; 57: 79–115. [DOI] [PubMed] [Google Scholar]
- 30.Woolf A, Burkhart K, Caraccio T, Litovitz T. Self-poisoning among adults using multiple transdermal nicotine patches. J Toxicol Clin Toxicol 1996; 34: 691–698. [DOI] [PubMed] [Google Scholar]
- 31.Murai M, Lau HK, Pereira BP, Pho RW. A cadaver study on volume and surface area of the fingertip. J Hand Surg Am 1997; 22(5): 935–941. [DOI] [PubMed] [Google Scholar]
- 32.EPA. A Laboratory Method to Determine the Retention of Liquids on the Surface of Hands US Environmental Protection Agency, 1992. EPA/747/R-92/003. [Google Scholar]
- 33.Engineering Toolbox. Liquids kinematic viscosities. The Engineering ToolBox http://www.engineeringtoolbox.com/kinematic-viscosity-d_397.html. Accessed 21 November 2016.
- 34.EPA. Dermal Exposure Factors. Exposure Factors Handbook: 2011 Edition Ch. 7 National Center for Environmental Assessment: Washington, DC, 2011. EPA/600/R-09/052F. [Google Scholar]


