Abstract
Valvular heart disease is the third-most common cause of heart problems in the United States. Malfunction of the valves can be acquired or congenital and each may lead either to stenosis or regurgitation, or even both in some cases. Heart valve disease is a progressive disease, which is irreversible and may be fatal if left untreated. Pharmacological agents cannot currently prevent valvular calcification or help repair damaged valves, as valve tissue is unable to regenerate spontaneously. Thus, heart valve replacement/repair is the only current available treatment. Heart valve research and development is currently focused on two parallel paths; first, research that aims to understand the underlying mechanisms for heart valve disease to emerge with an ultimate goal to devise medical treatment; and second, efforts to develop repair and replacement options for a diseased valve. Studies that focus on developmental malformation, genetic and disease epigenetics usually employ small animal models that are easy to access for in vivo imaging that minimally disturbs their environment during early stages of development. Alternatively, studies that aim to develop novel device for replacement and repair of diseased valves often employ large animals whose heart size and anatomy closely replicate human’s. This paper aims to briefly review the current state-of-the-art animal models, and justification to use an animal model for a particular heart valve related project.
Introduction
Heart valves are important components of the heart and their major function is to maintain the one-way direction of the blood flow from chamber to chamber and from chamber to major blood vessels. Mitral and tricuspid valves direct the blood flow from atria to ventricles while aortic and pulmonary valves control the flow from ventricles to aorta and pulmonary artery, respectively. Malfunction of the valves can be acquired or congenital and each may lead either to stenosis or regurgitation, or even both in some cases.[1] While degenerative valve disease is more common in the elderly population in industrialized countries, rheumatic valve disease is still one of the most common etiologies of valvular disease in developing countries.[2] In spite of that, the underlying mechanisms of valvular malfunction and disease progression in many acquired and congenital diseases is not yet known. Presently, there is no medical treatment to the valve disease other than procedures to repair or replace a heart valve.
Heart valve research and development is currently focused on two parallel paths: first, research that aims to understand the underlying mechanisms for heart valve disease to emerge with an ultimate goal to devise medical treatment; and second, efforts to develop repair and replacement options for a diseased valve.[3–5]
Since acquired heart valve disease is exclusive to human race, there is no naturally-occurring animal model for acquired heart valve disease. Congenital heart defects, however, have been reported to occur in animals. Studies that focus on developmental malformation, genetic and disease epigenetics usually employ small animals such chicken, mouse, and zebrafish to develop their models. This is because these embryos can be manipulated relatively easy to disrupt their valve formation. Alternatively, studies that aim to develop novel device for replacement and repair of diseased valves often employ large animals such as sheep and pig whose heart size, anatomy, material degradation and endogenous tissue growth closely replicate human’s. The present review is focused on small and large animal models currently used for heart valve research and development.
Small animal models for studying cardiac valve development
Heart formation in vertebrate involves a complex progression of finely orchestrated events. The heart begins as a tubular structure that soon, after formation, starts pumping blood flow.[6] During initial tubular stages, cardiac tissues consist of three distinct layers: the myocardium, a layer of contractile myocardial cells; the endocardium, a monolayer of endocardial cells; and the cardiac jelly layer, an extra-cellular matrix (ECM) layer in between the myocardium and endocardium. Along the tubular heart, distinct cardiac structures can be distinguished: the atrium, the atrio-ventricular (AV) canal, the ventricle, and the outflow tract (OFT). The initially-linear tubular heart then bends and twists forming a looping heart tube.[7, 8] Valve formation and chamber septation occur after looping.[9–11]
Endocardial cushions form in the AV canal and OFT early during tubular heart stages. [12–14] These cushions are the cardiac wall’s thickenings, initially composed of ECM that is rich in ground substance and largely devoid of fibrous proteins. They act as primitive valves by closing the lumen upon myocardial contraction. Cells from the endocardium, then, detach from the neighboring endocardial cells, acquire a mesenchymal phenotype and migrate into the cushions during the process of endocardial-mesenchymal-transition (EMT). In the cushions, EMT-derived mesenchymal cells proliferate, secrete fibrous ECM proteins such as type I collagen, and continue to migrate, while remodeling the endocardial cushion tissues.[14] EMT thus increases cell density in the cushions and changes their ECM composition. The AV canal cushions, which give rise to the mitral and the tricuspid valves, are exclusively populated by EMT-derived cells; whereas in the OFT cushions, from which the aortic and pulmonary valves originate, the cell population is mainly from EMT but neural crest and secondary heart field cells also contribute to the cushion cell population prior to valve formation. Cushion formation and the subsequent cushion cellularization and remodeling, determine valve formation.[15] In fact, a common deficit of the genetic anomalies and teratogen exposures is the formation of anomalous cushions that would lead to congenital valve disease and heart defect phenotypes.[16] Thus the cushions, and the contribution of different cells to the cushion population, have been the main focus of several studies aiming at understanding valve formation and congenital heart valve diseases.
Small animal models are typically used to determine how cardiac valves are formed. These models include mouse embryos, as mammalian model, as well as zebra fish and chicken embryo models. The main advantage of using mouse embryos is that genetic manipulations are common practice, allowing investigators to study the effects of different genes on cardiac (and valve) formation.[17] Further, mouse embryos can also be employed in lineage tracing studies, from which it is possible to determine the origin of cells that form the heart and the valves.[18] However, mouse embryos are difficult to access inside the uterus, precluding non-invasive in vivo imaging of cardiac function and blood flow conditions during early embryonic stages. Zebra fish and chicken embryos, on the other hand, are easy to access for in vivo imaging that minimally disturbs their environment during early stages of development,[19–27] making them ideal models to study embryonic changes that lead to cardiac defects. In particular, zebra fish and chicken embryos are extensively used to study the EMT that occurs prior to valve formation and is critical for proper valve development.[19, 28–31]
The advantage to study EMT using zebra fish and chicken embryo models is that their cardiac tissues are almost transparent at early stages of development. This enables high resolution imaging of the heart and vasculature, as well as monitoring of blood flow dynamics (blood flow velocity, blood pressure; e.g. see Figure 1). Genetic manipulations in zebra fish models further enable researchers to monitor the consequences of gene anomalies over time, and better understand early EMT dysregulation and its effect on valve formation. [30, 32] In chicken embryos, surgical interventions that alter blood flow through the heart are relatively easy to perform. [33] This allows studying the effects of blood flow dynamics during development of the heart and vasculature. In particular, results from in vitro and in vivo studies suggest that EMT is modulated by hemodynamic conditions. [29, 34–36] Thus valve formation depends on a finely orchestrated sequence of cellular signaling cascades that are modulated by blood flow conditions. Disruption of signaling or blood flow independently or together can lead to valve malformation. While the regulatory function of blood flow on valve formation is widely appreciated, the mechanisms behind this regulation are not yet understood and more research is needed to elucidate how blood flow influences cardiac and valve formation.
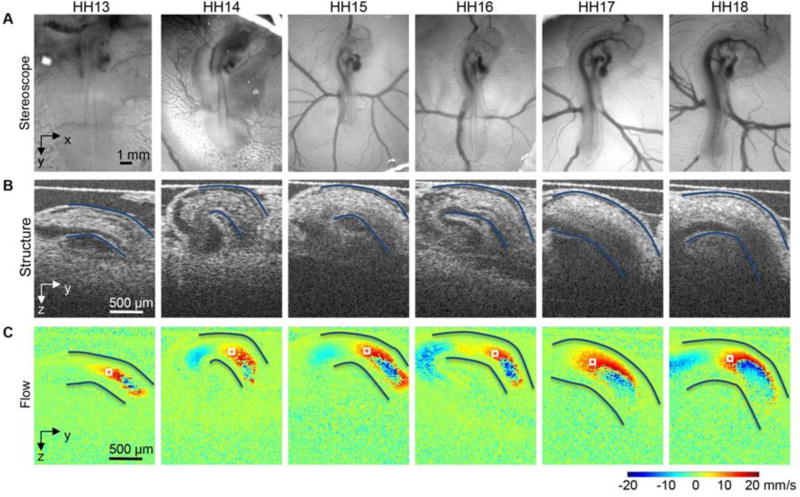
Figure 1. Images acquired from the heart of the HH13–HH18 chicken embryos, during tubular heart stages.
Example (A) optical images of the embryo in ovo on the top of the egg surface, (B) optical coherence tomography (OCT) structural 2D longitudinal images of the heart outflow tract (OFT) and neighboring structures, and (C) corresponding Doppler OCT images, which quantify flow velocity. The OFT myocardial walls are outlined in A and B, and approximately corresponding points to measure blood flow velocity are marked by a box in (C). Endocardial cushions, developing in the OFT at the stages shown, and affected by blood flow, will later give rise to semilunar valves. Reproduced from Midgett et al.[74]
Understanding the factors influencing valve formation is important, not only to understand congenital valve disease, but also because developmental programs take place during pathophysiological conditions that lead to valve degradation and malfunction. Therefore, understanding valve formation and malformation, can provide important clues on how to improve treatment and prevention strategies for heart valve disease. Table 1 compares the three small animal models for valve formation.
Table 1.
Comparison among the small animal models for heart valve research
| ZEBRAFISH | CHICKEN | MOUSE | |
|---|---|---|---|
| DEVELOPMENT | External AV valve starts to form by 105 hpf | In ovo AV valve tissue initiates at HH Stage 13. OFT valve cushions form at HH stage 16 | In utero AV Valve primordia starts to form around 9.5 dpc. OFT cushions from around 10 dpc. |
| CHAMBER FORMATION | 2 chambers (single atrium and ventricle) | 4 chambers | 4 chambers |
| VALVE FORMATION | AV valve | Aortic valve, pulmonary valve, mitral valve, tricuspid valve | Aortic valve, pulmonary valve, mitral valve, tricuspid valve |
| GENETIC MANIPULATION | Excellent for forward genetics. Rapid improvement in reverse genetics. | Rapid improvement in genetic editing with new CRISPR/Cas9 method | Strong in both forward and reverse genetics, including lineage tracing. |
| IN VIVO IMAGING | Strong: high-speed confocal microscopy & light sheet microscopy | Strong: Optical coherence tomography, confocal microscopy & optical microscopy | Less advantageous due to access (methodologies in progress) |
| SURGICAL AND PHARMACOLOGICAL MANIPULATION | Easy access for laser-targeted ablation and pharmacological interventions | Easy access for surgical, pharmacological manipulation and laser ablation | Less advantageous |
Large animal models for research and development of heart valve prosthetics
Preclinical studies to verify the safety and efficacy of new prosthetic heart valves require implantation in proper animal models whose hearts closely mimic human heart. During these studies, functionality, biocompatibility, durability and safety of valve devices are assessed before proceeding to human trials. Traditionally, ovine, swine and canine models have been used for prosthetic heart valve research and development. Although costly and time-consuming, these models help testing the biocompatibility of the devices and whether the living tissues are prone to any damage due to the implant in short- and long-term. Overall, the biological responses to implants in these mammalians are fairly similar to human, which can invoke comparable immunological response. However, in case of heart valve tissue engineering research, the way animal models may affect the degradation rate compared to endogenous tissue formation characteristic should be considered.[3, 37–40]
Choosing a right animal model for a particular experiment is very important and can significantly affect the cost and the outcome for a particular study. For example, adult sheep or pig are both suitable for acute heart valve implant studies and to check whether the device provides acceptable hemodynamics (e.g., desired pressure drop, no regurgitation, acceptable opening, etc.). The chronic animal studies aim to check whether the valve is durable and can maintain its function for a relatively long period of time. During chronic studies, valve developers usually look for sign of calcification, body’s adverse reaction to the implant, valve competency and hemodynamic performance in time. As a rule, if chronic study is the goal, sheep or minipigs are preferred since regular pigs grow faster, resulting in device-heart mismatch that will affect the chronic study outcome. Furthermore, keeping larger animals is more difficult and requires larger vivarium space that will add to the cost of the study.
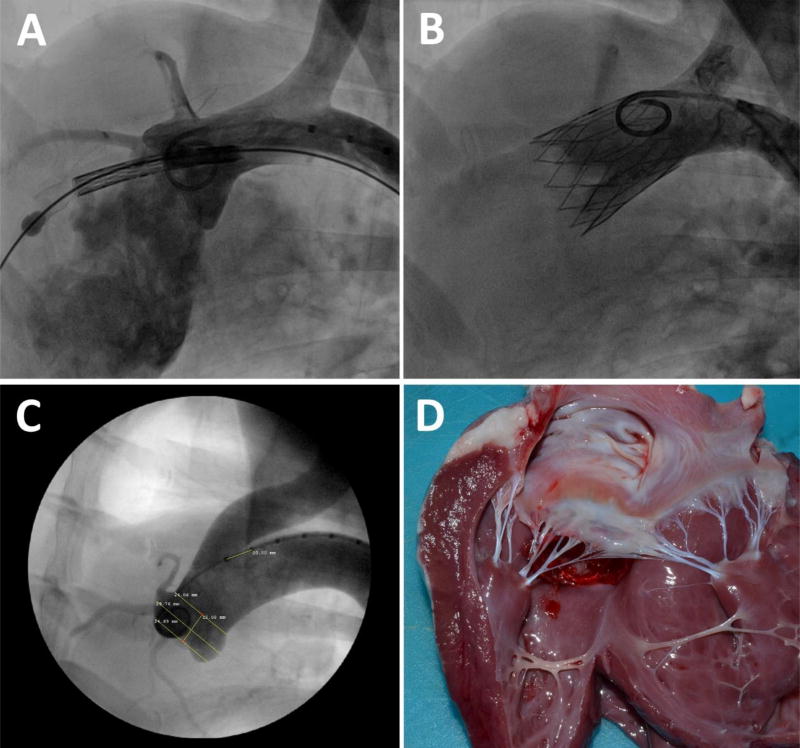
Overall, prosthetic heart valves are implanted to mitigate either a stenotic or a regurgitant native valve. Currently, there is no animal model that mimic either disease. Transcatheter aortic valves (TAVs) are implanted within a highly calcified native aortic valve. However, valvular calcification is merely a human disease and cannot be replicated in either of the ovine, canine or swine models. Calcified regions in the native valve help secure anchoring of the stented valve but technologies with staged deployment has overcome this limitation (Figure 2).[41] The anchoring limitation also pertains to transcatheter mitral valve (TMV) technologies. These valves are mainly aimed to alleviate native mitral valve incompetence. In most cases of mitral valve regurgitation, native valve annulus as well as the left atrium are significantly enlarged. Therefore, a very large prosthesis is needed to be implanted that requires a bulky delivery system. Imitation of enlarged native mitral annulus in animal models are close to impossible as such a disease does not naturally occur in large animal models. To summarize, the failure or success of both TAV and TMV implants in an animal model does not closely correlate with the human outcome. Having said that, many device features related to the transcatheter delivery and deployment can be safely studied and the results greatly mimic implantation in human.
Figure 2. Transcatheter implantation of FoldaValve in an ovine model’s aortic valve.
(A) zdvanced into the sheep’s left ventricle through aortic valve, (B) Foldavalve deployed and expanded within the sheep’s aortic valve. (C) Pre-procedural angiographic studies of sheep aortic annulus to measure the valve’s and aortic root’s dimensions, (D) post-procedural autopsy shows FoldaValve properly positioned within the sheep’s native aortic valve. The images are reproduced from Kheradvar et al.[41]
In conclusion, one needs to carefully select an animal model to test a specific aspect of their prosthetic valve to achieve the best result at lowest cost with minimal number of animal used. All acute and chronic conditions should be considered to select the best animal model mimicking human conditions. Here, we briefly describe in more details the three common large animal models (swine, canine, ovine) used for heart valve studies.
Swine model
There are many similarities between the porcine and human heart.[42, 43] The similarity among heart valves, major blood vessels, coronary arteries and cardiac conduction system of porcine heart to human is remarkable that justifies their use for acute testing of the prosthetic heart valves and other cardiac devices. While the similarity can be justified, some studies have shown that the swine model’s aortic valve can be different in size, geometry,[44] fibrous continuity,[45] and expression of metalloproteinase (MMP) I and proteoglycan.[46] As well, the wall thickness ratio between the left and right ventricle is much higher in the porcine heart compared to human’s.[45, 47] Moreover, human’s left atrium receives blood from four or five pulmonary veins in contrast to the porcine heart whose left atrium only received two pulmonary veins. [48]
Alternatively, there are several limitations in use of swine model to be considered; porcine heart is infamously sensitive to anesthesia and surgical manipulation that frequently leads to intra-surgical and post-surgical complications including arrhythmia and even death. [49] More specifically, there are several factors that may lead to fatal arrhythmia. For example, cardiac electrophysiology of human and swine models is quite similar with the exception of Ca2+-independent transient outward K+ current (Ito1) whose expression is absent, and only Ito2 is present in pig ventricular myocytes. [50, 51] As well, it has been shown that ‘hibernating’ the myocardium, commonly practiced during swine surgery, may lead to chronic arterial occlusion leading to cardiac death. [52] The difference in cardiac electrophysiology has led to higher heart rate in pigs compared to human heart. [53]
In addition, pigs are prone to post-surgical infection and overall need more regular follow up and attention. To avoid infection, special sterile techniques need to be used. Grehan et al. [49] reported difficulty in maintaining safe levels of anticoagulation with warfarin, when used in a study involving swine model for mechanical heart valve testing. Additionally, they reported marked fibrous formation and thrombosis around the valve as well as perivalvular defects, due to normal somatic growth occurring in young swine. [49]
Canine model
There are similarities between canine and human heart, but in comparison to human’s and ovine hearts, the canine heart has a more connected network of coronary collateralization between right and left coronary circulation that protects their heart from ischemia. Accordingly, canine heart was considered as a large animal model for myocardial ischemia studies. Generally, using catheters in canine model is easier than ovine and swine models during surgery. They have thinner skin, which makes it easier for interventionalist to advance catheters into the vasculatures. On top of that, imaging and cardiac monitoring are more accessible in canine compared to ovine and swine models. Although, canine tricuspid valve normally has two leaflets,[54] the lower risk of post-surgical infection after valve implantation was a main advantage to select canine for the valve replacement procedures in the past.[55] However, at the present time, canine models are not commonly used for heart valve implantations mainly due to restrictions in obtaining the necessary approval for performing experiments in this species, which is unique to canines.[56, 57]
Ovine model
The ovine model is currently considered the best animal model for valve replacement survival studies that satisfy Food and Drug Administration (FDA) and CE mark requirements.[58] The anatomy of the sheep heart as well as physiological parameters such as heart rate and blood pressure are similar to human’s.[59] More importantly, valves sizes of an adult sheep are comparable to human’s, which makes it an ideal model for heart valve replacement (Figure 2).[60] Alternatively, ovine aortic valves leaflets are thinner and more fragile in comparison with human,[61] and the fibrous continuity that is present in human’s mitral and aortic valve leaflets is absent in ovine heart valves.[62] As well, the coronary collateral network in ovine heart is low in compared to swine and human heart, which makes it an ideal animal model for myocardial ischemia research.[63, 64] Finally, ovine growth rate is generally lower than swine, which makes it an ideal model for overall heart valve prosthetics including regular and hybrid tissue-engineered heart valves.[65–69]
Since acquired human heart valve disease does not naturally occur in animals, many have tried developing their desired animal model through different methods. For example, Simmons and colleagues identified 584 genes as differentially expressed by the endothelium on the aortic side versus ventricular side of normal porcine aortic valves and used this model to study the correlation between phenotypic heterogeneity and regions of susceptibility in normal valvular endothelium.[70] Some studies show evidence that hypercholesterolemic diets in swine models would lead to human-type disease with small early calcific nodules observed in 6 to 7 months.[71] Others have used surgical banding to produce graded aortic and mitral valve stenosis.[72] As well, use of synthetic calcified polymeric valves has been suggested to imitate valve-in-valve transcatheter procedures.[73]
Conclusion
This paper aimed to briefly review the current state-of-the-art information on animal models used for heart valve research and development, and justification to use an animal model for a particular project. In summary, studies that aim to test novel device or procedures for replacement and repair of diseased valves may use large animals whose heart size and valve anatomy closely mimic human’s. As well, studies with a focus on developmental malformation, genetic and/or disease epigenetics usually employ small animal models that are easily accessible for in vivo imaging and can be genetically or physically modified as needed.
Acknowledgments
This work is partially supported by grants from National Institutes of Health: R21EB021513 to AK and R01HL094570 to SR.
Prof. Kheradvar is a co-founder of Folda LLC that commercializes FoldaValve™. He also and has an equity interest in Folda LLC, a company that may potentially benefit from the research results.
Footnotes
The terms of this arrangement have been reviewed and approved by the University of California, Irvine in accordance with its conflict of interest policies. The other authors have no conflicts of interest to declare.
References
- 1.Unger P, et al. Management of multiple valve disease. Heart. 2011;97(4):272–277. doi: 10.1136/hrt.2010.212282. [DOI] [PubMed] [Google Scholar]
- 2.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nature Reviews Cardiology. 2011;8:162. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 3.Kheradvar A, et al. Emerging Trends in Heart Valve Engineering: Part I. Solutions for Future. Annals of Biomedical Engineering. 2015;43(4):833–843. doi: 10.1007/s10439-014-1209-z. [DOI] [PubMed] [Google Scholar]
- 4.Kheradvar A, et al. Emerging Trends in Heart Valve Engineering: Part II. Novel and Standard Technologies for Aortic Valve Replacement. Annals of Biomedical Engineering. 2015;43(4):844–857. doi: 10.1007/s10439-014-1191-5. [DOI] [PubMed] [Google Scholar]
- 5.Kheradvar A, et al. Emerging Trends in Heart Valve Engineering: Part III. Novel Technologies for Mitral Valve Repair and Replacement. Annals of Biomedical Engineering. 2015;43(4):858–870. doi: 10.1007/s10439-014-1129-y. [DOI] [PubMed] [Google Scholar]
- 6.Wittig J, Münsterberg A. The Early Stages of Heart Development: Insights from Chicken Embryos. Journal of Cardiovascular Development and Disease. 2016;3(2):12. doi: 10.3390/jcdd3020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manner J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec. 2000;259(3):248–62. doi: 10.1002/1097-0185(20000701)259:3<248::AID-AR30>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Garita B, et al. Blood flow dynamics of one cardiac cycle and relationship to mechanotransduction and trabeculation during heart looping. American Journal of Physiology: Heart and Circulation Physiology. 2011;300:H879–H891. doi: 10.1152/ajpheart.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinsen BJ. Reference guide to the stages of chick heart embryology. Developmental Dynamics. 2005;233:1217–1237. doi: 10.1002/dvdy.20468. [DOI] [PubMed] [Google Scholar]
- 10.Schleiffarth JR, et al. Wnt5a Is Required for Cardiac Outflow Tract Septation in Mice. Pediatr Res. 2007;61(4):386–391. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407(6801):221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 12.Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. American Journal of Anatomy. 1976;148:85–120. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima Y, et al. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) The Anatomical Record. 2000;258:119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. International Review of Cytology. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- 15.Combs MD, Yutzey KE. Heart Valve Development: Regulatory Networks in Development and Disease. Circulation Research. 2009;105(5):408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rugonyi S. Genetic and flow anomalies in congenital heart disease. AIMS Genetics. 2016;3(3):157–166. doi: 10.3934/genet.2016.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon A. Chapter 4 Mouse Models of Congenital Cardiovascular Disease, in Current Topics in Developmental Biology. Academic Press; 2008. pp. 171–248. [DOI] [PubMed] [Google Scholar]
- 18.Meilhac SM, et al. Cardiac Cell Lineages that Form the Heart. Cold Spring Harbor Perspectives in Medicine. 2014;4(9):a013888. doi: 10.1101/cshperspect.a013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakkers J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovascular Research. 2011;91(2):279–288. doi: 10.1093/cvr/cvr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, et al. 4-Dimensional light-sheet microscopy to elucidate shear stress modulation of cardiac trabeculation. The Journal of Clinical Investigation. 2016;126(5):1679–1690. doi: 10.1172/JCI83496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nature Biotechnology. 2003;21:1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins MW, et al. Ultrahigh-speed optical coherence tomography imaging and visualization of the embryonic avian heart using a buffered fourier domain mode locked laser. Optics Express. 2007;15:6251–6267. doi: 10.1364/oe.15.006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rugonyi S, et al. Changes in wall motion and blood flow in the outflow tract of chick embryonic hearts observed with optical coherence tomography after outflow tract banding and vitelline-vein ligation. Physics in Medicine and Biology. 2008;53:5077–5091. doi: 10.1088/0031-9155/53/18/015. [DOI] [PubMed] [Google Scholar]
- 24.Li P, et al. In vivo imaging of blood flow and wall strain rate in outflow tract of embryonic chick heart using ultrafast spectral domain optical coherence tomography. Journal of Biomedical Optics. 2012;17:096006. doi: 10.1117/1.JBO.17.9.096006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins M, Watanabe M, Rollins A. Longitudinal imaging of heart development with optical coherence tomography. IEEE Journal of Selected Topics in Quantum Electronics. 2012;18:1166–1175. doi: 10.1109/JSTQE.2011.2166060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregg CL, Butcher JT. Quantitative in vivo imaging of embryonic development: Opportunities and challenges. Differentiation. 2012;84(1):149–162. doi: 10.1016/j.diff.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manner J, et al. High-resolution in vivo imaging of the cross-sectional deformations of contracting embryonic heart loops using optical coherence tomography. Developmental Dynamics. 2008;237(4):953–61. doi: 10.1002/dvdy.21483. [DOI] [PubMed] [Google Scholar]
- 28.Tavares ALP, et al. TGFβ-mediated RhoA expression is necessary for epithelial-mesenchymal transition in the embryonic chick heart. Developmental Dynamics. 2006;235(6):1589–1598. doi: 10.1002/dvdy.20771. [DOI] [PubMed] [Google Scholar]
- 29.Midgett M, et al. Increased Hemodynamic Load in Early Embryonic Stages Alters Endocardial to Mesenchymal Transition. Frontiers in Physiology. 2017;8(56) doi: 10.3389/fphys.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steed E, et al. klf2a couples mechanotransduction and zebrafish valve morphogenesis through fibronectin synthesis. 2016:11646. doi: 10.1038/ncomms11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavares ALP, et al. RUNX2-I is an early regulator of epithelial-mesenchymal cell transition in the chick embryo. Developmental Dynamics. 2017 doi: 10.1002/dvdy.24539. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YM, et al. Vascular endothelial growth factor receptor signaling is required for cardiac valve formation in zebrafish. Developmental Dynamics. 2006;235:29–37. doi: 10.1002/dvdy.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Midgett M, Rugonyi S. Congenital heart malformations induced by hemodynamic altering surgical interventions. Frontiers in Physiology. 2014;5 doi: 10.3389/fphys.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan H, et al. Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Developmental Biology. 2013;374(2):345–356. doi: 10.1016/j.ydbio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin RL, et al. The impact of flow-induced forces on the morphogenesis of the outflow tract. Frontiers in Physiology. 2014;5 doi: 10.3389/fphys.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menon V, et al. Altered Hemodynamics in the Embryonic Heart Affects Outflow Valve Development. Journal of Cardiovascular Development and Disease. 2015;2(2):108. doi: 10.3390/jcdd2020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung DY, Duan B, Butcher JT. Current Progress in Tissue Engineering of Heart Valves: Multiscale Problems, Multiscale Solutions. Expert opinion on biological therapy. 2015;15(8):1155–1172. doi: 10.1517/14712598.2015.1051527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez RA, et al. Naturally and synthetic smart composite biomaterials for tissue regeneration. Advanced drug delivery reviews. 2013;65(4):471–496. doi: 10.1016/j.addr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Sodian R, et al. Tissue engineering of heart valves: in vitro experiences. The Annals of thoracic surgery. 2000;70(1):140–144. doi: 10.1016/s0003-4975(00)01255-8. [DOI] [PubMed] [Google Scholar]
- 40.Sodian R, et al. Early In Vivo Experience With Tissue-Engineered Trileaflet Heart Valves. Circulation. 2000;102(suppl 3):Iii-22. doi: 10.1161/01.cir.102.suppl_3.iii-22. [DOI] [PubMed] [Google Scholar]
- 41.Kheradvar A, Groves EL, Tseng EE. Proof of Concept of FOLDAVALVE A Novel 14Fr Totally Repositionable and Retrievable Transcatheter Aortic Valve. Euro Intervention. 2015;10(11):591–6. doi: 10.4244/EIJY15M03_04. [DOI] [PubMed] [Google Scholar]
- 42.Naimark WA, et al. Correlation of structure and viscoelastic properties in the pericardia of four mammalian species. Am J Physiol. 1992;263(4 Pt 2):H1095–106. doi: 10.1152/ajpheart.1992.263.4.H1095. [DOI] [PubMed] [Google Scholar]
- 43.Kozlowski T, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67(1):18–30. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 44.Sim EKW, et al. Comparison of human and porcine aortic valves. Clinical Anatomy. 2003;16(3):193–196. doi: 10.1002/ca.10149. [DOI] [PubMed] [Google Scholar]
- 45.Crick SJ, et al. Anatomy of the pig heart: comparisons with normal human cardiac structure. Journal of Anatomy. 1998;193(Pt 1):105–119. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephens EH, Kearney DL, Grande-Allen KJ. Insight into pathologic abnormalities in congenital semilunar valve disease based on advances in understanding normal valve microstructure and extracellular matrix. Cardiovascular Pathology. 2012;21(1):46–58. doi: 10.1016/j.carpath.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lelovas PP, Kostomitsopoulos NG, Xanthos TT. A Comparative Anatomic and Physiologic Overview of the Porcine Heart. Journal of the American Association for Laboratory Animal Science : JAALAS. 2014;53(5):432–438. [PMC free article] [PubMed] [Google Scholar]
- 48.König HE, Ruberte J, Liebich J. Organs of the cardiovascular system (systema cardiovasculare) In: König HE, Liebich J, editors. Veterinary anatomy of domestic mammals. Schattauer; New York, NY: 2004. [Google Scholar]
- 49.Grehan JF, et al. Development and evaluation of a swine model to assess the preclinical safety of mechanical heart valves. J Heart Valve Dis. 2000;9(5):710–9. discussion 719–20. [PubMed] [Google Scholar]
- 50.Li G-R, et al. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovascular Research. 2003;58(1):89–98. doi: 10.1016/s0008-6363(02)00859-3. [DOI] [PubMed] [Google Scholar]
- 51.Schultz J-H, et al. Molecular and functional characterization of Kv4.2 and KChIP2 expressed in the porcine left ventricle. Pflügers Archiv - European Journal of Physiology. 2007;454(2):195–207. doi: 10.1007/s00424-006-0203-1. [DOI] [PubMed] [Google Scholar]
- 52.Canty JM, et al. Hibernating Myocardium. Circulation Research. 2004;94(8):1142. doi: 10.1161/01.RES.0000125628.57672.CF. [DOI] [PubMed] [Google Scholar]
- 53.Bianco RW, Callegos RP, Rivald Al, Voigt J, Dalmasso AP. Animal models for cardiac research. In: Laizzo PA, editor. Handbook of cardiac anatomy, physiology, and devices. Springer; Minneapolis, MN: 2009. [Google Scholar]
- 54.Evans HE. The heart and arteries. In: Evans HE, editor. Miller’s anatomy of the dog. Saunders; Philadelphia, PA: 1993. pp. 586–681. [Google Scholar]
- 55.Bianco RW, et al. Canine model for long-term evaluation of prosthetic mitral valves. J Surg Res. 1986;41(2):134–40. doi: 10.1016/0022-4804(86)90018-1. [DOI] [PubMed] [Google Scholar]
- 56.Milani-Nejad N, Janssen PML. Small and Large Animal Models in Cardiac Contraction Research: Advantages and Disadvantages. Pharmacology & therapeutics. 2014;141(3):235–249. doi: 10.1016/j.pharmthera.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camacho P, et al. Large Mammalian Animal Models of Heart Disease. Journal of Cardiovascular Development and Disease. 2016;3(4):30. doi: 10.3390/jcdd3040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iaizzo PA, et al. Heart Valves: From Design to Clinical Implantation. Springer; 2013. [Google Scholar]
- 59.Barnhart GR, et al. Bioprosthetic valvular failure. Clinical and pathological observations in an experimental animal model. J Thorac Cardiovasc Surg. 1982;83(4):618–31. [PubMed] [Google Scholar]
- 60.Sands MP, et al. An anatomical comparison of human pig, calf, and sheep aortic valves. Ann Thorac Surg. 1969;8(5):407–14. doi: 10.1016/s0003-4975(10)66071-7. [DOI] [PubMed] [Google Scholar]
- 61.Sands MP, Rittenhouse EA, Mohri H, Merendino KA. An anatomical comparison of human pig, calf, and sheep aortic valves. Ann Thorac Surg. 1969;8(5):407–14. doi: 10.1016/s0003-4975(10)66071-7. [DOI] [PubMed] [Google Scholar]
- 62.Hill AJ, Iaizzo PA. Comparative cardiac anatomy. In: Iaizzo PA, editor. Handbook of cardiac anatomy, physiology, and devices. Springer; New York, NY: 2009. [Google Scholar]
- 63.Schaper W, Flameng W, DeBrabander M. Comparative aspects of coronary collateral circulation. Adv Exp Med Biol. 1972;22:267–76. doi: 10.1007/978-1-4684-3213-8_15. [DOI] [PubMed] [Google Scholar]
- 64.DiVincenti L, Westcott R, Lee C. Sheep (Ovis aries) as a Model for Cardiovascular Surgery and Management before, during, and after Cardiopulmonary Bypass. Journal of the American Association for Laboratory Animal Science : JAALAS. 2014;53(5):439–448. [PMC free article] [PubMed] [Google Scholar]
- 65.Alavi SH, Kheradvar A. A Hybrid Tissue-Engineered Heart Valve. The Annals of thoracic surgery. 2015;99(6):2183–2187. doi: 10.1016/j.athoracsur.2015.02.058. [DOI] [PubMed] [Google Scholar]
- 66.Syedain Z, et al. Tubular Heart Valves from Decellularized Engineered Tissue. Annals of Biomedical Engineering. 2013;41(12):2645–2654. doi: 10.1007/s10439-013-0872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syedain ZH, et al. Implantation of a tissue-engineered heart valve from human fibroblasts exhibiting short term function in the sheep pulmonary artery. Cardiovascular Engineering and Technology. 2011;2(2):101–112. [Google Scholar]
- 68.Driessen-Mol A, et al. Transcatheter Implantation of Homologous “Off-the-Shelf” Tissue-Engineered Heart Valves With Self-Repair Capacity: Long-Term Functionality and Rapid In Vivo Remodeling in Sheep. Journal of the American College of Cardiology. 2014;63(13):1320–1329. doi: 10.1016/j.jacc.2013.09.082. [DOI] [PubMed] [Google Scholar]
- 69.Gottlieb D, et al. In vivo monitoring of function of autologous engineered pulmonary valve. The Journal of Thoracic and Cardiovascular Surgery. 2010;139(3):723–731. doi: 10.1016/j.jtcvs.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Simmons CA, et al. Spatial Heterogeneity of Endothelial Phenotypes Correlates With Side-Specific Vulnerability to Calcification in Normal Porcine Aortic Valves. Circulation Research. 2005;96(7):792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guerraty MA, et al. Hypercholesterolemia Induces Side-Specific Phenotypic Changes and Peroxisome Proliferator–Activated Receptor-γ Pathway Activation in Swine Aortic Valve Endothelium. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(2):225–231. doi: 10.1161/ATVBAHA.109.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor DE, Whamond JS. A method of producing graded stenosis of the aortic and mitral valves in sheep for fluid dynamic studies. J Physiol. 1975;244(1):16–17p. [PubMed] [Google Scholar]
- 73.Falahatpisheh A, et al. A calcified polymeric valve for valve-in-valve applications. Journal of Biomechanics. 2016 doi: 10.1016/j.jbiomech.2016.11.027. in press. [DOI] [PubMed] [Google Scholar]
- 74.Midgett M, et al. Blood flow through the embryonic heart outflow tract during cardiac looping in HH13–HH18 chicken embryos. Journal of The Royal Society Interface. 2015;12(111) doi: 10.1098/rsif.2015.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]