Abstract
Object.
Although traumatic brain injury (TBI) is the leading cause of death and morbidity in young adults, no effective pharmaceutical treatment is available. By inhibiting glycogen synthase kinase–3 (GSK-3) and histone deacetylases (HDACs), respectively, lithium and valproate (VPA) have beneficial effects in diverse neurodegenerative diseases. Furthermore, in an excitotoxic neuronal model and in animal models of amyotrophic lateral sclerosis, Huntington disease, and stroke, combined treatment with lithium and VPA produces more robust neuroprotective effects than treatment with either agent alone. Building on previous work that establishes that therapeutic doses of either lithium or VPA have beneficial effects in mouse models of TBI, this study evaluated the effects of combined treatment with subeffective doses of lithium and VPA in a mouse model of TBI.
Methods.
Male C57BL/6 mice underwent TBI and were subsequently treated with lithium, VPA, or a combination of lithium and VPA 15 minutes post-TBI and once daily thereafter for up to 3 weeks; all doses were subeffective (1 mEq/kg of lithium and 200 mg/kg of VPA). Assessed parameters included lesion volume via H & E staining; blood-brain barrier (BBB) integrity via immunoglobulin G extravasation; neurodegeneration via Fluoro-Jade B staining; motor coordination via a beam-walk test; and protein levels of acetylhistone H3, phospho-GSK-3β, and β-catenin via Western blotting.
Results.
Posttrauma treatment with combined subeffective doses of lithium and VPA significantly reduced lesion volume, attenuated BBB disruption, and mitigated hippocampal neurodegeneration 3 days after TBI. As expected, subeffective doses of lithium or VPA alone did not have these beneficial effects. Combined treatment also improved motor coordination starting from Day 7 and persisting at least 21 days after TBI. Acetylation of histone H3, an index of HDAC inhibition, was robustly increased by the combined treatment 3 days after TBI.
Conclusions.
Cotreatment with subeffective doses of lithium and VPA significantly attenuated TBI-induced brain lesion, BBB disruption, and neurodegeneration, and robustly improved long-term functional recovery. These findings suggest that potentiating histone acetylation by HDAC inhibition is probably part of the mechanism underlying the beneficial effects associated with this combined treatment for TBI. Because both lithium and VPA have a long history of safe clinical use, the results suggest that using a combination of these 2 agents at subtherapeutic doses to treat patients with TBI may also reduce side effects and enhance tolerability.
Keywords: traumatic brain injury, lithium, valproate, neuroprotection, acetylhistone H3, mouse
Traumatic brain injury is the leading cause of death and morbidity in young adults in developed countries,17 and is increasingly recognized as a “signature wound” in soldiers returning from Iraq and Afghanistan. In the US, an average of 1.7 million people sustain a TBI each year.9 The pathogenesis of TBI includes the primary injury caused by mechanical damage as well as a cascade of secondary injuries occurring hours or days after the initial event.15 These secondary injuries—including excitotoxicity, inflammation, oxidative stress, mitochondrial dysfunction, and BBB disruption—are responsible for further TBI pathology and are reversible.15 Despite extensive research, no FDA-approved pharmaceutical therapies currently exist to combat TBI.18 The complexity of TBI pathogenesis suggests that treatment will require a combination of drugs that target multiple pathways of cell survival or death.18
Lithium has been the mainstay of treatment for bipolar disorder for more than 60 years. It directly inhibits GSK-3, and can also indirectly inhibit this kinase by promoting serine phosphorylation by activating Akt, protein kinase A, and protein kinase C.2 Lithium’s neuroprotective effects via GSK-3 inhibition have been well established in diverse neurodegenerative conditions including stroke, Alzheimer disease, Parkinson disease, and Huntington disease.2,4,24 Notably, we and others recently demonstrated that lithium can reduce lesion volume, attenuate neuroinflammation, improve motor-behavioral outcome, decrease β-amyloid accumulation, and partially restore memory deficits in rodent models of TBI.6,19,25,26,28
Histone acetylation and deacetylation play important roles in the epigenetic regulation of cellular function, and histone hypoacetylation has been shown to be involved in neurodegenerative conditions.5 Mounting evidence suggests that epigenetic regulation by HDAC inhibition is neuroprotective by restoring histone acetylation levels and thereby correcting transcriptional deficits in various models of brain disorders, including TBI rodent models.5,7,20,27 In cellular and animal models of neurodegenerative diseases, VPA, another mood stabilizing drug and pan-HDAC inhibitor, has similarly been shown to have neuroprotective properties via HDAC inhibition.5,23 In a rat model of TBI, VPA improves BBB integrity, reduces contusion volume, and improves motor function and spatial memory.8
It is interesting to note that previous studies from our laboratory demonstrate that combined treatment with lithium and VPA produces more robust beneficial effects against neuronal excitotoxicity in culture, and in mouse models of amyotrophic lateral sclerosis and Huntington disease, than treatment with either agent alone.3,10,13 In addition, mesenchymal stem cells coprimed with lithium and VPA exert greater homing and migration capacity to lesion sites in a rat model of ischemic stroke; transplantation of these cells markedly improves functional outcome in these animals.21,22 However, the effect of combined treatment with lithium and VPA post-TBI has not been evaluated.
Building on previous work from our laboratory and others demonstrating that both lithium and VPA alone have significant benefits in rodent models of TBI, we decided to investigate whether subeffective doses of these 2 agents, administered simultaneously, will have a synergistic effect. Thus, the present study investigated the effects of posttrauma combined treatment with subeffective doses of lithium and VPA in a mouse model of TBI. This issue is of particular clinical relevance because, although therapeutic doses of either agent are putatively useful for treating TBI, subtherapeutic doses might be equally effective but minimize the side effects associated with these agents.
Methods
Animals and Surgery
Male C57BL/6 mice (126 total) were obtained from the Jackson Laboratory and housed in standard cages under a 12-hour light-dark cycle. Food and water were provided ad libitum. All animals were treated according to guidelines established by the Uniformed Services University of the Health Sciences and the National Research Council’s Guide for the Care and Use of Laboratory Animals.
The TBI was induced at 8 weeks of age via a CCI device (Leica) as previously described.25 Briefly, prior to TBI, mice were anesthetized with 2% isoflurane in O2 and mounted on a stereotactic frame with an adapter (Kopf Instruments). A craniotomy (approximately 4-mm diameter) was performed over the left parietal cortex between the lambda and bregma sutures. The skullcap was carefully removed without disrupting the dura mater. The point of impact was midway between the bregma and lambda sutures, as well as midway between the left temporalis muscle and central suture. A 3-mm-diameter convex tip set to compress the brain with a combination of velocity at 5 m/sec and deformation of 1.5 mm was used to perform the CCI injury. The bone flap was then repositioned to close the craniotomy. A heating pad coupled to a rectal probe was used to maintain body temperature at 37°C ± 0.5°C. A group of sham-operated mice underwent identical craniotomy procedures without CCI injury.
An effective dose of VPA (300 mg/kg dissolved in normal saline) was used to assess the neuroprotective effects of this drug on lesion volume. For all remaining experiments, subeffective doses of lithium (1 mEq/kg dissolved in normal saline), VPA (200 mg/kg dissolved in normal saline), or a combination of lithium (1 mEq/kg) and VPA (200 mg/kg) were used (all of the aforementioned drugs were obtained from Sigma, Inc.). All treatments were intraperitoneally administered 15 minutes after TBI, followed by daily injections for up to 3 weeks.
Lesion Volume Measurement
Lesion volume was measured 3 days after TBI. Animals were deeply anesthetized, and transcardially perfused with saline followed by 4% formaldehyde. Brains were fixed in the same fixative for 24–48 hours, and then subsequently immersed in 30% sucrose for another 24–48 hours. A cryostat (Leica) was used to cut 30-μm-thick sections starting 600 μm anterior to the bregma, and H & E was used to stain serial sections at 540-μm intervals; these were scanned with an Epson scanner. The ImageJ program (National Institutes of Health) was used to evaluate the lesion area of each section, and lesion volume for each given animal was calculated as previously described, with slight modifications,8,25 specifically, where A stands for the lesion area (in mm2) for each slice, and X stands for the distance (in mm) between 2 sequential slices: {0.5A1 + 0.5(A1 + A2) +...+ 0.5(An– 1 + An) + 0.5An} X.
Evaluation of BBB Integrity
Three days after TBI, 1-step immunohistochemical detection of IgG was used to assess BBB integrity. First, serial sections were incubated with 0.3% hydrogen peroxide, and then blocked with 5% normal donkey serum. A biotin-conjugated anti-IgG antibody (1:200; Vector Laboratories) was used to incubate sections overnight at 4°C, followed by incubation in avidin–biotin complex solution (Vector Laboratories). Sections were then stained with diamino benzidine (Vector Laboratories) and scanned with an Epson scanner. The ImageJ software was used to analyze the images, as previously described.23 Briefly, the optical density of ipsilateral and contralateral IgG staining was measured; relative optical density was calculated by subtracting the optical density of the ipsilateral hemisphere from that of the contralateral side.
Beam-Walk Test
The beam-walk test is a well-established and reliable method of evaluating fine motor coordination.11,16 The beam-walk apparatus comprises a narrow wooden beam 6 mm wide and 120 cm long suspended approximately 30 cm above a table. Over 2 consecutive days prior to TBI, each mouse was trained to walk from one end of the beam to the other over multiple trials. The number of foot faults for the right hind limb was counted over 50 steps. Animals with a basal level of < 10 faults per 50 steps were used in the experiment. The beam-walk test was performed on Days 1, 3, 7, 14, and 21 postinjury, and the number of foot faults per 50 steps was recorded. Observers were blinded to the treatment and control groups.
Fluoro-Jade B Staining
Fluoro-Jade B is a fluorescent dye that labels degenerating neurons. To perform FJB staining, serial sections at 540-μm intervals were first immersed in a solution containing 1% sodium hydroxide in 80% ethanol for 5 minutes, and then immersed for 2 minutes in 70% ethanol. Brain sections were subsequently transferred to a 0.06% potassium permanganate solution and gently shaken for 10 minutes. After immersion in a 0.0004% FJB solution, sections were air dried and cleared in xylene for at least 1 minute before mounting with DPX (Sigma). A magnification of 20 was used to count FJB-positive cells in the hippocampal dentate gyrus area; results were expressed as the number of FJB-positive cells per dentate gyrus.
Western Blot Analysis
Levels of Ac-H3, phospho-GSK-3β, and β-catenin were assessed via Western blot analysis. Three days postTBI, the ipsilateral cortex was collected and sonicated for 30 seconds in radioimmunoprecipitation assay buffer (Cell Signaling) containing protease inhibitor cocktail (Roche Diagnostics) as well as phosphatase inhibitors I and II (Sigma). Lysates were centrifuged at 12,000 rpm for 20 minutes; the bicinchoninic acid method was used to determine protein concentrations. An aliquot of 30 μg of protein was loaded onto each lane of a 4%–12% sodium dodecyl sulfate–polyacrylamide gel (Invitrogen). Proteins were separated by electrophoresis, and then transferred to a polyvinylidene difluoride membrane. The membrane was incubated overnight at 4°C with polyclonal rabbit antiphospho-GSK-3β serine 9 (1:1000; Cell Signaling), monoclonal mouse anti–Ac-H3 at Lys 9 and 14 (1:1000; Millipore), monoclonal mouse anti–β-catenin (1:1000; Cell Signaling), or monoclonal mouse anti–β-actin (1:5000; Sigma). Membranes were then incubated with a goat anti–mouse IRDye 800CW-conjugated secondary antibody or a goat anti–rabbit IRDye 700CW-conjugated secondary antibody, and then scanned using an Odyssey Infrared Imaging System (LI-COR Biosciences).
Statistical Analysis
GraphPad Prism software (GraphPad Software, Inc.) was used for all data analyses. Beam-walk test results were analyzed using 2-way repeated-measures ANOVA with post hoc Bonferroni comparison. One-way repeated-measures ANOVA with post hoc Bonferroni comparison was used when comparing multiple groups. Student t-tests were used to compare 2 groups. Results were quantified and expressed as the mean ± SEM. Statistical significance was established at p < 0.05.
Results
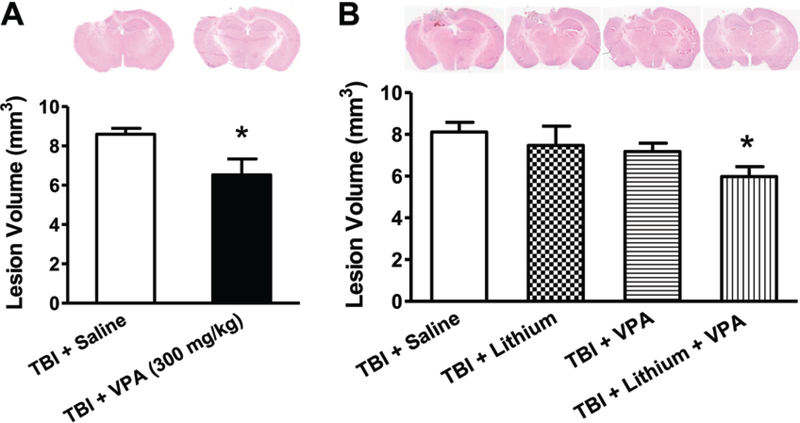
As an initial experiment, we examined the effect of VPA treatment on lesion volume 3 days after TBI. Results showed that posttrauma treatment with 300 mg/kg VPA significantly reduced lesion volume from 8.59 ± 0.30 mm3 to 6.52 ± 0.82 mm3 (Fig. 1A). In all subsequent experiments, only subeffective doses of all agents were used (1 mEq/kg of lithium, 200 mg/kg of VPA, or a combination of these 2 doses).
Fig. 1.
Combined treatment with subeffective doses of lithium and VPA reduced lesion volume 3 days post-TBI. H & E staining was performed to evaluate TBI-induced lesion volume. Representative H & E staining showed that TBI resulted in lesions in the ipsilateral cortex and hippocampus. A: Treatment with 300 mg/kg VPA significantly reduced lesion volume 3 days after TBI (n = 8/group; *p < 0.05). B: Treatment with lithium at 1 mEq/kg or VPA at 200 mg/kg had no effect on lesion volume. Combined treatment with these subeffective doses of lithium and VPA significantly reduced lesion volume (B; n = 9/group; *p < 0.05). Data throughout are expressed as the mean ± SEM.
As expected, neither 1 mEq/kg of lithium alone nor 200 mg/kg of VPA alone significantly reduced TBI-induced lesion volume (8.11 ± 0.46 mm3 vs 7.47 ± 0.92 mm3 or 7.18 ± 0.40 mm3) 3 days after TBI (Fig. 1B). However, combined subeffective doses of lithium and VPA markedly reduced lesion volume from 8.11 ± 0.46 mm3 to 5.97 ± 0.47 mm3, suggesting that combined treatment had a synergistic protective effect.
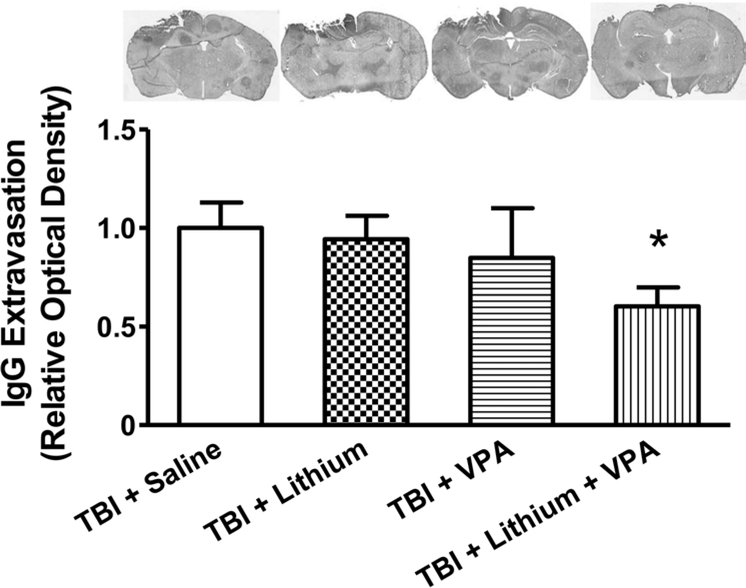
Because BBB disruption is an important secondary pathology after TBI, IgG extravasation in the brains of mice was examined 3 days after TBI to assess BBB integrity. The TBI caused marked IgG extravasation in the area close to the injury. As expected, neither 1 mEq/kg lithium nor 200 mg/kg VPA treatment alone significantly decreased TBI-induced IgG extravasation (the relative optical density was reduced by 5% and 15%, respectively). In contrast, combined treatment robustly reduced positive IgG staining by 40% (p < 0.05), again suggesting that combined treatment had a synergistic effect in preserving BBB integrity post-TBI (Fig. 2).
Fig. 2.
Combined treatment with subeffective doses of lithium and VPA attenuated BBB disruption 3 days after TBI. IgG staining was used to determine BBB disruption. Representative photographs (upper row) showing TBI increased the immune-positive signal in the area close to injury. The immune reactivity in monotreatment groups with either lithium or VPA was similar to that in the saline-treated group, whereas combined treatment significantly reduced the area of IgG-positive staining. Quantitative analysis showed that the relative optical density of IgG staining was robustly reduced after combined treatment, compared with the saline-treated group (n = 8/group; *p < 0.05), whereas treatment with lithium or VPA alone did not affect relative optical density.
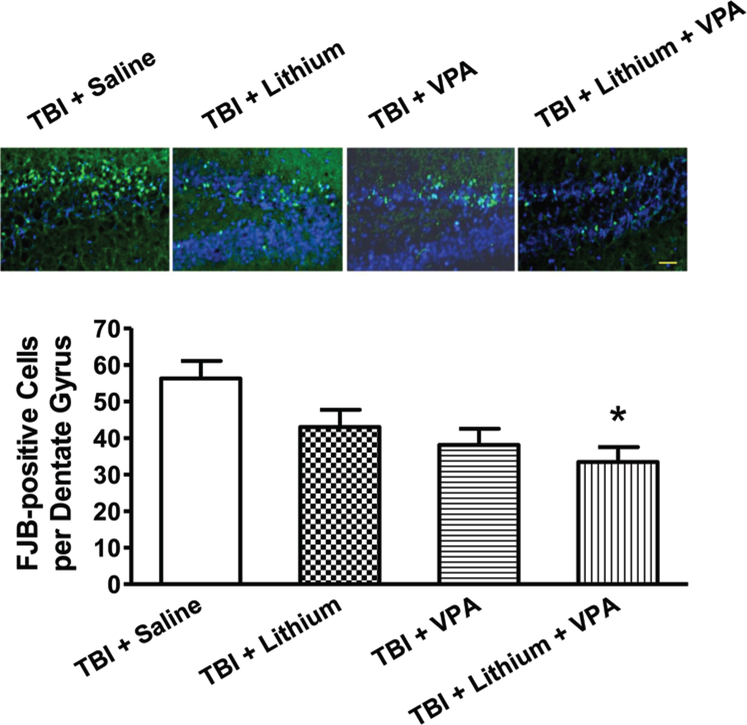
The FJB staining was used to assess TBI-induced neuronal degeneration. Widespread FJB-positive staining was observed in the hippocampal dentate gyrus area. Three days postinjury, the numbers of FJB-positive cells per dentate gyrus were 53 ± 5, 43 ± 5, 38 ± 4, and 33 ± 4 for the saline-, lithium-, VPA-, and combined-treatment groups, respectively. No significant difference was found between the saline-, lithium-, and VPA-treated groups. However, a significant reduction (p < 0.05) in the number of FJB-positive cells was noted in the combined-treatment group (1 mEq/kg lithium and 200 mg/kg VPA) versus the saline-treated group (Fig. 3).
Fig. 3.
Combined treatment with subeffective doses of lithium and VPA reduced the number of degenerating neurons 3 days post-TBI. Neuronal degeneration was evaluated using FJB staining. Representative microphotographs (upper row) showing FJB staining in the hippocampal dentate gyrus 3 days after injury. In the saline-treated group, many FJB-positive cells were visualized. Although fewer FJB-positive cells were observed in response to lithium or VPA treatment alone, the combined treatment group showed the fewest FJB-positive cells. Compared with the saline-treated group, quantitative analysis showed that the number of FJB-positive cells was significantly reduced in the combined treatment group (n = 9/group; *p < 0.05). Nuclei were stained with 4,6-diamino-2-phenylindole, as shown with blue fluorescence. Bar = 50 μm.
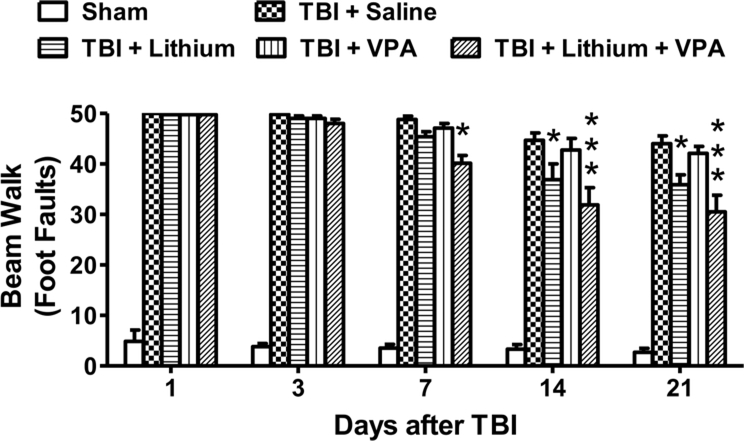
Consistent with our previous findings,25 TBI robustly (p < 0.001) increased the number of foot faults in all groups examined in the beam-walk test (Fig. 4). A trend toward reduction in number of foot faults was observed from 7 to 21 days postinjury in all groups. At 7 days after injury, only combined treatment with lithium and VPA significantly reduced the number of foot faults (saline: 48 ± 3; lithium: 45 ± 3; VPA: 47 ± 3; combined: 40 ± 2, p < 0.05). Fourteen days after injury, lithium treatment (1 mEq/kg) but not VPA treatment significantly reduced the number of foot faults (p < 0.05); notably, combined treatment produced a more robust and consistent effect (p < 0.001) (saline: 45 ± 3; lithium: 37 ± 2; VPA: 43 ± 3; combined: 32 ± 2). Similarly, 21 days after injury, lithium (1 mEq/kg) but not VPA significantly reduced the number of foot faults (p < 0.05). Again, combined treatment appeared to reduce the number of foot faults synergistically (p < 0.001) (saline: 44 ± 3; lithium: 36 ± 3; VPA: 42 ± 3; combined: 31 ± 2).
Fig. 4.
Combined treatment with subeffective doses of lithium and VPA improved beam-walk performance after TBI. The beam-walk test was performed 1, 3, 7, 14, and 21 days after injury to assess motor coordination. TBI markedly increased the number of foot faults in all groups examined. A slow recovery from 7 to 21 days postinjury was seen in all groups. Compared with the saline-treated group, a significant reduction in the number of foot faults was observed in the combined treatment group 7, 14, and 21 days after injury (n = 8/group for all drug treatment groups; n = 6/group for the sham and saline groups; *p < 0.05; ***p < 0.001). Treatment with VPA alone did not affect the number of foot faults at any time point, whereas the lithium-treated group showed a reduced number of foot faults compared with the saline-treated group on Days 14 and 21 after injury.
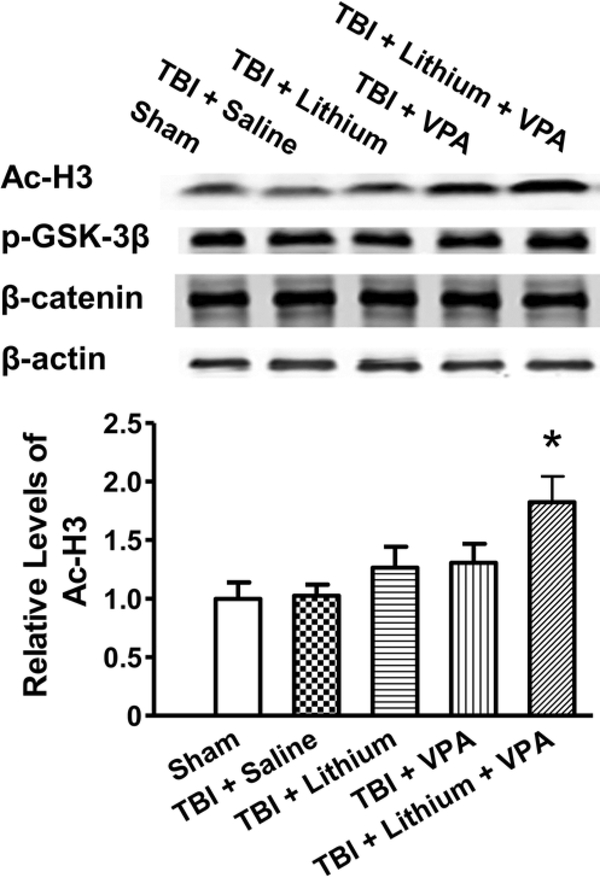
To study the possible mechanisms underlying the effect of this combined therapy, protein levels of Ac-H3, phospho-GSK-3β, and β-catenin in the ipsilateral cortex were measured 3 days after TBI. The Ac-H3 levels remained unchanged in the ipsilateral cortex in the salinetreated group compared with the sham-operated group. After treatment with lithium or VPA alone, levels of Ac-H3 increased slightly, but this increase did not reach statistical significance. In contrast, combined treatment significantly (p < 0.05) increased Ac-H3 levels compared with the sham-operated group by approximately 80% (Fig. 5). Phospho-GSK-3β and β-catenin levels remained unchanged after injury, regardless of drug treatment.
Fig. 5.
Combined treatment with subeffective doses of lithium and VPA increased levels of Ac-H3 3 days after TBI. The Ac-H3 levels remained unchanged in the ipsilateral cortex in the saline-treated group compared with the sham-operated group. After treatment with lithium or VPA alone, levels of Ac-H3 increased slightly. Combined treatment significantly increased Ac-H3 levels compared with the saline-treated group (upper). Quantified data showed a significant increase in Ac-H3 levels after combined treatment compared with the saline-treated group (lower; n = 6/group; *p < 0.05). Levels of phospho-GSK-3β (p-GSK-3β) and β-catenin were unchanged after injury or any treatment. The β-actin level was measured as a loading control.
Discussion
Building on previous work from our laboratory, this study is the first to demonstrate that combined treatment with subeffective doses of lithium and VPA significantly reduced lesion volume and preserved BBB integrity 3 days posttrauma in a mouse model of TBI. Combined treatment also significantly mitigated neurodegeneration in the hippocampal dentate gyrus. Furthermore, this combined treatment synergistically reduced the number of foot faults in a beam-walk motor coordination test 7, 14, and 21 days after TBI. As expected, treatment with subeffective doses of either lithium or VPA had no protective effects. The Ac-H3 levels were synergistically increased by the combined treatment, suggesting that potentiation of VPA-induced HDAC inhibition is the mechanism likely to be involved in these neuroprotective effects.
Both lithium and VPA are mood-stabilizing drugs used to treat bipolar disorder. Combined treatment with these 2 drugs can be prescribed when patients with bipolar disorder are resistant to monotherapy,14 and may be the first-line treatment for some subtypes (for example, patients experiencing rapid cycling or mixed episodes).14 The present study investigated the combination of subeffective doses of lithium and VPA for 3 reasons. First, despite their long history of safe clinical use, long-term lithium or VPA treatment has been associated with significant adverse effects, such as kidney and thyroid toxicity for lithium and liver toxicity for VPA.4 Thus, using subtherapeutic doses of these drugs has the potential advantage of reducing side effects. Second, the complexity of TBI pathogenesis may require a combination of drugs that target different cell survival pathways. In animal models both lithium and VPA have shown considerable beneficial effects in diverse neurodegenerative diseases, including TBI,4 by inhibiting GSK-3 and HDACs, respectively. Both GSK-3 and HDACs are strongly implicated in the pathophysiology of many brain disorders, and are considered promising targets for therapeutic intervention.2,4,5 Finally, previous studies from our laboratory found that combined treatment with lithium and VPA consistently produced additive/synergistic beneficial effects in cultured primary brain neurons and mesenchymal stem cells13,21 as well as in experimental animal models of amyotrophic lateral sclerosis, stroke, and Huntington disease.3,10,22
In the present study we found that subeffective doses of lithium or VPA alone were ineffective in reducing lesion volume or attenuating BBB disruption. In contrast, combined treatment of the same doses of these 2 drugs had significant neuroprotective effects. Although treatment with VPA alone reduced hippocampal neurodegeneration, this difference was not statistically significant compared with the saline-treated group. In contrast, significantly fewer FJB-positive cells were observed in the combined treatment group, indicating that neurodegeneration was attenuated. In the beam-walk test, which measures fine motor coordination, subeffective doses of lithium alone did have some beneficial effects 14 and 21 days after injury; nevertheless, combined treatment had more profound effects, and reduced the number of foot faults at 7, 14, and 21 days post-TBI.
Disruption of the BBB plays an important role in pathogenesis after TBI. The integrity of the BBB is maintained by tight junctions between endothelial cells, the basement membrane, and astrocytic end-feet. The protein MMP-9 digests extracellular matrix and tight junction proteins such as ZO-1 and occludin-5. Lithium (1.5 mEq/kg) has been shown to inhibit TBI-induced MMP-9 overexpression and attenuate BBB disruption,25 and VPA similarly attenuates BBB disruption in a focal cerebral ischemia model via MMP-9 and HDAC inhibition.23 The results of this study suggest that combined treatment with lithium and VPA could have a synergistic effect on MMP-9 inhibition. Additional studies examining the expression and activity of MMP-9 and its downstream targets, such as ZO-1 and occludin-5, could offer insights into any possible benefits of combined treatment with lithium and VPA.
It is interesting to note that a previous study indicated that posttrauma treatment with a subeffective dose of lithium (1 mEq/kg) has no beneficial effect in mice with TBI.28 Another recent study similarly found that this dose of lithium does not reduce lesion volume 3 days postinjury.25 However, the current study found that 1 mEq/kg of lithium improved long-term motor coordination in the beam-walk test 14 and 21 days after TBI. Several reasons may explain this discrepancy. First, it is well recognized that secondary injuries after TBI may last days or even weeks after insult,15 and that continued low-dose lithium treatment may have beneficial effects because of the sustained inhibition of neurodegeneration and neuroinflammation. Second, long-term treatment is necessary for the behavioral benefits of lithium to appear in diverse models of CNS disorders, including TBI.2,26 Finally, measurement of lesion volume alone has certain limitations in determining the extent of injury, and additional measurements such as biochemical and neurological should be considered.12
An in vitro study showed that combined treatment with lithium and VPA protects cerebellar granule cells from glutamate excitotoxicity by potentiating GSK-3 inhibition. The Ac-H3 levels also increase after VPA treatment, and are further potentiated by combined treatment with lithium.13 In transgenic mouse models of Huntington disease, combined treatment with lithium and VPA consistently inhibits GSK-3 and HDACs, and improves behavioral outcomes.3 In the present study, we found that combined treatment with subeffective doses of lithium and VPA was beneficial against TBI, and that the underlying mechanism was probably through enhanced HDAC inhibition, but not GSK-3 inhibition. The discrepancy could be due to the duration of treatment (3 days in this study compared with months in the Huntington disease study). The downstream targets of combined treatment with lithium and VPA include upregulation of brain-derived neurotrophic factor and heat shock protein 70.3 Further studies of long-term treatment with a combination of these 2 agents are warranted to explore the underlying mechanisms and possible involvement of brain-derived neurotrophic factor, heat shock protein 70, and other neuroprotective and/or neurotrophic proteins. Furthermore, because brain-derived neurotrophic factor can modulate neuroplasticity and memory,1 the possibility that long-term combined treatment with lithium and VPA can ameliorate the memory deficits induced by TBI should also be investigated.
Conclusions
Posttrauma treatment with a combination of subeffective doses of lithium and VPA synergistically reduced lesion volume, attenuated BBB disruption, ameliorated hippocampal neurodegeneration, and improved long-term motor coordination. These beneficial effects were probably mediated by potentiating HDAC inhibition. Both lithium and VPA have a long history of safe clinical use. Our findings provide evidence that this combination could putatively be used to treat patients with TBI, with the potential advantage of reduced side effects and enhanced tolerability.
Acknowledgments
The authors gratefully acknowledge the editorial assistance of Ioline Henter (NIMH). They also thank Dr. Oz Malkesman, Laura Tucker, and Dr. Amanda Fu (Center for Neuroscience and Regenerative Medicine) for help with behavioral tests and CCI surgery.
Disclosure
This work was funded in part by the Center for Neuroscience and Regenerative Medicine of the Department of Defense, the Blast Lethality Injury and Research Program, and the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). The authors have no conflicts of interest to disclose, financial or otherwise.
Abbreviations used in this paper:
- Ac-H3
acetylhistone H3
- BBB
blood-brain barrier
- CCI
controlled cortical impact
- FJB
Fluoro-Jade B
- GSK-3
glycogen synthase kinase–3
- HDAC
his-tone deacetylase
- IgG
immunoglobulin G
- MMP-9
matrix metalloproteinase–9
- TBI
traumatic brain injury
- VPA
valproate
References
- 1.Autry AE, Monteggia LM: Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu CT, Chuang DM: Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther 128:281–304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu CT, Liu G, Leeds P, Chuang DM: Combined treatment with the mood stabilizers lithium and valproate produces multiple beneficial effects in transgenic mouse models of Huntington’s disease. Neuropsychopharmacology 36:2406–2421, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu CT, Wang Z, Hunsberger JG, Chuang DM: Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65:105–142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT: Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci 32:591–601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dash PK, Johnson D, Clark J, Orsi SA, Zhang M, Zhao J, et al. : Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS ONE 6:e24648, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dash PK, Orsi SA, Moore AN: Histone deactylase inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience 163: 1–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, et al. : Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS ONE 5:e11383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faul M, Xu L, Wald MM, Coronado VG: Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2010 [Google Scholar]
- 10.Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM: Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience 155:567–572, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox GB, Fan L, Levasseur RA, Faden AI: Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma 15:599–614, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Hall ED, Bryant YD, Cho W, Sullivan PG: Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma 25:235–247, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM: Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci 28:2576–2588, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin D, Mok H, Yatham LN: Polytherapy in bipolar disorder. CNS Drugs 20:29–42, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Loane DJ, Faden AI: Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci 31:596–604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, et al. : Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med 15: 377–379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maas AI, Stocchetti N, Bullock R: Moderate and severe traumatic brain injury in adults. Lancet Neurol 7:728–741, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Margulies S, Hicks R: Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma 26: 925–939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapira M, Licht A, Milman A, Pick CG, Shohami E, EldarFinkelman H: Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci 34:571–577, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Shein NA, Grigoriadis N, Alexandrovich AG, Simeonidou C, Lourbopoulos A, Polyzoidou E, et al. : Histone deacetylase inhibitor ITF2357 is neuroprotective, improves functional recovery, and induces glial apoptosis following experimental traumatic brain injury. FASEB J 23:4266–4275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai LK, Leng Y, Wang Z, Leeds P, Chuang DM: The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology 35:2225–2237, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM: Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke 42:2932–2939, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM: Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 31:52–57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZF, Fessler EB, Chuang DM: Beneficial effects of mood stabilizers lithium, valproate and lamotrigine in experimental stroke models. Acta Pharmacol Sin 32:1433–1445, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, Chuang DM: Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury. J Neurotrauma 29:362–374, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu F, Zhang Y, Chuang DM: Lithium reduces BACE1 overexpression, beta amyloid accumulation, and spatial learning deficits in mice with traumatic brain injury. J Neurotrauma 29:2342–2351, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B, West EJ, Van KC, Gurkoff GG, Zhou J, Zhang XM, et al. : HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res 1226:181–191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu ZF, Wang QG, Han BJ, William CP: Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull 83:272–277, 2010 [DOI] [PubMed] [Google Scholar]