In the FLAURA trial Japanese subset, osimertinib significantly improved median PFS versus standard-of-care (gefitinib) in patients with previously untreated EGFR (exon 19 deletion or L858R) mutation-positive advanced or metastatic NSCLC.
Keywords: clinical trials, lung medicine, thoracic
Abstract
Background
The FLAURA study was a multicenter, double-blind, Phase 3 study in which patients with previously untreated epidermal growth factor receptor mutation-positive advanced non-small-cell lung carcinoma were randomized 1:1 to oral osimertinib 80 mg once daily or standard-of-care (gefitinib 250 mg or erlotinib 150 mg, once daily) to compare safety and efficacy. In the overall FLAURA study, significantly better progression-free survival was shown with osimertinib versus standard-of-care.
Methods
Selected endpoints, including progression-free survival (primary endpoint), overall survival, objective response rate, duration of response and safety were evaluated for the Japanese subset of the FLAURA study.
Results
In Japan, 120 eligible Japanese patients were randomized to osimertinib (65 patients) or gefitinib (55 patients) treatment from December 2014 to June 2017. Median progression-free survival was 19.1 (95% confidence interval, 12.6, 23.5) and 13.8 (95% confidence interval, 8.3, 16.6) months with osimertinib and gefitinib, respectively (hazard ratio, 0.61; 95% confidence interval, 0.38, 0.99). Median overall survival was not reached in either treatment arm (data were immature). In the osimertinib and gefitinib arms, objective response rate was 75.4% (49/65) and 76.4% (42/55), and median duration of response from onset was 18.4 (95% confidence interval, not calculated) and 9.5 (95% confidence interval, 6.2, 13.9) months, respectively. The incidence of adverse events was similar in the two groups. The frequency of Grade ≥3 interstitial lung disease and pneumonitis in the two groups were the same (one patient).
Conclusions
As the first-line therapy, osimertinib showed significantly improved efficacy versus gefitinib in the Japanese population of the FLAURA study. No new safety concerns were raised.
Clinical trial registration
NCT02296125 (ClinicalTrials.gov)
Introduction
First- and second-generation epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are the recommended first-line treatment for EGFR mutation-positive advanced non-small-cell lung cancer (NSCLC) (1,2). While treatment with gefitinib or erlotinib can significantly extend progression-free survival (PFS) compared with combination chemotherapy (3), more than 50% of patients treated with an EGFR-TKI become resistant within the first year of treatment (4–6).
Osimertinib is an oral, third-generation, central nervous system (CNS)-active, irreversible EGFR-TKI that potently and selectively inhibits both EGFR-TKI-sensitizing mutations (EGFRm; exon 19 and 21 mutations) and the T790M resistance mutation (7–11). Osimertinib has recently been approved in the USA and Europe as a first-line treatment for patients with EGFRm advanced NSCLC and for patients with T790M mutation-positive advanced NSCLC (12,13).
In the FLAURA study, a multicenter, double-blind, Phase 3 study with 556 patients with previously untreated EGFR mutation-positive advanced NSCLC, osimertinib was found to be statistically and clinically significant in prolonging PFS compared with gefitinib or erlotinib (18.9 months vs 10.2 months; hazard ratio [HR] for disease progression or death, 0.46; 95% confidence interval [CI], 0.37, 0.57; P < 0.0001) (14).
Based on the previous studies that demonstrated the usefulness of first- and second-generation EGFR-TKIs for the treatment of Japanese patients with EGFR mutation-positive NSCLC (15,16), these are now regarded as standard therapy in the clinical setting in Japan. However, Japanese NSCLC patients have a high rate of EGFR-TKI-sensitizing mutations (17,18). Furthermore, some issues of concern related to EGFR-TKIs remain, such as acquired resistance primarily involving the T790M mutation and the onset of interstitial lung disease (ILD) (19,20).
New first-line treatment options that circumvent the development of EGFR-TKI-sensitizing mutations in the Japanese population are needed to avoid the development of T790M resistance, delay clinical disease progression and improve treatment outcomes. This report describes a predefined subset analysis of the FLAURA study that compares the safety and efficacy of osimertinib versus standard-of-care in Japanese patients.
Materials and methods
Patients
Inclusion and exclusion criteria for the FLAURA study have been previously published (14). In brief, eligible patients had untreated advanced or metastatic NSCLC and were eligible for treatment with gefitinib or erlotinib. Patients with CNS metastases were eligible if their condition was stable or asymptomatic. Patients with locally or centrally confirmed EGFR exon 19 deletion or L858R mutation were eligible for the FLAURA study. Patients who were described as ‘Japanese’ on the case report form and were enrolled in a study site in Japan were included in the subset analyzed in the current study. All patients provided written informed consent.
Study design, treatments and blinding
The FLAURA study design, conducted between December 2014 and June 2017, has previously been published (14). The FLAURA study was conducted in accordance with the principles outlined in the Declaration of Helsinki, Good Clinical Practice and local regulatory requirements and was approved by the institutional review boards or independent ethics committees of the participating study centers. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
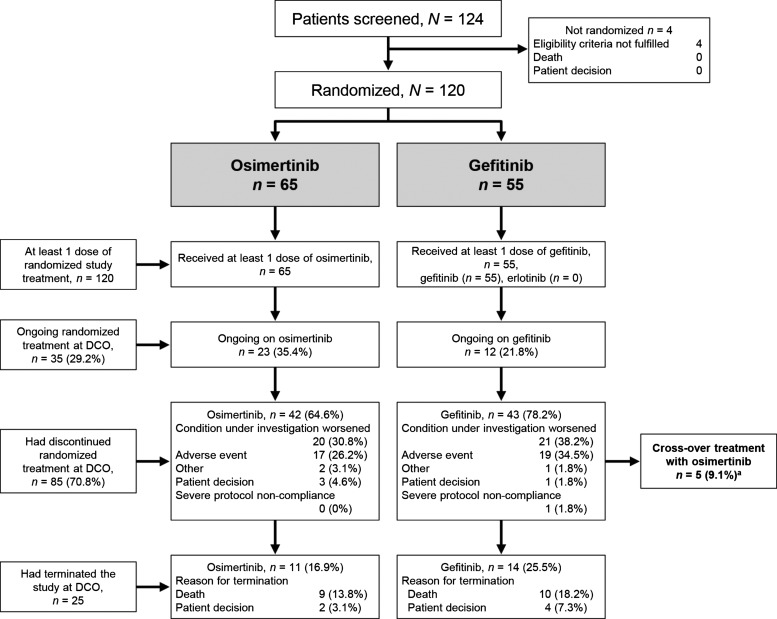
Patients eligible for the FLAURA study were stratified based on EGFR mutation status (EGFR exon 19 deletion or L858R mutation) and race (Asian and non-Asian) and then randomized 1:1 to either oral osimertinib 80 mg once daily or a standard EGFR-TKI (gefitinib 250 mg once daily or erlotinib 150 mg once daily) (Fig. 1). Each study site prespecified the EGFR-TKI to be used for the standard-of-care arm. In Japan, only gefitinib was chosen as the standard-of-care for comparison. The Japanese patients were enrolled from 18 study sites throughout Japan.
Figure 1.
Disposition of the Japanese subset in the FLAURA study. DCO, data cutoff. aAmong patients who requested dispensing of crossover treatment with osimertinib, five patients provided dosing information with osimertinib in the electronic case report form.
After investigator-assessed objective disease progression was confirmed, a protocol amendment allowed patients allocated to the standard-of-care arm to switch to osimertinib treatment if they had radiological disease progression, had not received interventional therapy after discontinuation of their randomized treatment and had the T790M mutation detected in either tissue or circulating tumor DNA collected after disease progression (Fig. 1).
Endpoints
The primary endpoint was the duration of PFS. Disease progression was assessed by the investigator according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Secondary endpoints were overall survival (OS), duration of response (DoR), disease-control rate (DCR), objective response rate (ORR) and safety. Adverse events (AEs) were assessed and graded by the investigator using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Tumors were assessed at baseline, every 6 weeks for the first 18 months and then every 12 weeks until disease progression was detected. Brain imaging was conducted at screening for those with known or suspected CNS metastases, and follow-up scans were conducted for those with confirmed CNS metastases.
The predefined post-progression outcome measures included the time from randomization to second progression or death after the start of subsequent therapy (PFS2), time from randomization to first subsequent therapy or death, time from randomization to discontinuation of treatment or death, and time to second subsequent treatment or death.
Statistical methods
Considering ~359 events of progression or death in a total of 530 randomly assigned patients, the FLAURA study had a 90% power to detect a HR of 0.71 (representing an improvement in median PFS from 10 to 14.1 months) at a two-sided alpha-level of 5%.
Efficacy was assessed in the full analysis set, including all randomized Japanese patients. AEs were assessed in the safety analysis set, which included all patients receiving at least one dose of the assigned treatment. A Kaplan–Meier survival analysis was performed. An adjusted log-rank test was conducted for an across-treatment comparison, stratified by EGFR mutation type (exon 19 deletion vs L858R), in which the Breslow approach was used to handle tied events. HRs were calculated along with 95% CIs. Adjusted analyses were conducted for DCR and ORR using logistic regression analysis stratified by EGFR mutation type (exon 19 deletion vs L858R). All P values are to be considered as nominal in this subgroup analysis, as the study was not powered for the Japanese subset. The data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
In the Japanese subset, a total of 120 eligible Japanese patients were randomized to treatment (osimertinib, 65 [54.2%]; gefitinib, 55 [45.8%]) (Fig. 1). Patients in Japan were randomized between February 2015 and March 2016. Baseline characteristics were well balanced between the two groups (Table 1). Overall, the patients had a median age of 67 years. There was a higher percentage of women in the osimertinib arm (66.2%) than in the gefitinib arm (50.9%). Slightly more than half of the patients were never-smokers (overall, 53.3%; osimertinib, 53.8% and gefitinib, 52.7%).
Table 1.
Patients’ baseline characteristics
| Osimertinib | Gefitinib | |
|---|---|---|
| (n = 65) | (n = 55) | |
| Sex, n (%) | ||
| Male | 22 (33.8) | 27 (49.1) |
| Female | 43 (66.2) | 28 (50.9) |
| Age, years, median (range) | 67.0 (34–82) | 67.0 (43–85) |
| Smoking status, n (%) | ||
| Never-smokers | 35 (53.8) | 29 (52.7) |
| Current/former smokers | 30 (46.2) | 26 (47.3) |
| CNS metastases at study entry, n (%) | 14 (21.5) | 13 (23.6) |
| WHO performance status, n (%) | ||
| 0 (normal activity) | 38 (58.5) | 34 (61.8) |
| 1 (restricted activity) | 27 (41.5) | 21 (38.2) |
| Overall disease classification, n (%) | ||
| Metastatic | 60 (92.3) | 53 (96.4) |
| Locally advanced | 5 (7.7) | 2 (3.6) |
| EGFR mutationa at baseline, n (%) | ||
| Exon 19 deletion | 33 (50.8) | 30 (54.5) |
| L858R | 32 (49.2) | 25 (45.5) |
CNS, central nervous system; EGFR, epidermal growth factor receptor; WHO, World Health Organization.
aEGFR mutations based on the test (local or central) used to determine randomization strata.
All randomized patients received at least one dose of the allocated treatment. The median duration of total treatment exposure was 15.3 months (range 0.5 to 25.5) in the osimertinib group and 11.0 months (range 0 to 25.1) in the gefitinib group.
All disease characteristics were similar in the two treatment groups (Table 1). The majority of patients had metastatic NSCLC (overall, 113 [94.2%]; osimertinib, 60 [92.3%] and gefitinib, 53 [96.4%]), with seven (5.8%) patients overall with locally advanced NSCLC. Overall, most patients had adenocarcinomas (116 [96.7%]), including 11 cases of papillary adenocarcinoma. Twenty-seven (22.5%) patients had a CNS metastasis at baseline (osimertinib, 14 [21.5%]; gefitinib, 13 [23.6%]). Overall, 63 (52.5%) patients had exon 19 deletions, and 57 (47.5%) patients had L858R mutations.
Efficacy
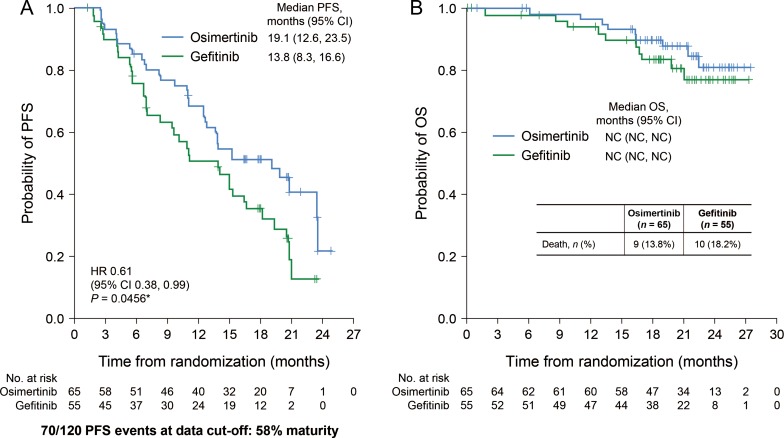
Disease progression occurred in 34 (52.3%) patients in the osimertinib group and 36 (65.5%) in the gefitinib group (Fig. 2A). Median PFS was 19.1 months (95% CI, 12.6, 23.5) in the osimertinib group and 13.8 months (95% CI, 8.3, 16.6) in the gefitinib group, with a HR of 0.61 (95% CI, 0.38, 0.99; P = 0.0456 [nominal P value; this subgroup analysis was not powered for the Japanese subset]). There were nine (13.8%) deaths in the osimertinib group and 10 (18.2%) in the gefitinib group. OS data were immature at the time of this report (Fig. 2B).
Figure 2.
PFS (A) and OS (B) in osimertinib and gefitinib treatment groups. *For reference purposes only. The P value is nominal as the present subgroup analysis was not powered for the Japanese subset analysis. CI, confidence interval; HR, hazard ratio; NC, not calculable; OS, overall survival; PFS, progression-free survival.
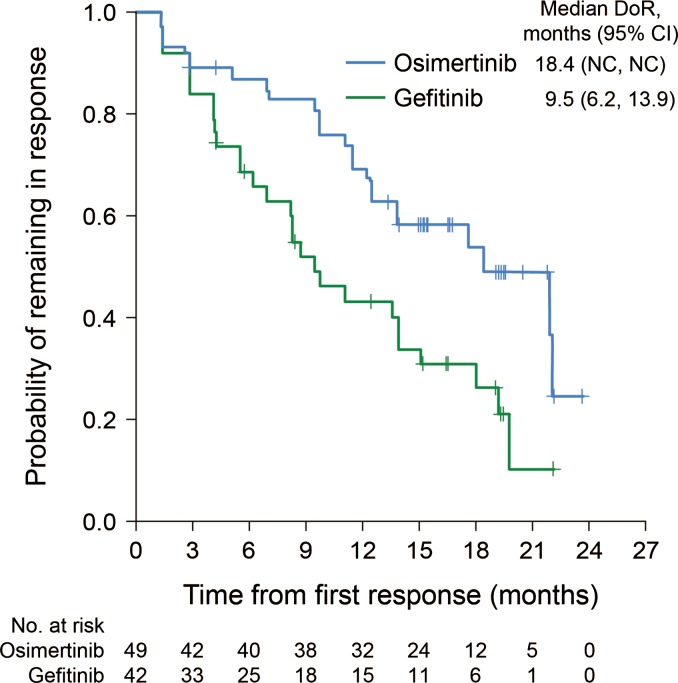
Results of the secondary endpoint analyses are summarized in Table 2. The ORR was similar in the two groups. In the osimertinib group, the ORR was 75.4% (49 of 65), with an adjusted response rate of 76.8%. In the gefitinib group, the ORR was 76.4% (42 of 55), with an adjusted rate of 77.1%. Two patients in the osimertinib group achieved a complete response, while no patients in the gefitinib group achieved a complete response. The median DoR from onset in the osimertinib group was almost twice as long as that in the gefitinib group: 18.4 months (no 95% CI) in the osimertinib group and 9.5 months (95% CI, 6.2, 13.9) for the gefitinib group (Fig. 3). At 6 months, an estimated 87.4% (95% CI, 74.1, 94.1) of patients were remaining in response in the osimertinib group and 69.0% (95% CI, 52.0, 81.1) in the gefitinib group. At 12 months, the numbers were 69.9% (95% CI, 54.5, 81.0) in the osimertinib group and 43.6% (95% CI, 27.4, 58.8) in the gefitinib group. The DCR was 96.9% (63 of 65) in the osimertinib group, with an adjusted DCR of 96.9%. In the gefitinib group, the DCR was 96.4% (53 of 55), with an adjusted DCR of 96.4%. The mean (standard deviation) best percent changes from baseline in target lesion size were −50.0 (25.3) mm and −45.6 (24.2) mm in the osimertinib and gefitinib groups, respectively.
Table 2.
Secondary efficacy endpoints
| Osimertinib | Gefitinib | |
|---|---|---|
| (n = 65) | (n = 55) | |
| OS, % (95% CI) | ||
| Survival at 12 months | 96.8 (87.7, 99.2) | 94.2 (83.0, 98.1) |
| Survival at 18 months | 90.1 (79.4, 95.5) | 83.7 (69.9, 91.5) |
| Objective responsea | ||
| Complete response, n (%) | 2 (3.1) | 0 |
| Partial response, n (%) | 47 (72.3) | 42 (76.4) |
| Adjusted response rateb, % | 76.8 | 77.1 |
| Odds ratio (95% CI) | 0.98 (0.41, 2.32) | |
| Median DoR from onsetc,d, months (95% CI) | 18.4 (NC, NC) | 9.5 (6.2, 13.9) |
| Disease controla | ||
| Patients under control, n (%) | 63 (96.9) | 53 (96.4) |
| Adjusted control rateb, % | 96.9 | 96.4 |
| Odds ratio (95% CI) | 1.19 (0.14, 10.23) | |
| Best percent change from baseline in target lesion size, unadjusted mean (SD) | −50.0 (25.3) | −45.6 (24.2) |
CI, confidence interval; EGFR, epidermal growth factor receptor; NC, not calculable; OS, overall survival; SD, standard deviation; DoR, duration of response.
aBased on investigator assessment.
bAdjusted using logistic regression analysis stratified by EGFR mutation type (exon 19 deletion vs L858R).
cDoR was the time from the first documentation of response until the date of progression or death in the absence of progression.
dCalculated using the Kaplan–Meier method.
Figure 3.
DoR in osimertinib and gefitinib treatment groups. CI, confidence interval; NC, not calculable; DoR, duration of response.
Of the 120 patients in the Japanese subset, 85 patients discontinued study treatment; 61 of 85 (71.8%) patients received several post-investigational anticancer therapies, including radiotherapy, and 58 of 85 (68.2%) patients had at least one post-investigational anticancer therapy. Eleven (20.0%) patients in the gefitinib group (including the five patients who were switched to open-label osimertinib) received osimertinib treatment as a post-investigational treatment therapy; and although no patients in the osimertinib group received osimertinib as a first post-treatment therapy, two (3.1%) patients in the osimertinib group received further osimertinib as a later line of therapy.
In the Japanese subset, there was a delay in the PFS2 in the osimertinib arm compared with the gefitinib group (HR, 0.73 [95% CI, 0.36, 1.48]). The median PFS2 values for osimertinib could not be calculated. Treatment with osimertinib was associated with a delay in the initiation of the first subsequent therapy or death (HR, 0.68 [95% CI, 0.41, 1.12]), a prolongation of the time from randomization to discontinuation of treatment or death (15.3 [95% CI, 11.6, 22.0] months vs 11.0 [95% CI, 5.3, 14.5] months in the osimertinib and gefitinib groups, respectively), and a prolongation of the time to second subsequent treatment or death (HR, 0.86 [95% CI, 0.45, 1.64]).
Safety
Among the Japanese subset of the FLAURA study, AEs were noted in 118 (98.3%) patients (Table 3). AEs Grade 3 or higher were reported in 31 (47.7%) patients in the osimertinib group and 31 (56.4%) patients in the gefitinib group. Serious AEs were reported in 14 (21.5%) patients in the osimertinib group and 12 (21.8%) patients in the gefitinib group. Only one AE leading to death was reported (in the gefitinib group). This was a case of endocarditis not considered related to treatment. AEs leading to discontinuation were reported in 17 (26.2%) patients in the osimertinib group and 19 (34.5%) patients in the gefitinib group.
Table 3.
Adverse events
| Osimertinib | Gefitinib | |
|---|---|---|
| (n = 65) | (n = 55) | |
| AE, any cause, n (%) | ||
| Any AE | 65 (100) | 53 (96.4) |
| Any AE Grade ≥3 | 31 (47.7) | 31 (56.4) |
| Any AE leading to death | 0 | 1 (1.8) |
| Any serious AE | 14 (21.5) | 12 (21.8) |
| Any AE leading to discontinuation | 17 (26.2) | 19 (34.5) |
| AE possibly causally related, n (%) | ||
| Any AE possibly causally related | 64 (98.5) | 53 (96.4) |
| Any AE possibly causally related Grade ≥3 | 18 (27.7) | 27 (49.1) |
| Any AE possibly causally related leading to death | 0 | 0 |
| Any possibly causally related serious AE | 12 (18.5) | 8 (14.5) |
AE, adverse event.
Dermatitis acneiform was more common in the gefitinib group, affecting 30 (46.2%) patients in the osimertinib group and 38 (69.1%) in the gefitinib group. Increases in liver enzymes were also more common in the gefitinib group. Aspartate aminotransferase increased was reported in seven (10.8%) patients in the osimertinib group and 25 (45.5%) in the gefitinib group. Alanine aminotransferase increase was reported in five (7.7%) patients in the osimertinib group and 27 (49.1%) in the gefitinib group. White blood cell (WBC) count decrease, QTc prolongation, ILD and pneumonitis were all more common in the osimertinib group. WBC count decrease was reported in 14 (21.5%) patients in the osimertinib group and one (1.8%) in the gefitinib group. QTc prolonged was reported in 14 (21.5%) and 5 (9.1%) patients in the osimertinib and gefitinib groups, respectively. There were no safety concerns identified in the underlying laboratory data. In the osimertinib group, the events of QTc prolongation and WBC count decrease were not associated with arrhythmias or infections. ILD and pneumonitis were reported in eight (12.3%) patients in the osimertinib group and one (1.8%) in the gefitinib group. The number of patients with Grade 1–2 ILD and pneumonitis reported was seven (10.8%) and zero in the osimertinib and gefitinib groups, respectively. The number of patients with Grade ≥3 ILD and pneumonitis reported was the same in both groups (one patient [2%] each). In most cases, no symptoms were reported, and these were the only radiological findings. In both arms, all patients with ILD discontinued treatment. All ILD events in the osimertinib arm were reported as recovered and patients were subsequently treated with another EGFR-TKI (Supplementary data, Table S1).
The number of dose interruptions was similar in the two treatment groups. Dose interruption due to an AE occurred in 26 (40.0%) patients in the osimertinib group and 25 (45.5%) in the gefitinib group. Dose reduction due to an AE occurred in nine (13.8%) patients in the osimertinib group and seven (12.7%) in the gefitinib group.
Discussion
The overall FLAURA study demonstrated that osimertinib had superior efficacy as a first-line treatment compared with treatment with gefitinib or erlotinib, without raising new safety concerns (14). The reduction in the risk of progression or death in the Japanese subset was consistent with that in the overall population (14). The PFS Kaplan–Meier curves for the Japanese subset show a clear separation between treatment arms within 3 months, which remain separated for the duration of the follow-up. Although PFS in the gefitinib group in the Japanese subset was longer than expected based on historical data (Supplementary data, Table S2), patients in the Japanese subset treated with osimertinib had a clinically meaningful improvement in PFS compared with the gefitinib group: median PFS was 5.3 months longer (osimertinib, 19.1 months [95% CI, 12.6, 23.5] vs gefitinib, 13.8 months [95% CI, 8.3, 16.6]). The HR for the Japanese subset was 0.61 (95% CI, 0.38, 0.99), indicating a 39% reduction in the risk of disease progression or death in the absence of RECIST progression in the osimertinib group compared with the gefitinib group. Afatinib was not included as a comparator in the FLAURA study; however, a recent meta-analysis concluded there is no difference in efficacy between afatinib, gefitinib and erlotinib in NSCLC (21). Historical studies of first-, second- and third-generation EGFR-TKIs show osimertinib has a superior efficacy over first- and second-generation EGFR-TKIs (Supplementary data, Table S2) (22–24).
A numerical improvement in OS in favor of osimertinib was observed in the Japanese subset and was consistent with that seen in the overall population. High responses rates (>75%) were seen in both arms, with a large majority of responses being partial responses. The responses in the osimertinib arm were durable: more patients treated with osimertinib remained in response at 6 months and beyond than patients treated with gefitinib. The improvement in DoR with osimertinib in the Japanese subset was consistent with the overall population.
The prespecified post-progression outcome measures were included in this study because the OS data were expected to be immature at the time of the primary PFS analysis. These measures provide information on whether any observed PFS advantage could be maintained during the administration of subsequent anticancer therapies. They also may provide preliminary evidence of any detrimental effect beyond PFS that could potentially be associated with a poorer OS, such as long-term toxicity, biological changes leading to a more aggressive disease or development of resistance that would affect the efficacy of the next line of therapy. Although the patient numbers are small, and the data are immature, all post-progression outcomes favor the osimertinib treatment group.
A delay was observed in the PFS2 in the osimertinib arm compared with the gefitinib group. This might have been because of the benefit accrued from the time from randomization to first progression (PFS). It might also be because of the limited number of PFS2 events among Japanese patients. Therefore, interpretation of the PFS2 results can be difficult and requires caution.
Treatment with osimertinib showed associations with a delay in terms of prolongation of the time to discontinuation of treatment or death and prolongation of the time to second subsequent treatment or death. These results suggest that the treatment benefit provided by osimertinib in prolonging PFS is maintained with longer follow-up and during subsequent therapy.
The tolerability and safety profile of osimertinib in the Japanese subset was generally consistent with that seen in the overall population (14). No new safety issues were identified for osimertinib in the Japanese subset. The safety findings of osimertinib in the Japanese subset were consistent with the known safety profile of osimertinib characterized in the AURA studies (11). While the frequency of Grade 1–2 ILD and pneumonitis was higher in the osimertinib group versus the gefitinib group, the frequency of Grade ≥3 ILD and pneumonitis in the osimertinib group was the same as that in the gefitinib group. The pattern of AEs reported in the treatment groups are as expected for a population of patients receiving an EGFR-TKI as first-line treatment for advanced NSCLC.
The incidence of AEs was broadly similar in the two treatment groups in the Japanese subset, despite the longer exposure in the osimertinib arm. Rates of Grade ≥3 AEs were lower in the osimertinib group than in the gefitinib group. Common AEs, including diarrhea, paronychia, stomatitis, dermatitis acneiform, dry skin and decreased appetite were reported in at least 20% of patients in both treatment groups. Dermatitis acneiform was more common in the gefitinib group, likely because osimertinib has higher selectivity against wild-type EGFR than gefitinib. Increases in liver enzymes were also more common in the gefitinib group. In the osimertinib arm, these AEs were generally mild, with only three Grade 3 or higher events. In the gefitinib group, 15 Grade 3 or 4 hepatic-related AEs were reported. ILD, QTc prolongation and decreases in WBC counts were all more common in the osimertinib group but still infrequent and generally Grade 1 or 2. The events of QTc prolongation and decreases in WBC counts reported in the osimertinib group were not associated with clinical sequelae such as arrhythmias or infections. Laboratory data for QTc prolongation and WBC count decrease in the Japanese subset were consistent with that observed in the overall population (14). In the Japanese subset, comparison of the safety profiles between the two treatment arms in FLAURA confirms that osimertinib is generally well tolerated and has an acceptable safety profile compared with gefitinib.
In Japan, EGFR-TKI therapy is currently recommended for first-line treatment of EGFRm advanced NSCLC, but patients treated with first- and second-generation EGFR-TKIs typically develop resistance due to T790M mutations. In contrast, osimertinib can effectively target T790M mutations. The availability of osimertinib as a first-line treatment for patients with EGFR exon 19 deletions or L858R mutations could prevent resistance and provide potent and durable targeting of EGFR mutations.
Future studies are needed to address the mechanisms of resistance to osimertinib. As the PFS survival curves separate within the first 3 months of treatment, the initial evidence suggests that resistance to osimertinib does not arise early in treatment as it does in some patients receiving gefitinib.
The T790M mutation arises in about 50–60% of patients treated with standard EGFR-TKIs. Twenty-eight Japanese patients in the gefitinib group had a first post-investigational treatment in the FLAURA study. Of these, about 14–17 would be expected to have the T790M mutation. Eleven (20.0%) patients in the gefitinib group received osimertinib treatment as a post-investigational treatment therapy. Thus, the number of patients who received osimertinib as post-therapy in the gefitinib group was reasonable.
The FLAURA study was not powered for the subanalysis of the Japanese patients; thus, the P value presented in this report is for reference purposes only. Although OS analysis was performed, the HR could not be evaluated because the data were immature. The number of patients with CNS metastases in the Japanese subset did not reach the minimum threshold for a separate analysis. Furthermore, brain imaging was limited to patients with known or suspected CNS metastases, so the study did not assess asymptomatic CNS metastases. The study does not compare osimertinib as a first-line treatment to sequential treatment with gefitinib followed by osimertinib. However, the treatment effect of osimertinib on PFS observed in this study suggests that starting treatment with osimertinib could potentially be more effective than sequential EGFR-TKIs.
As a first-line therapy, osimertinib demonstrated a clinically meaningful improvement in efficacy versus gefitinib in the Japanese subset of the FLAURA study. The safety profile in this study was consistent with the known AEs characteristic of respective EGFR-TKIs (such as low-grade ILD-like events and QTc prolongation in the osimertinib group and hepatic and skin toxicities in the gefitinib group). These data support osimertinib as a first-line treatment for Japanese patients with EGFR mutation-positive metastatic NSCLC.
Supplementary Material
Acknowledgements
The authors are grateful to all of the patients and families involved in this study. The authors thank all of the principal investigators: Toshiaki Takahashi from Shizuoka Cancer Center, Shizuoka, Japan; Kazuo Kasahara from Kanazawa University Hospital, Kanazawa, Japan; Kiyotaka Yoh from National Cancer Center Hospital East, Chiba, Japan; Masaharu Shinkai from Yokohama City University Medical Center, Kanagawa, Japan; Tsuneo Shimokawa from Yokohama Municipal Citizen’s Hospital, Kanagawa, Japan; Nobuyuki Katakami from Institute of Biomedical Research and Innovation Hospital, Hyogo, Japan; Makoto Maemondo from Miyagi Cancer Center, Miyagi, Japan; Shuji Murakami from Kanagawa Cancer Center, Kanagawa, Japan; Shinji Atagi from National Hospital Organization Kinki-chuo Chest Medical Center, Osaka, Japan; and Noriyuki Masuda from Kitasato University Hospital, Kanagawa, Japan. The authors thank Susan Cottrell, PhD, of Edanz Medical Writing for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice three guidelines (www.ismpp.org/gpp3). The results of this study have previously been presented at ESMO 2017 and the Japan Lung Cancer Society 2017 conference.
Funding
This work was supported by AstraZeneca. Osimertinib is being developed by AstraZeneca. The study sponsor contributed to the study design; the data collection, analysis and interpretation; and the decision to submit the article for publication.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest statement
This study and the preparation of the manuscript were funded by AstraZeneca. Hirohiko Uchida, Rachel Hodge, Sarah L. Vowler, and Andrew Walding declare consultancy for AstraZeneca. Yuichiro Ohe, Fumio Imamura, and Shunichi Sugawara declare honoria from AstraZeneca. Yuichiro Ohe, Fumio Imamura, Naoyuki Nogami, Isamu Okamoto, Takayasu Kurata, Terufumi Kato, Shunichi Sugawara, Suresh S. Ramalingam, and Kazuhiko Nakagawa have received research funding from AstraZeneca. The remaining authors declare no conflicts of interest.
References
- 1. Hanna N, Johnson D, Temin S, et al. . Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 2017;35:3484–515. [DOI] [PubMed] [Google Scholar]
- 2. Novello S, Barlesi F, Califano R, et al. . Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1–27. [DOI] [PubMed] [Google Scholar]
- 3. Lee CK, Davies L, Wu YL, et al. . Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J Natl Cancer Inst 2017;109: 10.1093/jnci/djw279. [DOI] [PubMed] [Google Scholar]
- 4. Oxnard GR, Arcila ME, Sima CS, et al. . Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu HA, Arcila ME, Rekhtman N, et al. . Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenkins S, Yang JC, Ramalingam SS, et al. . Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol 2017;12:1061–70. [DOI] [PubMed] [Google Scholar]
- 7. Yang JC, Ahn MJ, Kim DW, et al. . Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 2017;35:1288–96. [DOI] [PubMed] [Google Scholar]
- 8. Ballard P, Yates JW, Yang Z, et al. . Preclinical comparison of osimertinib with Other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130–40. [DOI] [PubMed] [Google Scholar]
- 9. Cross DA, Ashton SE, Ghiorghiu S, et al. . AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mok T, Ahn MJ, Hanet JY, et al. . CNS response to osimertinib in patients (pts) with T790M-positive advanced NSCLC: data from a randomized phase III trial (AURA3). J Clin Oncol 2017;35:9005. [Google Scholar]
- 11. Mok TS, Wu Y-L, Ahn M-J, et al. . Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA TAGRISSO (osimertinib). Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208065s008lbl.pdf (26 April 2018, date last accessed).
- 13. EMA Tagrisso (osimertinib). Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004124/WC500202022.pdf (June 2018, date last accessed).
- 14. Soria JC, Ohe Y, Vansteenkiste J, et al. . Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 15. Maemondo M, Inoue A, Kobayashi K, et al. . Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 16. Mitsudomi T, Morita S, Yatabe Y, et al. . Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- 17. Han B, Tjulandin S, Hagiwara K, et al. . EGFR mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: The IGNITE study. Lung Cancer 2017;113:37–44. [DOI] [PubMed] [Google Scholar]
- 18. Liu L, Liu J, Shao D, et al. . Comprehensive genomic profiling of lung cancer using a validated panel to explore therapeutic targets in East Asian patients. Cancer Sci 2017;108:2487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ando M, Okamoto I, Yamamoto N, et al. . Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2006;24:2549–56. [DOI] [PubMed] [Google Scholar]
- 20. Kudoh S, Kato H, Nishiwaki Y, et al. . Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008;177:1348–57. [DOI] [PubMed] [Google Scholar]
- 21. Batson S, Mitchel SA, Windisch R, et al. . Tyrosine kinase inhibitor combination therapy in first-line treatment of non-small-cell lung cancer: systematic review and network meta-analysis. Onco Targets Ther 2017;10:2473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang JC, Schuler MH, Yamamoto N, et al. . LUX-Lung 3: a randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J Clin Oncol 2012;30: 10.1200/jco.2012.30.18_suppl.lba7500. [DOI] [Google Scholar]
- 23. Wu YL, Zhou C, Hu CP, et al. . Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- 24. Park K, Tan EH, O’Byrne K, et al. . Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.