OsPIP1;2 overexpression enhances rice growth and grain yield by facilitating leaf CO2 diffusion, which increases the net CO2 assimilation rate and phloem sucrose transport.
Keywords: Aquaporin, elevated CO2, grain yield, mesophyll conductance, rice, sucrose
Abstract
Aquaporins are involved in CO2 transport from the leaf intercellular air space to the chloroplast, which contributes to CO2 assimilation. However, the mechanism of CO2 transport by rice (Oryza sativa L.) aquaporins is unknown. Here, we investigated the function of the aquaporin OsPIP1;2 in CO2 diffusion-associated photosynthesis and phloem sucrose transport. Moreover, the grain yield of rice lines overexpressing OsPIP1;2 was determined. OsPIP1;2 was localized to the plasma membrane and the relative expression of OsPIP1;2 was approximately 5-fold higher in leaves in the presence of an elevated CO2 concentration. Overexpression of OsPIP1;2 increased mesophyll conductance by approximately 150% compared with wild-type (WT) rice. The OsPIP1;2-overexpressing lines had higher biomass than the WT, possibly due to increased phloem sucrose transport. In addition, the grain yield of OsPIP1;2-overexpressing lines was approximately 25% higher than that of the WT in three-season field experiments, due to the increased numbers of effective tillers and spikelets per panicle. Our results suggest that OsPIP1;2 modulates rice growth and grain yield by facilitating leaf CO2 diffusion, which increases both the net CO2 assimilation rate and sucrose transport.
Introduction
The atmospheric CO2 concentration ([CO2]) has increased significantly from 318 to >400 ppm since 1959 (Meinshausen et al., 2011). The rate of increase in atmospheric [CO2] may accelerate (Tripati et al., 2009), and is predicted to reach 550–700 ppm by 2050 (Meinshausen et al., 2011). Therefore, the effect of elevated [CO2] on crop production has been intensively investigated in recent decades (Kimball, 2016). Rice (Oryza sativa L.) is a major staple food crop for almost half the global population (Kurai et al., 2011). The yield of various rice cultivars is reportedly improved by an elevated [CO2], as indicated by increased growth, tiller number, and leaf area (Kimball, 2016; Hasegawa et al., 2013). An elevated [CO2] had a positive effect on leaf gas exchange and net photosynthetic rate (Norby et al., 2016), and thus is important for plant growth and development.
In general, CO2 entering chloroplasts must pass through leaf stomata, plasma membranes, cytoplasm, and chloroplast membranes; these steps are collectively reflected by stomatal conductance (gs) and leaf mesophyll conductance to CO2 (gm) (Evans and Loreto, 2000; Evans et al., 2009). In rice, gs is a limiting factor in photosynthesis (Kusumi et al., 2012), and enhanced gs increased biomass in Arabidopsis (Wang et al., 2014). Although gm was long considered to be constant, it is now known to vary according to environmental conditions (Montpied et al., 2009; Singh et al., 2014). Flexas et al. (2008) reported that gm is an important determinant of the photosynthetic rate, indicating that CO2 diffusion from the leaf intercellular air space to the chloroplast is a limiting factor in photosynthesis. Aquaporin NtAQP1 from tobacco leaf facilitates CO2 transport across the plasma membrane in vivo, which in turn modulates membrane permeability to CO2 and mesophyll conductance (Uehlein et al., 2003). In addition, NtAQP1 is located in the inner chloroplast membrane, and reduced expression of NtAQP1 resulted in a 20% decrease in CO2 conductance (Uehlein et al., 2008). By contrast, overexpression of NtAQP1 in tobacco significantly increased gm. In Arabidopsis, T-DNA insertion of atpip1;2 reduced leaf CO2 conductivity, indicating that AtPIP1;2 facilitates CO2 transport (Heckwolf et al., 2011). Moreover, overexpression of the barley aquaporin HvPIP2;1 in rice plants resulted in an increased gm value (Hanba et al., 2004).
OsPIP1;2 is a plasma membrane intrinsic protein (PIP) localized to cellular plasma membranes (Lian et al., 2006). Sakurai et al. (2005) reported that rice plants possess 33 aquaporin-encoding genes and stop-flow spectrophotometry analysis revealed that OsPIP2;4 and OsPIP2;5, but not OsPIP1;2, have high water-channel activity. In addition, OsPIP1 members are localized mainly to mesophyll cells (Sakurai et al., 2005). According to the sequence homology of PIP genes in various plant species, the role of OsPIP1;2 may be related to CO2 diffusion in rice plants; however, the evidence is sparse. In this study, we evaluated the function of OsPIP1;2 in CO2 permeability using OsPIP1;2-overexpressing (OE) rice lines under ambient and elevated [CO2] by determining the biomass, photosynthesis-related physiological parameters, and phloem sucrose-transport rate. Moreover, the contribution of OsPIP1;2 to rice yield was analysed in a field experiment. We aimed to determine the function of OsPIP1;2 in rice plants and its potential for agriculture.
Materials and methods
Plant growth conditions
Rice seeds were sterilized as described by Zhu et al. (2009) for hydroponic experiments. After 10 d, seedlings of OE lines and the wild type (WT) were transplanted into 7-litre plastic containers. Rice plants were grown in a growth chamber (Saifu DRX-680E-DG-CO2, Ningbo, China) under a light intensity of 300 μmol m−2 s−1 at shoot height, a relative humidity of ca 70%, and a 14 h light (26 °C)/10 h dark (22 °C) photoperiod. Each experiment was randomized and involved three replicates of five plants each at ambient [CO2] (400 μmol mol−1) and elevated [CO2] (800 μmol mol−1). The nutrient solution contained: 1.25 mM NH4NO3, 0.3 mM K2SO4, 0.3 mM NaH2PO4, 1 mM CaCl2, 1 mM MgSO4, 9 μM MnCl2, 0.39 μM Na2MoO4, 20 μM H3BO4, 0.77 μM ZnSO4, 0.32 μM CuSO4, and 20 μM EDTA-Fe. Nutrient solution was exchanged every 3 d and its pH was maintained at 5.5. The expression level of OsPIP1;2 was determined in cv. Nipponbare at the tillering, booting, flowering, and grain-filling stages grown in soil pots from June to October 2016; three replications were used.
Homology modeling and sequence alignment
We performed homology modeling using the workspace at the Swiss-Model website (http://swissmodel.expasy.org/). The X-ray crystal structures of SoPIP2;1 (Protein Data Bank [PDB] codes: 2D5F and 1Z98) served as templates for homology modeling (Törnroth-Horsefield et al., 2006). The amino acid sequence of aquaporin OsPIP1;2 was aligned using Jalview software version 1.6 (http://www.jalview.org/).
Construction of OsPIP1;2-transgenic rice plants
For β-glucuronidase (GUS) expression analysis, the OsPIP1;2 (Os04g47220) promoter (1977 bp) was amplified from rice (Oryza sativa L. cv Nipponbare) genomic DNA using the primers listed in Supplementary Table S1 at JXB online. The fragment was ligated into the SalI/KpnI sites of the vector pS1aG3 to replace the cauliflower mosaic virus 35S promoter (Tang et al., 2012). The open reading frame (ORF) sequence of OsPIP1;2 was amplified using the primers listed in Supplementary Table S1. Generation of the OsPIP1;2-OE vector was described by Patron et al. (2015).
Histochemical localization of GUS expression
Histochemical analysis was performed as described previously (Ai et al., 2009). Leaves and roots of rice plants were collected in triplicate after 3 weeks. Inflorescences were selected prior to flowering, and seeds were selected 30 d after pollination. Tissues were immersed in GUS reaction mix for 30 min and subsequently incubated at 37 °C for 2 h. GUS-stained tissues were visualized using an Olympus BX51T stereomicroscope equipped with a color charge-coupled device camera.
Transient expression of OsPIP1;2 and fluorescence microscopy
The ORF of OsPIP1;2 without the stop codon was cloned into the C-terminus of the pCAMBIA (GFP) vector at HindIII/PstI sites. Next, the 35S::OsPIP1;2::GFP expression vector was transferred into rice protoplasts using polyethylene glycol-mediated transformation. Rice protoplasts were obtained from etiolated seedlings and transfected as described previously (Jia et al., 2011). OsMCA1 was used as a plasma membrane localization marker (Takamitsu et al., 2012). A confocal laser scanning microscope (LSM410; Carl Zeiss, Oberkochen, Germany) was used to obtain fluorescence images.
Reverse transcription-polymerase chain reaction and real-time quantitative PCR
To investigate the expression pattern of OsPIP1;2, samples were taken from rice plants at different growth stages (Yamaji et al., 2013). To determine the expression level of OsPIP1;2 in leaf and the effect of CO2 on its expression, rice seedlings (2 weeks old) were exposed to ambient [CO2] (400 μmol mol−1) or elevated [CO2] (800 μmol mol−1) for 1 week in a growth chamber (Saifu DRX-680E-DG-CO2).
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). OsPIP1;2 and OsActin were subjected to reverse transcription-polymerase chain reaction (RT-PCR) and real-time quantitative RT-PCR using the primers in Supplementary Tables S2 and S3 and the protocol of Zeng et al. (2012).
Field experiments
Transgenic T4- and T6-generation rice plants were cultivated in plots at the Nanjing Agricultural University experimental site from June to October in 2016 and 2017. T5-generation rice plants were grown in plots of the experimental site of Sanya Nanjing Agricultural University (tropical climate) from December 2016 to April 2017. The soil at the experimental site contained 29.42 g kg−1 organic carbon, 25.78 mg kg−1 Olsen-P, and 140 mg kg−1 exchangeable potassium. The pH of the soil was 6.4. Nitrogen (urea, 200 kg ha−1), phosphorus (P2O5, 90 kg ha−1), and potassium (K2O, 150 kg ha−1) were applied for the present experiment. Nitrogen fertilizer was split into basal dressing, panicle initiation, and initial spikelet differentiation during the growing season at a ratio of 50:30:20. Phosphorus and potassium fertilizer was applied only as a basal dressing prior to transplanting. The field experiment was set up in triplicate randomized plots of 2 × 2.5 m. Four randomly selected samples were taken from plants in each plot at the flowering and harvest stages, and the data for 12 plants were calculated. Effective tiller number, spikelets per panicle, and grain yield were calculated at the harvest stage.
Gas exchange and chlorophyll fluorescence measurements
The LI-6400 system (LI-COR, Lincoln, NE, USA) was used for measuring gas exchange and chlorophyll fluorescence in plants grown in the growth chamber and the field. The temperature of the leaf chamber was maintained at 25 °C, with a photosynthetically active radiation (PPFD) of 1500 μmol m−2 s−1. The ambient CO2 concentration was adjusted to 400 μmol m−2 s−1. The relative humidity in the leaf chamber was maintained at 50–60%. After equilibration to a steady state, the gas-exchange parameters, steady-state fluorescence (Fs), and maximum fluorescence (Fm′) were recorded. For Anet–Ci curves, the leaf was adjusted to 1500 μmol m−2 s−1 PPFD, 400 μmol CO2 mol−1, and 25 °C. The relative humidity in the leaf chamber was maintained at 50–60%. Before the measurement, three rice leaves were heated until they did not have chlorophyll fluorescence. They were used to correct the leakage of the measured Anet–Ci curves (Flexas et al., 2007). Measurements were started when the net rate of CO2 assimilation became constant at 400 μmol CO2 mol−1 under saturating light (1500 μmol m−2 s−1). The ambient [CO2] was increased stepwise from 50 to 1000 μmol CO2 mol−1 at intervals of 20 min. After dark adaptation for 30 min, light-response curves were recorded in steps of more than 20 min duration (stable statue) at nine PPFDs (0, 50, 100, 150, 200, 400, 600, 1000, and 1500 μmol m−2 s−1). The measurement conditions were as described above, except that the light intensity was increased stepwise. Newly and fully expanded leaves were selected for measurement at 09.00–15.00 h daily.
Efficiency of photosystem II electron transport (ΦPSII) was calculated as ΦPSII=1−Fs/Fm′. The electron transport rate (J) was calculated as follows:
| (1) |
Where α is leaf absorption and β is the proportion of quanta between PSI and PSII. The product αβ was obtained by varying the PPFDs under non-photorespiratory conditions in the presence of less than 2% O2 (Valentini et al., 1995). The gm and chloroplast CO2 concentration (Cc) were calculated by the variable J method (Harley et al., 1992) as follows:
| (2) |
| (3) |
where A is net rate of CO2 assimilation, Ci is the substomatal CO2 concentration and Г* is the CO2 compensation point in the absence of respiration. In the present study, a Г* value of 40 μmol mol−1, typical for rice plants, was used based on Franks and Farquhar (2001) and Giuliani et al. (2013). Daytime respiration rate (Rd) was calculated as the intercept of the linear regression of the photosynthetic rate against PPFD×ΦPSII/4 using light-response curve data (Yin et al., 2009). The Rd values of transgenic plants are listed in Supplementary Fig. S1.
A curve-fitting method (Ethier and Livingston, 2004; Ethier et al., 2006) was used to calculate the maximum Rubisco activity (Vcmax) and maximum electron transport rate (Jmax) as described by Sharkey et al. (2007).
The leaf net rate of CO2 assimilation (Anet) in the paddy field was measured on August 10–11 (flowering stage), August 20–21 (middle grain-filling stage), and September 4–5 (end grain-filling stage). The temperature of the leaf chamber was maintained at 25 °C, with a PPFD of 1500 μmol m−2 s−1. The relative humidity in the leaf chamber was maintained at 50–60%. Four random samples from three plots per group (n=12) were selected for measurement. Newly and fully expanded leaves were selected for measurement at 09.00–11.00 h daily.
Measurement of leaf chlorophyll content, stomatal density, stomatal size, relative water content, dry mass per unit area and water use efficiency
Chlorophyll was extracted with ethanol and quantified using a SpectraMax M5 spectrometer (Molecular Devices, Sunnyvale, CA, USA) (Sartory and Grobbelaar, 1984; Xiong et al., 2015). The stomatal density (n=15) and size (n=30) of leaf adaxial and abaxial surfaces were measured as described by Wang et al. (2011, 2014). Briefly, three fully expanded leaves of rice plants were selected, and the stomatal density and size were determined in five randomly selected microphotographs of the adaxial or abaxial surface of the lamina. Leaf number of three plants was measured. In the field experiment, after the leaf net rate of CO2 assimilation was measured for plants, the leaf chlorophyll content was measured using a chlorophyll meter (SPAD 502 Plus; Spectrum Technologies, Japan). The leaf relative water content and dry mass per unit area were measured according to Heckwolf et al. (2011). Water us efficiency (WUE) was calculated as the ratio between net rate of CO2 assimilation and transpiration rate.
Measurement of leaf N content
Leaf samples were harvested, heated at 105 °C for 30 min, and dried at 80 °C for 3 d. The leaf samples were digested with 18.4 M H2SO4 at 260–270 °C and their N contents were determined using an Auto Analyzer 3 digital colorimeter (Bran + Luebbe GmbH, Germany).
Measurement of phloem sucrose transport and sucrose content in tissues
Phloem exudates were collected as described previously (King and Zeevaart, 1974). Petioles were cut in 10 M EDTA (pH 6.0), transferred to a cup containing 1.0 ml EDTA, and incubated in the dark for 1 h for exudation.
The sucrose contents of rice-plant tissues were measured in five biological replicates according to Stitt et al. (1989). Samples (0.1 g) were extracted three times with 4 ml of 80% v/v ethanol for 20 min at 80 °C. Next, the samples were incubated for 10 min in boiling water. The sucrose content was measured using a SpectraMax M5 spectrometer.
Statistical analysis
The data were subjected to analysis of variance and Duncan’s multiple-range test using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). A value of P<0.05 was considered indicative of statistical significance.
Results
Localization and expression pattern of OsPIP1;2 in rice plants
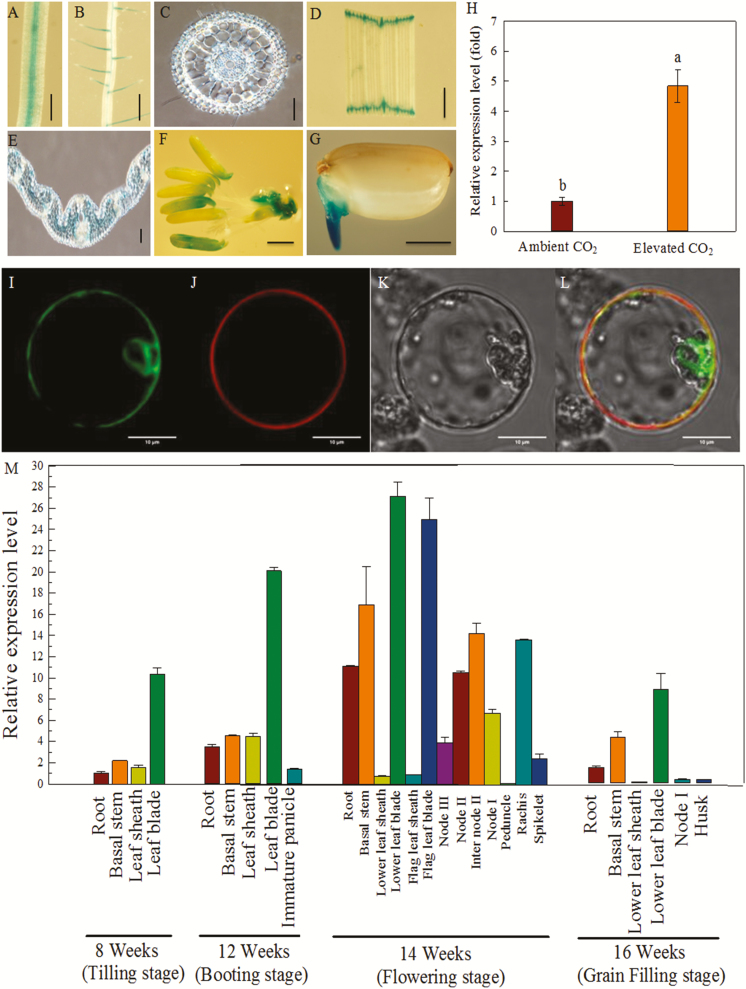
The amino acid sequences of OsPIP1;2 and other aquaporins (NtAQP1, AtPIP1;2, HvPIP2;1, and HvPIP2;3) are highly conserved. A homology model of OsPIP1;2 was generated using the crystal structure of SoPIP2;1 (PDB codes: 2D5F and 1Z98) as a template (Supplementary Fig. S2). To analyse the expression of OsPIP1;2 in rice tissue, a 1977 bp fragment immediately upstream of the translation start site of OsPIP1;2 was used for GUS reporter (Fig. 1A–G). OsPIP1;2 was expressed in the roots of rice plants (Fig. 1A–C). GUS activity was high in the leaf, panicle, and embryonic primary tissue (Fig. 1D, F, G). In the cross-section of the leaf blade, GUS activity was detected in mesophyll cells (Fig. 1E).
Fig. 1.
OsPIP1;2 expression pattern and subcellular localization. (A–G) Rice transformed with OsPIP1;2 promoter::GUS. GUS activity in the root (A), lateral root elongation zone (B), cross-section of the root tip (C), leaf blade (D), cross-section of the leaf blade (E), floret (F), and germinating seeds (G). Scale bars: 100 μm (C, E) and 1 mm (A, B, D, F, G). (H) Response of OsPIP1;2 expression to CO2 in leaf blade by quantitative real-time PCR. The internal reference gene was OsActin. Values are means ±SD (n=3); different letters indicate significant differences (P<0.05). (I–L) Subcellular localization of OsPIP1;2 in rice protoplasts. Scale bars: 10 μm. (I) Fluorescence signal of 35S::OsPIP1;2-GFP. (J) fluorescence signal of OsMCA1 (Takamitsu et al., 2012). (K) bright-field image. (L) merged image. (M) Expression levels of OsPIP1;2 in the indicated tissues and at the indicated growth stages of the wild type (WT) (cv. Nipponbare) grown in soil, as determined by quantitative real-time PCR with OsActin as an internal reference. Values are means ±SD (n=3).
By real-time quantitative RT-PCR, the relative expression level of OsPIP1;2 in the rice leaf blade was 5-fold higher in the presence of an elevated [CO2] (Fig. 1H). The expression of OsPIP1;2 was next investigated in different organs and growth stages (Fig. 1M). OsPIP1;2 showed the highest expression in the leaf blade at all growth stages. In addition, the expression of OsPIP1;2 in the leaf blade was highest at the flowering stage. OsPIP1;2 was also expressed in other organs at various levels (Fig. 1M).
To determine the subcellular localization of OsPIP1;2, an OsPIP1;2–GFP fusion was expressed in mature rice protoplasts isolated from culms of rice seedlings grown in the dark. The GFP fluorescence signal was detected at the plasma membrane (Fig. 1I–L), indicating co-localization with the plasma membrane marker Ca2+-permeable mechanosensitive channel OsMAC1 (Takamitsu et al., 2012). Therefore, OsPIP1;2 is localized to the plasma membrane.
Gas-exchange parameters of rice plants
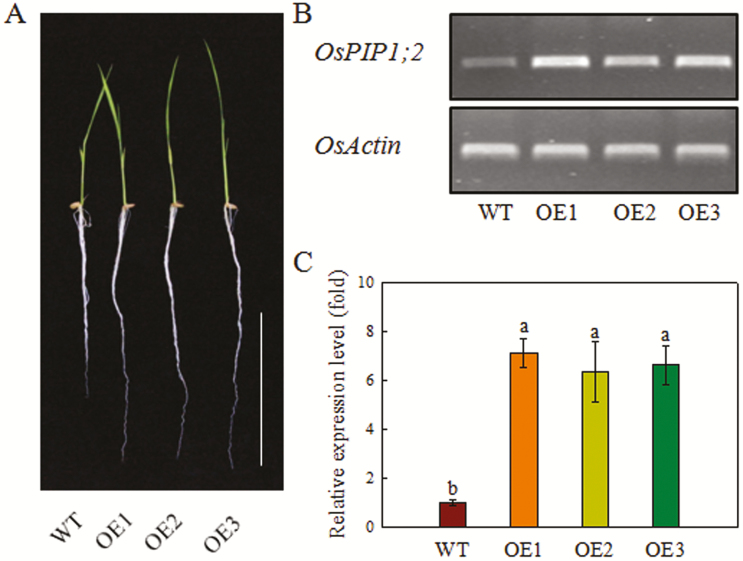
To characterize the physiological function of OsPIP1;2 in rice plants, OE lines were constructed. The relative expression level of OsPIP1;2 was significantly 6.3–7.1-fold higher in the OE lines than in the WT (Fig. 2).
Fig. 2.
Expression level of OsPIP1;2 in selected rice transgenic lines. (A) Phenotype of 7-day-old transgenic lines [overexpressing (OE) 1, OE2, and OE3]. Scale bar: 10 cm. (B, C) Expression level of OsPIP1;2 in the OE lines by RT-PCR (B) and real-time quantitative PCR (C) with OsActin as an internal reference. Values are means ±SD (n=3); different letters indicate significant differences (P<0.05).
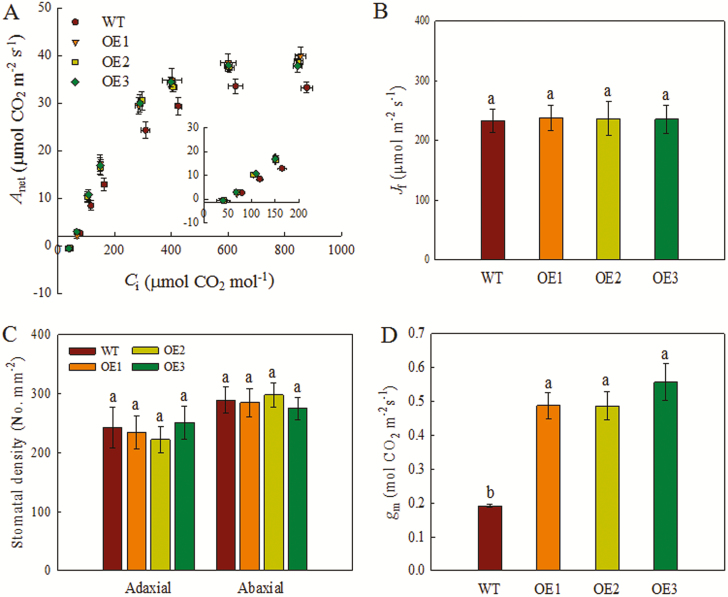
We next examined the net rate of CO2 assimilation (Anet) at different substomatal CO2 concentrations (Ci) and PPFDs using a gas-exchange system (Fig. 3; Supplementary Fig. S3). The Anet values revealed that the response of the OE lines to a low PPFD (<200 μmol m−2 s−1) was similar to that of the WT (Supplementary Fig. S3). However, at a PPFD of 1500 μmol m−2 s−1, the Anet of the OE lines was 17–19% higher than that of the WT (Supplementary Fig. S3). Under these conditions, Anet was light-saturated and limited by the carboxylation rate. The light-use efficiency of the OE lines was higher than that of the WT under light-saturated conditions. The Anet of the OE lines was also higher than that of the WT at a Ci of >150 μmol CO2 mol−1 (Fig. 3A). The maximum Anet of the OE lines was ~37 μmol CO2 m−2 s−1, compared with ~31 μmol m−2 s−1 in the WT. Therefore, the OE lines had greater CO2 for photosynthesis. Based on the chlorophyll fluorescence and gas exchange data, the leaf gm of the OE lines increased by approximately 150% that of the WT (Fig. 3D). The gs values of the OE lines were 26–38% higher than that of the WT (Supplementary Fig. S4). By contrast, water-use efficiency did not differ significantly between the OE lines and the WT (Supplementary Fig. S5). Further, there was no significant difference between the OE lines and the WT in the electron transport rate (Jf) and maximum electron transport rate (Jmax) (Fig. 3B; Table 1). The Cc and maximum Rubisco activity (Vcmax) of the OE lines were 25–30% and 28–33%, respectively, higher than those of the WT (Table 1).
Fig. 3.
Net rate of CO2 assimilation response curves and parameters. Rice plants were grown under ambient [CO2] in growth chambers for 4 weeks. (A) The response of the net rate of CO2 assimilation to the substomatal CO2 concentration (Ci) (n=5). (B) Electron transport rate (Jf) (n=5). (C) Stomatal density on the adaxial and abaxial surfaces of leaves (n=15). (D) Mesophyll CO2 conductance (gm) of rice plants (n=5). All values are means ±SD; different letters indicate significant differences (P<0.05).
Table 1.
Net rate of CO2 assimilation parameters of rice plants under ambient [CO2]
| Anet (μmol CO2 m−2 s−1) |
Ci (μmol CO2 mol−1) |
Cc (μmol CO2 mol−1) |
Vcmax (μmol CO2 m−2 s−1) |
Jmax (μmol photons m−2 s−1) |
|
|---|---|---|---|---|---|
| WT | 28.05 ± 1.3b | 305 ± 9a | 158 ± 16b | 55.9 ± 4.8b | 242 ± 11a |
| OE1 | 33.43 ± 1.6a | 267 ± 18b | 198 ± 15a | 71.8 ± 4.6a | 251 ± 9a |
| OE2 | 32.95 ± 1.5a | 272 ± 16b | 204 ± 8a | 74.1 ± 5.8a | 246 ± 17a |
| OE3 | 33.19 ± 1.1a | 265 ± 14b | 205 ± 10a | 72.4 ± 6.5a | 248 ± 26a |
Rice plants were grown under ambient [CO2] in the chamber for 4 weeks. Values are means ±SD (n=5). Different letters indicate significant differences at the P<0.05 level in rice plants. Anet, net rate of CO2 assimilation; Cc, chloroplastic CO2 concentration; Ci, substomata CO2 concentration; Jmax, maximum electron transport rate; Vcmax, maximum Rubisco activity.
In the field experiment, the Anet values of the OE lines were 9–15%, 13–42%, and 19–34% higher than those of the WT at the flowering, middle-filling, and end-filling stages, respectively. In addition, the Anet values of the OE lines and the WT decreased from the flowering stage to the end of the grain-filling stage. There was no significant difference in chlorophyll content at any growth stage between the OE lines and the WT (Supplementary Fig. S6).
The OE lines and the WT did not display significant differences in chlorophyll content, number of leaves per plant, relative water content, leaf dry mass per unit area (Supplementary Table S4), or stomatal density and size on the adaxial or abaxial surface (Fig. 3C; Supplementary Table S5). In addition, the leaf N content was similar between the OE lines and the WT (Supplementary Fig. S7).
Response of OsPIP1;2 to ambient and elevated [CO2]
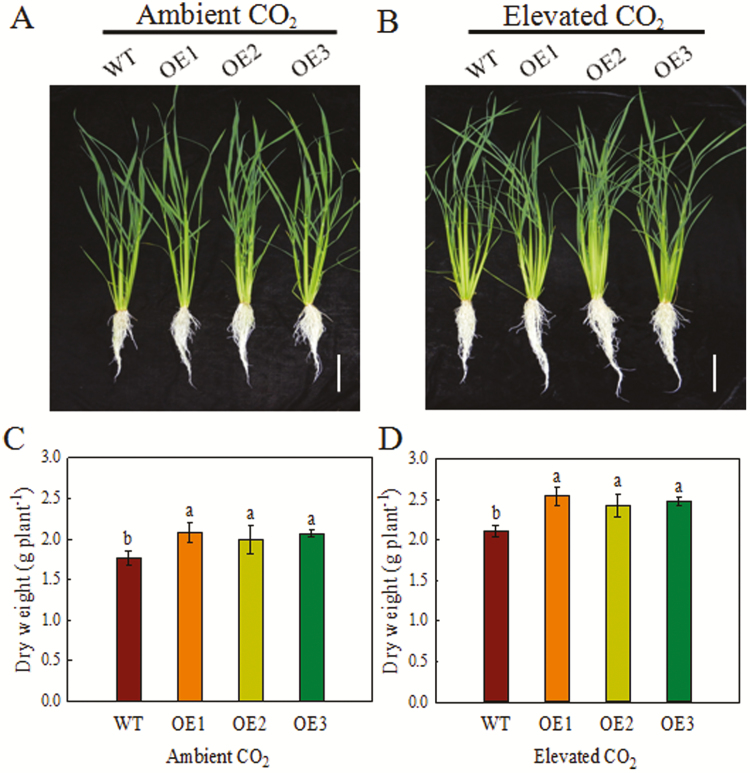
We determined the growth of the OE lines and the WT under ambient and elevated [CO2] over 4 weeks (Fig. 4). Compared with ambient [CO2], the total dry weight of the OE lines and the WT were significantly higher in the presence of an elevated [CO2]. Moreover, the total dry weight of the OE lines was 13–18% and 15–20% higher than that of the WT under ambient and elevated [CO2], respectively (Fig. 4C, D).
Fig. 4.
Growth of rice plants under ambient and elevated [CO2]. Seedlings were grown under ambient [CO2] (400 ppm) or elevated [CO2] (800 ppm) in growth chambers for 4 weeks. (A, B) Growth phenotypes of the OE lines and the WT under ambient [CO2] (400 ppm) or elevated [CO2] (800 ppm) for 4 weeks. Scale bar: 10 cm. (C, D) Total dry weight of the OE lines and the WT grown under ambient [CO2] or elevated [CO2] for 4 weeks. Values are means ±SD (n=5); different letters indicate significant differences (P<0.05).
Carbohydrate content and sucrose transport under ambient and elevated [CO2]
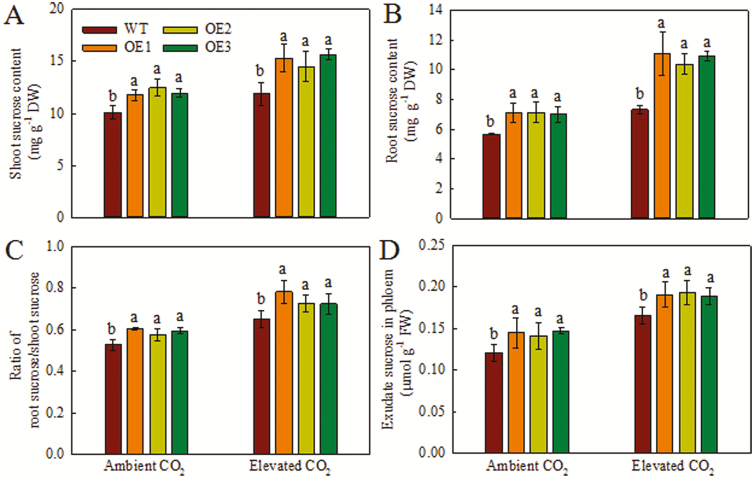
Phloem sucrose transport and sucrose content were measured in the WT and OE lines under ambient and elevated [CO2] (Fig. 5). The shoot sucrose content, root sucrose content, ratio of root/shoot sucrose, and phloem sucrose transport activity in the OE lines were 10–18%, 17–20%, 10–15%, and 18–21% higher, respectively, than those of the WT under ambient [CO2]. Under elevated [CO2], the shoot sucrose content, root sucrose content, and ratio of root/shoot sucrose of the OE lines were 19–28%, 24–34%, 11–20%, and 13–16%, respectively, higher than those of the WT. Phloem sucrose transport activity in the OE lines was 13–16% higher than that in the WT (Fig. 5), indicating that the OE lines allocate more carbon from source to sink. Therefore, we investigated phloem sucrose transport from shoot (source) to panicle (sink). The panicle sucrose content of the T4-, T5-, and T6-generation OE lines was 41–49%, 51–66%, and 37–50% higher than that of the WT, respectively (Table 2); this may influence the rice yield.
Fig. 5.
Effect of OsPIP1;2 overexpression on phloem sucrose transport in rice plants. (A, B) Sucrose contents of the shoot (A) and root (B) of the OE lines and the WT grown under ambient [CO2] (400 ppm) or elevated [CO2] (800 ppm) in growth chambers for 4 weeks. (C) Ratio of root sucrose/shoot sucrose in the OE lines and the WT grown under ambient [CO2] or elevated [CO2] for 4 weeks. (D) Phloem sucrose contents of the OE lines and the WT grown under ambient [CO2] or elevated [CO2] for 4 weeks. Values are means ±SD (n=5); different letters indicate significant differences (P<0.05).
Table 2.
Comparison of agronomic traits and panicle sucrose content of rice plants grown in paddy field in 2016 and 2017
| Effective tiller No. per plant | Spikelets per panicle | Panicle sucrose content (mg g−1) | |
|---|---|---|---|
| 2016 Nanjing | |||
| WT | 18.7 ± 0.9b | 85 ± 8.4b | 10.2 ± 0.3b |
| OE1 | 22.3 ± 1.4a | 97 ± 11a | 14.4 ± 0.5a |
| OE2 | 24.5 ± 0.7a | 101 ± 14a | 14.8 ± 1.1a |
| OE3 | 21.9 ± 1.5a | 95 ± 8.8a | 15.2 ± 1.5a |
| 2017 Sanya | |||
| WT | 21.3 ± 0.5b | 79 ± 8.3b | 9.5 ± 0.8b |
| OE1 | 27.7 ± 1.2a | 95 ± 4.1a | 14.3 ± 1.5a |
| OE2 | 25.3 ± 1.6a | 97 ± 8.2a | 15.8 ± 1.2a |
| OE3 | 25.9 ± 1.7a | 92 ± 5.8a | 15.2 ± 0.5a |
| 2017 Nanjing | |||
| WT | 15.0 ± 2.4b | 88 ± 6.5b | 11.5 ± 0.8b |
| OE1 | 21.0 ± 1.7a | 98 ± 7.3a | 15.7 ± 0.6a |
| OE2 | 17.7 ± 3.1a | 95 ± 6.7a | 17.3 ± 1.2a |
| OE3 | 18.9 ± 1.6a | 96 ± 7.2a | 16.1 ± 0.9a |
Statistical analysis of data is from T4–T6 generations. Values are means ±SD (n=12). Different letters indicate significant differences at the P<0.05 level in rice plants.
Alteration of OsPIP1;2 expression affects rice grain yield
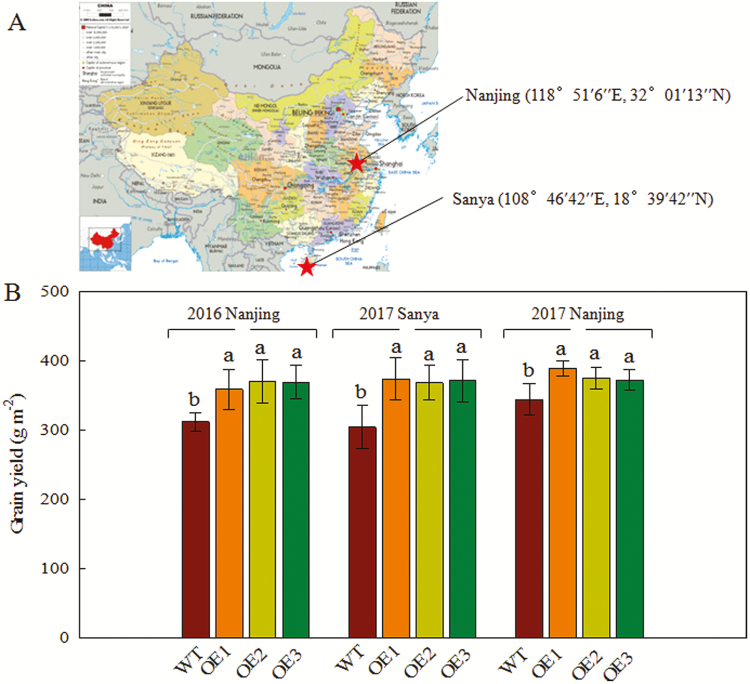
To examine the influence of OsPIP1;2 on rice grain yield, OsPIP1;2 WT and OE lines were cultivated in a field (Fig. 6; Table 2). The agricultural traits of the T4–T6 generations of the OE lines and the WT were investigated at Nanjing City, Jiangsu Province and Sanya City, Hainan Province from 2016 to 2017 (Table 2). The effective tiller number and spikelets per panicle of the OE lines were 17–40% and 12–23% higher than those of the WT. The grain yield of the OE lines was enhanced by 13–25% (T4 generation) at Nanjing, by 18–23% (T5 generation) at Sanya, and by 13–36% (T6 generation) at Nanjing, relative to the WT (Fig. 6). Therefore, overexpression of OsPIP1;2 enhances rice yield in the field.
Fig. 6.
Grain yield of transgenic rice plants grown in a paddy field in 2016 and 2017. (A) The locations of the field experiments in 2016 (Nanjing) and 2017 (Nanjing and Sanya). (B) Grain yield of transgenic rice plants. Values are means ±SD (n=3); different letters indicate significant differences (P<0.05).
Discussion
We report here that overexpression of OsPIP1;2 enhances the net CO2 assimilation rate by improving CO2 diffusion in the leaf, which increases the growth and yield of rice plants. Rice OsPIP1;2 belongs to the PIP1 family, among which PIP1;2 had a function in CO2 diffusion in tobacco and Arabidopsis (Uehlein et al., 2003; Heckwolf et al., 2011; Sade et al., 2014). We reported previously that oocytes transfected with OsPIP1;2 did not show water-transport activity (Ding et al., 2016), suggesting that water transport activity was not enhanced in the OE lines. Interestingly, the amino acid sequences of OsPIP1;2 and other aquaporins related to CO2 permeability are highly conserved (Supplementary Fig. S2). According to Mori et al. (2014), OsPIP1;2 had the same amino acid residue at the C-terminal end of the E-loop as barley aquaporin, which was permeable to CO2 in the Xenopus laevis oocyte expression system. In addition, the relative expression of OsPIP1;2 was significantly increased under elevated [CO2] (Fig. 1). Therefore, OsPIP1;2 may be associated with CO2 permeability. In addition, gm related closely to the modification of aquaporins in tobacco and Arabidopsis (Uehlein et al., 2003; Heckwolf et al., 2011; Sade et al., 2014). In our study, OsPIP1;2 expression was significantly up-regulated in transgenic plants relative to the WT (Fig. 2). The OsPIP1;2-OE transgenic rice lines had higher gm and Anet, and the gm of OE lines was 1.5-fold higher than that of the WT (Fig. 3D; Table 1). Our results are consistent with the observation in NtAQP1 (NtAQP1;2) OE tobacco plants (Uehlein et al., 2003). Although, gm and gs are in general correlated (Lauteri et al., 1997; Loreto et al., 2003), the gs of the OE lines was observed to be only 0.3-fold higher than that of the WT (Supplementary Fig. S4), indicating that a high Anet is mainly contributed by increased gm. The cholorplastic CO2 concentration was also higher in the OE lines than in the WT (Table 1), indicating that OsPIP1;2 influences mesophyll CO2 conductance. In general, high gs usually causes lower WUE (Lawson and Blatt, 2014). However, in our study, the OsPIP1;2 OE lines did not show decreased WUE even though the gs increased. The reason is the enhanced net rate of CO2 assimilation in overexpression of OsPIP1;2, which showed high gm (Supplementary Figs S4 and S5). Therefore, a potential approach for crop plants is to increase gs, maintaining WUE without substantial cost in overexpression of OsPIP1;2.
g s, but not stomatal density or size, differed between the OE lines and the WT (Fig. 3; Supplementary Fig. S4; Supplementary Table S5). These results are partly consistent with the findings of Hanba et al. (2004), who reported that overexpression of barley aquaporin HvPIP2;1 in rice increased gs by 27%. The Anet–Ci curves revealed that the response to increasing Ci was enhanced during the first phase in the OE lines (Fig. 3A), consistent with the reduced Ci in atpip1;2 lines reported by Heckwolf et al. (2011). According to the Anet–Ci curves, Vcmax and CO2 assimilation rate of the OE lines were increased (Table 1), but this is not due to the difference in leaf N content (Supplementary Fig. S7). In addition, under the FACE system, Vcmax decreases as an acclimatory response to long-term elevated [CO2] (Ainsworth and Long, 2005). In the present study, gm and Vcmax were increased in OE lines, which suggested that gm enhancement may increase Vcmax as the early response to CO2 in rice plants.
Sucrose is the major translocated photosynthetic product and the main form of carbon on which plant sinks grow (Sung et al., 1989). In this study, the shoot sucrose content of the OE lines was higher than that of the WT (Fig. 5A). Sucrose acts as a signaling molecule and provides energy for root growth and development (Chiou and Bush, 1998). Efficient sucrose transport from shoot to root via phloem plays a key role in root growth (Salerno and Curatti, 2003). Plant acclimation to elevated [CO2] has been associated with an increase in carbohydrate content (Weigel and Manderscheid, 2012). In the present study, the OE lines had higher biomass than the WT under both ambient and elevated [CO2] (Fig. 4), consistent with the results of Kim et al. (2001). This is because the OE lines showed higher carbohydrate content than the WT (Fig. 5). Our results suggest that OsPIP1;2 is involved in the response to elevated [CO2] and increases the root/shoot sucrose concentration ratio (Figs 4 and 5). Although the root/shoot sucrose concentration ratio is not a good proxy for phloem export, it is also affected by the sucrose consumption rates in organs. Thus, we examined phloem transport in rice plants. Under ambient [CO2], the OE lines showed a higher root sucrose content than the WT (Fig. 5D). Therefore, the OE lines exhibited higher phloem sucrose transport activity compared with the WT, suggesting greater carbon allocation from source to sink (Fig. 5D).
Sucrose is an important determinant of the number of grains in rice spikelets, and an increased number of spikelets per panicle is key for enhancing grain yield (Kato et al., 2007). In the field experiment, the panicle sucrose content of the OE lines was markedly higher (Table 2), suggesting greater sucrose export from leaves to seeds, relative to the WT. This suggests that the OE lines were source limited, but not sink limited, in grain yield increase. Further, the OE lines had a larger number of spikelets per panicle than the WT (Table 2), which may be due to their greater sink capacity. This finding is consistent with prior reports that the yield potential of rice is enhanced by its large sink capacity, itself related to the large number of spikelets per panicle (Peng et al., 2008). Therefore, overexpression of OsPIP1;2 increased the number of spikelets per panicle in rice plants by enhancing sucrose transport from leaf to panicle by increasing the net CO2 assimilation rate. This likely contributed to the increased yield of the OE lines in the field experiment (Fig. 6).
In addition, the leaf relative water content and dry mass per unit area reflect the water status of the plant (Jones, 2007). The leaf relative water content and dry mass per unit area did not differ between the OE lines and the WT (Supplementary Table S4), indicating that water transport in leaves was unaffected by overexpression of OsPIP1;2. The number of leaves per plant also did not differ between the OE lines and the WT (Supplementary Table S4), suggesting that the OE lines have the same developmental rates. Our results indicate that the effect of OsPIP1;2 on rice growth and yield is largely due to the facilitation of CO2 transport rather than the modulation of water transport and developmental rate. In conclusion, our results indicate that overexpression of OsPIP1;2 modulates the number of spikelets per panicle by increasing leaf CO2 diffusion, photosynthetic performance, and phloem sucrose transport; together, these effects have a positive effect on rice yield.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Daytime respiration rate (Rd) of the transgenic rice plants.
Fig. S2. Homology modeling of OsPIP1;2.
Fig. S3. Net rate of CO2 assimilation (Anet) to PPFD of rice plants under 400 ppm CO2.
Fig. S4. Stomatal conductance (gs) of rice plants under 400 ppm CO2.
Fig. S5. Water-use efficiency of rice plants under 400 ppm CO2.
Fig. S6. Chlorophyll content and net rate of CO2 assimilation (Anet, μmol m−2 s−1) of newly and fully expanded leaves of rice plants at the flowering stage (Flowering), middle grain-filling stage (Mid-Fill), and end grain-filling stage (End-Fill).
Fig. S7. Leaf N content of rice plants under 400 ppm CO2.
Table S1. Primers used for construction of vectors.
Table S2. Primers used for RT-PCR analysis.
Table S3. Primers used for real-time quantitative PCR analysis.
Table S4. Morphological and physiological parameters of the rice plants.
Table S5. Stomatal size in leaves of the transgenic rice plants.
Supplementary Material
Acknowledgements
We are grateful for grant support from the National Key R&D Program of China (2018YFD0200302 and 2018YFD020044), National Natural Science Foundation of China (31471937 and 31761130073).Furthermore, we thank Professor Ian Dodd (Lancaster University) for useful discussion on this work.
References
- Ai PH, Sun SJ, Fan XR, Xin W, Guo Q, Yu L, Xu GH. 2009. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. The Plant Journal 57, 798–809. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–371. [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. 1998. Sucrose is a signal molecule in assimilate partitioning. Proceedings of the National Academy of Sciences, USA 95, 4784–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gao L, Liu W, Wang M, Gu M, Ren B, Xu G, Shen Q, Guo S. 2016. Aquaporin plays an important role in mediating chloroplastic CO2 concentration under high-N supply in rice (Oryza sativa) plants. Physiologia Plantarum 156, 215–226. [DOI] [PubMed] [Google Scholar]
- Ethier GJ, Livingston NJ. 2004. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant Cell & Environment 27, 137–153. [Google Scholar]
- Ethier GJ, Livingston NJ, Harrison DL, Black TA, Moran JA. 2006. Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant, Cell & Environment 29, 2168–2184. [DOI] [PubMed] [Google Scholar]
- Evans JR, Loreto F. 2000. Acquisition and diffusion of CO2, in higher plant leaves. In Photosynthesis: Physiology and metabolism, Leegood RC, Sharkey TD, von Caemmerer S, eds. Dordrecht, The Netherlands: Kluwer Academic Publishers, 321–351. [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. 2009. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60, 2235–2248. [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Berry JA, Cifre J, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbó M. 2007. Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. Journal of Experimental Botany 58, 1533–1543. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell & Environment 31, 602–621. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD. 2001. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiology 125, 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE. 2013. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiology 162, 1632–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M. 2004. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant & Cell Physiology 45, 521–529. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Sakai H, Tokida T. 2013. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Functional Plant Biology 40, 148–159. [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 Flux by Analysis of the Response of Photosynthesis to CO2. Plant Physiology 98, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckwolf M, Pater D, Hanson DT, Kaldenhoff R. 2011. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. The Plant Journal 67, 795–804. [DOI] [PubMed] [Google Scholar]
- Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, Chen J, Wu P, Xu G. 2011. The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiology 156, 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG. 2007. Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. Journal of Experimental Botany 58, 119–130. [DOI] [PubMed] [Google Scholar]
- Kato T, Shinmura D, Taniguchi A. 2007. Activities of enzymes for sucrose-starch conversion in developing endosperm of rice and their association with grain filling in extra-heavy panicle types. Plant Production Science 10, 442–450. [Google Scholar]
- Kim HY, Lieffering M, Miura S, Kobayashi K, Okada M. 2001. Growth and nitrogen uptake of CO2-enriched rice under field conditions. New Phytologist 150, 223–229. [Google Scholar]
- Kimball BA. 2016. Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Current Opinion in Plant Biology 31, 36–43. [DOI] [PubMed] [Google Scholar]
- King RW, Zeevaart JA. 1974. Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiology 53, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R. 2011. Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnology Journal 9, 826–837. [DOI] [PubMed] [Google Scholar]
- Kusumi K, Hirotsuka S, Kumamaru T, Iba K. 2012. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. Journal of Experimental Botany 63, 5635–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauteri M, Scartazza A, Guido MC, Brugnoli E. 1997. Genetic variation in photosynthetic capacity, carbon isotope discrimination and mesophyll conductance in provenances of Castanea sativa adapted to different environments. Functional Ecology 11, 675–683. [Google Scholar]
- Lawson T, Blatt MR. 2014. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiology 164, 1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian HL, Yu X, Lane D, Sun WN, Tang ZC, Su WA. 2006. Upland rice and lowland rice exhibited different PIP expression under water deficit and ABA treatment. Cell Research 16, 651–660. [DOI] [PubMed] [Google Scholar]
- Loreto F, Centritto M, Chartzoulakis K. 2003. Photosynthetic limitations in olive cultivars with different sensitivity to salt stress. Plant Cell & Environment 26, 595–601. [Google Scholar]
- Meinshausen M, Smith SJ, Calvin K, Daniel JS, Kainuma MLT, Lamarque J. 2011. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Climatic Change 109, 213–241. [Google Scholar]
- Montpied P, Granier A, Dreyer E. 2009. Seasonal time-course of gradients of photosynthetic capacity and mesophyll conductance to CO2 across a beech (Fagus sylvatica L.) canopy. Journal of Experimental Botany 60, 2407–2418. [DOI] [PubMed] [Google Scholar]
- Mori IC, Rhee J, Shibasaka M, Sasano S, Kaneko T, Horie T, Katsuhara M. 2014. CO2 transport by PIP2 aquaporins of barley. Plant & Cell Physiology 55, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby RJ, De Kauwe MG, Domingues TF, et al. . 2016. Model-data synthesis for the next generation of forest free-air CO2 enrichment (FACE) experiments. New Phytologist 209, 17–28. [DOI] [PubMed] [Google Scholar]
- Patron NJ, Orzaez D, Marillonnet S, et al. . 2015. Standards for plant synthetic biology: a common syntax for exchange of DNA parts. New Phytologist 208, 13–19. [DOI] [PubMed] [Google Scholar]
- Peng S, Gurdevs K, Parminder V, Tang Q, Zou Y. 2008. Progress in ideotype breeding to increase rice yield potential. Field Crops Research 108, 32–38. [Google Scholar]
- Sade N, Gallé A, Flexas J, Lerner S, Peleg G, Yaaran A, Moshelion M. 2014. Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta 239, 357–366. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. 2005. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant & Cell Physiology 46, 1568–1577. [DOI] [PubMed] [Google Scholar]
- Salerno GL, Curatti L. 2003. Origin of sucrose metabolism in higher plants: when, how and why?Trends in Plant Science 8, 63–69. [DOI] [PubMed] [Google Scholar]
- Sartory DP, Grobbelaar JU. 1984. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114, 177–187. [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. 2007. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell & Environment 30, 1035–1040. [DOI] [PubMed] [Google Scholar]
- Singh J, Pandey P, James D, Chandrasekhar K, Achary VM, Kaul T, Tripathy BC, Reddy MK. 2014. Enhancing C3 photosynthesis: an outlook on feasible interventions for crop improvement. Plant Biotechnology Journal 12, 1217–1230. [DOI] [PubMed] [Google Scholar]
- Stitt M, Lilley RMC, Gerhardt R, Heldt HW. 1989. Metabolites in specific cells and subcellular compartments of plant leaves. Methods in Enzymology 174, 518–552. [Google Scholar]
- Sung SJ, Xu DP, Black CC. 1989. Identification of actively filling sucrose sinks. Plant Physiology 89, 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamitsu K, Daisuke N, Yukari Y, et al. . 2012. Plasma membrane protein osmca1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biology 12, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Fan X, Li Q, Feng H, Miller AJ, Shen Q, Xu G. 2012. Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx. Plant Physiology 160, 2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P. 2006. Structural mechanism of plant aquaporin gating. Nature 439, 688–694. [DOI] [PubMed] [Google Scholar]
- Tripati AK, Roberts CD, Eagle RA. 2009. Coupling of CO2 and ice sheet stability over major climate transitions of the last 20 million years. Science 326, 1394–1397. [DOI] [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. 2003. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425, 734–737. [DOI] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. 2008. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. The Plant Cell 20, 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini R, Epron D, Angelis PD, Matteucci G, Dreyer E. 1995. In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water supply. Plant, Cell & Environment 18, 631–640. [Google Scholar]
- Wang Y, Noguchi K, Ono N, Inoue S, Terashima I, Kinoshita T. 2014. Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proceedings of the National Academy of Sciences, USA 111, 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Noguchi K, Terashima I. 2011. Photosynthesis-dependent and -independent responses of stomata to blue, red and green monochromatic light: differences between the normally oriented and inverted leaves of sunflower. Plant & Cell Physiology 52, 479–489. [DOI] [PubMed] [Google Scholar]
- Weigel HJ, Manderscheid R. 2012. Crop growth responses to free air CO2 enrichment and nitrogen fertilization: rotating barley, ryegrass, sugar beet and wheat. European Journal of Agronomy 43, 97–107. [Google Scholar]
- Xiong D, Liu X, Liu L, Douthe C, Li Y, Peng S, Huang J. 2015. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant, Cell & Environment 38, 2541–2550. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Sasaki A, Xia JX, Yokosho K, Ma JF. 2013. A node-based switch for preferential distribution of manganese in rice. Nature Communications 4, 2442. [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC, Romero P, Harbinson J, Evers JB, Van Der Putten PE, Vos J. 2009. Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant, Cell & Environment 32, 448–464. [DOI] [PubMed] [Google Scholar]
- Zeng HQ, Liu G, Toshinori K, Zhang RP, Zhu YY, Shen QR, Xu GH. 2012. Stimulation of phosphorus uptake by ammonium nutrition involves plasma membrane H+-ATPase in rice roots. Plant and Soil 35, 205–214. [Google Scholar]
- Zhu Y, DI T, Xu G, Chen X, Zeng H, Yan F, Shen Q. 2009. Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant, Cell & Environment 32, 1428–1440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.