Abstract
α-Mannosidosis (AMD) is an autosomal recessively inherited lysosomal storage disorder affecting brain function and structure. We performed ex vivo and in vivo diffusion tensor imaging (DTI) on the brains of AMD-affected cats to assess gray and white matter abnormalities. A multi-atlas approach was used to generate a brain template to process the ex vivo DTI data. The probabilistic label method was used to measure fractional anisotropy (FA), mean diffusivity, axial diffusivity, and radial diffusivity values from gray and white matter regions from ex vivo DTI. Regional analysis from various regions of the gray matter (frontal cortex, cingulate gyrus, caudate nucleus, hippocampus, thalamus, and occipital cortex), and white matter (corpus callosum, corticospinal tract, cerebral peduncle, external and internal capsule) was also performed on both ex vivo and in vivo DTI. Ex vivo DTI revealed significantly reduced FA from both gray and white matter regions in AMD-affected cats compared to controls. Significantly reduced FA was also observed from in vivo DTI of AMD-affected cats compared to controls, with lower FA values observed in all white matter regions. We also observed significantly increased axial and radial diffusivity values in various gray and white matter regions in AMD cats from both ex vivo and in vivo DTI data. Imaging findings were correlated with histopathologic analyses suggesting that DTI studies can further aid in the characterization of AMD by assessing the microstructural abnormalities in both white and gray matter.
Keywords: Diffusion tensor imaging, Lysosomal storage diseases, Mannosidosis, MRI, Myelination, Neuropathology.
INTRODUCTION
α-Mannosidosis (AMD) is a lysosomal storage disorder (LSD) with autosomal recessive inheritance caused by a deficiency of the lysosomal α-mannosidase enzyme gene (1, 2). This enzyme is involved in the degradation of N-linked glycoproteins through sequential degradation of mannose and complex oligosaccharides. Its deficiency leads to intralysosomal accumulation of undigested mannose-rich oligosaccharides in the cells before birth as well as through early life in various organs including brain, kidney, and liver, resulting in mental retardation and progressive skeletal abnormalities (3).
Central nervous system (CNS) abnormalities observed in AMD-affected humans as well as in feline models of AMD include accumulation of oligosaccharides within cells resulting in swelling of neurons and glia (4–6), immun-odeficiency, and progressive psychiatric and neurological manifestations, particularly ataxia, and mental retardation (1). Dysmyelination has also been reported in the cat model of AMD, although the mechanism for the deficiency has not yet been identified (4–6). AMD-affected feline models exhibit clinical, biochemical, and neuropathological abnormalities similar to those in children with AMD and are useful in the evaluation of experimental therapies including bone marrow transplantation and gene therapy (2, 7–9). The effect of treatment on cerebral and extracerebral functions has been assessed by histological analysis and clinical disease progression in AMD-affected cats (5, 8).
An ability to monitor disease progression and abnormalities in the gray and white matter noninvasively would facilitate the diagnosis of LSDs and provide a method for monitoring responses to therapy. Magnetic resonance imaging (MRI) has been employed to examine morphological changes in the cat model of AMD (5, 6, 8). These studies include magnetization transfer ratio, demonstrating abnormalities in white matter (5, 8), diffusion-weighted MRI, showing abnormalities in both gray and white matter (6), and single voxel magnetic resonance spectroscopy, reporting the accumulation of the undegraded carbohydrate substrates in the cerebral cortex of AMD-affected cats (4). Additional information about the microstructural brain abnormalities can be obtained by diffusion tensor imaging (DTI), because this analysis provides the directionality of diffusion as well as the magnitude of water diffusion (10). Diffusion tensor imaging parameters in neuroimaging, including fractional anisotropy (FA) and mean diffusivity (MD), have been used extensively for detecting changes in myelination in the developing brain in humans and in mouse models of neurodevelopmental disorders (11–14), for assessing microstructural morphology in demyelinating diseases (15, 16), for neurological disorders (17), and in characterizing LSD such as mucopolysaccharidosis in a murine model (18). There are, however, very few studies evaluating the utility of axial diffusivity (AD) and radial diffusivity (RD) in LSDs. The present study was thus performed with an aim to further characterize the gray and white matter abnormalities in the feline model of AMD using ex vivo and in vivo DTI so as to develop surrogate noninvasive imaging biomarkers for studying LSD in general.
MATERIALS AND METHODS
Experimental Animals
The study was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Wild-type (+/+) and heterozygous (+/-) controls, both of which have normal phenotypes, and AMD-affected (-/-) cats were raised and maintained in the animal colony at the School of Veterinary Medicine under National Institutes of Health (NIH) and United States Department of Agriculture (USDA) guidelines for the care and use of animals in research. The animals were housed in a facility maintained at 21°C with food and water ad libitum, 12-hour light cycles and with 12 to 15 air changes per hour. AMD-affected cats were bred from carrier cats and were genotyped for the mutant allele. Peripheral blood leukocytes from all cats were tested at 1 day of age for the 4 base pair deletion causing AMD using PCR and restriction enzyme (19). Physical and neurological examinations were performed monthly from birth until the animals were killed with an intravenous injection of barbiturate; the thoracic cavity was opened under aseptic conditions, and the animals were then transcardially perfused with cold saline. After perfusion, the brain was removed from the skull and stored at 4 °C in 4% paraformaldehyde solution for at least 7 days.
Prior to imaging, the brain samples were removed from the fixative and immersed in phosphate buffered saline (PBS) for 48 hours at 4 °C to rehydrate the tissue. After 48 hours, the samples were removed and rinsed twice with fresh phosphate-buffered saline and blotted using a soft tissue paper towel to remove any remaining PBS. The brains were then placed in a custom-designed acrylic container and the remaining space was filled with a proton-free susceptibility-matching fluid (Fomblin®, Ausimount, Thorofare, NJ). Care was taken to remove all air bubbles from the container before sealing to minimize image distortions due to magnetic susceptibility effects.
Ex Vivo DTI
For the ex vivo study, 5 AMD-affected and 6 normal age-matched cats were euthanized at 5 months of age, when CNS pathological alterations are severe in AMD. DTI data were acquired on an Agilent 9.4T horizontal bore magnet equipped with 40 gauss/cm gradient tube and interfaced to a Agilent Direct Drive console (Agilent, Palo Alto, CA), running the version vnmrj 2.3.C software. All ex vivo DTI studies employed a 70-mm inner diameter transmit-receive quadrature birdcage radio-frequency (RF) coil (M2M Imaging, Cleveland, OH). The specimen container was positioned inside the RF coil, which was then mounted inside the magnet. Scout images were acquired in 3 orthogonal planes to localize the position and orientation of the specimen. A multislice 2D diffusion-weighted fast spin echo pulse sequence was used to generate diffusion-weighted images spanning the entire specimen. The diffusion gradients were placed at the beginning of the fast spin echo readout. The relevant acquisition parameters were as follows: orientation = axial, field of view (FOV) = 40 x 40 mm; slice thickness = 1 mm; matrix =128 x 128, slices = 51; number of averages = 12; echo time (TE) =35 ms; repetition time (TR) = 2.3 s; and b-value = 1027.36 s/mm2. Fourteen data sets were acquired using 6 diffusion directions with positive and negative diffusion-weighting gradients and 2 reference data sets (b = 0 s/mm2) resulting in a total acquisition time of slightly less than 14 hours.
In Vivo DTI
For in vivo MRI studies, a separate cohort of age and gender-matched 8 to 10-week-old AMD-affected (n = 3) and wild type (n = 3) cats were studied. Cats were fasted and received subcutaneous atropine (0.02 mg/kg) and bupren-orphine (0.01 mg/kg), and then intravenous propofol (4 mg/kg) to induce anesthesia. The animals were then intubated with a 3 to 3.5-mm endotracheal tube and maintained on 2% to 3.5% isoflurane and oxygen throughout the scan. During the scan, body temperature was maintained at 37 °C by using a heating water pad with a pumping device to circulate the heated water. in vivo DTI studies were performed on a 3T Tim Trio MRI scanner (Siemens, Erlangen, Germany) equipped with a single-channel 11-cm internal diameter transmit-receive birdcage coil (M2M). Initially, localizer images were acquired to determine orientation and position of the brain. Anatomical axial T1- and T2-weighted images were acquired with an FOV = 80 x 100 mm2, number of slices = 20, slice thickness = 2 mm. DTI images were acquired using a 30-direction single shot spin echo echo planar imaging (EPI)-based sequence with following parameters: FOV = 80 x 100 mm2, slice thickness = 2 mm, number of slices = 20, TR = 3000 ms, TE = 75 ms, Avg = 8, concatenations = 1, b-values: 0 and 1000 s/mm2 with approximately 13-minute acquisition time. Physiological monitoring including pulse oximetry and vital signs (oxygen saturation and heart rate) were recorded before and during scanning.
Histopathology
AMD-affected and wild type cat brain samples were randomly chosen from each group for histological examination after performing the ex vivo DTI experiment. Tissues were paraffin embedded and 5-µm sections were cut. For routine histology, sections were stained with hematoxylin and eosin (5) or Luxol-fast blue (LFB) by the pathology core at the Children’s Hospital of Philadelphia. For immunoh-istochemistry, sections were deparaffinized, permeabilized, and blocked for 30 minutes in 4% goat or horse serum in PBS-T (PBS containing 0.3% Triton™ X-100). The sections were then incubated overnight at 4 °C with the following primary antibodies: chicken anti-glial fibrillary acidic protein (GFAP) (1:500, AB5541, Millipore, Temecula, CA) or goat anti-Iba1 (1:500, AB5076, Abcam, Cambridge, MA). After 3 washes in PBS-T, sections were incubated with the appropriate biotinylated secondary antibodies (goat anti-chicken or horse anti-goat, 1:250, Vector Laboratories, Burlingame, CA) for 45 minutes followed by PBS-T washes. The antibody binding was visualized using VECTASTAIN Elite ABC reagent and 3, 3’-diaminobenzidine substrate kit for peroxidase (Vector Laboratories). Sections were then dehydrated and mounted in Cytoseal™ 60 mounting medium (Richard Allen Scientific, Kalamazoo, MI) with glass coverslips. Whole slides were scanned at 20x or 40x magnification using the Aperio Scan Scope® (Aperio, Vista, CA). To evaluate the degenerative process, Fluoro-Jade® C staining (AG325, Millipore, Temecula, CA) was used. Sections were dehydrated in basic alcohol solution containing 80% ethanol, followed by 70% ethanol and rinsed in distilled water. Slides were then incubated for 10 minutes in 0.06% potassium permanganate, washed for 1 minute in distilled water and incubated in 0.0001% Fluoro-Jade C staining solution for 10 minutes. Images were visualized under an Fluorescein isothiocyanate (FITC) filter using a Leica AF6000 LX microscope (Leica, Heerbrugg, Switzerland) and acquired using a DFC 360FX camera (Leica). The digital images used identical intensity scales for all images.
Image Processing and Quantification
For the ex vivo DTI data, image reconstruction was performed offline using in-house custom software developed in the interface definition language (IDL) programming environment (ITT Visual Information Solutions, Boulder, CO). Briefly, the process included application of a Gaussian filter and 2D Fourier transformation. The magnitude images from each echo train were then summed to improve the signal-to-noise ratio of the images. Data sets acquired with opposite polarity diffusion gradients were combined as described elsewhere (20) to minimize contributions of background gradients. The resulting group of images were saved in DTI studio (21) file format for further processing. The Camino toolkit (22) was used to reconstruct the diffusion tensors from the data set along with relevant scalar metrics including FA, MD, AD, and RD. In order to define a reference anatomical space to define regions of interest (ROIs), a template image was created using the ANTs toolkit (23) and the b = 0 image from each subject. An initial template was defined by averaging all inputs. Each input was then aligned to the template using the cross-correlation metric and a diffeomorphic transformation. The set of all warped inputs were then averaged to create a new template. This process was iterated 3 times to define a final template image. A threshold signal intensity was chosen to create a brain segmentation template that excluded background signal. For each sample, the FA image was thresholded at 0.2 and the resulting binary image was used as a set of seeds for whole-brain deterministic approach using Camino. Each set of fibers was used to create an image of streamline density. These streamline density images were then warped into the template space, averaged, and normalized to create a probabilistic segmentation of the total white matter. A corresponding probabilistic gray matter mask was created by subtracting the white matter from the whole brain area (Fig. 1A–C). To assess regional differences in individual gray and white matter areas, ROI based analyses were performed separately. The brain template was used to draw bilateral ROIs on the left and right side of the brain in each region except the corpus callosum (where a single ROI on the mid axial slice of the color-coded brain map was drawn). Six gray matter (frontal cortex, cingulate gyrus, caudate nucleus, hippocampus, thalamus, and occipital cortex) and 5 white matter regions (corpus callosum, corticospinal tract, cerebral peduncle, external and internal capsule) were chosen from the template image. The individual ROI files were saved as image files to extract the FA, MD, AD, and RD values from individual data sets from both controls and affected samples.
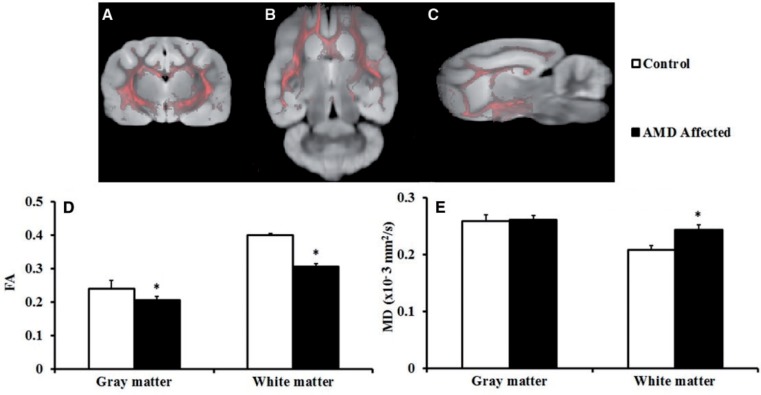
FIGURE 1.
Segmentation of gray and white matter from whole brain. (A–C) Pink color represents white matter; gray color represents gray matter. Image orientations are axial (A), sagittal (B), and coronal (C). (D, E) Bar graphs demonstrate average ex vivo diffusion tensor imaging-derived fractional anisotropy (FA) and mean diffusivity (MD) values from gray and white matter of the α-mannosidosis (AMD)-affected and normal control cat brains. *p < 0.05. Error bars represent ± SE.
The in vivo images were transferred offline for further processing and analysis. DicomWorks (version 1.3.5) was used to organize and rename the raw data files. After renaming, the in vivo DTI data were processed and analyzed using DTI studio software (Johns Hopkins School of Medicine, www.mristudio.org) (21). ROI-based analysis was performed on same regions as mentioned above for ex vivo DTI to compute FA, MD, AD, and RD values for normal control and AMD-affected cats (Fig. 2).
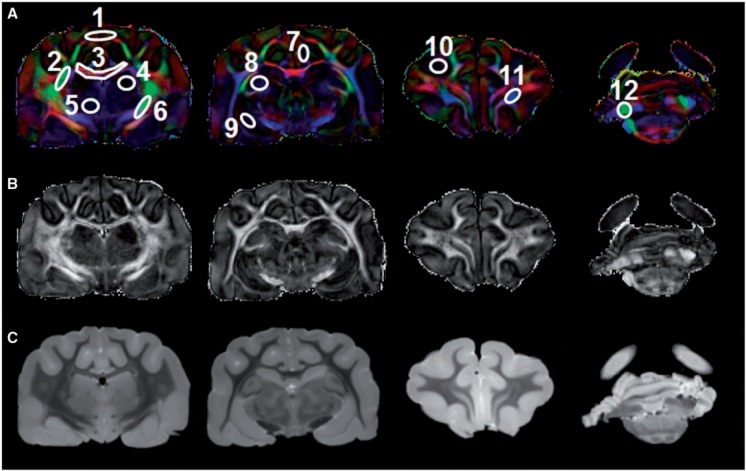
FIGURE 2.
Placement of regions of interest (ROI) in the gray and white matter areas used for both ex vivo and in vivo diffusion tensor imaging (DTI) data analysis. (A–C) The ROIs are shown overlaid on fractional inosotropy (FA)-weighted color maps (A) in which red is right-left, green is anterior-posterior, and blue denotes the superior-inferior direction. Representative images from a multislice imaging sequence showing gray scale FA maps (B) and mean diffusivity maps (C). 1 = frontal cortex; 2 = external capsule; 3 = corpus callosum; 4 = hippocampus; 5 = thalamus; 6 = internal capsule; 7 = cingulate gyrus; 8 = caudate nucleus; 9 = putamen; 10 = occipital cortex; 11 = corticospinal tract; 12 = cerebral peduncle.
Statistical Analysis
All statistical computations were performed using statistical package for social sciences (SPSS, version 16.0 SPSS, Inc., Chicago, IL). An independent Student t-test was performed on ex vivo DTI data to assess differences in FA, MD, AD, and RD values from the entire gray and white matter between control and AMD-affected cats. Ex vivo and in vivo DTI data from ROI-based analysis were also compared using independent Student t-test between AMD-affected and control cats. A p value of ≤ 0.05 was considered to be statistically significant.
RESULTS
Ex vivo DTI analysis was performed to maximize detection of differences between affected AMD cat brains and normal control brains. The ex vivo DTI analysis showed significantly lower FA from both the gray (0.21 ± 0.01, p = 0.04) and white matter (0.31 ± 0.01, p = ≤ 0.001) in AMD-affected compared to normal control cat brains (gray matter = 0.24 ± 0.03 and white matter = 0.40 ± 0.01), respectively (Fig. 1D). Along with significantly lower FA values, we also observed significantly higher AD and RD values in gray and white matter regions in AMD-affected cat brains (Table 1). The reduced FA with increased AD and RD values in AMD-affected cats are suggestive of demyelination or myelin loss, axonal loss, and gliosis, which are frequently seen on histological examination in AMD (24). When individual areas within the gray and white matter regions were analyzed, significantly lower FA and higher AD and RD values were observed in all analyzed white matter regions including the corpus callosum, corticospinal tract, cerebral peduncle, external and internal capsule in AMD-affected cats compared to normal controls (Table 1).
TABLE 1.
Ex Vivo Diffusion Tensor Imaging Indices From Various Gray and White Matter Regions of the Brain From Normal Healthy Controls and AMD-Affected Cats
| White Matter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Side | FA |
MD x10−3mm2/s |
AD x10−3mm2/s |
RD x10−3mm2/s |
||||
| Control | AMD | Control | AMD | Control | AMD | Control | AMD | ||
| 1 | 0.42 ± 0.04 | 0.33 ± 0.02** | 0.21 ± 0.02 | 0.28 ± 0.02* | 0.31 ± 0.04 | 0.38 ± 0.04* | 0.17 ± 0.02 | 0.24 ± 0.03** | |
| 2 | L | 0.43 ± 0.02 | 0.36 ± 0.02** | 0.19 ± 0.02 | 0.24 ± 0.02** | 0.27 ± 0.03 | 0.32 ± 0.02* | 0.14 ± 0.02 | 0.19 ± 0.02** |
| R | 0.41 ± 0.02 | 0.33 ± 0.03* | 0.18 ± 0.02 | 0.24 ± 0.02* | 0.28 ± 0.03 | 0.32 ± 0.02* | 0.14 ± 0.02 | 0.19 ± 0.02* | |
| 3 | L | 0.48 ± 0.05 | 0.38 ± 0.02** | 0.18 ± 0.02 | 0.22 ± 0.01** | 0.28 ± 0.03 | 0.32 ± 0.01** | 0.13 ± 0.02 | 0.17 ± 0.01** |
| R | 0.46 ± 0.05 | 0.36 ± 0.02** | 0.18 ± 0.03 | 0.22 ± 0.01** | 0.27 ± 0.03 | 0.31 ± 0.01* | 0.13 ± 0.02 | 0.18 ± 0.01** | |
| 4 | L | 0.41 ± 0.03 | 0.32 ± 0.01** | 0.19 ± 0.02 | 0.24 ± 0.01** | 0.27 ± 0.02 | 0.31 ± 0.02* | 0.15 ± 0.02 | 0.20 ± 0.01** |
| R | 0.41 ± 0.03 | 0.31 ± 0.02** | 0.18 ± 0.02 | 0.24 ± 0.02* | 0.27 ± 0.03 | 0.32 ± 0.03* | 0.14 ± 0.02 | 0.20 ± 0.01** | |
| 5 | L | 0.41 ± 0.04 | 0.31 ± 0.04* | 0.19 ± 0.02 | 0.25 ± 0.04 | 0.28 ± 0.04 | 0.33 ± 0.04 | 0.15 ± 0.02 | 0.21 ± 0.04 |
| R | 0.40 ± 0.06 | 0.32 ± 0.03** | 0.18 ± 0.02 | 0.23 ± 0.02* | 0.27 ± 0.04 | 0.32 ± 0.04 | 0.14 ± 0.02 | 0.19 ± 0.03* | |
| Gray Matter | |||||||||
| 6 | L | 0.21 ± 0.06 | 0.17 ± 0.02 | 0.22 ± 0.02 | 0.26 ± 0.01 | 0.27 ± 0.02 | 0.30 ± 0.08 | 0.20 ± 0.02 | 0.24 ± 0.07 |
| R | 0.22 ± 0.08 | 0.19 ± 0.02 | 0.22 ± 0.03 | 0.23 ± 0.05 | 0.26 ± 0.04 | 0.27 ± 0.06 | 0.19 ± 0.03 | 0.21 ± 0.05 | |
| 7 | L | 0.22 ± 0.03 | 0.20 ± 0.02 | 0.24 ± 0.02 | 0.25 ± 0.02 | 0.29 ± 0.02 | 0.30 ± 0.02 | 0.22 ± 0.02 | 0.23 ± 0.02 |
| R | 0.21 ± 0.03 | 0.21 ± 0.03 | 0.25 ± 0.02 | 0.26 ± 0.01 | 0.30 ± 0.03 | 0.32 ± 0.02 | 0.23 ± 0.02 | 0.24 ± 0.01 | |
| 8 | L | 0.22 ± 0.03 | 0.22 ± 0.03 | 0.25 ± 0.02 | 0.27 ± 0.03 | 0.30 ± 0.02 | 0.33 ± 0.03 | 0.22 ± 0.02 | 0.24 ± 0.03 |
| R | 0.24 ± 0.03 | 0.25 ± 0.04 | 0.25 ± 0.02 | 0.26 ± 0.02 | 0.31 ± 0.02 | 0.33 ± 0.02 | 0.22 ± 0.02 | 0.23 ± 0.02 | |
| 9 | L | 0.22 ± 0.04 | 0.20 ± 0.03 | 0.25 ± 0.02 | 0.32 ± 0.05 | 0.31 ± 0.03 | 0.38 ± 0.05* | 0.22 ± 0.02 | 0.29 ± 0.05 |
| R | 0.25 ± 0.04 | 0.22 ± 0.04 | 0.24 ± 0.02 | 0.29 ± 0.02** | 0.30 ± 0.03 | 0.35 ± 0.03* | 0.21 ± 0.02 | 0.26 ± 0.01** | |
| 10 | L | 0.19 ± 0.05 | 0.14 ± 0.04 | 0.22 ± 0.04 | 0.25 ± 0.02 | 0.26 ± 0.04 | 0.28 ± 0.02 | 0.20 ± 0.03 | 0.23 ± 0.02 |
| R | 0.20 ± 0.05 | 0.16 ± 0.03 | 0.22 ± 0.03 | 0.26 ± 0.03 | 0.27 ± 0.04 | 0.30 ± 0.03 | 0.20 ± 0.03 | 0.24 ± 0.02* | |
| 11 | L | 0.21 ± 0.01 | 0.12 ± 0.01** | 0.21 ± 0.02 | 0.25 ± 0.01** | 0.26 ± 0.03 | 0.28 ± 0.01* | 0.19 ± 0.02 | 0.23 ± 0.01** |
| R | 0.26 ± 0.03 | 0.19 ± 0.02** | 0.21 ± 0.02 | 0.24 ± 0.02* | 0.26 ± 0.03 | 0.28 ± 0.02 | 0.18 ± 0.02 | 0.23 ± 0.01* | |
| 12 | L | 0.20 ± 0.01 | 0.13 ± 0.01** | 0.21 ± 0.02 | 0.25 ± 0.01** | 0.26 ± 0.03 | 0.28 ± 0.01* | 0.19 ± 0.02 | 0.23 ± 0.01** |
| R | 0.22 ± 0.04 | 0.19 ± 0.05 | 0.21 ± 0.02 | 0.23 ± 0.02 | 0.25 ± 0.02 | 0.27 ± 0.01 | 0.19 ± 0.02 | 0.21 ± 0.01 | |
1 = corpus callosum; 2 = external capsule; 3 = internal capsule; 4 = corticospinal tract; 5 = cerebral peduncle; 6 = cerebral cortex; 7 = cingulate gyrus; 8 = occipital cortex; 9 = hippocampus; 10 = caudate nucleus; 11 = putamen; 12 = thalamus.
L, left side of brain; R, right side of the brain; AD, axial diffusivity; FA, fractional inosotropy; MD, mean diffusivity; RD, radial diffusivity; ROI, region of interest; AMD, α-mannosidosis.
p ≤ 0.05.
p ≤ 0.005.
The ex vivo DTI data also revealed significantly higher MD values from the whole brain white matter in AMD-affected cat brains (0.24 ± 0.01, p = 0.02) compared to controls (0.21 ± 0.01), while no significant differences in MD values were observed in gray matter regions between AMD-affected (0.26 ± 0.01) compared to wild type control (0.26 ± 0.01, p = 0.87) cats (Fig. 1E). On regional analysis, significantly higher MD values were observed in all white matter regions including corticospinal tract, cerebral peduncle, external and internal capsules in AMD-affected cats (Table 1). Some of the gray matter regions also demonstrated significantly higher MD values in AMD-affected cats including left and right putamen, right hippocampus, and left thalamus (Table 1).
In vivo DTI was performed at 3T to determine if changes in the diseased brain could be detected in a clinical magnet, as a potential adjunct to therapy and diagnosis. The in vivo DTI data (Table 2) demonstrated similar results in FA, AD, and RD values as were observed in the ex vivo DTI studies. Lower FA and higher AD and RD values were observed in all the analyzed white matter regions in AMD-affected compared to normal control cats. Specifically, significantly lower FA (p = 0.01) and higher AD (p = 0.04) and RD (p = 0.04) values were observed in the corpus callosum of AMD-affected cats (Table 2). We also observed significantly reduced FA and increased AD values in left and right cerebral peduncle in AMD-affected cats compared to controls (Table 2). However, only a slight but nonsignificant reduction in FA value was noted in all gray matter regions of AMD-affected compared to control cats. Similar to white matter regions, higher but nonsignificant differences in AD and RD values were noted from most of the gray matter regions in AMD-affected compared to normal controls (Table 2).
TABLE 2.
In Vivo Diffusion Tensor Imaging Indices From Various Gray and White Matter Regions of the Brain From Normal Healthy Controls and AMD-Affected Cats
| White Matter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Side | FA |
MD x10−3mm2/s |
AD x10−3mm2/s |
RD x10−3mm2/s |
||||
| Control | AMD | Control | AMD | Control | AMD | Control | AMD | ||
| 1 | 0.26 ± 0.03 | 0.16 ± 0.02* | 0.48 ± 0.08 | 0.43 ± 0.06 | 0.97 ± 0.03 | 1.28 ± 0.13* | 0.83 ± 0.08 | 1.04 ± 0.09* | |
| 2 | L | 0.56 ± 0.06 | 0.39 ± 0.05* | 0.35 ± 0.01 | 0.34 ± 0.01 | 0.94 ± 0.03 | 1.10 ± 0.03* | 0.49 ± 0.01 | 0.61 ± 0.03* |
| R | 0.57 ± 0.08 | 0.39 ± 0.06* | 0.37 ± 0.03 | 0.37 ± 0.03 | 0.95 ± 0.03 | 1.20 ± 0.03* | 0.46 ± 0.02 | 0.53 ± 0.01* | |
| 3 | L | 0.53 ± 0.02 | 0.42 ± 0.07* | 0.37 ± 0.01 | 0.37 ± 0.04 | 0.91 ± 0.06 | 1.07 ± 0.01* | 0.48 ± 0.03 | 0.62 ± 0.06* |
| R | 0.60 ± 0.05 | 0.44 ± 0.06* | 0.36 ± 0.01 | 0.35 ± 0.01 | 0.93 ± 0.05 | 1.23 ± 0.09* | 0.43 ± 0.03 | 0.55 ± 0.04* | |
| 4 | L | 0.38 ± 0.11 | 0.30 ± 0.06 | 0.36 ± 0.01 | 0.35 ± 0.01 | 0.87 ± 0.02 | 1.03 ± 0.15 | 0.76 ± 0.21 | 0.67 ± 0.05 |
| R | 0.40 ± 0.10 | 0.26 ± 0.05 | 0.35 ± 0.01 | 0.36 ± 0.01 | 0.90 ± 0.07 | 1.12 ± 0.06* | 0.71 ± 0.07 | 0.69 ± 0.11 | |
| 5 | L | 0.38 ± 0.04 | 0.29 ± 0.02* | 0.36 ± 0.05 | 0.35 ± 0.01 | 0.81 ± 0.03 | 1.16 ± 0.11** | 0.56 ± 0.08 | 0.73 ± 0.28 |
| R | 0.44 ± 0.04 | 0.31 ± 0.05* | 0.47 ± 0.14 | 0.41 ± 0.05 | 0.83 ± 0.06 | 1.19 ± 0.05* | 0.63 ± 0.05 | 0.78 ± 0.17 | |
| Gray Matter | |||||||||
| 6 | L | 0.16 ± 0.02 | 0.14 ± 0.04 | 0.53 ± 0.17 | 0.46 ± 0.11 | 1.00 ± 0.14 | 1.18 ± 0.23 | 1.00 ± 0.26 | 0.86 ± 0.11 |
| R | 0.17 ± 0.03 | 0.12 ± 0.02 | 0.48 ± 0.01 | 0.46 ± 0.08 | 0.99 ± 0.06 | 1.13 ± 0.22 | 0.94 ± 0.21 | 0.85 ± 0.07 | |
| 7 | L | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.54 ± 0.10 | 0.49 ± 0.07 | 0.88 ± 0.03 | 0.93 ± 0.09 | 0.79 ± 0.06 | 0.70 ± 0.09 |
| R | 0.16 ± 0.01 | 0.16 ± 0.02 | 0.43 ± 0.02 | 0.40 ± 0.03 | 1.30 ± 0.62 | 1.08 ± 0.20 | 0.86 ± 0.15 | 1.01 ± 0.59 | |
| 8 | L | 0.15 ± 0.02 | 0.12 ± 0.02 | 0.48 ± 0.01 | 0.41 ± 0.01* | 0.95 ± 0.15 | 1.19 ± 0.09 | 1.02 ± 0.10 | 0.78 ± 0.16 |
| R | 0.14 ± 0.03 | 0.13 ± 0.05 | 0.47 ± 0.08 | 0.47 ± 0.07 | 0.88 ± 0.01 | 1.04 ± 0.05* | 0.97 ± 0.06 | 0.74 ± 0.05 | |
| 9 | L | 0.18 ± 0.05 | 0.16 ± 0.06 | 0.54 ± 0.14 | 0.42 ± 0.07 | 0.82 ± 0.22 | 0.85 ± 0.01 | 0.68 ± 0.01 | 0.82 ± 0.09 |
| R | 0.19 ± 0.04 | 0.12 ± 0.04 | 0.47 ± 0.10 | 0.46 ± 0.07 | 1.09 ± 0.22 | 0.88 ± 0.02 | 0.67 ± 0.02 | 1.04 ± 0.30 | |
| 10 | L | 0.21 ± 0.04 | 0.16 ± 0.03 | 0.60 ± 0.12 | 0.36 ± 0.01 | 0.89 ± 0.04 | 1.04 ± 0.22 | 0.82 ± 0.24 | 0.72 ± 0.06 |
| R | 0.22 ± 0.03 | 0.17 ± 0.02 | 0.51 ± 0.01 | 0.36 ± 0.01* | 1.09 ± 0.34 | 1.12 ± 0.28 | 0.92 ± 0.28 | 0.92 ± 0.33 | |
| 11 | L | 0.17 ± 0.05 | 0.17 ± 0.03 | 0.43 ± 0.05 | 0.37 ± 0.01 | 0.85 ± 0.05 | 0.96 ± 0.11 | 0.76 ± 0.09 | 0.72 ± 0.05 |
| R | 0.19 ± 0.02 | 0.17 ± 0.01 | 0.51 ± 0.12 | 0.40 ± 0.06 | 0.92 ± 0.19 | 1.04 ± 0.16 | 0.77 ± 0.17 | 0.77 ± 0.18 | |
| 12 | L | 0.22 ± 0.06 | 0.21 ± 0.02 | 0.39 ± 0.05 | 0.44 ± 0.07 | 1.02 ± 0.05 | 0.91 ± 0.06 | 0.69 ± 0.03 | 0.74 ± 0.03 |
| R | 0.22 ± 0.02 | 0.21 ± 0.01 | 0.38 ± 0.05 | 0.44 ± 0.02 | 1.05 ± 0.16 | 0.90 ± 0.03 | 0.74 ± 0.12 | 0.81 ± 0.12 | |
1 = corpus callosum, 2 = external capsule, 3 = internal capsule, 4 = corticospinal tract, 5 = cerebral peduncle, 6 = cerebral cortex, 7 = cingulate gyrus, 8 = occipital cortex, 9 = hippocampus, 10 = caudate nucleus, 11 = putamen, 12 = thalamus.
L, left side of brain; R, right side of the brain; AD, axial diffusivity; FA, fractional inosotropy; MD, mean diffusivity; RD, radial diffusivity; ROI, region of interest; AMD, α-mannosidosis.
p ≤ 0.05
p ≤ 0.005
Lower MD values from in vivo DTI data were observed in all gray and white matter regions of AMD cats, which is consistent with previous diffusion-weighted imaging (DWI) studies from our group (6). These differences, however, were only significant from the left occipital cortex (p = 0.02) and right caudate nucleus (p = 0.03), respectively (Table 2).
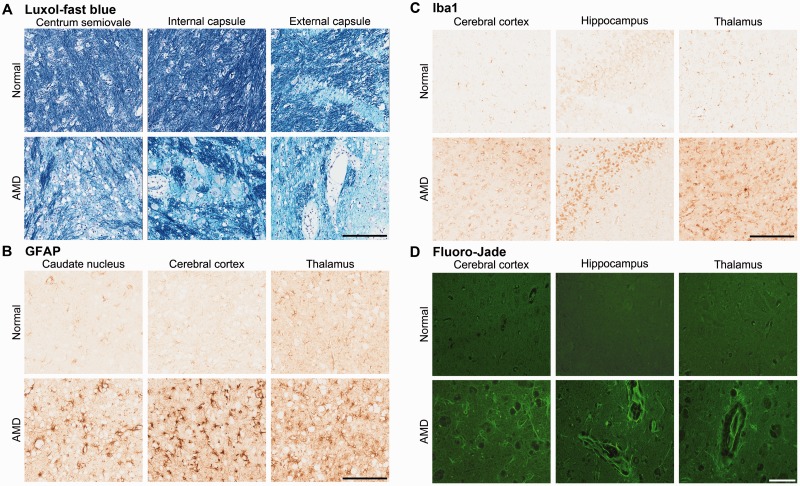
Neuropathological alterations in the brains of AMD cats were also observed. Hematoxylin and eosin-stained sections showed cytoplasmic vacuolation and distension of neurons and astrocytes caused by lysosomal storage throughout the brain in the AMD-affected cats (Fig. 3). LFB staining showed abnormalities in myelin with decreased stain intensity and distention and splitting of myelin sheaths in all white matter regions (Fig. 4A). GFAP immunohistochemistry showed reactive astrogliosis (Fig. 4B), and Iba1 immunohistoch-emistry showed increased microglia, indicative of the reaction commonly observed in neurogenetic diseases (Fig. 4C). Fluoro-Jade C staining of the neuron cell bodies and neurites indicated neurodegeneration in the AMD cats (Fig. 4D).
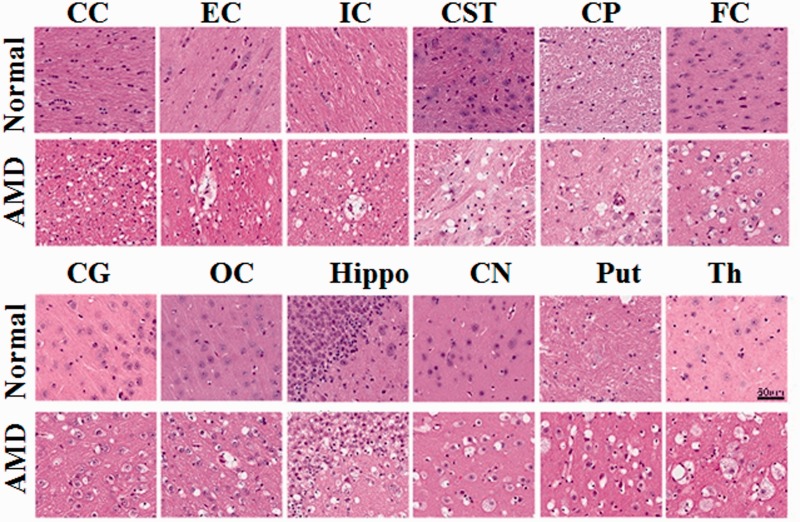
FIGURE 3.
Hematoxylin and eosin staining of brain sections from α-mannosidosis (AMD)-affected (top rows) and wild type normal (bottom rows) cats using 5-µm-thick paraffin sections. The vacuolation in the AMD brain sections represents distended lysosomes, which are present throughout the brains of AMD cats. Regions corresponding to areas of image analysis used in MRI experiments are shown. Scale bars are 60 µm for each histology micrograph. CC = corpus callosum; EC = external capsule; IC = internal capsule; CST = corticospinal tract; CP = cerebral peduncle; FC = frontal cortex; CG = cingulate gyrus; OC = occipital cortex; Hippo = hippocampus; CN = caudate nucleus; Put = putamen; Th = thalamus.
FIGURE 4.
Histopathology and immunohistochemistry. (A–D) Pathology of the AMD cat brains. Luxol-fast blue staining of white matter regions (A), Gray matter regions immunostained for glial fibrillary acidic protein (GFAP) for reactive astrocytes (B), Iba1 immunohistochemistry for microglia (C), and Fluoro-Jade C staining for neurodegeneration (D). Scale bars: A–C, 200 μm; D, 60 μm.
DISCUSSION
In the current study, DTI demonstrated significantly lower FA and higher AD and RD values from most of the white matter regions suggestive of increased gliosis and neurodegeneration, demyelination, myelin loss, and axonal loss in AMD-affected cats, as expected in this model (4, 8, 25, 26). Pathological evaluation of the brain tissue from AMD-affected cats demonstrated cytoplasmic vacuolation, myelin alterations, reactive gliosis, and the presence of neurodegeneration. These findings qualitatively correlated with the imaging findings indicating that the use of DTI in vivo may further help in understanding the pathophy-siological conditions and the brain structural abnormalities in this disease (18, 25).
Our previous study on diffusion-weighted MRI found lower apparent diffusion coefficient (ADC) or MD in both the white and gray matter of AMD cats (6). Although not significantly different, the MD values from the present in vivo studies in AMD cats were also lower (except the thalamus, which were nonsignificantly higher) than the control cats (Table 2). We note that these differences are smaller than our previously published study. However, there were technical differences between the 2 studies including differences in the field strength (4.7 T vs 3.0 T), data acquisition sequence (ADCav measured using a spin echo DWI sequence with 4 b-values versus MD measured from an echo planar spin echo based DTI sequence with only 2 b-values. Despite the differences in acquisition parameters and field strengths used, it is encouraging to note that the trend of decreased ADC (MD) values is similar in the 2 studies.
In contrast, our ex vivo DTI studies showed signi-ficantly higher MD values from all the white matter regions and a trend towards higher MD in most of the gray matter regions in AMD-affected brains. The differences between the in vivo and ex vivo data are probably due to the difference in measuring ADC in fixed tissue versus live animals (27) because the fixatives used and the time of fixation alter the mobility of water molecules, which imparts variability in the measurements of ADC values (28). It is interesting to note that the differences in FA, AD, and RD values between AMD and normal controls were similar between in vivo and ex vivo studies suggesting that these parameters are more robust for detecting changes in this model.
MRI studies including diffusion-weighted MRI and magnetization transfer ratio studies have shown white matter abnormalities in AMD cats indicative of dysmyelination (5, 6). DTI provides additional information about the tissue microstructural integrity and orienation of white matter fiber tracts at the microscopic level. Alterations in DTI measures such as FA may reflect changes at the level of the axon, or in oligodendrocytes and myelin sheath. Normal myelination was differentially disrupted in AMD-affected cats, being particularly reduced across the external and internal capsule, corticospinal tract, callosum, and cerebellar white matter as observed in LFB stains. This is consistent with our imaging findings of widespread reduction in FA, which may be the result of perturbed axonal structure and organization, and reduced myelin (29). The directional diffusivities (AD and RD) derived from DTI hold promise as specific markers of axonal damage and demyelination in various neurological disorders. RD, the diffusion of water perpendicular to white matter fibers, increases in response to demyelination (30) and dysmyelination (31, 32). AD measures the diffusion of water molecule parallel to white matter, specifically to axonal fibers, and has been shown to be sensitive in assessing neurodegeneration. The increases in RD values in most regions of the brain in AMD-affected cats suggest widespread dysmyelination (30), whereas altered AD suggests axonal degeneration and/or reorganization (33), both of which are consistent with the white matter alterations in AMD (5, 6).
Alterations in FA, AD, and RD values in AMD-affected cats probably reflect structural perturbations due to accumulation of undegraded oligosaccharides, which is seen in MR spectroscopy (4). The intracellular accumulation of undigested oligosaccharides may also lead to diffuse gliosis and cytoplasmic vacuolation throughout the brains of AMD-affected cats. These cytoplasmic vacuoles represent lysosomes distended with the stored, undegraded mannose-containing oligosaccharides. We believe that the myelin abnormalities and gliosis may be reflected in alternations in the DTI parameters. The accumulation of undegraded substrate throughout the brain may be related to the cellular dysfunction that accompanies LSDs, although the precise mechanisms underlying the neurodegeneration observed are incompletely understood.
The clinical signs of feline AMD can also be partly explained with our DTI finding. Psychomotor dysfunction with variable degree of ataxia has been reported in humans as well in various animal models of AMD (34). The corticospinal tracts, including external and internal capsules, are mainly responsible for locomotion and coordination (35), and our imaging findings of reduced FA and increased AD and RD values along with immunohistological staining from these regions in AMD-affected cats support the corticopinal tract involvement. Similarly, significantly reduced FA in the corpus callosum in AMD-affected cats indicates reduction in the myelin sheath, reduced axonal fibers, or reduced density in the connecting fibers, which suggests that there are abnormalities in the structural connectivity across the brain hemispheres involved with functional deficits in motor ability and impaired cognition (36).
Activation of astrocytes and microglia is a common feature of neurodegenerative diseases including LSDs (37, 38), as well as in AMD-affected individuals (6, 24). Microglial activation due to the accumulation of primary or secondary storage material as well as exogenous stimuli of inflammation and/or phagocytosis may contribute to this (39). Significant reduction in FA values in the putamen and thalamus regions may be due to cell swelling and astrogliosis in AMD-affected cats; myelin abnormalities may also affect the gray matter structures (40). Our findings indicate that DTI may further aid in understanding the gray matter abnormalities in AMD.
In summary, the reduced FA and elevated AD and RD values from AMD-affected cats compared to controls suggest that the abnormalities in both the white and gray matter can be assessed in this model and that DTI studies can aid in further characterization of this disease model. Because DTI is commonly performed in the clinic, these studies may also be useful in clinical assessment of AMD, and potentially other LSDs, as well as be used to monitor disease progression and response to therapy.
REFERENCES
- 1. Thomas GH. Disorders of glycoprotein degradation: α-Mannosidosis, β-mannosidosis, Fucosidosis and sialidosis In: Scriver AR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001:3507–34 [Google Scholar]
- 2. Sun H, Wolfe JH. Recent progress in lysosomal α-mannosidase and its deficiency. Exp Mol Med 2001:33;1–7 [DOI] [PubMed] [Google Scholar]
- 3. Michalski JC, Klein A. Glycoprotein lysosomal storage disorders: α- and β-mannosidosis, fucosidosis and α-N-acetylgalactosaminidase deficiency. Biochim Biophys Acta 1999:1455;69–84 [DOI] [PubMed] [Google Scholar]
- 4. Magnitsky S, Vite CH, Delikatny EJ, et al. Magnetic resonance spectroscopy of the occipital cortex and the cerebellar vermis distinguishes individual cats affected with α-mannosidosis from normal cats. NMR Biomed 2010:23;74–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vite CH, McGowan JC, Braund KG, et al. Histopathology, electrodiagnostic testing, and magnetic resonance imaging show significant peripheral and central nervous system myelin abnormalities in the cat model of α-mannosidosis. J Neuropathol Exp Neurol 2001:60;817–28 [DOI] [PubMed] [Google Scholar]
- 6. Vite CH, Magnitsky S, Aleman D, et al. Apparent diffusion coefficient reveals gray and white matter disease, and T2 mapping detects white matter disease in the brain in feline α-mannosidosis. Am J Neuroradiol 2008:29;308–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walkley SU, Thrall MA, Dobrenis K, et al. Bone marrow transplantation corrects the enzyme defect in neurons of the central nervous system in a lysosomal storage disease. Proc Natl Acad Sci USA 1994:91;2970–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vite CH, McGowan JC, Niogi SN, et al. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol 2005:57;355–64 [DOI] [PubMed] [Google Scholar]
- 9. Sun H, Yang M, Haskins ME, et al. Retrovirus vector-mediated correction and cross-correction of lysosomal α-mannosidase deficiency in human and feline fibroblasts. Hum Gene Ther 1999:10;1311–9 [DOI] [PubMed] [Google Scholar]
- 10. Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics 2007:4;316–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: Apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 1998:209;57–66 [DOI] [PubMed] [Google Scholar]
- 12. Aggarwal M, Mori S, Shimogori T, et al. Three-dimensional diffusion tensor microimaging for anatomical characterization of the mouse brain. Magn Reson Med 2010:64;249–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar M, Gupta RK, Saksena S, et al. A diffusion tensor imaging study of deep gray and white matter brain maturation differences between patients with spina bifida cystica and healthy controls. J Clin Neurosci 2010:17;879–85 [DOI] [PubMed] [Google Scholar]
- 14. Kumar M, Kim S, Pickup S, et al. Longitudinal in-vivo diffusion tensor imaging for assessing brain developmental changes in BALB/cJ mice, a model of reduced sociability relevant to autism. Brain Res 2012:1455;56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo AC, Petrella JR, Kurtzberg J, et al. Evaluation of white matter anisotropy in Krabbe disease with diffusion tensor MR imaging: Initial experience. Radiology 2001:218;809–15 [DOI] [PubMed] [Google Scholar]
- 16. Larsson EM, Englund E, Sjobeck M, et al. MRI with diffusion tensor imaging post-mortem at 3.0 T in a patient with frontotemporal dementia. Dement Geriatr Cogn Disord 2004:17;316–9 [DOI] [PubMed] [Google Scholar]
- 17. Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J Mol Neurosci 2008:34;51–61 [DOI] [PubMed] [Google Scholar]
- 18. Kumar M, Nasrallah IM, Kim S, et al. High-resolution magnetic resonance microscopy and diffusion tensor imaging to assess brain structural abnormalities in the murine mucopolysaccharidosis VII model. J Neuropathol Exp Neurol 2014:73;39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berg T, Tollersrud OK, Walkley SU, et al. Purification of feline lysosomal α-mannosidase, determination of its cDNA sequence and identification of a mutation causing α-mannosidosis in Persian cats. Biochem J 1997:328;863–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neeman M, Freyer JP, Sillerud LO. A simple method for obtaining cross-term-free images for diffusion anisotropy studies in NMR microimaging. Magn Reson Med 1991:21;138–43 [DOI] [PubMed] [Google Scholar]
- 21. Jiang H, van Zijl PC, Kim J, et al. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 2006:81;106–16 [DOI] [PubMed] [Google Scholar]
- 22. Cook PA BY, Nedjati-Gilani S, et al. Camino: Open-Source Diffusion-MRI Reconstruction and Processing. International Society for Magentic Resonance in Medicine. Seattle, WA, USA: International Society for Magentic Resonance in Medicine; 2006:P2759 [Google Scholar]
- 23. Avants BB, Yushkevich P, Pluta J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage 2010:49;2457–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vite CH, Cross JR. Correlating magnetic resonance findings with neuropathology and clinical signs in dogs and cats. Vet Radiol Ultrasound 2011:52;S23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001:13;534–46 [DOI] [PubMed] [Google Scholar]
- 26. Kumar M, Rathore RK, Srivastava A, Yadav SK, Behari S, Gupta RK. Correlation of diffusion tensor imaging metrics with neurocognitive function in Chiari I malformation. World Neurosurg 2011:76;189–94 [DOI] [PubMed] [Google Scholar]
- 27. Sun SW, Neil JJ, Liang HF, et al. Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infracted brain. Magn Reson Med. 2005;53:1447–51 [DOI] [PubMed] [Google Scholar]
- 28. Sun SW, Neil JJ, Song SK. Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains. Magn Reson Med 2003:50;743–8 [DOI] [PubMed] [Google Scholar]
- 29. Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36:893–906 [DOI] [PubMed] [Google Scholar]
- 30. Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005; 26:132–40 [DOI] [PubMed] [Google Scholar]
- 31. Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–36 [DOI] [PubMed] [Google Scholar]
- 32. Nair G, Tanahashi Y, Low HP, et al. Myelination and long diffusion times alter diffusion-tensor imaging contrast in myelin-deficient shiverer mice. Neuroimage 2005;28:165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axons and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–22 [DOI] [PubMed] [Google Scholar]
- 34. D'Hooge R, Lullmann-Rauch R, Beckers T, et al. Neurocognitive and psychotiform behavioral alterations and enhanced hippocampal long-term potentiation in transgenic mice displaying neuropathological features of human α-mannosidosis. J Neurosci 2005:25;6539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simonato M, Bennett J, Boulis NM, et al. Gene therapy for neurological disorders. Nat Rev Neurol 2013:9;277–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steelman AJ, Thompson JP, Li J. Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication. Neurosci Res 2012:72;32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Damme M, Stroobants S, Walkley SU, et al. Cerebellar alterations and gait defects as therapeutic outcome measures for enzyme replacement therapy in α-mannosidosis. J Neuropathol Exp Neurol 2011;70:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeyakumar M, Dwek RA, Butters TD, et al. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci 2005;6:713–25 [DOI] [PubMed] [Google Scholar]
- 39. Walterfang M, Fahey M, Desmond P, et al. White and gray matter alterations in adults with Niemann-Pick disease type C: A cross-sectional study. Neurology 2010;75:49–56 [DOI] [PubMed] [Google Scholar]
- 40. Walterfang M, Fahey M, Abel L, et al. Size and shape of the corpus callosum in adult Niemann-Pick type C reflects state and trait illness variables. Am J Neuroradiol 2011:32;1340–6 [DOI] [PMC free article] [PubMed] [Google Scholar]