Abstract
Background/Aims
There are wide variations in colorectal cancer (CRC) incidence across the world. Historically, the highest incidence rates have been reported historically in more developed countries; however, increasing trends have been seen in developing countries. Here, we present the CRC incidence pattern in Cyprus, Israel, Jordan, and İzmir, Turkey, which are countries of the Middle East Cancer Consortium (MECC).
Materials and Methods
We analyzed 2005–2010 CRC data from population-based registries and calculated crude and age standardized rates for CRC, colon and rectum subsites, and annual percent changes (APCs) for trends.
Results
The age-adjusted incidence rates (AAIRs) for CRC were the highest in Israeli Jews (IJ) (46.7 for males and 35.5 for females), which exceeded those of the USA Surveillance, Epidemiology, and End Result (SEER) program registries. In both sexes, AAIRs in Cyprus and Israeli Arabs (IA) were close to those in SEER registries. For both sexes, AAIRs in İzmir and Jordan were substantially lower than those in other registries. Statistically significant decreasing trends over time were observed in AAIRs for both sexes in the SEER program (APCs: males, −3.24% and females, −2.54%), whereas the trends varied within the MECC registries. There were decreasing AAIR trends for males in IJ and IA and for females in Cyprus and IJ; APC for females in IJ (−4.29%) was significant. Conversely, increasing trends with the significant APCs were observed in males in İzmir (2.43%) and Jordan (7.57%).
Conclusion
MECC countries comprise both high- and low-risk populations for CRCs. However, increasing trends in low-risk populations have been alarming. Thus, the need for implementing tailored primary and secondary prevention programs in the region is essential.
Keywords: Colorectal cancer, incidence, Cyprus, Turkey, Jordan, Israel
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide. There is a 23-fold variation in the rates of CRC worldwide between low-incidence areas in Asia/ Africa and Western Europe, Australia, New Zealand, and North America. Historically high CRC incidence and mortality rates were observed in the more developed populations. However, despite the stable or slightly increasing (in France, Italy, and England) or even decreasing (in USA) incidence rates in developed countries, in the past few decades, there have been remarkable increases in CRC incidence in less-developed areas such as Eastern European countries, certain Asian countries, and several South American countries (1,2).
As for the subsites, geographic distributions of the incidence rates of colon and rectal cancers incidences separately maintain the same pattern which presents higher incidence rates in developed countries and lower rates in developing ones, but the variations for colon cancer are more striking. In high-risk populations, the ratio of colon to rectal cancer incidence is 2:1 or higher (more in females than in males). In low-risk countries, colon and rectal cancer rates are generally of the same magnitude. Epidemiological studies have shown that the CRC risk is significantly associated with family history, diet, and lifestyle (3). Historically, CRC has not been considered to be one of the major cancers in the Middle East, except among Jews in Israel (1,4,5). Knowing that CRC has been significantly increasing in the developing world (2) and that the populations of the Middle East are changing, we aimed to present descriptive data regarding the current trends of CRC incidence rates in the Middle East for identifying opportunities for interventions that are aimed at prevention and control.
MATERIALS AND METHODS
This study involved the analysis of population-based cancer registry data from National Cancer Institute/United States of America (NCI/USA) supported four registries in Middle East Cancer Consortium (MECC): Cyprus, Israel (Israel Arabs (IA) and Jews (IJ), C (Jordan), and D (Turkey/İzmir) for the period of 2005–2010. All registries use the MECC standards, which are comparable with international standards (6,7) for collecting, processing, and analyzing data. Detailed information on the work of registries can be found elsewhere (8,9). For comparative purposes, we also analyzed data from the USA Surveillance, Epidemiology, and End Result (US SEER) program (SEER 18 Registries; www.seer.cancer.gov; SEER*Stat Database). The International Classification of Diseases for Oncology, Third Edition (ICD-O-3) (10) was used for coding topography and histology. International Agency for Research on Cancer/European Network of Cancer Registries (IARC/ENCR) multiple primary rules (http://www.iacr.com.fr/images/doc/MPrules_july2004.pdf) were applied to identify multiple primaries for all data, including those from the SEER registries. Histology was categorized using ICD-O3 codes.
Crude incidence rates, age-adjusted incidence rates (AAIRs; per 100 000), and annual percent changes (APCs) were analyzed for 2005–2010. For Israel, cancer incidence rates were calculated separately for the Jewish and Arab populations to compare cancer rates between them. AAIRs and their 95% confidence intervals were calculated using the direct method the WHO World Standard Population 2000–2025 (http://www.who.int/healthinfo/paper31.pdf accessed on 15.12.2017). APCs for trend were examined using the SEER Join-point software, version 7.0.4. (National Cancer Institute SEER*Stat software; www.seer.cancer.gov/seerstat).
The analysis was restricted to invasive behavior code (3) and site codes of C18.0–C20.9 and C26.0. We also calculated the incidence rates for colon only (C18.0-C18.9 and C26.0) and the rectum & rectosigmoid junction (C19.9-C20.9). All statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
We used the data from the routine sources (cancer registries) which do not contain any individual information that could be used to identify the patients and the study process did not have any direct involvement of human subjects. Thus, according to WMA (World Medical Association) Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” amended in October 2013 informed consent and ethical committee approval are not required for our study.
RESULTS
In total, 21,427 CRC cases in MECC regions and 221,844 CRC cases in 18 SEER registries were diagnosed during the study period. The percentage of cases with microscopic confirmation ranged from 85.9% (IJ) to 99.6% (Jordan) in the MECC region compared with 97.0% from the SEER registries (Table 1).
Table 1.
Some characteristics of CRC data, 2005–2010
| Histological distribution* (%) | Grade distribution* (%) | SEER summary stage at diagnosis* (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| Registry, population | Number of CRC cases | Microscopically confirmedcases ** (%) | DCO*** (%) | Carcinomas* | Adenocarcinomas (within carcinomas) | Unspecified | Well differentiated | Moderately differentiated | Poorly differentiated | Undifferentiated | Grade unknown | Localized | Regional | Distant | Stage unknown |
| Cyprus | 1949 | 91.3 | 7.7 | 99.9 | 98.7 | 9 | 24.9 | 61.6 | 13.5 | 0.1 | 13.6 | 21.8 | 58.4 | 19.8 | 17.7 |
| IJ | 17870 | 93.3 | 3.6 | 99.8 | 98.8 | 5.9 | 19.9 | 64.5 | 15.1 | 0.4 | 24.4 | 28.1 | 59.2 | 12.8 | 24.4 |
| IA | 1411 | 95.0 | 2.9 | 99.8 | 99.1 | 4.7 | 27.3 | 54.8 | 17.3 | 0.7 | 24.6 | 24.4 | 63.2 | 12.4 | 22.9 |
| İzmir | 4800 | 93.3 | 2.3 | 99.7 | 98.3 | 9.8 | 15.9 | 74.3 | 7.6 | 2.2 | 36.1 | 22.9 | 53.4 | 23.7 | 21.6 |
| Jordan | 2917 | 99.6 | 0.1 | 99.8 | 98.9 | 1.3 | 7.0 | 80.8 | 11.8 | 0.5 | 21.2 | 17.9 | 57.9 | 24.2 | 36.4 |
| SEER 18 | 221844 | 97.0 | 1.4 | 99.9 | 95.2 | 2.4 | 10.4 | 69.1 | 18.7 | 1.9 | 17.1 | 42.3 | 37.0 | 20.6 | 5.6 |
Where known,
with in CRC cases,
DCO: death-certificate-only cases, within all cancers
CRC: colorectal cancer; SEER: Surveillance, Epidemiology, and End Results; IJ: Israel Jews; IA: Israel Arabs
The proportion of cases with unspecified histology changed from 1.3% (Jordan) to 9.8% (İzmir) in the MECC regions compared with 2.4% in the SEER registries. Carcinomas dominate the histological pattern (Cyprus, 99.9%; IJ, 99.8%; IA, 99.7%; İzmir, 99,2%; Jordan, 99.6%; and SEER, 99.7%), and within carcinomas, adenocarcinomas comprise majority of cases both in the MECC registries (98.4%, 96.6%, 97.2%, 97.0%, 96.3%, and 93.5%, respectively) and in the SEER registry. The proportions of cases with unknown grade are higher in the MECC countries (with the highest proportion of 36.1% in İzmir) than in the SEER registries (17.1%), except in Cyprus (13.6%). There was a greater proportion of well-differentiated cancers observed in the MECC Ys (registry/registries) in a range of 15.9% in İzmir to 27.3% in IA, except in Jordan (7.0%), than in the SEER registries (10.4%). Overall, 94.4% of the cases in the SEER registries had a known stage at diagnosis, whereas the number of known grade cases were much lower in the MECC registries, with the lowest 63.6% in Jordan and highest 82.3% in Cyprus. Within the known stage cases, considerably higher proportions of cases were localized at diagnosis in the SEER registry (42.3%) than in the MECC countries with the highest in IJ (28.1%). Conversely, the proportions of cases diagnosed at the regional stage were greater in the MECC registries (from 53.4% in İzmir to 63.24% in IA) than in SEER 18 Registries (37.0%; Table 1).
AAIRs were the highest in the MECC countries (for IJ) (46.7 for males and 35.5 for females) and exceeded those of the SEER registries (36.5 and 27.3, respectively). In both sexes, AAIRs in Cyprus and IA were slightly lower to those in SEER, lower generally except slightly higher rate seen for females in IA (male, female: Cyprus, 34.1, 24.4; IA, 34.9, 29.0). In İzmir and Jordan, AAIRs were substantially lower than those in other Ys for both sexes (male, female: İzmir, 25.8, 15.7; Jordan, 19.6, 14.9; Table 2, Fig. 1). The male-to-female ratios for AAIRs were close to the ratio in the SEER registry (1.3) and in the MECC Ys (registry/registries), except in İzmir (1.6). The male-to-female ratios for AAIRs are quite similar for all the populations except İzmir. Age-specific rates increased with age in all registries for both sexes, except the slight decreases in rates in Jordan for males and females after the age of 79 years (Fig. 2). AAIRs for subsites (colon, rectum) are presented in Table 3 with the AAIR ratios for males, females, and both sexes. In İzmir, the lowest AAIR ratios for colon/rectum (C/R) ratio were observed (1.6), whereas in Cyprus (2.5) and IJ (2.5), the ratios were higher than those in the SEER registries (2.3).
Table 2.
CRC incidence rates (per 100,000) and male-to-female ratios for AAIRs, 2005–2010
| Registry | Number of cases (%) | Crude rate | AAIR** | AAIR | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| Male | Female | Male | Female | Male | Female | M/F ratio | |
| Cyprus | 1067 (54.7%) | 882 (45.3%) | 46.1 | 36.5 | 34.1 (27.7 40.8) | 24.4(19.3 29.8) | 1.39 |
| Israel Jews | 9171 (51.3%) | 8699 (48.7%) | 56.4 | 52.0 | 46.7 (45.8 47.4) | 35.5 (34.8 36.3) | 1.32 |
| Israel Arabs | 730 (51.7%) | 681 (48.3%) | 16.5 | 15.9 | 34.9 (32.3 37.5) | 29.0 (26.8 31.2) | 1.20 |
| İzmir | 2807 (58.5%) | 1993 (41.5%) | 24.8 | 17.6 | 25.8 (25.6 26.0) | 15.7 (15.6 15.7) | 1.64 |
| Jordan | 1637 (56.1%) | 1280 (43.9%) | 9.1 | 7.6 | 19.6 (19.4 19.7) | 14.9 (14.8 15.1) | 1.31 |
| SEER 18 | 113659 | 108185 | 45.5 | 42.2 | 36.5 (36.46 36.53) | 27.3 (27.31 27.37) | 1.34 |
per 100 000,
WHO World Standard Population 2000–2025, CRC: colorectal cancer; AAIR: age-adjusted incidence rates; CI: confidence intervals are provided in brachets; M: male; F: female; SEER: Surveillance, Epidemiology, and End Results
Figure 1.
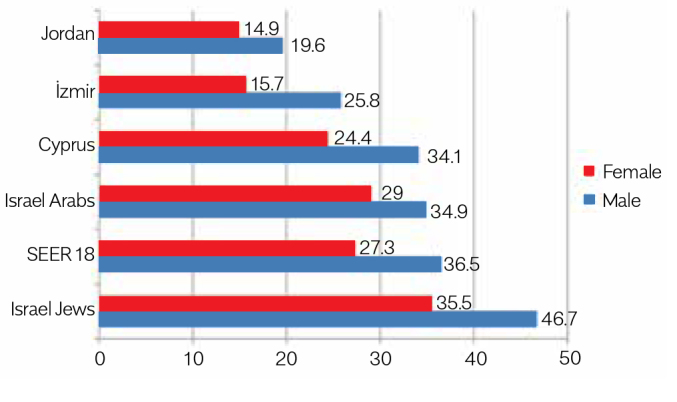
Colorectal cancer AAIRs* for 2005–2010
*per 100,000; WHO World Standard Population, 2001
Figure 2.
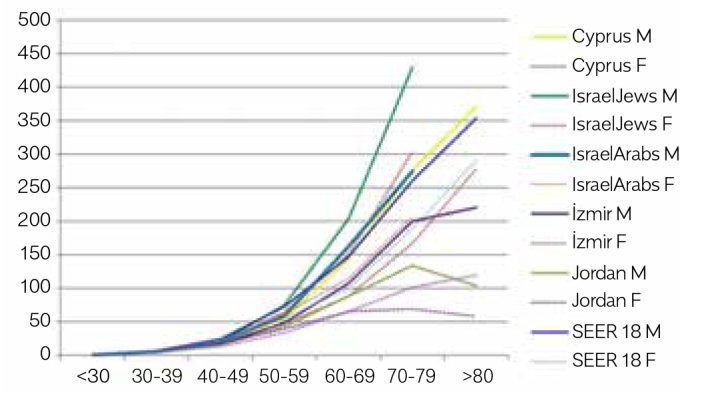
Age-specific incidence rates*of colorectal cancer with regard to sexes, 2005–2010
*per 100,000; Israel data, 0–70+; M, Male; F, Female
Table 3.
Subsites (colon and rectum) AAIRs, male/female and colon/rectum ratios, 2005–2010
| Colon | Rectum | Colon/rectum | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Male | Female | M/F | Male | Female | M/F | Male | Female | M+F | |
| Cyprus | 23.8 | 17.9 | 1.33 | 9.9 | 6.5 | 1.52 | 2.4 | 2.8 | 2.5 |
| Israel Jews | 32.6 | 25.8 | 1.26 | 14.1 | 9.8 | 1.44 | 2.3 | 2.6 | 2.5 |
| Israel Arabs | 22.4 | 20.9 | 1.07 | 12.5 | 8.2 | 1.52 | 1.8 | 2.5 | 2.1 |
| İzmir | 15.5 | 9.7 | 1.60 | 10.3 | 6.0 | 1.72 | 1.5 | 1.6 | 1.6 |
| Jordan | 13.2 | 10.1 | 1.31 | 6.4 | 4.8 | 1.33 | 2.1 | 2.1 | 2.1 |
| SEER 18 | 24.6 | 19.8 | 1.24 | 11.9 | 7.5 | 1.59 | 2.07 | 2.64 | 2.3 |
per 100,000,
WHO World Standard Population 2000–2025
CRC: colorectal cancer; M: male; F: female; SEER: Surveillance, Epidemiology, and End Results; AAIR: age-adjusted incidence rates
Statistically significant decreasing trends over time were observed in AAIRs for both sexes in the SEER registry for 2005–2010 (APCs: male, −3.24%; p=0.0008; female, −2.54%; p=0.0003), whereas the trends varied within the MECC registries. Decreasing trends of AAIRs were observed for male in IJ and IA, and decreasing trends of AAIRs were observed for females in Cyprus and IJ; however, only the APC(–) for females in IJ (−4.29%) was statistically significant. Conversely, increasing trends were observed for the remaining, with APCs for males in İzmir (2.43%) and Jordan (7.57%; Table 4, Fig. 3) being statistically significant.
Table 4.
APCs (%), 2005–2010, male and female
| Male | Colorectal | Colon | Rectum |
|---|---|---|---|
| Cyprus | 1.74 | 3.64 | −3.20 |
| IJ | −4.17 | −4.69* (p=0.017) | −2.90 |
| IA | −0.01 | 3.43 | −6.06 |
| İzmir | 2.43* (p=0.005) | 1.83 | 3.60 |
| Jordan | 7.57* (p=0.028) | 6.75 | 8.88* (p=0.021) |
| SEER 18 | −3.24* (p=0.0008) | −3.48* (p=0.002) | −2.50* (p=0.005) |
| Female | Colorectal | Colon | Rectum |
| Cyprus | −1.50 | 1.11 | −9.12* (p=0.007) |
| IA | −4.29* (p=0.016) | −3.63* (p=0.017) | −6.02* (p=0.032) |
| IA | 0.13 | −0.15 | −0.45 |
| İzmir | 2.27 | 2.11 | 2.64 |
| Jordan | 4.87 | 5.36 | 3.71 |
| SEER 18 | −2.54* (p=0.0003) | −2.86* (p<0.0001) | −1.67* (p=0.03) |
Statistically significant,
CRC: colorectal cancer; IJ: Israel Jews; IA: Israel Arabs; SEER: Surveillance, Epidemiology, and End Results; APCs: annual percent changes
Figure 3.
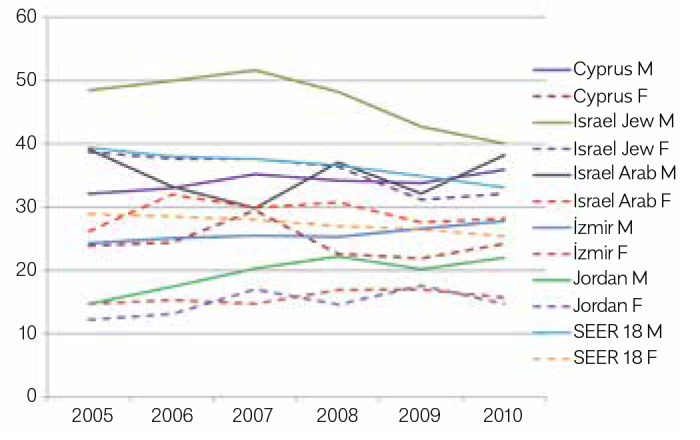
Age-adjusted incidence rates* of colorectal cancer, per 100,000 (2005–2010), males and females
*per 100,000; WHO World Standard Population 2001; M, Male; F, Female
For subsites, significant decreasing trends were observed in both sexes in the SEER registries. Comparably, in Israel, significant decreasing trends were seen in both sexes for all subsites, except the rectum in males and the distal colon in females in which the decreasing trends were not significant. In Jordan, significant increasing trends were calculated for the rectum in males (Table 4).
DISCUSSION
This study describes CRC incidence patterns using data from four population-based cancer registries of the Middle East. The quality of the data was considered sufficiently high for usage in our study. The data from Turkey/İzmir, Israel, and Cyprus were evaluated as high quality to be accepted for publication in Cancer Incidence in Five Continent series (1). Indicators such as proportions of microscopically verified and death-certificate-only cases, unknown histology, stage, and grade ratios suggest that the Jordan Cancer Registry data also have sufficient quality to be used for comparisons (Table 1).
The changes in the prevalence of cancer risk factors and the implementation of national screening programs have resulted in changes in the pattern of CRC incidence worldwide, which was characterized with high rates in developed countries and quite lower rates in developing countries. The CRC incidence is decreasing in the USA and some other developed countries, and it may be stabilizing in certain parts of Western and Northern Europe. In contrast, particularly in economically transitioning countries such as Japan, Taiwan, Singapore, Thailand, urban China, and countries from the Eastern Europe, the incidence rates continue to sharply increase, approaching those reported in the western countries (11). Likewise, similar increases in CRC incidence rates have also been reported in many other developing countries (Iran, Yemen, Egypt, and Saudi Arabia) (12). In our study, the high incidence rates calculated for both men and women in IJ and IA populations and Cyprus resemble the higher rates reported by developed countries, whereas the rates were relatively lower in both sexes in İzmir and Jordan, similar to those reported in developing countries.
We found significant decreasing trends in the SEER data for both sexes and in IJ for females. AAIRs were stabilized in IA, increasing for both sexes in İzmir and Jordan with significant APCs in males. In summary, high rates and decreasing trends were observed in IJ for both sexes as reported by other developed countries, whereas lower rates and increasing trends were found in İzmir and Jordan. In Cyprus and IA, the rates were relatively high, but the trends varied. In IA, time trends were stabilized, whereas in Cyprus, despite being non-significant, a small increase in males and a small decrease in females were observed.
Age; a history of adenomatous polyps and inflammatory bowel disease; family history of CRC or adenomatous polyps; and inherited conditions such as familial adenomatous polyposis and hereditary non-polyposis CRC (HNPCC) were reported to be non-modifiable risk factors, whereas the critical role environmental risk factors play in CRC is also widely acknowledged. In fact, most of the international variation in rates may be owing to the differences in diet, exercise, obesity, and other lifestyle and prevailing screening activities (13). In males, colon cancer contributed to the largest number of cancer cases and were attributable to high body mass index (BMI), the estimated population attributable fractions for colon and rectum were 13% and 6.2%, respectively, in males and 7.6% and 3.6, respectively, in females across the world (14). Diets high in animal fat and red meat and low in vegetables, fruit, and fiber are strongly associated with an increased risk for CRC. Widespread utilization of screening tests such as the fecal occult blood test, which also detects asymptomatic cases, may also lead to an initial increase in incidence rates (15).
In this regard, the differences in incidence and time trends among the different MECC countries and the SEER registries can be mainly owing to the variation in diet and lifestyle/obesity, while acknowledging that different screening practices and genetic factors may also play a role. The estimated population attributable fraction (PAFs) associated with high BMIs for colon and rectum cancers were 19% and 10%, respectively, for Israel, 20% and 10%, respectively, for Jordan, 20% and 11%, respectively, for Cyprus, 17% and 9% for Turkey, 21% and 11% for the USA in males, respectively. The corresponding PAFs in females were 7.6% and 3.6% for Israel, 13% and 7% for Jordan, 11% and 6% for Cyprus, 12% and 6%, respectively, for Turkey, 11% and 6%, respectively, for the USA (14). Genetic factors regarding high prevalence of genetic mutations and HNPCC or Lynch syndrome in Askenazi Jews (16) might be considered for interpreting the higher incidence rates in IJ than those in the SEER registries. However, the difference can be explained by the differences in lifestyles besides genetic influences as well. Indeed, IA may be at a lower risk for CRC than IJ owing to their less westernized lifestyle characterized with a traditional diet rich in fruits, vegetables, and olive oil and more physical activity (17,18; The World Factbook, https://www.cia.gov/library/publications/the-world-factbook/geos/is.html)
The different trends over time also could be explained with the changing lifestyle and dietary factors along different time periods in these countries. In the recent times, a considerable proportion of the populations of MECC countries were engaged in agriculture and hence most of the population followed a Mediterranean diet that is rich in vegetables, fruits, and olive oil and low in red meat and fat. Recently, because of a greater prosperity, particularly in Israel and Cyprus, and immigration from rural areas to towns, there has been an increase in the adoption of a westernized diet which contains high calorie, higher red meat intake and more fast-food. Change to a more sedentary lifestyle with less exercise as a result of the adaption to the westernized lifestyle as a whole package along with the changes in diet may be responsible for the significant difference in CRC incidences between generations. This difference between generations is obvious in Israel and Cyprus where older generations have been raised with a true Mediterranean diet rich in vegetables and low in red meat, while the younger generations are raised with a western diet, with increased red meat and processed fast food (2,4,18). At present, İzmir and Jordan seem at the earlier stages of this transition. According to 2003 WHO World Health Survey (http://www.who.int/healthinfo/survey/whsresults/en/index3.html) in Turkey, the prevalence of respondents who consume insufficient fruit and vegetables (less than five servings of fruit/vegetables per one typical day) was 80.3% for males and 81.4% for females. In Cyprus, 37% of children in a cross-sectional study of 1140 children (average age=10.7 years) had a poor Mediterranean Diet Quality Index for children and teenagers (KIDMED test) score which indicates poor adherence to the Mediterranean diet and only 6.7% were compliant of the Mediterranean diet which is considered to be a healthy diet (19). A case-control study in Jordan reported CRC cases had lower dietary intake of fiber, vegetables, and fruits, while the frequency of consumption of red meat and saturated fats was higher as compared to controls (20).
In 2010, the proportion of having insufficient activity (less than 150 minutes per week [last 7 days] spent on walking/moderate activity/vigorous activity) in 18+ years have been reported as 28.1% in males and 37.5% in females in Turkey; 28.9% in males and 40.4% in females in Cyprus, 15.6% in males and 15.6% in females in Jordan; and 25.4% in males and 39.3 in females in the USA Global Health Observatory (GHO) (http://apps.who.int/gho/data/node.main.A867?lang=en). The proportion was 15.2% in males and 19.9% in females in Turkey in 2003 (WHO World Health Survey). In Israel, it was reported that the rate of compliance with recommendations for physical activity, considering both targeted exercise and activities of daily living were 42.5% among Jewish males, 41.7% among Jewish females, 32.5% among Arab males, and 31.9% among Arab females from the 2011–2012 physical activity survey, and 38% of males and 35.6% of females reported that they engage in at least 30 minutes of physical activity at least three times a week by Knowledge, Attitudes and Practices 2011 survey (21). In a study done in Turkey, elevated risks were observed for colon and rectal cancers among workers who held sedentary jobs (22).
In terms of obesity, the figures show clear increasing trends of the prevalence of overweight/obese people in all study populations. According to WHO GHO 2010 and 2014 data (24), 63.8% and 66.3% in Turkey, 61.8% and 63.5% in Israel, 58.7% and 60.3% in Cyprus, 63.4% and 65.9% in Jordan, 65.5% and 67.3% in the USA, respectively, of the populations over 18 years were overweight Body Mass Index (BMI), ≥25 kg/m2, while the same proportions were 27.3 and 29.5 in Turkey, 23.5 and 25.5 in Israel, 22.0 and 23.8 in Cyprus, 28.1 and 30.5 in Jordan, and 31.2 and 33.7 in the USA for obesity (BMI, ≥30 kg/m2). According to the 2003 and 2008 European Health Surveys, in Cyprus, 33.7% of the population was overweight in 2003 (BMI, 25–30 kg/m2) and 12.3% was obese (BMI, >30 kg/m2), with the 2008 survey showing further increase, with 34.1% of the population being overweight and 14.8% obese (23).
The figures suggest a worsening dietary pattern/lifestyle in all populations including the USA. Thus, we suggest that the decrease in the CRC incidence rates in the USA may be attributed to the influence of screening programs used in recent years rather than changes in diet; i.e., those mainly performed by colonoscopy (24) because colonoscopy screening procedures involve the removal of precancerous polyps as well (25,26).
The striking diversities we found in the distribution of stage at diagnosis (Table 1) with a much higher percentage of localized cancers in the SEER registries compared to the MECC registries can be interpreted as an indication of the success of early detection and screening programs. Despite the approach being largely opportunistic, screening with guaiac fecal occult blood test, fecal immunochemical test for hemoglobin, and colonoscopy was reported as highly endorsed in the USA (24,25). In Israel, organized screening program has prevailed since 1990, while in Turkey and Jordan opportunistic screening programs have been implemented into practice recently (27). In the absence of the opportunistic screening programs in Cyprus, a pilot program was set up in 2012 in a small population with the plan to expand the program across the country in the following years (23). The absence of organized screening programs in the MECC countries except Israel is likely to result in a relative increase of cancers being diagnosed at an advanced stage compared to countries where screening programs are in place.
In developed countries, the male-to-female ratio of age-adjusted cancer incidence (ASIRR) is about 1.5, while it is about 1.3 in less-developed countries (1). In our data, ASIRRs were 1.24 for the USA SEER, 1.31 for Jordanians, 1.07 for IA, 1.26 for IJ, 1.33 for Cypriots, and 1.6 for İzmir. These ratios were 1.38, 1.03, 1.27, 1.25, 1.19, and 1.25, respectively, in different years within 1996–2001 period (28,5). It seems that in the low-risk populations AAIRs in males and females are quite similar due to the low exposure to risk factors. Yet, when CRC AAIRs tend to rise, we observe that the rates in males increase more rapidly. The differences in male-to-female ASIRRs among the MECC countries might be considered mainly due to the fact that the smaller increases have occurred in CRC incidence rates in females than in males, which may reflect the slower adaptation of hazardous life style changes in females associated with CRC (29,30).
In males, the APCs for CRC were statistically significant as 2.43% in İzmir and 7.57% in Jordan versus non-significant APCs in females. In Cyprus, there was a decrease in females, while a non-significant increase seen in males. Thus, the male-to-female ratios increased in Jordan, Cyprus, and İzmir, the populations that were at the very beginning of the transition period in the 1990s, reflecting more rapid increases in CRC incidence rates in males due to the quicker adaptation to the westernized lifestyle among males.
Consistent with the findings in the literature, high-risk westernized populations were observed to have higher ratios for colon:rectal cancers (1,4). We found higher ratios for colon to rectal cancer AAIRs in Cyprus (2.4 in males and 2.8 in females) and among IJ (2.3 and 2.6, respectively) in both sexes as for the SEER registry (2.1 and 2.6 respectively) and low ratios in İzmir (1.5 and 1.6 respectively). However, our findings with relatively high colon:rectal cancer AAIR ratios (2.1) in both sexes in Jordan despite the low risks for CRC, is incompatible with literature. The lower ratio in males (1.8) than in females (2.5) in IA is also contradictory findings (Table 3).
In areas with historically high CRC incidence rates, rectal cancer incidence has been decreasing (e.g., USA, France, and Denmark) or staying stable (e.g., Australia and Italy). In contrast, in historically low-risk areas, colon cancer incidences are rising sharply, accompanied by modest increases in rectal cancer (1). Our findings for time trends in colon and rectal cancer incidence rates are compatible with literature.
Although the 5-year period might not be sufficient for applying time trends in APC calculations, we still preferred to have the calculations based upon the consistent trends reported from the aforementioned countries in literature and specific reports (1,2,4,5,12,28).
We found decreasing trends for rectal cancer incidence in high-risk populations (IJ, IA, and Cyprus) in both sexes as in the SEER registry, while there were increasing trends in İzmir and Jordan in both sexes. The APCs were statistically significant in both sexes in the SEER program, in females among IJ and Cyprus and among males in Jordan. Among IJ, decreasing trends were revealed both in males and females with significant APCs for colon cancer incidence as well as in the SEER registry, while increasing trends were noted in İzmir and Jordan in both sexes. Among IA, the trends were contradictory as decreasing in females and increasing in males (Table 4).
In the MECC area, there are both high- and low-risk populations for CRCs. However, increasing trends in low-risk populations have been alarming. The essential need for the implementation of prevention programs to promote a healthy lifestyle including healthy diet, increasing physical activity, and controlling the body weight, is obvious. Tailored screening programs may also be adopted for secondary prevention.
Acknowledgements
The authors would like to thank Dr. Freddie Bray and Dr. Ariana Znaor for their support and send their best regards and appreciation to each member of each cancer registry for their part in the creation of the data of our research.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: Informed consent is not necessary due to the retrospective nature of this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - S.E., H.A.C., B.S., P.P.; Design - S.E., H.A.C.; Supervision - S.E.; Resource - S.E., J.C., H.C., B.S., A.D., C.Y., O.N., P.P., S.Ö., A.Z., L.S., K.W., H.A.C.; Data Collection and/or Processing - S.E., B.S., P.P., O.N., C.Y., S.Ö., A.D.; Analysis and/or Interpretation - S.E., H.A.C., J.C., H.C., B.S., A.D., C.Y., O.N., P.P., S.Ö., A.Z., L.S., K.W.; Literature Search - S.E., B.S., H.C.; Writing - S.E.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents (CI5) I–X (cited 2016 December 15) Avaible from: URL: http://ci5.iarc.fr/Default.aspx. [Google Scholar]
- 2.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–94. doi: 10.1158/1055-9965.EPI-09-0090. https://doi.org/10.1158/1055-9965.EPI-09-0090 [DOI] [PubMed] [Google Scholar]
- 3.Boyle P, Leon ME. Epidemiology of colorectal cancer. Br Med Bull. 2002;64:1–25. doi: 10.1093/bmb/64.1.1. https://doi.org/10.1093/bmb/64.1.1 [DOI] [PubMed] [Google Scholar]
- 4.Freedman LS, Edwards BK, Ries LG, et al. NIH Pub. 2006. Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared with US SEER; pp. 60–5873. [Google Scholar]
- 5.Fidaner C, Eser SY, Parkin DM. Incidence in Izmir in 1993–1994: First results from Izmir Cancer Registry. Eur J Cancer. 2001;37:83–92. doi: 10.1016/s0959-8049(00)00355-5. https://doi.org/10.1016/S0959-8049(00)00355-5 [DOI] [PubMed] [Google Scholar]
- 6.Anton-Culver H, Young JL. Standard Operating Procedures for MECC Registries. (Second Edition) 2008 [Google Scholar]
- 7.Young JL, Ward K. MECC Manual of Coding and Staging, Version 5.1. Jul, 2009. [Google Scholar]
- 8.Anton-Culver H, Chang J, Bray F, et al. Cancer burden in four countries of the Middle East Cancer Consortium (Cyprus; Jordan; Israel; Izmir (Turkey)) with comparison to the United States surveillance; epidemiology and end results program. Cancer Epidemiol. 2016;44:195–202. doi: 10.1016/j.canep.2016.06.004. https://doi.org/10.1016/j.canep.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charalambous H, Pavlou P, Chang J, et al. Lung Cancer Incidence Patterns in Four Countries of the Middle East Cancer Consortium (Cyprus, Jordan, Israel, Izmir (Turkey)) with Comparison to the United States Surveillance, Epidemiology and End Results (SEER) Program. Int J Cancer Res Ther. 2016;1:2–7. doi: 10.1016/j.canep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz A, Percy C, Jack A, et al., editors. ICD-O, International Classification of Diseases for Oncology. Third edition. Geneva: World Health Organization; 2000. [Google Scholar]
- 11.Cress RD, Morris C, Ellison GL, Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ ethnicity, 1992–2001. Cancer. 2006;107:1142–52. doi: 10.1002/cncr.22011. https://doi.org/10.1002/cncr.22011 [DOI] [PubMed] [Google Scholar]
- 12.Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20:6055–72. doi: 10.3748/wjg.v20.i20.6055. https://doi.org/10.3748/wjg.v20.i20.6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–7. doi: 10.1055/s-0029-1242458. https://doi.org/10.1055/s-0029-1242458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold M, Pandeya N, Bynes G, et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. https://doi.org/10.1016/S1470-2045(14)71123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122:1357–67. doi: 10.1002/ijc.23273. https://doi.org/10.1002/ijc.23273 [DOI] [PubMed] [Google Scholar]
- 16.Locker GY, Lynch HT. Genetic factors and colorectal cancer in Ashkenazi Jews. Fam Cancer. 2004;3:215–21. doi: 10.1007/s10689-004-9547-x. https://doi.org/10.1007/s10689-004-9547-x [DOI] [PubMed] [Google Scholar]
- 17.Fireman Z, Sandler E, Kopelman Y, Segal A, Sternberg A. Ethnic differences in colorectal cancer among Arab and Jewish neighbors in Israel. Am J Gastroenterol. 2001;96:204–7. doi: 10.1111/j.1572-0241.2001.03476.x. https://doi.org/10.1111/j.1572-0241.2001.03476.x [DOI] [PubMed] [Google Scholar]
- 18.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. doi: 10.1136/gutjnl-2015-310912. https://doi.org/10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 19.Lazarou C, Panagiotakos DB, Matalas AL. Level of adherence to the Mediterranean diet among children from Cyprus: The CYKIDS Study. Public Health Nutr. 2009;12:991–1000. doi: 10.1017/S1368980008003431. https://doi.org/10.1017/S1368980008003431 [DOI] [PubMed] [Google Scholar]
- 20.Arafa MA, Waly MI, Jriesat S, Al Khafajei A, Sallam S. Dietary and lifestyle characteristics of colorectal cancer in Jordan: a case-control study. Asian Pacific J Cancer Prev. 2011;12:1931–36. [PubMed] [Google Scholar]
- 21.Health 2013, Israel Ministry of Health, Center for Disease Control. Health 2013 [Hebrew] 2014. (cited 2015 December 17): Avaible from: URL: http://www.health.gov.il/PublicationsFiles/health2013.pdf.
- 22.Dosemeci M, Hayes RB, Vetter R, et al. Occupational physical activity, socioeconomic status, and risks of 15 cancer sites in Turkey. Cancer Causes Control. 1993;4:313–21. doi: 10.1007/BF00051333. https://doi.org/10.1007/BF00051333 [DOI] [PubMed] [Google Scholar]
- 23.Farazi PA. Cancer trends and risk factors in Cyprus. Ecancermedicalscience. 2014;8:389. doi: 10.3332/ecancer.2014.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–94. doi: 10.1158/1055-9965.EPI-05-0678. https://doi.org/10.1158/1055-9965.EPI-05-0678 [DOI] [PubMed] [Google Scholar]
- 25.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. doi: 10.1002/cncr.23044. https://doi.org/10.1002/cncr.23044 [DOI] [PubMed] [Google Scholar]
- 26.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. https://doi.org/10.3322/CA.2007.0018 [DOI] [PubMed] [Google Scholar]
- 27.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–49. doi: 10.1136/gutjnl-2014-309086. https://doi.org/10.1136/gutjnl-2014-309086 [DOI] [PubMed] [Google Scholar]
- 28.Barchana M. Colorectal Cancer. In: Freedman LS, Edwards BK, Ries LAG, Young JL, editors. Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared with US SEER. National Cancer Institute; NIH Pub. No. 06-5873. [Google Scholar]
- 29.Mackay J, Amos A. Women and tobacco. Respirology. 2003;8:123–30. doi: 10.1046/j.1440-1843.2003.00464.x. https://doi.org/10.1046/j.1440-1843.2003.00464.x [DOI] [PubMed] [Google Scholar]
- 30.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20. doi: 10.1056/NEJMoa0801891. https://doi.org/10.1056/NEJMoa0801891 [DOI] [PubMed] [Google Scholar]


