Abstract
The innate myeloid immune system is a complex network of cells that protect against disease by identifying and killing pathogens and tumor cells, but it is also implicated in homeostatic mechanisms like tissue remodeling and wound healing. Myeloid phagocytes such as monocytes, macrophages, or dendritic cells are at the basis of controlling these immune responses in all tissues of the body. In this review we summarize recent studies demonstrating that the mechanistic target of rapamycin (mTOR) regulates innate immune reactions in macrophages and dendritic cells. The mTOR pathway serves as a decision maker to control the cellular response to pathogens and tumors by regulating the expression of inflammatory mediators such as cytokines, chemokines or interferons. In addition to various in vivo mouse models, kidney transplant patients under mTOR inhibitor therapy allowed the elucidation of important innate immune functions regulated by mTOR in humans. The role of the mTOR pathway in macrophages and dendritic cells enhances our understanding of the immune system and suggests new therapeutic avenues for the regulation of pro- versus anti-inflammatory mediators with potential relevance to cancer therapy, the design of novel adjuvants, and the control of distinct infectious and autoimmune diseases.
Keywords: mTOR, macrophages, dendritic cells, immune regulation
The mechanistic (or mammalian) target of rapamycin (mTOR) is an evolutionary conserved serine/threonine kinase that senses environmental and cellular input signals to control an enormous amount of diverse processes depending on the cell type including the immune system [1–5]. This short review focuses on the most recent studies that have identified a role of mTOR signaling in macrophages and dendritic cells (DCs), two cardinal phagocytes of the innate immune system, to regulate immune responses and tissue homeostasis. The function of mTOR in T-cells has been recently covered by several excellent reviews [6, 7].
The mTOR signaling pathway
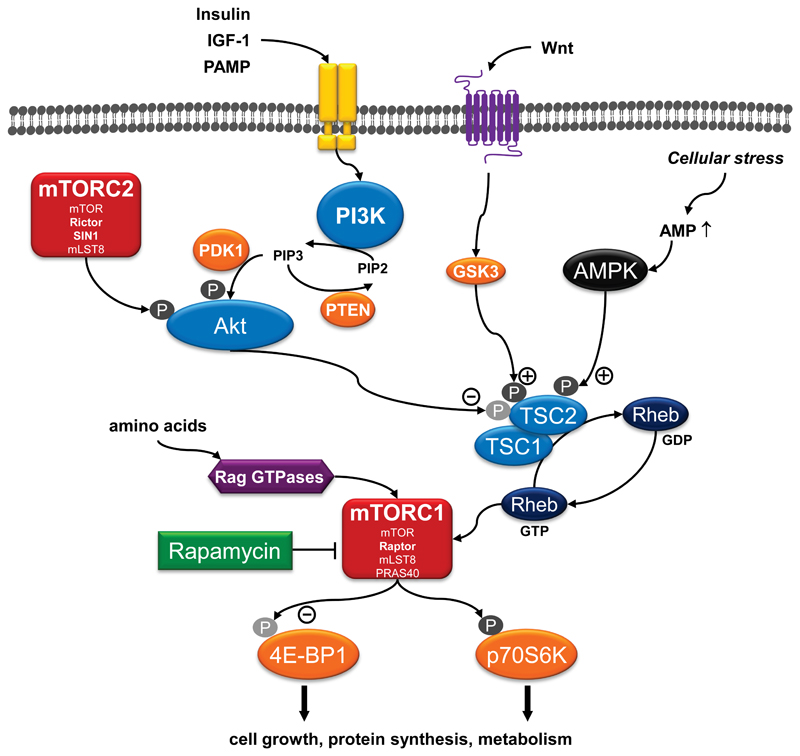
mTOR is part of two known multi-protein complexes designated mTOR complex 1 (mTORC1) and mTORC2 [2]. Raptor is the defining protein for mTORC1, whereas Rictor and Sin1 are specific for mTORC2 [8]. The prototypical allosteric mTOR inhibitor rapamycin blocks mTORC1, while mTORC2 is usually insensitive to rapamycin [8]. Recently, a number of novel ATP-competitive mTOR inhibitors that block both mTOR complexes have been developed [9]. The mTOR pathway constitutes a central rheostat integrating diverse extra- and intracellular signals including nutrients and growth factors to control cell growth, translation and metabolism. For example, mTORC1 senses cellular energy levels by monitoring cellular ATP:AMP levels via AMPK, amino acids via Rag GTPases, signals from the Wnt family via GSK3 and growth factors such as insulin and IGF-1 via the insulin receptor and the IGF-1 receptor, respectively (Figure 1) [2, 8]. mTORC2 is believed to be activated by growth factors alone [2]. Stimulation of antigen receptors, cytokine receptors (e.g. interleukin [IL]-2 receptor), or costimulatory receptors (e.g. CD28) promotes mTORC1 activation in T-cells [6, 7]. In macrophages and DCs, stimulation of Toll-like receptors (TLR) by pathogen-associated molecular patterns (PAMPs) activates mTORC1 and mTORC2 [10–12]. The growth factors GM-CSF and Flt3l, which are important for DC differentiation, can also induce mTORC1 activation [13, 14].
Figure 1. mTOR signaling.
A simplified view on the mTOR signaling pathway is shown. For details see the discussion in the main text. Comprehensive reviews of the mTOR pathway have been recently published by Cornu et al [2] and Laplante & Sabatini [8].
Activation via PI3K is the best documented example of activating mTORC1 [8]. Stimulation of a growth factor receptor or TLR leads to the recruitment of PI3K to the receptor. PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3) as a second messenger. PIP3 then recruits Akt to the plasma membrane and allows its phosphorylation and activation by PDK1 (Figure 1). A main effector of Akt is TSC2, which is a tumor suppressor that forms a heterodimeric complex with TSC1. TSC2 is phosphorylated and inactivated by Akt leading to a loss of suppression of mTORC1 by the TSC1-TSC2 complex [2, 8]. The active TSC1-TSC2 complex serves as a GPTase-activating protein that converts the small GTPase Rheb into its inactive GDP-bound state to prevent mTORC1 activation (Figure 1). Inactivation of the TSC1-TSC2 complex allows the accumulation of the GTP-loaded form of Rheb, which directly interacts with and activates mTORC1 [2]. Amino-acid-induced activation of the Rag GTPases promotes the translocation of mTORC1 to lysosomal compartments that contain Rheb to allow the activation of mTORC1 via growth factors [8]. The best known mTORC1 substrates are eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and S6 kinase (S6K1 or p70S6K), two regulators of protein synthesis.
Regulation of DC development by mTORC1
DCs provide a critical link between innate and adaptive immunity by efficiently priming pathogen-specific adaptive T-cell responses. Three subsets of DCs can be defined in the steady state in the mouse: CD8- conventional DCs (cDCs), CD8+ cDCs, and plasmacytoid DCs (pDCs). The growth factor Flt3l controls the development of DCs from a common bone marrow-derived DC precursor and is particularly important for the differentiation of pDCs and CD8+ cDCs [14]. Injection of recombinant Flt3l into mice induces mTORC1 activation in DCs but not in macrophages and other cell types [14]. Inhibition of mTORC1 with rapamycin impairs Flt3l-driven DC development in vitro, with the pDCs and CD8+ cDCs most profoundly affected. Conversely, genetic activation of the PI3K-mTOR pathway by deletion of the PI3K negative regulator PTEN facilitates Flt3l-driven DC development in vitro and in vivo [14]. Langerhans cells (LCs) are a specialized subset of DCs that populate the epidermal layer of the skin. DC-specific loss of Raptor (lack of mTORC1 activity) but not of Rictor (lack of mTORC2 activity) leads to a progressive decrease of LCs in the skin of mice [15]. Raptor-deficient LCs have an increased tendency to leave the skin and show increased apoptosis suggesting that mTORC1 is critical for the preservation of the LC network in mice [15].
Analogous results have been observed for human DC development. Here, a CD34+ hematopoietic progenitor cell can develop into myeloid CD11c+ DCs (homologous to mouse CD8- cDCs), myeloid CD141+ DCs (homologous to mouse CD8+ cDCs), pDCs and LCs. Precursor proliferation and development of all the human DC subsets requires intact PI3K-Akt-mTOR signaling [16, 17]. In contrast, the survival of terminally differentiated myeloid DCs is not dependent on PI3K or mTOR activity [16]. The differentiation of monocytes to monocyte-derived DCs (moDCs) by GM-CSF and IL-4 depends on mTORC1 [4, 13, 18]. Inhibition of mTOR by rapamycin during the entire moDC differentiation period induces apoptosis and generates a tolerance-promoting DC phenotype [4, 13]. However, renal transplant patients treated with the mTOR inhibitor rapamycin have similar numbers of myeloid DCs and pDCs in their blood compared to control patients [13]. These results show that mTOR inhibition at doses ranging from 5-10 ng/ml, which is far beyond the concentrations used for murine studies, does not compromise the DC compartment in humans in vivo.
Regulation of inflammatory cytokines by mTOR signaling in macrophages and dendritic cells
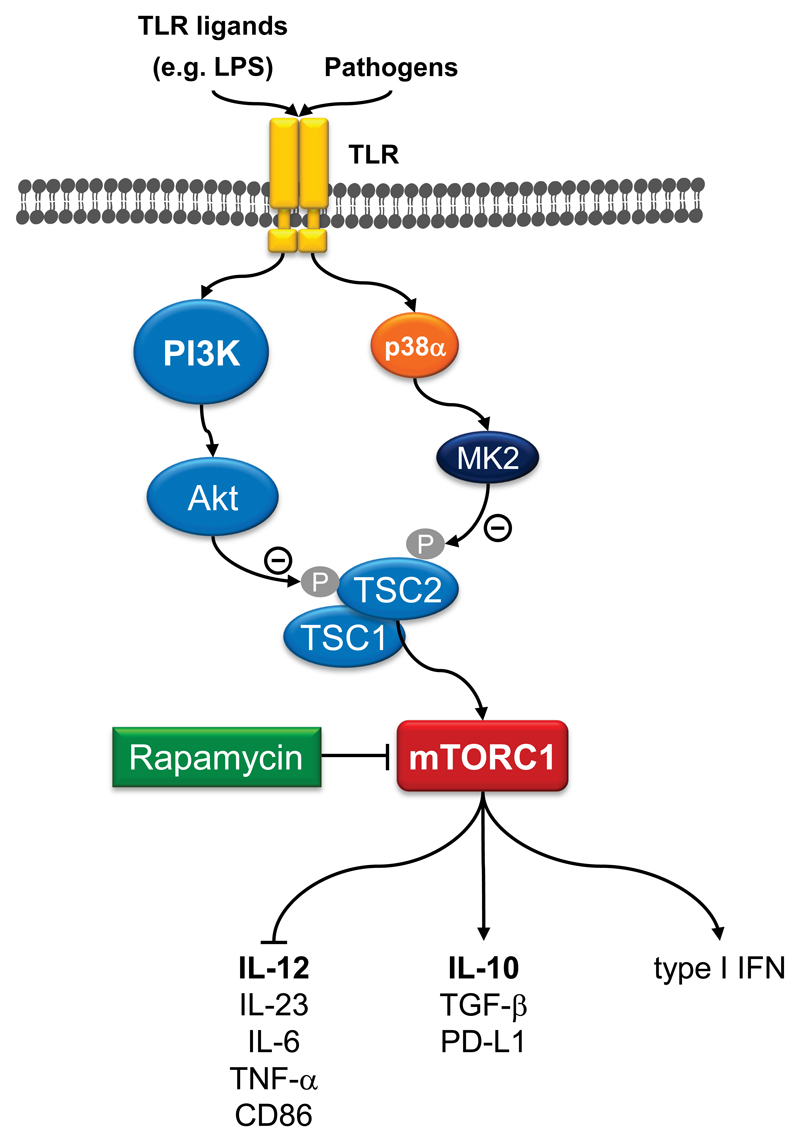
The coordinated secretion of pro- versus anti-inflammatory cytokines by monocytes, macrophages, and DCs is indispensable for effective immunity and tissue homeostasis. Recently, the mTOR pathway emerged as critical regulator of the inflammatory cytokine response in innate immune cells [3–6]. Stimulation of human or murine monocytes, macrophages and DCs by TLR ligands such as LPS activates the mTORC1 pathway [10, 13, 19–23]. Inhibition of mTORC1 with rapamycin during TLR stimulation enhances the expression of the proinflammatory cytokine IL-12 via the transcription factor NF-κB, while the release of the anti-inflammatory cytokine IL-10 is inhibited via reduced Stat3 activation (Figure 2) [10]. Conversely, knockdown of TSC2 results in chronic activation of mTORC1 in human monocytes and enhances IL-10 but inhibits IL-12 production via transcriptional events [10, 24]. In addition, mTORC1 promotes the expression of antiviral type I IFN in mDCs and pDCs depending on the transcription factors IRF5 and IRF7 [11]. The pathogens Leishmania donovani and Listeria monocytogenes activate the mTORC1 pathway to modulate the IL-12/IL-10 balance [10, 25]. Similarly, cytokine-stimulated T-cells induce IL-10 production in macrophages via PI3K and mTORC1 [26]. In line, mice with a DC-specific deletion of Raptor show strongly suppressed IL-10 production in intestinal DCs and are highly susceptible to dextran sodium sulfate-induced colitis [27]. Moreover, rapamycin enhances the induction of tissue factor and the proinflammatory cytokine TNF-α in LPS-stimulated peritoneal macrophages potentially by reducing IL-10 expression [28]. Chronic stimulation of the intracellular nucleotide oligomerization domain 2 (Nod2), a bacterial-sensing intracellular protein, augments the secretion of the anti-inflammatory molecules IL-10, TGF-β, and IL-1 receptor antagonist in a rapamycin-sensitive manner [29]. mTORC1 inhibition increases inflammation and pulmonary injury by enhancing NF-κB activity in the lung of tobacco-exposed mice and promotes the recruitment of inflammatory macrophages [30]. HIV infects macrophages and impairs innate immune signaling in part by activation of mTOR [31]. Inhibition of mTOR restores LPS-mediated TNF-α expression in HIV-infected macrophages. Moreover, HIV-1 blocks autophagy by mTOR activation and impedes immune functions of DCs [32]. Therefore, inhibition of mTORC1 may serve as a potential therapeutic target to upregulate macrophage and DC innate immune responsiveness in HIV+ persons.
Figure 2. mTOR-dependent inflammatory signaling in macrophages and DCs.
Diverse pathogens and TLR ligands activate mTORC1 in macrophages and dendritic cells. Activation of mTORC1 limits the production of proinflammatory cytokines such as IL-12, IL-23, IL-6, and TNF-α via reducing the activity of the transcription factor NF-κB. mTORC1 stimulation promotes the expression of anti-inflammatory cytokines such as IL-10 or TGF-β and of type I interferons in macrophages. These activities are mediate by the signal transducer and activator of transcription 3 (STAT3) and by the interferon regulated factors (IRF)-5 and IRF-7.
In addition, it has been suggested that mTORC2 also negatively regulates proinflammatory cytokine production in DCs [33]. Knockdown of Rictor in DCs augments the production of IL-12, TNF-α and IL-8 in LPS-stimulated cells [33]. The study suggested that mTORC2-dependent phosphorylation of Akt in DCs promotes the phosphorylation and cytoplasmic retention of the transcription factor FoxO1 resulting in a reduced inflammatory response. Inactivation of FoxO1 subsequent to TLR4-dependent Akt activation has been previously reported to limit the overactivation of the innate immune response [34]. Curiously, TSC1-deficient macrophages produce elevated proinflammatory cytokines in response to multiple TLR stimuli [35]. As the activity of Akt was strongly reduced in these cells, it might be possible that some of these effects were actually mediated via mTORC2. Interestingly, a positive regulatory role of the TSC1-TSC2 complex for mTORC2 activation has been proposed [36]. In general, many studies in myeloid immune cells have been performed with pharmaceutical inhibitors of the mTOR pathway, as these cells are notoriously difficult to transfect. Since rapamycin inhibits mTORC2 in some cell types, it is possible that some of the rapamycin effects are actually due to inhibition of mTORC2 signaling. Further studies with tissue-specific knockout mice will be required to unwire the role of mTORC1 vs. mTORC2 in the innate immune system.
Inflammatory events in patients under mTOR inhibitor therapy
The general proinflammatory effects of mTORC1 inhibition in experimental models are supported by the immunological analysis of kidney transplant patients on rapamycin therapy. These patients display an increased inflammatory and immunostimulatory potential of myeloid DCs ex vivo compared with controls [13, 21]. These data were supported by an unbiased transcriptional analysis of rapamycin-treated kidney transplant recipients [37]. Hence, the most prominent transcriptional alterations affected the innate immune cell system and NF-κB-mediated proinflammatory pathways [37]. The proinflammatory and immunostimulatory properties of rapamycin on myeloid immune cells in kidney transplant patients can be observed even in the presence of steroids, one of the strongest anti-inflammatory agents used in clinical medicine [21]. The use of rapamycin in kidney transplantation is problematic due to the frequent occurrence of various side effects including inflammatory manifestations such as pneumonitis, glomerulonephritis, or chronic inflammatory anemia [38]. In a prospective observational study that analyzed these inflammatory complications, 66% of the patients displayed at least one inflammatory symptom [39]. After switching the patients to rapamycin, there was an increase in IL-6 and TNF-α expression in the peripheral blood without the usual compensation of negative feedback loops depending on IL-10 and soluble TNF receptors [39]. IL6 and TNF-α changes correlated with the intensity of the clinical inflammatory manifestations suggesting that mTOR inhibition triggers a destabilization of the inflammatory cytokine balance in transplanted patients [39]. Proteinuria, which is another frequently observed side effect in patients treated with mTOR inhibitors, seems to originate from the activation of the innate immune system [40]. In contrast, kidney transplant patients on rapamycin rarely experience cytomegalovirus infection and reactivation, which is normally a serious problem in this patient cohort. The virus sustains mTORC1 activity during late phases of the viral cycle to promote viral protein expression and replication in macrophages and fibroblasts [41, 42].
Upstream regulators of mTOR in innate immune cells
As elucidated above, PI3K is a major activator of mTORC1 and it plays a prominent role transducing the TLR activating signal to mTORC1. Hence, similarly to mTORC1 inhibition, genetic disruption or pharmacological inhibition of PI3K augments IL-12 production in isolated murine splenic DCs [19]. Pharmacological inhibition of PI3K in a murine cecal ligation and puncture-induced polymicrobial sepsis model increases mortality caused by an amplified production of proinflammatory cytokines including IL-1β, IL-6, IL-12, and TNF-α, supporting the PI3K pathway to be a negative feedback regulator for inflammatory cytokines [43]. Furthermore, stimulation of human monocytes with Porphyromonas gingivalis LPS during suppression of PI3K activity increases IL-12, but suppresses IL-10 synthesis [44]. Therefore, it is possible to integrate the TSC-mTOR pathway downstream to PI3K in monocytes, macrophages and DCs for the regulation of inflammatory responses (Figure 2) [10].
Recently, it was shown that the mitogen-activated protein kinase (MAPK) p38α activates the mTOR pathway in TLR-stimulated monocytes and macrophages [45]. Mechanistically, p38α phosphorylates the p38 substrate MK2, which then phosphorylates and inactivates TSC2 to promote mTORC1 activation [45]. p38α-mediated mTORC1 activation is independent of PI3K, and the data suggest that p38α and PI3K concurrently modulate mTORC1 to balance IL-12 and IL-10 expression (Figure 2) [45].
Another upstream regulator of mTORC1 constitutes Cot/tpl2, a kinase that is responsible for the activation of the MKK1-Erk1/2 pathway after activation of TLRs. Deficiency of Cot/tpl2 in LPS-stimulated peritoneal or bone marrow-derived macrophages strongly reduces the activation of mTORC1 and increases the expression of IL-12p40 and of inducible nitric oxide synthase (iNOS) [46]. Moreover, Cot/tpl2 controls mRNA translation in TLR-activated macrophages through the mTORC1 pathway [47]. The dissociation of the 4E-BP-eIF4E complex, a key event in cap-dependent mRNA translation initiation, is dependent on Cot/tpl2 and increases cap-dependent translation of TNF-α and IL-6 [47]. Translational control of IRF7 via the mTORC1-4E-BP1/2 pathway is also important to regulate the expression of type I interferons [48]. Leishmania parasites cleave mTOR by a protease to inhibit host translation as a survival mechanism to promote Leishmania proliferation [49].
Inhibition of mTOR in myeloid cells as therapeutic principle against cancer
Vaccination strategies involving DCs to induce potent tumor-specific T-cell responses that reduce the tumor mass and control tumor relapses are actively pursued. Inhibition of mTOR in TLR-stimulated DCs promotes the expression of the costimulatory molecule CD86, whereas PD-L1, a negative regulator for T-cell activation, is decreased (Figure 2) [10, 13, 27]. Moreover, rapamycin augments autophagy, which is important for the presentation of endogenous and exogenous proteins on MHC class I and class II molecules, thereby promoting activation of CD8+ and CD4+ T-cells, respectively [50]. Short-term inhibition of mTOR in monocytes or DCs during TLR stimulation augments the differentiation of proinflammatory, anti-tumor CD4+ T helper 1 (Th1) cells in vitro [10, 20, 21]. Mice immunized with rapamycin-treated DCs and the tuberculosis-vaccine strain BCG show enhanced Th1-mediated protection against virulent Mycobacterium tuberculosis [50].
These T-cell promoting effects of mTOR inhibition in DCs have been exploited in various murine cancer models. Therapeutic autologous vaccination using DCs treated with TLR agonists plus rapamycin results in improved generation of CD8+ T-cells in vivo and improved anti-tumor immunity in a murine melanoma model [51]. Treatment of mice with an immunostimulatory agonistic CD40 antibody together with AZD8055, an ATP-competitive mTOR inhibitor, elicits synergistic anti-tumor responses in a metastatic renal cell carcinoma model [23]. Double-treated mice have a higher number of matured macrophages and DCs producing the anti-tumor cytokines IL-12, IFN-γ and TNF-α [23]. Interestingly, the effects were only observed with AZD8055 but not with rapamycin [23]. The mTOR pathway may be also critical for reprogramming monocytes/macrophages in the tumor microenvironment into tumor-associated macrophages (TAM) that support tumor growth and progression by augmenting tumor angiogenesis [24]. mTORC1 activation by TSC2 knockdown causes monocytes to differentiate into efficient TAM releasing less IL-12 and more IL-10 and to adopt pro-angiogenic properties in vitro. Tumor angiogenesis and growth of murine xenografts is promoted by infusion of mice with TSC2-deficient monocytes. Macrophage depletion is sufficient to block the anti-angiogenic effects of rapamycin on tumors [24].
Concluding remarks
In the last years, an important role of the mTOR pathway for macrophage and DC biology has been elucidated. These results are underscored by the evaluation of the immune system of organ transplant patients receiving an mTOR inhibitor. Future studies with tissue-specific knockout mice that target individual components of the mTOR pathway proteins will decipher the exact role of mTORC1 and mTORC2 in macrophages as well as in DCs for immune responses and tissue homeostasis.
Funding
Thomas Weichhart is supported by the Else Kröner-Fresensius-Stiftung.
Abbreviations
- AMP
adenosine monophosphate
- AMPK
AMP-activated protein kinase
- ATP
adenosine-5'-triphosphate
- Flt3l
Fms-like tyrosine kinase 3 ligand
- FoxO1
forkhead box O1
- GSK3
glycogen synthase kinase 3
- GDP
guanosine diphosphate
- GM-CSF
granulocyte macrophage colony-stimulating factor
- GTP
guanosine-5'-triphosphate
- IGF-1
insulin-like growth factor 1
- IRF
interferon regulatory factor
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PDK1
phosphoinositide-dependent kinase-1
- PI3K
phosphatidylinositol-3 kinases
- PTEN
phosphatase and tensin homolog
- Raptor
regulatory-associated protein of mTOR
- Rheb
Ras homolog enriched in brain
- Rictor
rapamycin-insensitive companion of mTOR
- Stat3
signal transducer and activator of transcription 3
- TNF-α
tumor necrosis factor-α
- TSC1
tuberous sclerosis protein 1
- TSC2
tuberous sclerosis protein 2
References
- 1.Weichhart T. Mammalian target of rapamycin: a signaling kinase for every aspect of cellular life. Methods Mol Biol. 2012;821:1–14. doi: 10.1007/978-1-61779-430-8_1. [DOI] [PubMed] [Google Scholar]
- 2.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013 doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23:707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR Mediated Anti-Cancer Drug Discovery. Drug Discov Today Ther Strateg. 2009;6:47–55. doi: 10.1016/j.ddstr.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz F, Heit A, Dreher S, Eisenacher K, Mages J, Haas T, Krug A, Janssen KP, Kirschning CJ, Wagner H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol. 2008;38:2981–2992. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 12.Weintz G, Olsen JV, Fruhauf K, Niedzielska M, Amit I, Jantsch J, Mages J, Frech C, Dolken L, Mann M, et al. The phosphoproteome of toll-like receptor-activated macrophages. Mol Syst Biol. 2010;6:371. doi: 10.1038/msb.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Horl WH, Saemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 14.Sathaliyawala T, O'Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellersch B, Brocker T. Langerhans cell homeostasis in mice is dependent on mTORC1 but not mTORC2 function. Blood. 2013;121:298–307. doi: 10.1182/blood-2012-06-439786. [DOI] [PubMed] [Google Scholar]
- 16.van de Laar L, Buitenhuis M, Wensveen FM, Janssen HL, Coffer PJ, Woltman AM. Human CD34-derived myeloid dendritic cell development requires intact phosphatidylinositol 3-kinase-protein kinase B-mammalian target of rapamycin signaling. J Immunol. 2010;184:6600–6611. doi: 10.4049/jimmunol.0903089. [DOI] [PubMed] [Google Scholar]
- 17.van de Laar L, van den Bosch A, Boonstra A, Binda RS, Buitenhuis M, Janssen HL, Coffer PJ, Woltman AM. PI3K-PKB hyperactivation augments human plasmacytoid dendritic cell development and function. Blood. 2012;120:4982–4991. doi: 10.1182/blood-2012-02-413229. [DOI] [PubMed] [Google Scholar]
- 18.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 19.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 20.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weichhart T, Haidinger M, Katholnig K, Kopecky C, Poglitsch M, Lassnig C, Rosner M, Zlabinger GJ, Hengstschlager M, Muller M, et al. Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood. 2011;117:4273–4283. doi: 10.1182/blood-2010-09-310888. [DOI] [PubMed] [Google Scholar]
- 22.Turnquist HR, Cardinal J, Macedo C, Rosborough BR, Sumpter TL, Geller DA, Metes D, Thomson AW. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood. 2010;115:4758–4769. doi: 10.1182/blood-2009-10-251488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Q, Weiss JM, Back T, Chan T, Ortaldo JR, Guichard S, Wiltrout RH. mTOR kinase inhibitor AZD8055 enhances the immunotherapeutic activity of an agonist CD40 antibody in cancer treatment. Cancer Res. 2011;71:4074–4084. doi: 10.1158/0008-5472.CAN-10-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Ma T, Shen XN, Xia XF, Xu GD, Bai XL, Liang TB. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res. 2012;72:1363–1372. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
- 25.Cheekatla SS, Aggarwal A, Naik S. mTOR signaling pathway regulates the IL-12/IL-10 axis in Leishmania donovani infection. Med Microbiol Immunol. 2012;201:37–46. doi: 10.1007/s00430-011-0202-5. [DOI] [PubMed] [Google Scholar]
- 26.Foey A, Green P, Foxwell B, Feldmann M, Brennan F. Cytokine-stimulated T cells induce macrophage IL-10 production dependent on phosphatidylinositol 3-kinase and p70S6K: implications for rheumatoid arthritis. Arthritis Res. 2002;4:64–70. doi: 10.1186/ar385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtani M, Hoshii T, Fujii H, Koyasu S, Hirao A, Matsuda S. Cutting Edge: mTORC1 in Intestinal CD11c+CD11b+ Dendritic Cells Regulates Intestinal Homeostasis by Promoting IL-10 Production. J Immunol. 2012;188:4736–4740. doi: 10.4049/jimmunol.1200069. [DOI] [PubMed] [Google Scholar]
- 28.Baker AK, Wang R, Mackman N, Luyendyk JP. Rapamycin enhances LPS induction of tissue factor and tumor necrosis factor-alpha expression in macrophages by reducing IL-10 expression. Mol Immunol. 2009;46:2249–2255. doi: 10.1016/j.molimm.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedl M, Abraham C. Secretory mediators regulate Nod2-induced tolerance in human macrophages. Gastroenterology. 2011;140:231–241. doi: 10.1053/j.gastro.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, Gandjeva A, Zhen L, Chukwueke U, Mao T, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med. 2010;16:767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Han X, Llano J, Bole M, Zhou X, Swan K, Anandaiah A, Nelson B, Patel NR, Reinach PS, et al. Mammalian target of rapamycin inhibition in macrophages of asymptomatic HIV+ persons reverses the decrease in TLR-4-mediated TNF-alpha release through prolongation of MAPK pathway activation. J Immunol. 2011;187:6052–6058. doi: 10.4049/jimmunol.1101532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchet FP, Moris A, Nikolic DS, Lehmann M, Cardinaud S, Stalder R, Garcia E, Dinkins C, Leuba F, Wu L, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–669. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown J, Wang H, Suttles J, Graves DT, Martin M. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286:44295–44305. doi: 10.1074/jbc.M111.258053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan W, Morinaga H, Kim JJ, Bae E, Spann NJ, Heinz S, Glass CK, Olefsky JM. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29:4223–4236. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan H, O'Brien TF, Zhang P, Zhong XP. The role of tuberous sclerosis complex 1 in regulating innate immunity. J Immunol. 2012;188:3658–3666. doi: 10.4049/jimmunol.1102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouard S, Puig-Pey I, Lozano JJ, Pallier A, Braud C, Giral M, Guillet M, Londoño MC, Oppenheimer F, Campistol JM, et al. Comparative Transcriptional and Phenotypic Peripheral Blood Analysis of Kidney Recipients under Cyclosporin A or Sirolimus Monotherapy. Am J Transplant. 2010 doi: 10.1111/j.1600-6143.2010.03302.x. [DOI] [PubMed] [Google Scholar]
- 38.Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 39.Buron F, Malvezzi P, Villar E, Chauvet C, Janbon B, Denis L, Brunet M, Daoud S, Cahen R, Pouteil-Noble C, et al. Profiling sirolimus-induced inflammatory syndrome: a prospective tricentric observational study. PLoS One. 2013;8:e53078. doi: 10.1371/journal.pone.0053078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirsch AH, Riegelbauer V, Tagwerker A, Rudnicki M, Rosenkranz AR, Eller K. The mTOR-inhibitor rapamycin mediates proteinuria in nephrotoxic serum nephritis by activating the innate immune response. Am J Physiol Renal Physiol. 2012;303:F569–575. doi: 10.1152/ajprenal.00180.2012. [DOI] [PubMed] [Google Scholar]
- 41.Poglitsch M, Weichhart T, Hecking M, Werzowa J, Katholnig K, Antlanger M, Krmpotic A, Jonjic S, Horl WH, Zlabinger GJ, et al. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am J Transplant. 2012;12:1458–1468. doi: 10.1111/j.1600-6143.2012.04002.x. [DOI] [PubMed] [Google Scholar]
- 42.Buchkovich NJ, Yu Y, Zampieri CA, Alwine JC. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat Rev Microbiol. 2008;6:266–275. doi: 10.1038/nrmicro1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 44.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 45.Katholnig K, Kaltenecker CC, Hayakawa H, Rosner M, Lassnig C, Zlabinger GJ, Gaestel M, Muller M, Hengstschlager M, Horl WH, et al. p38alpha Senses Environmental Stress To Control Innate Immune Responses via Mechanistic Target of Rapamycin. J Immunol. 2013;190:1519–1527. doi: 10.4049/jimmunol.1202683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Pelaez M, Soria-Castro I, Bosca L, Fernandez M, Alemany S. Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: Implications for NO synthase 2 expression. Eur J Immunol. 2011;41:1733–1741. doi: 10.1002/eji.201041101. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Pelaez M, Fumagalli S, Sanz C, Herrero C, Guerra S, Fernandez M, Alemany S. Cot/tpl2-MKK1/2-Erk1/2 controls mTORC1-mediated mRNA translation in Toll-like receptor-activated macrophages. Mol Biol Cell. 2012;23:2982–2992. doi: 10.1091/mbc.E12-02-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colina R, Costa-Mattioli M, Dowling RJ, Jaramillo M, Tai LH, Breitbach CJ, Martineau Y, Larsson O, Rong L, Svitkin YV, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 49.Jaramillo M, Gomez MA, Larsson O, Shio MT, Topisirovic I, Contreras I, Luxenburg R, Rosenfeld A, Colina R, McMaster RW, et al. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell Host Microbe. 2011;9:331–341. doi: 10.1016/j.chom.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 51.Amiel E, Everts B, Freitas TC, King IL, Curtis JD, Pearce EL, Pearce EJ. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol. 2012;189:2151–2158. doi: 10.4049/jimmunol.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]