Brief Summary:
Treatment of the autoimmune and immune-dysregulatory features of patients with STAT1 GOF or STAT3 GOF disease remains challenging. Jakinibs have been used to treat the severe immune-dysregulation in patients with either STAT1 GOF or STAT3 GOF mutations.
Keywords: STAT1, STAT3, Jakinib, Tofacitinib, Ruxolitinib, Tocilizumab, HLH
To the editor,
Genetic variants in proteins involved in cytokine signaling are now recognized as a cause of overwhelming human immune dysregulation. Autosomal dominant gain of function (GOF) mutations in Signal Transducer and Activator of Transcription (STAT) 1 cause a variable clinical phenotype that can include chronic mucocutaneous candidiasis (CMC), susceptibility to dimorphic fungal and invasive viral infections, combined immunodeficiency, autoinflammation and organ specific autoimmunity1, 2. STAT3 GOF mutations cause early-onset lymphoproliferation with lymphadenopathy and hepatosplenomegaly and multi-organ autoimmunity including cytopenias, hepatitis, inflammatory lung disease, enteropathy, hypothyroidism, and diabetes mellitus (DM)3, 4.
STAT1 and STAT3 belong to a 7-member family of transcription factors that help transmit key cytokine signals from cell membrane receptors to the nucleus using Janus kinases (JAKs). Classically, after a cytokine binds to its receptor, one of four JAKs is activated and phosphorylates the cytokine receptor. STAT molecules are then recruited to the activated receptor, are phosphorylated, dimerize, and translocate to the nucleus where they alter gene expression. Activation of STAT1 and STAT3 occurs downstream of numerous cytokines including: types I, II and III interferons for STAT1 and IL-6, γ chain cytokines, IL-10 family cytokines, and IL-23 for STAT31, 3. Jakinibs are small molecule inhibitors that block cytokine induced JAK activation; tofacitinib preferentially blocks JAK1 and JAK3 activation while ruxolitinib preferentially blocks JAK1 and JAK2. Tofacitinib and ruxolitinib are approved for the treatment of rheumatoid arthritis and myelofibrosis, respectively.
Treatment of the autoimmune and autoinflammatory manifestations of STAT1 GOF or STAT3 GOF disease remains challenging and many patients fail treatment with multiple immunosuppressants. JAK inhibition provides precision directed therapy for these two disease states where more broad immunosuppression is ineffective. Herein, we describe 17 patients with either a STAT1 GOF or STAT3 GOF mutation who were treated with jakinibs, most exhibiting profound symptomatic improvement.
Patients with experimentally confirmed gain-of-function mutations in STAT1 or STAT3 who were treated with a jakinib (ruxolitinib or tofacitinib) were identified from eleven centers internationally. All patients provided written informed consent to site specific Institutional Review Board approved protocols. Retrospective chart reviews were performed to determine clinical manifestations, indication for treatment, dosage, length of treatment, and treatment associated complications (Online Repository Table Ia and Table Ib).
Eleven patients with STAT1 GOF mutations were treated with a jakinib. All 11 patients had previous infections that had resolved by the time of treatment, with the exception of coccidiomycosis present in P5. CMC was present in patients 1, 2, 5, and 6–11. Autoimmune cytopenias and/or autoimmune hepatitis was present in 6 patients (P1, 3–5, 9–10). In 8 patients (P3–5, P7–11), growth failure was prominent; 5 of those patients had severe enteropathy and 3 required total parenteral nutrition (TPN). Endocrinopathies were present in five. In 6 patients bronchiectasis and chronic lung disease related to infection was the most prominent pulmonary complication.
Six patients with STAT3 GOF mutations were treated with a jakinib. Most infectious complications were from respiratory viral illness. All patients had severe autoimmunity including hepatitis, enteropathy (P14 and P15 were TPN dependent), cytopenias, lymphoproliferation (including hemophagocytic lymphohistocytosis (HLH)), serositis, and severe growth failure. One patient had polyarticular arthritis (P16) and two patients had thyroid disease (P13,17). Lymphocytic interstitial pneumonia (LIP)/interstitial lung disease (ILD) was present in 2 patients (P15 and P17), both of whom were oxygen-dependent.
Ruxolitinib is broadly available in 5mg, 10mg, 15mg, 20mg, or 25mg tablets and is currently approved for the treatment of adults with polycythemia vera and myelofibrosis5, 6. There is little pediatric dosing guidance and no pharmacokinetic data in patients with STAT1 GOF or STAT3 GOF disease. In the patients included in this cohort, ruxolitinib was dosed at 0.4–2mg/kg/day or 15mg/m2/dose BID as described in Loh et al. Sixteen patients received ruxolitinib (P1–13, P15,17) and one received tofacitinib (P16). Tofacitinib was used in P16 according to the recommended dosing per the package insert for treatment of rheumatoid arthritis.
All patients had received prior immunosuppression and several were receiving concomitant immunosuppression at the time of jakinib treatment (Online Repository Table Ia and Table Ib). Indications for jakinib therapy included autoimmunity or immunodysregulation not controlled with other therapies (P1–5, P7–17), immunosuppression prior to hematopoietic stem cell transplant (HSCT) (P8), HLH (P14) and/or as adjuvant to chronic progressive infection (P1–3, P5–6, 8–9,11).
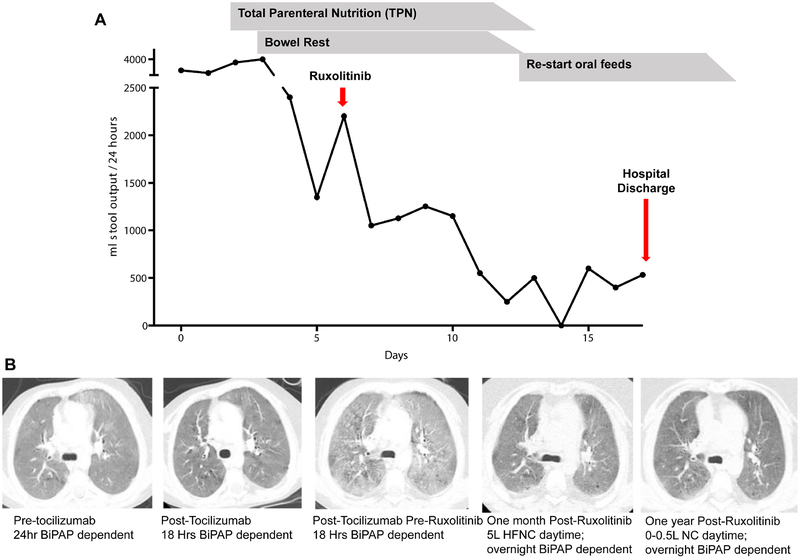
Fourteen patients had significant clinical improvement with the addition of tofacitinib or ruxolitinib to their therapy. The two patients with TPN-dependent enteropathy became TPN independent (Figure Ia), one patient with LIP had radiographic resolution with improved lung function and cessation of daytime supplemental oxygen (P15) (Figure Ib), autoimmune cytopenias improved in four patients with STAT1 GOF and four patients with STAT3 GOF, and HLH entered into remission in P14 prior to HSCT.
Figure I:
Improvement in Diseases Manifestations in STAT11 GOF and STAT3 GOF after treatment with jakinib and tocilzumab.
A. Patient 3 with severe TPN-dependent enteropathy. Stool output significantly declined with intitiation of ruxolitinib.
B. Patient 12 with lympocytic interstitial pneumonia. a. Chest CT imaging Pre-Tocilizumab shows diffuse ground glass pulmonary opacities and a few tiny cysts. b. Post-Tocilizumab chest CT imaging shows a slight overall decrease in the diffuse ground glass pulmonary opacities. c. Pre-Ruxolitinib chest CT imaging shows an increase in the diffuse ground glass pulmonary opacities compared to both prior CT exams. d. One month after starting Ruxolitinib shows a considerable decrease in the diffuse ground glass pulmonary opacities. e. One year after treatment with both tocilizumab, chest CT and ruxolitinib shows a further decrease in the diffuse ground glass pulmonary opacities. BiPAP, bilevel positive airway pressure ventilation. HFNC, high flow nasal cannula. NC, nasal cannula.
In all patients with STAT3 GOF, tocilizumab, a humanized IL-6 receptor blocking monoclonal antibody, was used as adjunctive therapy and was initiated either preceding (P13, P15,16), concurrently (P12,14), or following (P17) jakinib therapy. Patients 12, and 14–16, had significant clinical improvement; specifically in arthritis (P16), autoimmune hepatitis (P13, P15–16), and interstitial lung disease (P15). In the patients who started tocilizumab preceding a jakinib, they failed to obtain complete disease control which led to the addition of a jakinib. Ruxolitinib and tocilizumab were initiated concurrently in patient 14 for treatment of HLH, which was able to induce remission prior to HSCT. Patient 17 was first treated with ruxolitinib with mild improvement in enteropathy, but without pulmonary improvement. Thus, tocilizumab was subsequently added.
Three patients (P6, P13, P17) had no or minimal response to jakinib therapy ultimately succumbing to fatal disease progression. P6 had disseminated coccidiomycosis when ruxolitinib was started. In an effort to balance her inflammation without worsening her progressive fungal disease, a low dose of ruxolitinib was started. She ultimately succumbed to her disease despite jakinib therapy. P13 developed Pneumocystis jirovecii pneumonia complicated by gram negative sepsis, disseminated intravascular coagulopathy, and multi-organ failure. Ruxolitinib was added to tocilizumab treatment during this event in an effort to diminish the inflammatory response; however, she ultimately died. Patient 17 was first treated with ruxolitinib with transient, mild improvement in enteropathy, but failed to improve his end-stage lung disease. Therefore, tocilizumab was added, but his respiratory failure progressed leading to his demise.
In the remaining fourteen patients, adverse effects of jakinib therapy included transient thrombocytopenia, transiently elevated transaminases and bilirubin, and concurrent infection with rhinovirus, human metapneumovirus, parainfluenza virus type I, norovirus, and adenovirus. All of these infections cleared with supportive care alone while on jakinib therapy. Two patients (P3 and P11) developed Herpes zoster while receiving ruxolitinib. Ruxolitinib was held during acute infection and patients were treated with anti-viral medications (acyclovir and famvir). Patient 3 re-started ruxolitinib after this episode and has remained well with continued resolution of enteropathy and CMC. Patient 11 continued off ruxolitinib based on parental preference and had recurrence of severe CMC and a few months later developed Pneumocystis jirovecii and Cryptococcal pneumonia.
Fourteen patients responded favorably to treatment with ruxolitinib or tofacitinib and long term treatment led to clinical improvement; most effectively for immune dysregulation features. In all patients with STAT1 GOF disease, the CMC resolved. There were few medication side effects and most patients tolerated the jakinibs well.
Use of jakinibs to treat rheumatoid arthritis has been associated with an increase in herpes zoster infections. We noticed a trend toward increased viral infections in our cohort including 2 patients with herpes family viruses. However, P15 with ILD but stable minimal oxygen requirements was able to clear both rhinovirus and parainfluenza virus infections while receiving immunomodulation from ruxolitinib. In the case of Patients 3 and 11 who had Herpes zoster, the infection cleared with treatment with anti-virals and while ruxolitinib was held. Neither patient was on acyclovir prophylaxis while on the jakinib.
Tocilizumab was used as additional treatment in all 6 STAT3 GOF patients, 3 prior to, 2 concurrently, and 1 following a jakinib. After an initial robust response to tocilizumab in three patients, the effect appeared to plateau, necessitating the addition of a jakinib. The addition of a jakinib completely resolved some features of immune dysregulation in P12 and P14–16, suggesting that the combination of IL-6 blockade plus a jakinib may be an effective treatment strategy for STAT3 GOF disease.
Four of the seventeen patients died (P6, P13, P14, and P17). In three of the four, ruxolitinib was ineffective at reversing disease progression and infectious burden in critically ill patients with severe, invasive infection or with progressive lung disease and disseminated infection suggesting that jakinibs may be most effective when started before patients become severely ill when the combination was started, concluding that further immunosuppression was ineffective. The experience with these three patients demonstrates that there may be a point in which disease burden is so great that jakinib therapy is no longer effective. The fourth death (P14) was due to post-transplant complications and not related to pre-transplant jakinib therapy; his HLH and enteropathy resolved prior to transplant on therapy.
Treatment of systemic autoimmunity and immune dysregulation is challenging, however the identification of monogenic causes of disease such as STAT1 and STAT3 GOF has provided the opportunity to apply targeted precision therapies in identified patients. Both of these disorders have significant morbidity and mortality, and lack effective treatments1, 3, 4, 7, 8. Use of multiple chronic immunosuppressant medications often does not result in clinical resolution of autoimmune manifestations and can be complicated by significant side effects. Hematopoietic stem cell transplantation has been attempted in STAT1 GOF but is complicated by low survival and high rates of secondary graft loss9; whereas, in STAT3 GOF disease there is slightly better survival after transplant (data not shown). In both diseases, survival and engrafment may be dependent on disease stability at the time of transplant.
The findings in this cohort suggest that jakinibs can be both safe and effective in the treatment of STAT1 or STAT3 GOF patients with severe autoimmunity and immune dysregulation. It is also evident from this limited study that initiation of jakinib therapy late in the course of disease may not be sufficient to induce disease remission or to prevent life-threatening immune dysregulation. Based on our experience, we initiate jakinib therapy when patients present with evidence of immune dysregulation, organ specific autoimmunity, chronic autoimmune cytopenias, lymphoproliferation, refractory CMC, or recurrent severe infections. The starting dose for ruxolitinib was extrapolated from data on the use of ruxolitinib treatment in pediatric myelofibrosis6. A dose escalation is employed starting with half of the goal dose and increasing by 25% weekly until the goal dose is achieved. Transaminase levels and complete blood count are monitored frequently and if abnormalities occur, the dose is adjusted. We monitor for invasive viral infections quarterly. Because of the risk of herpes virus infections, we also prescribe acyclovir prophylaxis. Further investigation of the most effective dose, bioavailability, kinetics and side effects of jakinibs for treatment in STAT1 GOF and STAT3 GOF disease are needed.
Supplementary Material
Acknowledgements
This work was supported in part by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have reviewed the manuscript and have no conflicts of interest to disclose.
References
- 1.Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood 2016; 127:3154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vargas-Hernandez A, Mace EM, Zimmerman O, Zerbe CS, Freeman AF, Rosenzweig S, et al. Ruxolitinib partially reverses functional NK cell deficiency in patients with STAT1 gain-of-function mutations. J Allergy Clin Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Allen HL, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet 2014; 46:812–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood 2015; 125:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstovsek S, Passamonti F, Rambaldi A, Barosi G, Rosen PJ, Rumi E, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014; 120:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loh ML, Tasian SK, Rabin KR, Brown P, Magoon D, Reid JM, et al. A phase 1 dosing study of ruxolitinib in children with relapsed or refractory solid tumors, leukemias, or myeloproliferative neoplasms: A Children’s Oncology Group phase 1 consortium study (ADVL1011). Pediatr Blood Cancer 2015; 62:1717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinacht KG, Charbonnier LM, Alroqi F, Plant A, Qiao Q, Wu H, et al. Ruxolitinib reverses dysregulated T helper cell responses and controls autoimmunity caused by a novel signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J Allergy Clin Immunol 2017; 139:1629–40.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol 2015; 135:551–3. [DOI] [PubMed] [Google Scholar]
- 9.Leiding JW, Okada S, Hagin D, Abinun M, Shcherbina A, Balashov DN, et al. Hematopoietic stem cell transplantation in patients with gain-of-function signal transducer and activator of transcription 1 mutations. J Allergy Clin Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.