Abstract
Introduction:
Protein kinases are involved in various cellular functions including metabolism, cell cycle regulation, survival, and differentiation. Dysregulation of protein kinases is implicated in various processes of carcinogenesis. The advent of protein kinase inhibitors in cancer therapy has led to a paradigm shift in how we treat cancer. There are several protein kinase inhibitors that have been approved by FDA in the last few decades.
Areas Covered:
This article provides a review of the clinical benefits and side effect profiles of FDA approved protein kinase inhibitors as of December 2017 for the well-known oncogenic protein kinases. The role of the respective oncogenic protein kinases in carcinogenesis and cancer progression were searched in PubMed and discussed. The relevant and landmark clinical trials mostly phase III trials of protein kinase inhibitors leading up to the FDA approval were PubMed searched and discussed.
Expert Commentary:
Further understanding of the molecular origin of cancers would help us identify new targets, while clinical trials trying to identify the appropriate sequence of available kinase inhibitor would make better use of current protein kinase inhibitor armamentarium. Also, testing these drugs in the adjuvant setting in patients with high risk of recurrence might offer some clinical benefit. Development of resistance, side effects and cost are major limitations of protein kinase inhibitors, therefore understanding of the molecular mechanisms of resistance and designing protein kinase inhibitors to obviate the resistance would help overcome the resistance. Finally, collaboration between international organizations for cancer research and voluntary and charity organizations might help reduce the cost.
Keywords: cancer therapy, targeted therapy, medicinal chemistry, kinase inhibitors, tyrosine kinase inhibitors
1. Introduction to kinases
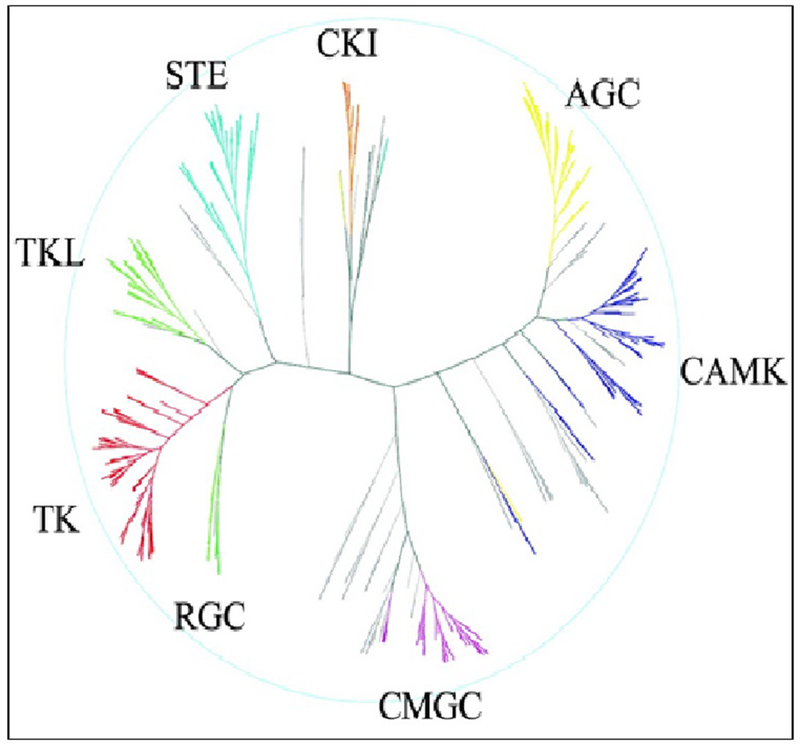
Ever since the first phosphorylation reaction was identified in glycogen metabolism and glycogen phosphorylase was identified in the 1960s, kinases have been of interest to scientists. A protein kinase is an enzyme that modifies other proteins by chemically adding the terminal γ-phosphate group of adenosine triphosphate (ATP) to serine, threonine or tyrosine residues which is also known as phosphorylation. Phosphorylation results in a functional change of the target protein (substrate) by regulating signaling pathways by amplification (common) or cellular location, or by interactions with regulatory proteins. Human genome sequencing has revealed that about 2% of the human genome encodes for protein kinases [1, 2]. They are further subdivided into groups, families, and subfamilies (Fig 1). There >500 protein kinases known and the structures of these kinases in various conformations have been determined by x-ray protein crystallography [2]. Kinases are involved in various cellular functions including metabolism, cell cycle regulation, survival, and differentiation. Once activated kinases typically phosphorylate serine, threonine or tyrosine residues on the target protein, leading to conformational change and thereby the functionality of the target proteins [3]. Please see the figure I for the human kinome represented as a phylogenetic tree as listed in the scientific database.
Figure 1:
The ‘Human Kinome’ as adapted from ‘ The Protein Kinase Complement of the Human Genome’ [2]. Human kinome represented as a phylogenetic tree listed in the scientific database. In addition to the eight protein kinase groups depicted in the main dendrogram, lipid, atypical and clinically-relevant mutant kinases are also annotated to the human kinome. The classic kinase dendrogram includes the following eight kinase groups: TK - Tyrosine kinase; TKL - Tyrosine kinase-like; STE - Homologs of yeast Sterile 7, Sterile 11, Sterile kinases; CK1 - Casein kinase 1; AGC - Containing PKA, PKG, PKC families; CAMK - Calcium/calmodulin-dependent protein kinase; CMGC - Containing CDK, MAPK, GSK3, CLK families; OTHER - Divergent kinases not represented in other groups.
2. Oncogenic Protein Kinases
Phosphorylation of the target proteins by kinases is tightly regulated and any perturbation to this regulation may lead to a diseased state. There are several kinases that are found to be dysregulated in various cancers. In fact, the first proto-oncogene to be identified in 1978, c-Src codes a non-receptor tyrosine kinase [4]. Multiple mechanisms lead to the dysregulation of kinases enhancing oncogenic potential which includes over-expression, relocation, and fusion, point mutations, or dysregulation of upstream signaling [5–7]. Exploring the role of kinases has not only helped advance the field of cancer biology but also led to the advent of ‘targeted therapy’ or ‘personalized medicine’ in cancer leading to a paradigm shift in cancer therapy.
In this review, we will focus on the protein kinases which are implicated in carcinogenesis, the progression of cancer and for which there is a US FDA (the United States Food and Drug Administration) approved kinase inhibitors.
3. Kinase Inhibitors
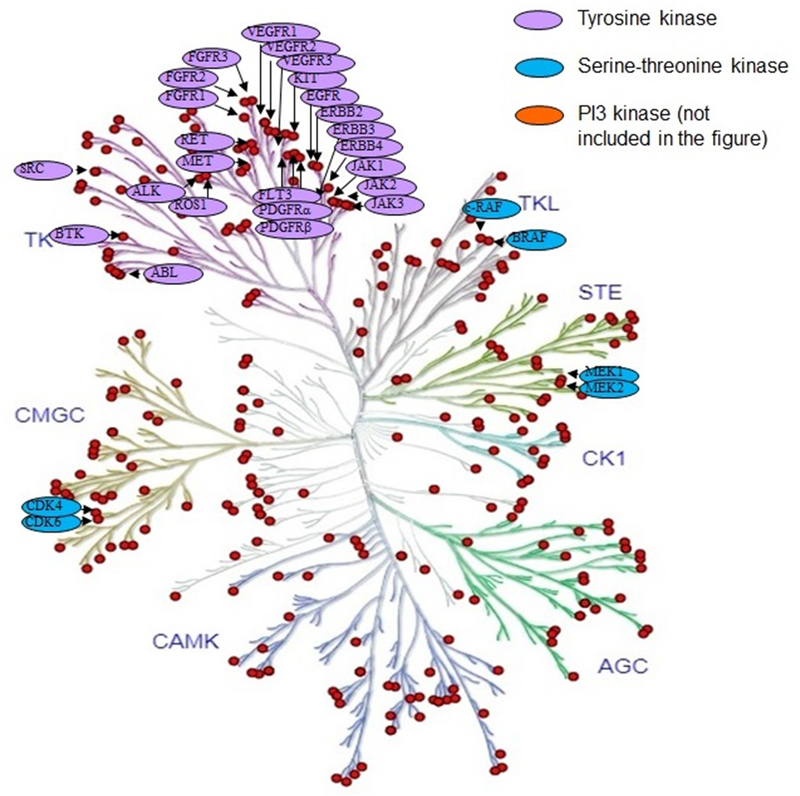
The spectacular results demonstrated with imatinib mesylate (aka Gleevec) targeting break point cluster (Bcr)-Abelson (Abl) fusion protein in chronic myeloid leukemia (CML) lead to the development of a flurry kinase inhibitors against several oncogenic kinases offering clinical benefit. The potential benefit of kinase inhibitors depends on the degree of oncogenic addiction to the specific kinase, to the pharmacokinetic and pharmacodynamic properties of the kinase inhibitor. Please see the figure II for the small molecule inhibitors of various kinases approved by FDA matched to the phylogenetic map of the respective kinases. Chemical structure of the prototype tyrosine kinase inhibitor (TKI) imatinib, the epidermal growth factor receptor (EGFR) TKI gefitinib, serine/threonine kinase inhibitor vemurafenib are shown in figure III–V. A list of FDA approved KIs, their targets and their clinical indications is shown in table 1.
Figure 2:
The phylogenetic map of the human kinome demonstrating development and US FDA approval of small molecule kinase inhibitors to tyrosine kinases and serine/threonine kinases for a variety of solid and hematologic malignancies. Other kinome family members under active clinical investigation include NTRK-fusion activated kinase, CDK8, and ERK1/2.
Figure 3:
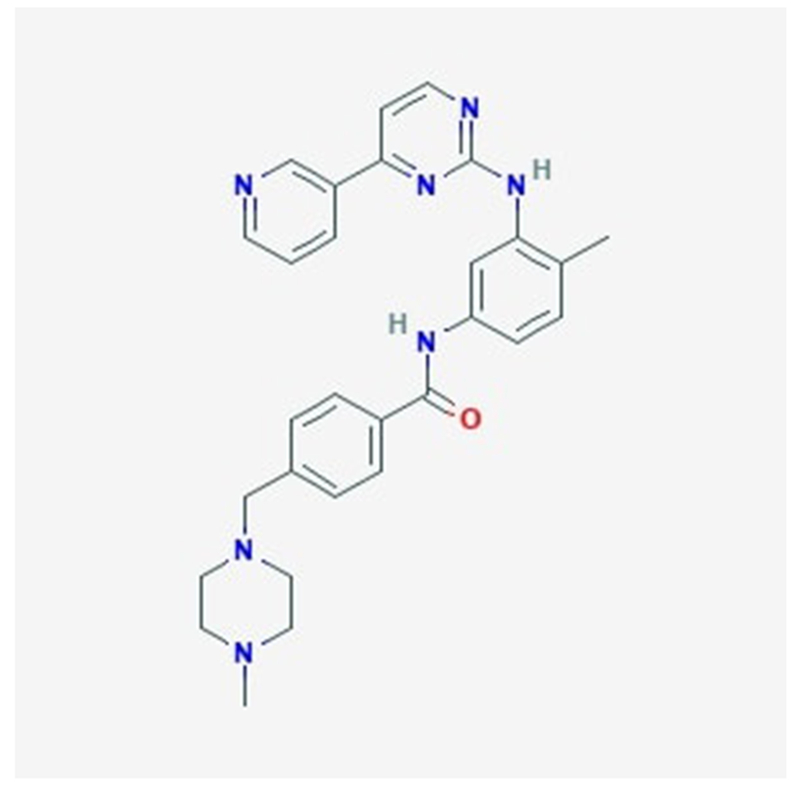
Chemical structure of a prototype TKI imatinib as adapted from National Center for Biotechnology Information.
Figure 5:
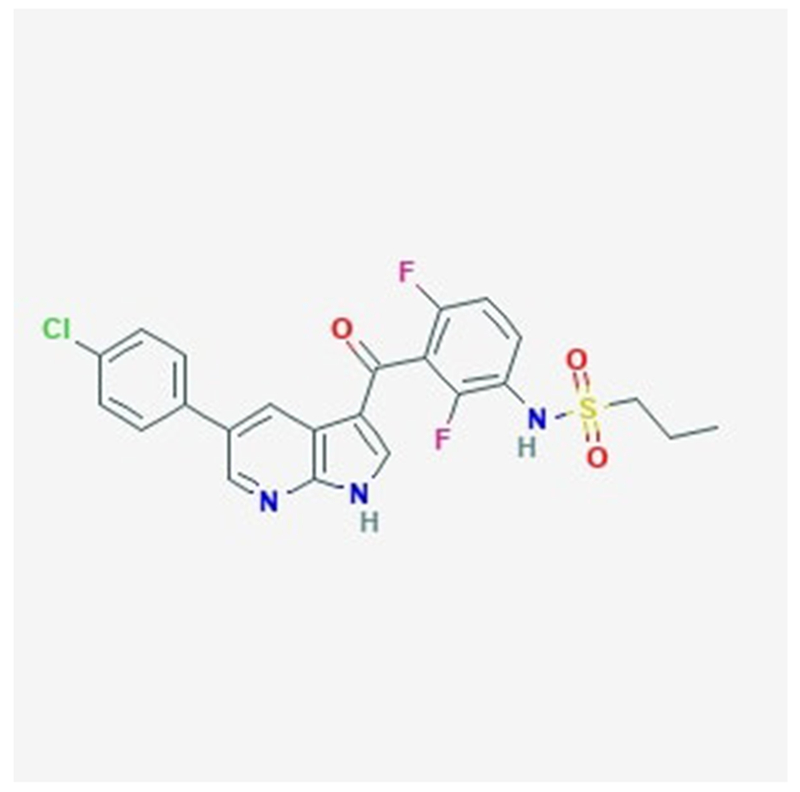
Chemical structure of a serine/threonine kinase inhibitor vemurafenib as adapted from National Center for Biotechnology Information.
Table 1.
Cellular targets and clinical indications of FDA-approved kinase inhibitors. The list of KI is not comprehensive, and these drugs might also inhibit additional proteins that are not listed here.
| No | Name of the TKI | Mechanism of Action: Target(s) | FDA Approved Indications |
|---|---|---|---|
| 1. | Imatinib | Bcr-Abl kinase, c-Kit, PDGFR | 1. Ph+ CML 2. Adult and pediatric patients Ph+ ALL 3. Myelodysplastic syndrome or myelo-proliferative syndrome with PDGFR gene re-arrangemetns 4. Chronic eosinophilic leukemia 5. Aggressive systemic mastocytosis 6. Dermatofibrosarcoma protubarans 7. c-Kit postive GIST 8. Adjuvant Treatment of GIST |
| 2. | Nilotinib | Bcr-Abl kinase, c-Kit, PDGFR | 1. Ph+ CML as a first line therapy 2. Treatment can be discontinued after sustained response |
| 3. | Dasatinib | Bcr-Abl kinase, c-Kit, PDGFR, Src | 1.Ph+ CML and ALL 2. Ph+ CML who are resistant and intolerant to imatinib |
| 4. | Bosutinib | Bcr-Abl, Src | 1. Ph+ CML |
| 5. | Ponatinib | Bcr-Abl including T315I mutant kinase | 1.T3I5I+ CML or ALL 2. Ph+ CML or ALL in whom no other TKI can be used |
| 6. | Gefitinib | EGFR | 1. EGFR mutation positive metastatic NSCLC as a first line therapy |
| 7. | Erlotinib | EGFR | 1. EGFR mutation positive metastatic NSCLC as a first line therapy 2. Combination with gemcitabine in advanced pancreatic cancer |
| 8. | Afatinib | EGFR | 1.EGFR mutation positive metastatic NSCLC as a first line therapy 2. Advanced squamous cell carcinoma as a second line therapy. |
| 9. | Osimertinib | EGFR, including EGFR T790M mutant kinase | 1. EGFR T790M mutation positive advanced NSCLC in patients who had progressed on or after TKI therapy |
| 10. | Lapatinib | HER2 | 1. HR-positive, HER2-positive advanced breast cancer in combination with letrozole 2. HER2-positive advanced breast cancer patients who had at least received one anthracyclcline, taxane and trastuzumab |
| 11. | Neratinib | HER1, HER2, HER3, HER4 | 1. Extended adjuvant treatment of HER-positive early stage breast cancer after trastuzumab based adjuvant therapy in stage I-III breast cancer |
| 12. | Sorafenib | VEGFR, PDGFR, BRAF, FTL3, RET, c-Kit | 1. Metastatic RCC 2. Advanced HCC 3. Radioactive-iodine refractory differentiated thyroid cancer |
| 13. | Sunitinib | VEGFR, PDGFR, FLT3R, c-Kit, RET | 1. Metastatic RCC 2. Imatinib resistant GIST 3. Adjuvant therapy for RCC after nephrectomy |
| 14. | Pazopanib | VEGFR, PDGFR, c-Kit, FGFR3 | 1. Metastatic RCC 2. Advanced soft tissue sarcoma |
| 15. | Axitinib | VEGFR | 1. Second line therapy for metastatic RCC |
| 16. | Lenvatinib | VEGFR, FGFR, PDGFR, RET, c-Kit | 1. Progressive, radioactive iodine refractory differentiated thyroid cancer 2. Metastatic RCC as a second line agent in combination with everolimus |
| 17. | Cabozatinib | VEGFR, PDGFR, c-Kit, MET, FLT3, RET | 1. Advanced MCT 2. First line therapy for metastatic RCC |
| 18. | Vandetanib | VEGFR, RET, MET | 1. Advanced medullary thyroid carcinoma |
| 19. | Regorafenib | VEGFR, PDGFR, FGFR, BRAF, c-Kit and RET | 1. Advanced CRC 2. Advanced GIST 3. Advanced HCC |
| 20. | Vemurafenib | BRAFV600E mutant kinase | 1. BRAFV600E positive advanced melanoma |
| 21. | Dabrafenib | BRAFV600E mutant kinase | 1.BRAFV600E positive advanced melanoma 2. BRAFV600E positive advanced melanoma in combination with trametinib |
| 22. | Trametinib | MEK | 1.BRAFV600E positive advanced melanoma 2. BRAFV600E positive advanced melanoma in combination with dabrafenib |
| 23. | Cobimetinib | MEK | 1. BRAFV600E positive advanced melanoma in combination with vemurafenib |
| 24. | Crizotinib | ALK-EML4, MET, ROS-1 | 1. ALK-EML4 positive NSCLC 2. ROS1 gene re-arrangement positive NSCLC |
| 25. | Certinib | ALK-EML4 | 1. ALK-EML4 positive NSCLC for first line therapy |
| 26. | Alectinib | ALK-EML4 | 1. ALK-EML4 positive NSCLC for first line therapy |
| 27. | Brigatinib | ALK-EML4 | 1. ALK-EML4 positive NSCLC who are resistant or intolerant to crizotinib |
| 28. | Lorlatinib | ALK-EML4, ROS-1 | 1. ALK-EML4 positive NSCLC who had progressed on one or more ALK TKIs |
| 29. | Ibrutinib | BTK | 1. Relapsed and refractory MCL 2. Relapsed and refractory WM 3. First line therapy for CLL and SLL 4. Relapsed and refractory MZL |
| 30. | Acalibrutinib | BTK | 1. Relapsed and refractory MCL |
| 31. | Midostaurin | PKC, FLT3 | In combination with chemotherapy for AML |
| 32. | Ruxolitinib | JAK1 and JAK2 | 1. Intermediate to high risk MF 2. Hydroxyurea resistant or intolerant PV |
| 33. | Idelalisib | PI3Kδ | 1. Relapsed CLL 2. Relapsed follicular B-cell NHL 3. Relapsed SLL |
| 34. | Copanlisib | pan-class I PI3K | 1. Relapsed FL |
| 35. | Palbociclib | CDK4/CDK6 | 1. Postmenopausal women with advanced and metastatic breast cancer in HR+, HER2-ve, combination with letrozole (2015). 2. Postmenopausal women with advanced and metastatic breast cancer in HR+, HER2-ve, combination with aromatase inhibitors |
| 36. | Ribociclib | CDK4/CDK6 | 1. Postmenopausal women with advanced and metastatic breast cancer in HR+, HER2-ve, combination with aromatase inhibitors |
| 37. | Abemaciclib | CDK4/CDK6 | 1. Postmenopausal women with advanced and metastatic breast cancer in HR+, HER2-ve, combination with aromatase inhibitors. 2. As a single agent in postmenopausal women HR+, HER2-ve breast cancer who had progressed on hormone therapy or who progressed on chemotherapy in case of the metastatic setting |
3.1. Bcr-Abl Tyrosine kinase inhibitors
The discovery of the Bcr-Abl fusion in CML has revolutionized the understanding of the molecular origin of cancer [8]. Imatinib, a TKI that was designed to inhibit the Bcr-Abl kinase activity led to the paradigm shift in cancer therapeutics [9]. Since then multiple other Bcr-Abl kinase inhibitors have been developed as detailed below to overcome the shortcomings of imatinib.
3.1.1. Imatinib
Imatinib was initially discovered by Ciba-Geigy while they were screening for a protein kinase inhibitor against platelet-derived growth factor receptor (PDGFR). Interestingly, a compound named CGP53,716 also had significant activity against Abl kinase. Further modifications of this compound to better target Bcr-Abl kinase lead to the discovery of imatinib (STI571) [9]. Based on previous studies showing the efficacy of imatinib in patients who were failed on the first line therapy, imatinib was tested in patients with previously untreated CML against the then standard therapy interferon and cytarabine [10, 11]. FDA approved imatinib for the treatment of chronic phase CML as front-line therapy in 2001. Since then, it has been approved for the treatment of rare hematologic malignancies and proto-oncogene c-Kit or tyrosine-protein kinase Kit (c-Kit) mutated gastrointestinal stromal tumors (GIST) [12].
International Randomized study of Interferon and STI571 (IRIS) is a landmark phase III, multi-center, crossover, international randomized control trial that compared imatinib vs interferon plus cytarabine in chronic phase CML. In the median follow up of 19 months, in the intention to treat analysis, imatinib group achieved significantly higher complete hematologic responses in 95.3 % [95% confidence interval (CI) 93.2-96.9] of patients compared to 27.3 % (95% CI 6.0-61.0) of patients in the interferon plus cytarabine group. A major cytogenetic response was observed in 85.2 % (95% CI 81.9-88.0) of patients when compared to 22.1 % (95% CI 18.7-25.8) of patients in interferon plus cytarabine group. Not surprisingly, there was no significant difference in the overall survival (OS) noted as many patients in combination group crossed over to the imatinib group [13]. At 60 months follow up only 3% of the group assigned to interferon plus cytarabine group remained in the combination group. At 60 months follow up, the imatinib group achieved 98% a complete hematologic remission, 92% major cytogenetic response, and 89% (95% CI 86-92) overall survival rate (OSR) [14]. In 120 months follow up, 48.3% of patients who had randomly assigned to imatinib had completed the treatment regimen, whereas only 1.3 % in the combination group had completed the assigned treatment. The imatinib group had achieved OSR of 83.3% (95% CI 80.1-86.6) [15].
Grade 3 or 4 adverse events reported were neutropenia, thrombocytopenia, anemia, elevated liver enzymes, and other drug-related adverse events [14]. No new cumulative toxic side effects were found in 10 years follow up [15].
3.1.2. Nilotinib
Nilotinib is a second generation Bcr-Abl kinase inhibitor. It has a similar spectrum of kinase targets that includes c-Abl, Bcr-Abl, Arg, c-Kit and PDGFR. It was designed to overcome the imatinib resistance by binding to the kinase domain of imatinib-resistant mutants of Bcr-Abl and imatinib sensitive Bcr-Abl with higher affinity. In vitro nilotinib inhibited the kinase activity of most of the 15 common Bcr-Abl mutants except for T315I gatekeeper mutant enzyme [16]. It is 20-fold more potent than imatinib in inhibiting Bcr-Abl TK activity, expected to be more efficacious than imatinib and to cause the decreased frequency of resistance [17]. Nilotinib is currently approved by FDA as front-line therapy for chronic phase CML and for patients who are resistant or intolerant to imatinib.
Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients (ENESTnd) is a phase III, randomized, open-label, multi-center study which compared the efficacy and safety of nilotinib 300 mg bid and 400 mg bid vs imatinib 400 mg daily in patients with chronic phase CML. At 12 months, 300 mg bid of nilotinib achieved a two-fold high rate of response at 44% compared to 22% with imatinib (p<0.001); 300 mg bid of nilotinib achieved 80% complete cytogenetic response vs 65% with imatinib (p<0.001). At 24 months 300 mg bid of nilotinib achieved a two-fold high response rate of 71% compared to 44% with imatinib (p<0.0001). Nilotinib at 300 mg bid achieved a 26% complete cytogenetic response compared to 10% with imatinib (p<0.0001). In addition, during 12 months and 24 months follow up either 300 mg bid of nilotinib group or 400 mg bid of nilotinib achieved significant improvement in the time to progression to the accelerated phase or to blast crisis compared to imatinib [18, 19]. Interestingly, there was no OS advantage with nilotinib, although CML related death was lower in the nilotinib group [19]. At 60 months follow up, 300 mg nilotinib bid again had achieved a significantly higher molecular response [20]. Studies are underway to identify predictive markers for achieving molecular response [21].
Nilotinib was tested in patients with CML who had a suboptimal cytogenetic response on a standard dose of imatinib 400 mg daily against dose escalation of imatinib to 800 mg daily in the Randomized Phase III study of Imatinib Dose Optimization Compared With Nilotinib in Patients With Chronic Myelogenous Leukemia and Supotimal Resonose to Standard-dose Imatinib (LASOR) trial. At 6 months, when the responses achieved after cross-over were included there was no statistically significant difference in molecular response rate achieved, although long-term follow up in this study is needed to make meaningful conclusions [22]. ENESTfreedom is a phase II clinical trial Evaluating Nilotinib Efficacy and Saftety in Clincal Trials assessing the treatment-free remission rate in patients treated with nilotinib for chronic phase CML. At 48 weeks after stopping nilotinib, about half of the patients remained in a major molecular response [23]. Following this study FDA has approved the label for nilotinib stating the discontinuation therapy can be attempted after attaining a sustained molecular response.
Grade 3 or 4 adverse events include rash, headache, diarrhea, fluid retention, cardiovascular events, cytopenia and biochemical abnormalities [20].
3.1.3. Dasatinib
Dasatinib is an orally available second generation TKI. When compared to imatinib and nilotinib, Dasatinib is an extremely potent (350-fold compared to imatinib) Bcr-Abl kinase inhibitor. It preferentially binds to active conformation of the Bcr-Abl kinase domain. Dasatinib not only inhibits Bcr-Abl and c-Kit, PDGFRA and B, and ephrin receptor kinase but also Src kinase family members. Src kinase is implicated in the imatinib resistance. In vitro, dasatinib inhibited the kinase activity of 14 of 15 Bcr-Abl mutant proteins except for the gatekeeper T315I mutant kinase. So, dasatinib was expected to have better and durable efficacy than imatinib and was also expected to be useful in imatinib-resistant CML [24]. It is currently FDA approved for the treatment of Ph-positive CML that is resistant or intolerant to previous Bcr-Abl kinase inhibitors.
Multiple phase III trials in patients with imatinib-resistant or intolerant chronic phase CML and blast crisis have established the beneficial effect of dasatinib [25–27]. When dasatinib was compared with imatinib as first-line therapy in chronic phase CML, like nilotinib, dasatinib achieved better cytogenic responses and lesser transformation to accelerated or blast phase. Once again, there was no significant difference in the progression free survival (PFS) or OSR. Pleural effusions and grade 3/4 thrombocytopenia were common drugs related adverse events. Pulmonary hypertension occurred in 1.2% of patients treated with dasatinib [28].
3.1.4. Bosutinib
Bosutinib is a third generation TKI, which is orally available. Like dasatinib, it is a dual Src-Abl kinase inhibitor, more potent than imatinib in inhibiting Bcr-Abl and has activity against almost all imatinib-resistant Bcr-Abl except for T315I mutant kinase. However, unlike dasatinib, it does not inhibit the kinase activity of c-Kit or PDGFR. As most of the side effects of imatinib were attributed to its c-Kit and PDGFR inhibitor activity, bosutinib was developed to offer similar efficacy like dasatinib and better safety profile than the earlier Bcr-Abl kinase inhibitors [28, 29].
In phase I and phase II clinical trials bosutinib was shown to be effective in patients who were resistant or intolerant to imatinib [30]. However, when compared to imatinib as frontline therapy in an open-label, randomized, multinational, Phase III Bosutinib Efficacy and Safety in chronic myeloid Leukemia (BELA) trial, it did not meet the primary endpoint of complete cytogenic response. It did offer deeper and durable molecular response and less transformation to accelerated and blast phase [31, 32]. In another multinational, phase III trial Bosutinib Trial in First-Line Chronic Myelogenous Leukemia Treatment (BEFORE) comparing the efficacy of imatinib vs bosutinib in newly diagnosed CML, during the interim analysis at 12 months bosutinib was found to be superior in achieving better major molecular response rate (47.2% in bosutinib group vs 36.9%, in imatinib group (P =.02) as well as complete cytogenic response 77.2% in bosutinib group vs 66.4% in imatinib group, (P = .0075). Based on this trial, FDA has approved bosutinib in December 2017 for the treatment of newly diagnosed CML [33].
The side effect profile is distinct with bosutinib when compared with other TKIs as expected. The most common side effect is diarrhea which mostly resolved with supportive measures and elevated liver enzymes, whereas cardiac and vascular toxicities were uncommon [32, 33].
3.1.5. Ponatinib
Previous Bcr-Abl kinase inhibitors including second and third generation inhibitors were not able to bind to the T315I mutant kinase due to steric hindrance caused by the bulky isoleucine residue at position 315 in the T315I mutant kinase. Ponatinib (AP24534) a fourth generation TKI was designed based on a computational and structure-based approach to overcome the steric hindrance [34]. As expected it was active against all the mutant forms of Bcr-Abl in vitro [35].
Ponatinib was found to show response in patients in whom previous therapy had failed, in patients who harbour a T315I mutation, and in patients who are refractory to therapy with multiple TKIs in the absence of detectable Bcr-Abl mutations. Ponatinib offered deeper and durable responses [36]. It had a robust response in all the three phases of CML. Interestingly patients who eventually developed resistance to ponatinib did not have Bcr-Abl point mutations, they rather had compound mutations especially patients in blast phase, raising an important question as to whether ponatinib should be used as the front line to avoid the development of resistant clones [37]. The utility of ponatinib in newly diagnosed patients with chronic phase CML is yet to be established as the study open-label, phase III Evaluation of Ponatinib versus Imatinib in Chronic Myeloid Leukemia (EPIC) trial designed to assess the efficacy and safety of ponatinib, compared with imatinib, had to be terminated due to arterial thrombotic events reported in earlier studies [38]. Ponatinib Philadelphia-Positive Acute Lymphoblastic Leukemia and Chronic Myeloid Leukemia Evaluation (PACE) trial compared ponatinib vs Allo-SCT. At 24 months and at 48 months, ponatinib showed significantly higher OSR in patients with chronic phase CML (24 months: 84% vs 60.5 %; P = 0.004; 48 months 72.7 % vs 55.8%; P = 0.013), HR -0.37 (95% CI 0.16 - 0.84; P =0.017) [39]. Further understanding of the mechanisms of resistance to ponatinib will help overcome the resistance [40–42].
The common side effects observed were rash, dry skin, and abdominal pain and cytopenias of varying degree. Serious adverse events were pancreatitis, abdominal pain, increased lipase levels, diarrhea, pyrexia, myocardial infarction, thrombocytopenia, anemia, neutropenia, febrile neutropenia, and pancytopenia. Arterial thrombotic events that were considered by the site investigator to be at least possibly related to treatment were observed in 2.2%, 0.7%, and 1.6% of patients, respectively. [37].
Due to the risk of arterial embolism, FDA has narrowed the recommended use of ponatinib in 2014 to adult patients with T3I5I-positive CML or ALL and in patients with Ph-positive CML or ALL in whom no other TKI can be used.
3.2. Epidermal Growth Factor receptor tyrosine kinase inhibitors
EGFR belongs to a family of receptor tyrosine kinases (RTKs) that includes EGFR (HER1), ERBB2 (HER2), ERBB3 (HER3) and ERBB4 (HER4) respectively. Binding of the ligand to the extracellular domain leads to receptor dimerization, activation of the tyrosine kinase and signal transduction resulting in activation of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinases (ERK) and PI3K/AKT/mammalian target of rapamycin (mTOR) pathways among other pathways involved in cell proliferation and survival. EGFR pathway is dysregulated in multiple cancers: EGFR is overexpressed in ~80% of non-small cell lung cancer (NSCLC) and mutated in 20% of NSCLC [43, 44]. In addition, EGFR2 (ERBB2, HER2) is overexpressed in breast cancer [45]. Ever since trastuzumab, a monoclonal antibody showed significant benefit in patients with HER2-positive breast cancer, tyrosine kinase inhibitors targeting HER2 have been actively pursued in the treatment of HER2-positive breast cancer [46].
3.2.1. Gefitinib
Gefitinib was the first EGFR TKI to be developed. As many NSCLCs overexpress EGFR, gefitinib was expected to have significant response in patients with NSCLC. However, only limited number of patients were sensitive to gefitinib [47]. Following this finding FDA retracted its approval for the treatment of NSCL. Two landmark articles showed that patients who responded to gefitinib had mutated EGFR genes specifically exon 19 deletions or exon 21 (L858R) substitution mutation, thus these EGFR mutations became the predictive marker for EGFR TKI therapies [43, 48]. Currently, gefitinib is approved as first-line therapy in patients with EGFR exon 19 deletions or exon 21 (L858R) substitution mutation-positive metastatic NSCLC.
The grade 3 and grade 4 side effects include diarrhea, skin rash and interstitial pneumonia [47, 49].
3.2.2. Erlotinib
Erlotinib is another first-generation EGFR TKI. Erlotinib was originally approved for the treatment of locally advanced or metastatic NSCLC after failing at least one line of chemotherapy in 2004. This approval was regardless of the EGFR mutation status as stratifying NSCLC based on EGFR mutation was not the standard of care in 2004 [50]. Erlotinib is no longer approved for the use in this setting. Currently, erlotinib is approved as first-line therapy in patients with EGFR exon 19 deletions or exon 21 (L858R) substitution mutation-positive NSCLC.
Two randomized controlled trials Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive Non-small-cell Lung Cancer (EURTAC) and Erlotinib vs Chemotherapy as first-line Treatment for Patients with advanced EGFR mutation-positive Non-small-cell lung cancer (OPTIMAL) compared erlotinib with the standard of care cisplatin plus docetaxel and erlotinib with gemcitabine plus carboplatin respectively in patients with EGFR mutated NSCLC. OPTIMAL study conducted in China showed that erlotinib significantly increased PFS when compared to chemotherapy (13.1 months vs 4.6 months); hazard ratio (HR) 0.16, (95% CI 0.10-0.26; p<0.0001) with a better side effect profile. EURTAC study conducted in Europe showed similar findings with a median PFS of 9.7 months (95% CI 8·4-12·3) vs 5.2 months (4.5–5.8); HR-0.37 (95% CI 0.25-0.54; p<0·0001) with erlotinib vs chemotherapy respectively, again with a better side effect profile [51, 52]. In 2013, erlotinib was approved as first-line therapy for EGFR mutation harboring NSCLC. It is also approved as a second or third line therapy in EGFR mutated NSCLC patients who had failed previous chemotherapy [53].
Studies comparing erlotinib and gefitinib in other setting have not shown any significant difference in the PFS and OS. This can be explained by the fact these two molecules share similar chemical structures [54]. Based on a study comparing erlotinib with gemcitabine vs gemcitabine alone, FDA approved erlotinib to be used in combination with gemcitabine in advanced pancreatic cancer [55].
The most common grade 3 or 4 toxic effects with erlotinib were diarrhea, elevated liver enzymes and skin rash [51, 52].
3.2.3. Afatinib
Afatinib is an orally available second-generation EGFR TKI. Unlike earlier EGFR TKIs gefitinib and erlotinib, afatinib not only inhibits HER2, but also inhibits HER3 and HER4 and it is an irreversible inhibitor and was proposed to have better efficacy than first generation TKIs. [56].
LUX-LUNG 3 is a randomized control trial that compared afatinib with cisplatin plus pemetrexed as first-line therapy in patients with EGFR mutated adenocarcinoma of the lung. Afatinib increased the PFS 11.1 vs 6.9 months (HR-0.58; 95% CI 0.43-0.78; p=0.001) significantly compared to combination chemotherapy. The objective response rate was also significantly higher for afatinib when compared to cisplatin plus pemetrexed (56 vs 23%; p =0.001) [57]. In another randomized control trial LUX-LUNG 6 that compared afatinib to gemcitabine plus cisplatin, afatinib again proved to offer significantly longer PFS 11.0 months vs 5.6 months (HR-0.28; 95% CI 0.2-0.39; p < .0001) and objective response rate 66.9% vs 23.0% [58]. Although, in either study afatinib did not increase the OS 28.2 months vs 28.2 months; HR-0.88 (95% CI 0.66-1.17; p = .39) and 23.1 vs 23.5 months; HR-0.93; (95% CI 0.72-1.22; p = .61) respectively, the quality of life was significantly better with afatinib [59]. These studies lead to the FDA approval of afatinib as a first-line agent in the treatment of advanced adenocarcinoma of the lung harboring EGFR exon 19 deletions or exon 21 (L858R) substitution mutations in 2013.
LUX-LUNG 5 is a phase III randomized control trial in patients with NSCLC who had progressed with more than one line of chemotherapy (including platinum and pemetrexed) and erlotinib/gefitinib after ≥12 weeks of treatment, and who had attained ≥12 weeks’ clinical benefit on afatinib monotherapy. This study compared the efficacy of afatinib plus paclitaxel or investigator’s choice of single-agent chemotherapy. The PFS was significantly higher in afatinib plus paclitaxel group over the single agent monotherapy 5.6 vs 2.8 months; HR 0.60, (95% CI 0.43-0.85, p= 0.003). Although serious drug-related adverse events leading to discontinuation were more common in the investigational arm 32.6 % vs 11.7%, this did not significantly affect the global health and quality of life [60].
LUX-LUNG 8 is a phase III randomized control trial comparing afatinib vs erlotinib in advanced squamous cell carcinoma of the lung as second-line therapy. Afatinib had significantly improved median PFS 2.6 months (95% CI 2.0-2.9) vs 1.9 months (95% CI 1.9-2.1) and median OS 7·9 months (95% CI 7.2-8.7) vs 6.8 months (95% CI 5.9-7.8) with a comparable safety profile. Following this study afatinib was approved for second-line agent in patients with advanced squamous cell carcinoma of the lung [61].
Diarrhea, skin rash/acne, and fatigue are the most frequent treatment-related adverse events [62].
3.2.4. Osimertinib
NSCLC patients eventually develop resistance to both first and second-generation EGFR TKIs. Multiple investigations have revealed that a secondary mutation EGFR T790M lead to treatment failure in ~60% of the patients. This understanding has led to the development of third-generation EGFR TKIs osimertinib and other irreversible EGFR TKI selective for EGFR-TKI-sensitizing and T790M mutation to overcome resistance mediated by EGFR T790M [63]. AZD9291 Versus Platinum-Based Doublet-Chemotherapy in Locally Advanced or Metastatic Non-Small Cell Lung Cancer (AURA3) osimertinib was compared to carboplatin plus pemetrexed in patients who had progressed after first-line TKI therapy. Osimertinib significantly increased PFS (10.1 months vs 4.4 months; HR 0.30; (95% CI 0.23-0.41; p<0.001) when compared to combination chemotherapy. The ORR was also significantly better with osimertinib (71%; 95% CI 65-76) than with cisplatin plus pemetrexed (31%; 95% CI 24–40) [64].
Osimertinib was approved by FDA in 2015 for EGFR T790M mutation-positive advanced NSCLC in patients who had progressed on or after TKI therapy [65].
Grade 3 drug-related adverse events are diarrhea, rash and interstitial pneumonia [64].
3.2.5. Lapatinib
Lapatinib is a selective HER2 TK inhibitor. It was designed to target HER2 TK activity in patients with HER2-positive breast cancer, as monoclonal antibody trastuzumab that targeted HER2 showed significant benefit in the treatment of HER2-positive breast cancer [46]. Lapatinib was originally approved by FDA in 2007 for the treatment of HER2-positive advanced and metastatic breast cancer which had progressed on at least anthracycline, taxane, and trastuzumab. It was approved as the first-line agent in combination with letrozole in 2010 in HR-positive, HER2-positive patients with advanced breast cancer.
The original FDA approval was followed by a pre-specified interim analysis of phase III, randomized, open-label study comparing lapatinib plus capecitabine with capecitabine alone in women with progressive, HER2-positive, locally advanced or metastatic breast cancer who had previously been treated with a minimum of an anthracycline, a taxane, and trastuzumab. Time to progression was considered as the primary end-point which was 0.49 (95% CI 0.34 to 0.71; p<0.001), with 49 events in the combination-therapy group and 72 events in the monotherapy group. The median time to progression increased significantly in the combination group 8.4 months vs 4.4 months in the monotherapy group without an increase in serious toxic effects or symptomatic cardiac events [66].
The approval for frontline therapy was followed by a randomized, double-blind, controlled, parallel-group, multi-center, phase III study of post-menopausal women with advanced breast cancer. In HR-positive, HER2-positive patients, the combination therapy significantly decreased the risk for disease progression compared to letrozole alone; HR-0.71, (95% CI 0.53-0.96). The PFS was significantly prolonged in the combination therapy arm vs letrozole and placebo group 8.2 months vs 3.0 months as well as overall response rate (ORR) 28% in the combination group to 15% in the letrozole and placebo group. The clinical benefit rate was 48% in the combination group and 29% in the letrozole alone group.
The common side effects in the combination group were diarrhea (68%) and rash (46%). Interestingly, unlike trastuzumab, no cardiac side effects were reported in lapatinib group [67].
3.2.6. Neratinib
Neratinib is a second-generation irreversible pan-EGFR receptor tyrosine kinase inhibitor that also has activity against EGFR tyrosine kinase. Neratinib is FDA approved for the extended adjuvant treatment of HER2-positive early stage breast cancer after trastuzumab-based adjuvant therapy.
Neratinib After Trastuzumab-based Adjuvant Therapy in HER2-positive Breast Cancer (ExteNET) is randomized, multi-center, double-blind, phase III randomized clinical trial that studied the efficacy and safety of 12 months of neratinib in patients with stage I-III HER2-positive breast cancer that had completed neoadjuvant and adjuvant trastuzumab therapy up to 2 years. The 2-year invasive disease-free survival rate was 93·9% (CI 92·4-95·2) in the neratinib group and 91·6% (CI 90·0-93·0) in the placebo group. The HR for invasive disease-free survival events 0·67, (95% CI 0·50-0·91; p=0·0091).
The common side effects encountered are diarrhea, nausea, and vomiting [68]. Following this study, FDA approved this drug for the above-mentioned indication.
3.3. Vascular Endothelial Growth Factor Receptor (VEGFR) Tyrosine Kinase Inhibitors
Vascular endothelial growth factors (VEGFs) are a secreted family of polypeptides with a highly conserved receptor-binding domain composed of a disulfide-knot structure similar to that of the PDGFRs. There are two VEGFs; VEGF-A and VEGF-B. They bind to VEGFR which are RTKs located on vascular endothelial cells. VGEF-A binds to VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1). VEGF-A is the major angiogenic factor. Pro-angiogenic signals are mediated through VEGFR-2 whereas the soluble VEGFR-1 inhibits angiogenesis. The role of VEGFR-B is not well understood [69]. Soon after the studies showing that the inhibition of VEGF-VEGFR signaling inhibited angiogenesis [70], multiple monoclonal antibodies and tyrosine kinase inhibitors that target VEGF-VEGFR axis have entered the clinical trials targeting various cancers. The multi-kinase inhibitors like sorafenib and sunitinib at least partly exert their anti-tumor activity by inhibiting the TK activity of VEGFR-2 [71].
The elucidation of the implications of von Hippel-Lindau (pVHL) tumor suppressor protein in the pathogenesis of the renal cell carcinoma (RCC) identified VEGF-VEGFR axis of signaling as a potential target in the treatment of RCC [72, 73]. Approximately 80% of the patients with RCC carries an inactivated vHL gene. Monoclonal antibody against VEGF-R significantly prolonged the PFS in patients with metastatic RCC [74]. However, interest shifted in testing small molecule inhibitors targeting VGEG-VEGFR pathway in RCC.
3.3.1. Sorafenib
Sorafenib is a multi-kinase inhibitor that inhibits various kinases including VEGFRs, PDGFRs, FLT3R (Fms-related tyrosine kinase/Flk2/Stk-2-Receptor), murine sarcoma viral oncogene homolog B (BRAF), rearranged during transfection (RET), and c-Kit. Sorafenib, the first TKI approved for the treatment of metastatic RCC (2005) is currently used as a second line agent in patients with metastatic RCC. It was later approved for hepatocellular carcinoma (HCC) in 2007 and for radioactive-iodine refractory differentiated locally recurrent or metastatic thyroid cancer in 2013.
The Treatment Approaches In Renal Cancer Global Evaluation Trial (TARGET) a multi-center, phase III, randomized, double-blind, placebo-controlled trial of advanced RCC compared the efficacy of sorafenib vs placebo. Sorafenib significantly improved the median PFS, 5.5 months vs 2.8 months for placebo; HR-0.44 (95% CI 0.35 to 0.55; p<0.01) favoured sorafenib [75]. In the final efficacy and safety analysis, the intention-to-treat group did not show a survival benefit. However, when post-cross-over placebo survival data were censored, sorafenib group showed significant improvement in OS (17.8 sorafenib vs 14.3 control group, HR-0.78; p- .029) [76].
In the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) multi-center, phase III, double-blind, placebo-controlled trial, sorafenib was compared to placebo in patients with advanced HCC. Sorafenib significantly increased the median OS 10.7 months vs 7.9 months in the placebo group. The HR of 0.69 (95% CI, 0.55 to 0.87; p<0.001) favoured sorafenib [77]. The results were reproducible in another study conducted in Asia [78].
In the Nexavar vs Placebo In Locally Advanced/Metastatic Radio-active Iodine Refractory Differentiated Thyroid Cancer (DECISION) multicentre, randomized, double-blind, placebo-controlled, phase III trial, the efficacy of sorafenib in patients with radioactive iodine-refractory locally advanced or metastatic differentiated thyroid cancer was evaluated. Median PFS was significantly longer in the sorafenib group; 10·8 months vs 5·8 months in the placebo group; HR-0·59 (95% CI 0·45-0·76; p<0·0001) [79].
Common side effects noted were hypertension, anemia, fatigue, nausea, vomiting, and diarrhea [75, 76, 79].
3.3.2. Sunitinib
Sunitinib is a multi-targeted tyrosine kinase inhibitor that inhibits PDGFR (A and B), VEGFR1, VEGFR2, FLT3R, c-Kit, and RET-mediated signaling [80–82]. Sunitinib is approved by FDA for the treatment of metastatic RCC and imatinib-resistant gastrointestinal stromal tumor (GIST). In 2017 sunitinib was also approved as an adjuvant therapy for adult patients with high risk of recurrent RCC following nephrectomy. In a randomized, double-blind, phase III trial in patients with loco-regional RCC who have undergone nephrectomy, sunitinib significantly increased the median duration of disease-free survival when compared to placebo; 6.8 years (95% CI 5.8 to not reached) in the sunitinib group vs 5.6 years (95% CI 3.8-6.6) in the placebo group; HR-0.76; (95% CI 0.59-0.98; P=0.03) [83].
In a phase III landmark trial in patients with advanced metastatic RCC comparing the efficacy of sunitinib vs interferon-α, sunitinib group showed significantly longer median PFS (11 months) compared to interferon-α (5 months); HR-0.42 (95% CI 0.32 -0.54; p<0.001) [84]. In a prospective, placebo-controlled, randomised phase III clinical trial comparing the safety and efficacy of sunitinib vs placebo in advanced GIST patients who did not respond to imatinib, sunitinib significantly decreased the median time to tumour progression 27·3 weeks (95% CI 16·0-32·1) when compared to 6·4 weeks (4·4-10·0) in the placebo group; HR-0·33 (p<0·0001) [85].
Grade 3 neutropenia, leukopenia, diarrhea, fatigue, nausea, hypertension, and hand-foot-mouth syndrome were the common side effects [83–85]
3.3.3. Pazopanib
Pazopanib is a second generation multi-TKI that inhibits VEGFR, PDGFR (A and B), c-Kit and fibroblast growth factor receptor (FGFR) [86]. It was approved by FDA for metastatic RCC (2009) and advanced soft tissue sarcoma (2012).
In a placebo-controlled, randomized, double-blind, global, multi-center, phase III study in patients with locally advanced and metastatic RCC, pazopanib significantly improved the PFS 9.2 v 4.2 months; HR-0.46 (95% CI 0.34-0.62; p< .0001). The median duration of response was longer than 1 year [87]. Followed by the results of the study FDA approved pazopanib for first-line treatment of patients with metastatic RCC. However, in ‘the Adjuvant Sunitinib or Sorafenib for High-risk, Non-metastatic RCC (ASSURE)’ a double-blind, placebo-controlled, randomised, phase III trial that tested pazopanib in the adjuvant setting, pazopanib did not show any benefit over placebo [88].
The randomized, Phase III, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib vs sunitinib in patients with metastatic RCC (PISCES) Study compared the quality of life and patient preference of pazopanib vs sunitinib. More patients preferred pazopanib (70%) over sunitinib (22%); 8% expressed no preference (p< .001) [89]. This study illustrates the importance of the clinical utility of drugs with same efficacy and different side effect profiles.
Diarrhea, hypertension, hair color changes and elevated liver enzymes were common side effects [87].
3.3.4. Axitinib
Axitinib is a highly potent second-generation VEGFR TKI. Axitinib is relatively specific for VEGFR and does not have effects on PDGFR, c-Kit, FGFR or B-RAF in the concentrations that affect VEGFR [90]. Axitinib was approved by FDA in 2012 for the treatment of metastatic RCC as a second line therapy.
Comparative Effectiveness of Axitinib vs Sorafenib in Advanced Renal Cell Carcinoma (AXIS) a phase III randomized control trial that tested axitinib against sorafenib in the setting of metastatic RCC as a second line therapy. Axitinib significantly increased the median PFS 6·7 months with axitinib vs 4·7 months with sorafenib; HR-0·665; (95% CI 0·544-0·812; one-sided p<0·0001) [91]. In updated efficacy, quality of life, and safety results, there was no survival advantage for using axitinib over sorafenib, although axitinib significantly increased the median investigator-assessed PFS of 8·3 months (95% CI 6·7-9·2) vs sorafenib of 5·7 months (4·7-6·5); HR-0·656 (95% CI 0·552-0·779; one-sided p<0·0001) [92].
Like other VEGFR TKIs, diarrhea, hypertension, fatigue and hand-foot syndrome are the common side effects [91, 92].
3.3.5. Cabozantinib
Cabozantinib is a multi-target TKI that inhibits a wide range of RTKs including VEGFR, PDGFR, c-Kit, tyrosine protein kinase MET (MET), FLT3, RET etc [93, 94]. Because of its pleiotropic effects, cabozantinib has been tested in various cancers. Cabozantinib was approved by FDA in 2012 for the treatment of metastatic medullary carcinoma (MCT) of the thyroid gland. FDA also approved cabozantinib in 2017 for the treatment of advanced RCC for first-line therapy.
A Study of Cabozantinib (XL184) vs Everolimus in Subjects With Metastatic Renal Cell Carcinoma (METEOR) (METEOR), a randomized, open-label, phase III trial compared the efficacy of cabozantinib with everolimus, in patients with RCC that had progressed after VEGFR-targeted therapy. Cabozantinib significantly increased the median PFS 7.4 months vs everolimus 3.8 months; HR-0.58; (95% CI 0.45-0.75; p<0.001). The rate of progression or death was 42% lower with cabozantinib vs everolimus; HR- 0.58; (95% CI 0.45-0.75; p<0.001) [95]. An unplanned second interim analysis from this study revealed that cabozantinib increased the median OS 21·4 months (95% CI 18·7- not estimable) vs 16·5 months (14·7-18·8) with everolimus; HR-0·66; (95% CI 0·53-0·83, p=0·00026) [96].
Study of Cabozantinib (XL184) Versus Prednisone in Men With Metastatic Castration-resistant Prostate Cancer Previously Treated With Docetaxel and Abiraterone or MDV3100 (COMET-1) is a multi-center, randomized, double-blind controlled trial that compared the efficacy of cabozantinib against prednisone in a heavily pre-treated population of patients with castration-resistant prostate cancer and cabozantinib did not improve the primary outcome which was OS. However, cabozantinib significantly improved the median radiographic PFS of 5.6 months vs 2.8 months for prednisone; HR-0.48; (95% CI 0.40-0.57; p< .001) [97].
Cabozantinib vs sunitinib as initial targeted therapy for patients with metastatic RCC of poor or intermediate Risk (The alliance A031203 CABOSUN trial) is a randomized phase II multi-center control trial that compared the efficacy of cabozantinib vs sunitinib as a first-line therapy in patients with metastatic renal cell carcinoma. Cabozantinib significantly increase the median PFS of 8.2 months (95% CI 6.2-8.8 months) vs 5.6 months (95% CI 3.4-8.1 months) for sunitinib. Following this study, FDA expanded its approval to first-line therapy in patients with metastatic renal cell carcinoma [98].
A phase III, randomized control trial evaluated the efficacy of cabozantinib in patients with radiographic progression of metastatic MTC. Cabozantinib significantly prolonged the median PFS 11.2 months vs placebo 4.0 months; HR-0.28; (95% CI 0.19-0.40; p<.001). Interestingly, prolonged PFS with cabozantinib was observed across all subgroups including age, prior TKI treatment, and RET mutation status (hereditary or sporadic). The response rate was 28% with cabozantinib and 0% for placebo and responses were seen regardless of RET mutational status [99].
The most common grade 3 or 4 adverse events with cabozantinib were hypertension, diarrhea, fatigue, hemorrhage, venous thrombosis, gastrointestinal fistula [95, 96, 99].
3.3.6. Lenvatinib
Lenvatinib is another multi-TKI that inhibits VEGFR with inhibitory activity also against fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3, and FGFR4), PDGFRs, RET, and Kit [100].
FDA approved lenvatinib in 2015 for the treatment of radioactive iodine-refractory differentiated thyroid cancer. It was approved for metastatic RCC as a second line therapy in combination with everolimus in 2016 after anti-angiogenic therapy.
In phase II randomized, open-label, multicentre trial comparing the efficacy of everolimus against lenvatinib as monotherapy and with the combination of everolimus and lenvatinib in patients with metastatic RCC as a second line therapy in patients who previously were treated with an anti-angiogenic therapy. Lenvatinib plus everolimus combination therapy significantly increased the median PFS 14·6 months (95% CI 5·9-20·1) compared with everolimus alone 5·5 months (3·5-7·1); HR-0·40 (95% CI 0·24-0·68; p=0·0005). Lenvatinib alone significantly improved the median PFS 7·4 months (95% CI 5·6-10·2); HR-0·61, (95% CI 0·38-0·98; p=0·048) when compared to everolimus alone [101].
In phase III, randomized, double-blind, multi-center study involving patients with progressive thyroid cancer that was refractory to radioactive iodine, lenvatinib was evaluated against the placebo. The median PFS was 18.3 months in the lenvatinib group vs 3.6 months in the placebo group; HR for progression or death was 0.21; (p<0.001) [102].
Hypertension, fatigue, nausea, vomiting, lack of appetite, diarrhea, hand-foot syndrome are the commonly reported side effects [101, 102].
3.3.7. Vandetanib
Vandetanib is another multi-kinase inhibitor that inhibits VEGFR, RET, MET [103]. It is the first kinase inhibitor to be approved for the treatment of advanced medullary thyroid carcinoma (MTC) by FDA in 2011.
In a multi-center phase III randomized, placebo control trial the efficacy of vandetanib was evaluated in patients with advanced MTC. Vandetanib significantly prolonged the median PFS; the median PFS was not achieved with vandetanib vs placebo 19.3 months; HR-0.46 (95% CI 0.31-0.69; p < .001) [104].
Diarrhea, rash, nausea, hypertension, fatigue, and headache were the common side effects noted [104].
3.3.8. Regorafenib
Regorafenib is a multi-kinase inhibitor that inhibits the kinase activity of VEGFR, PDGFR, FGFR, BRAF, Kit, and RET. It is FDA approved for the treatment of advanced colorectal carcinoma (CRC) (2012), advanced GIST (2013) and advanced HCC (2017). In a landmark, phase III randomized control trial ‘Study of Regorafenib After Sorafenib in Patients With Hepatocellular Carcinoma (RESORCE)’ patients with advanced HCC who had progressed on sorafenib, regorafenib was investigated vs placebo. Regorafenib significantly improved OS with HR of 0·63 (95% CI 0·50-0·79; one-sided p<0·0001); median OS was 10·6 months (95% CI 9·1-12·1) for regorafenib vs 7·8 months (6·3-8·8) for placebo leading to FDA approval for this indication after 10 years of negative clinical trials [105].
In an international, multicentre, randomized, placebo-controlled, phase III trial Patients with Metastatic Colorectal Cancer Treated With Regorafenib or Placebo After Failure of Standard Therapy (CORRECT) the efficacy of regorafenib was evaluated in patients with CRC who had progressed on all approved therapies. Regorafenib significantly improved the median OS 6.4 months vs the control group (5.0 months); HR-0.77; (95% CI 0.64-0.94; one-sided p=0.0052) [106].
The efficacy of regorafenib was evaluated in an international, multicentre, randomized, placebo-controlled, phase III trial ‘Study of Regorafenib as a 3rd-line or Beyond Treatment for Gastrointestinal Stromal Tumors (GRID)’ in patients with GIST who had progressed on at least imatinib and sunitinib. Regorafenib significantly improved median PFS 4.8 months vs placebo 0.9 months; HR-0.27 (95% CI 0.19-0.39; p =0.0001) [107].
Common adverse events were the hand-foot syndrome, fatigue, diarrhea, fatigue, and hypertension [105–107].
3.4. BRAF Kinase Inhibitors
BRAF is a member of the RAF family of serine/threonine kinases and is frequently activated in patients with cancer through genetic aberrations. It signals downstream of RAS in the MAPK pathway. Interestingly, almost 50% of patients with melanoma have BRAF activation by V600E mutation and are susceptible to BRAF or MAPK/ERK kinase (MEK) inhibition [101, 108]. BRAF kinase inhibitors have changed the landscape of therapy in melanoma.
3.4.1. Vemurafenib
Vemurafenib is the first BRAF kinase inhibitor designed to inhibit the mutant BRAF V600E kinase in patients with advanced melanoma. Vemurafenib was approved by FDA in 2011 for the treatment of patients with BRAF V600E bearing metastatic melanoma.
A Study of Vemurafenib (RO5185426) in Comparison With Dacarbazine in Previously Untreated Patients With Metastatic Melanoma (BRIM 3) (BRIM3) is a landmark phase III randomized control trial that compared vemurafenib vs dacarbazine in patients with previously untreated melanoma with the BRAF V600E mutation. At 6 months, vemurafenib significantly prolonged OS compared to dacarbazine; 84% (95% CI 78-89) in the vemurafenib group vs 64% (95% CI 56-73) in the dacarbazine group; HR-0.37; (95% CI 0.26-0.55; p<0.001). The median PFS was 5.3 months for vemurafenib vs 1.6 months for dacarbazine; HR-0.26; (95% CI 0.20-0.33; p<0.001) [109]. In an extended follow up of this trial median OS was significantly longer in the vemurafenib group 13·6 months (95% CI 12·0-15·2) vs dacarbazine group 9·7 months (95% CI 7·9-12·8); HR-0·70; (95% CI 0·57-0·87; p=0·0008). Median PFS was significantly prolonged in vemurafenib group 6·9 months (95% CI 6·1-7·0) vs dacarbazine group 1·6 months (95% CI 1·6-2·1); HR-0·38 (95% CI 0·32-0·46, p<0·0001) [110].
The common side effects encountered were arthralgia, rash, fatigue, photosensitivity and cutaneous squamous cell carcinoma [109, 110].
3.4.2. Dabrafenib
Dabrafenib is the second BRAF kinase inhibitor designed to inhibit the mutant BRAF V600E kinase in patients with advanced melanoma.
Dabrafenib was also approved in 2014 as a single agent for treatment of BRAF V600E mutation-positive unresectable or metastatic melanoma. In 2015, FDA approved dabrafenib in combination with trametinib to treat patients with unresectable or metastatic melanoma with a BRAF V600E mutation in patients who had received at least one platinum-based chemotherapy. This combination is currently approved for first-line therapy for the same indication.
An open-label, phase III randomized control trial compared dabrafenib to dacarbazine in patients with BRAFV600E mutant metastatic melanoma. Similar to vemurafenib, dabrafenib significantly improved the median PFS 5·1 months vs dacarbazine 2·7 months; HR-0·30 (95% CI 0·18-0·51; p<0·0001) [111].
The commonly reported side effects were hyperkeratosis, palmoplantar hyperkeratosis, squamous cell carcinoma, fatigue, arthralgia and fever [111].
3.5. MEK inhibitors
The understanding of the implication BRAF mutation in melanoma prompted scientists to study its upstream and downstream signaling mediators [112]. BRAF, a serine-threonine kinase, when activated, activates its downstream kinases MEK1/2 which in turn activates MAP kinase. MAPK pathway is involved in cell proliferation, survival and is implicated in carcinogenesis [113]. Furthermore, BRAF mutation in cancer cells predicted response to MEK inhibition [114]. This lead to the development of MEK inhibitors in the treatment of unresectable melanoma.
Further understanding of the molecular mechanisms of resistance to BRAF kinase inhibitors revealed that MEK may be activated in patients who acquire resistance to BRAF kinase inhibitors [115]. This lead to the clinical trials testing the combination of BRAF kinase inhibitor plus MEK inhibitor in patients with BRAF mutated metastatic melanoma.
3.5.1. Trametinib
Trametinib is a MEK inhibitor developed to target MEK in the treatment of BRAF mutated metastatic melanoma. It is FDA approved for the treatment of BRAF V600E mutated metastatic melanoma as a monotherapy in 2013 as well as in combination with dabrafenib in 2014. Currently, the combination is approved for first-line therapy for the treatment of BRAF V600E mutant metastatic melanoma.
GSK1120212 vs Chemotherapy In Advanced or Metastatic BRAF V600E/K Mutation-positive Melanoma (METRIC) is a phase III randomized control trial that compared the efficacy of trametinib vs dacarbazine in patients with BRAF V600E mutant metastatic melanoma. Trametinib significantly prolonged the median PFS vs dacarbazine, 4.8 months in the trametinib group vs 1.5 months in the dacarbazine group; HR for disease progression or death in the trametinib group was 0.45; (95% CI 0.33-0.63; p<0.001) [116].
In ‘A Study Comparing the Trametinib to Dabrafenib Monotherapy in Subjects With BRAF-mutant Melanoma (COMBI-d)’, a double-blind, phase III randomized control trial, compared the combination of dabrafenib plus trametinib vs dabrafenib monotherapy in patients with BRAF V600E mutant metastatic melanoma. In the primary analysis of COMBI-d, with a median follow-up of 9 months (range 0-16), median PFS was 9·3 months for the combination and 8·8 months for dabrafenib monotherapy; HR-0·75 (95% CI 0·57-0·99; p=0·0348) [117]. In the final OS analysis, 47% patients in the dabrafenib plus trametinib group had died vs 58% in the dabrafenib the only group; HR-0·71 (95% CI 0·55-0·92; p=0·0107). Median OS was 25·1 months (95% CI 19·2-not reached) for the combination group vs 18·7 months (15·2-23·7) for the dabrafenib group [118].
Similar results were observed in ‘Dabrafenib Plus Trametinib vs Vemurafenib Alone in Unresectable or Metastatic BRAF V600E/K Cutaneous Melanoma (COMBI-v)’, a double-blind, phase III randomized control trial, compared the combination of dabrafenib plus trametinib vs vemurafenib in patients with BRAF V600E mutant metastatic melanoma. The combination group showed significantly prolonged OS and median PFS at 12 months of 72% (95% CI 67-77) in the combination-therapy group vs 65% (95% CI 59-70) in the vemurafenib group; HR for death in the combination therapy group was 0.69; (95% CI 0.53-0.89; p=0.005). Median PFS of 11.4 months in the combination group vs 7.3 months in the vemurafenib group; HR-0.56; (95% CI 0.46-0.69; p<0.001) [119].
‘A study of BRAF Inhibitor Dabrafenib in Combination with MEK inhibitor Trametinib in the Adjuvant Treatment of High-risk BRAF V600 Mutation-positive Melanoma After Surgical Resection (COMBI-AD)’, another double-blind, placebo-controlled, phase III randomized control trial, evaluated the combination of dabrafenib plus trametinib as an adjuvant therapy in patients with resected, stage III melanoma with BRAFV600E mutations. At a median follow-up of 2.8 years, the estimated 3-year rate of relapse-free survival was 58% in the combination-therapy group vs 39% in the placebo group; HR for relapse or death was 0.47; (95% CI 0.39-0.58; p=0.0006) [120].
Common reported side effects with trametinib were rash, diarrhea, and peripheral edema. Asymptomatic and reversible reduction in the cardiac ejection fraction and ocular toxic effects occurred infrequently. Interestingly, secondary skin neoplasms that were noticed in patients with BRAF kinase inhibitor were not observed with MEK inhibitor combination [116].
In the studies that tested the combination BRAF inhibitor plus MEK inhibitor vs BRAF inhibitor alone adverse events related to paradoxical activation of the MAPK pathway including hyperkeratosis, cutaneous squamous cell carcinoma, new primary melanomas, and non-cutaneous treatment-emergent cancers were reduced with the combination compared with BRAF inhibitor monotherapy, however, pyrexia was more common and more severe [117–119].
3.5.2. Cobimetinib
Cobimetinib is a MEK inhibitor that the FDA approved in 2015 for the treatment of patients with BRAFV600E mutant metastatic melanoma in combination with vemurafenib.
‘A Study Comparing Vemurafenib vs Vemurafenib plus Cobimetinib in Participants with Metastatic Melanoma (coBRIM)’ is a phase III double-blind, placebo, randomized control trial that compared the combination of vemurafenib plus cobimetinib vs vemurafenib monotherapy. Like the other trials that tested the combination BRAF inhibitor with MEK inhibitor, the combination group showed prolonged median PFS of 9.9 months in the combination vs 6.2 months in the control; HR for death or disease progression was 0.51; (95% CI 0.39-0.68; p<0.001) [121].
In the updated efficacy study with a median follow-up of 14·2 months, median PFS was 12·3 months (95% CI 9·5-13·4) for the combination vs 7·2 months (5·6-7·5) for placebo plus vemurafenib; HR-0·58, (95% CI 0·46-0·72, p<0·0001). Median OS was 22·3 months (95% CI 20·3-not estimable) for the combination group vs 17·4 months (95% CI 15·0-19·8) for vemurafenib monotherapy; HR-0·70, (95% CI 0·55-0·90; p=0·005) [122].
Elevated liver enzymes and pyrexia were the common side effects reported [121, 122].
3.6. Anaplastic Lymphoma Kinase (ALK) TKIs
3.6.1. Crizotinib
In addition to pathogenic EGFR mutations, gene rearrangement involving ALK and echinoderm microtubule-associated protein-like 4 (EML4) resulting in EML4-ALK fusion gene also acts as driver mutation in adenocarcinoma of the lung (NSCLC) [123]. The EML4-ALK fusion occurs in 2-7% of patients with NSCLC [124]. Originally developed to target c-met, crizotinib was also found to inhibit ALK tyrosine kinase activity and was tested on NCSLC patients with ALK fusion gene with significant benefits [125]. AKL and c-ros oncogene (ROS1) tyrosine kinases have high sequence similarity and crizotinib has also been found to have beneficial effects on ROS-1 gene rearrangement-positive NSCLC [126].
FDA has approved crizotinib for EML4-ALK fusion gene-positive NSCLC in 2011 and ROS 1 gene rearrangement-positive NSCLC in 2016.
Crizotinib was investigated vs pemetrexed in EML4-ALK fusion gene-positive NSCLC patients as a second line therapy in phase III, open-label trial. Crizotinib significantly increased the median PFS 7.7 months vs pemetrexed at 3.0 months; HR for progression or death with crizotinib was 0.49 (95% CI 0.37-0.64; p<0.001). The response rates were 65% (95% CI 58-72) with crizotinib vs 20% (95% CI 14-26) with pemetrexed (p<0.001) [127].
Crizotinib was evaluated vs chemotherapy in an international multi-center, randomized, open-label, phase III study ‘A Clinical Trial Testing the Efficacy of Criozotinib vs Standard Chemotherapy Pemetrexed plus Cisplatin or Carboplatin in Patients with ALK-positive NSCLC (PROFILE 1014) as a first-line therapy for patients with EML4-ALK fusion gene-positive NSCLC. Crizotinib significantly improved the median PFS of 10.9 months (95% CI 8.3-13.9) vs chemotherapy of 7.0 months (95% CI 6.8-8.2). HR for progression or death with crizotinib was 0.45; (95% CI 0.35-0.60; p<0.001) [128].
The side effect profile included vision disorders, nausea, vomiting, diarrhea, constipation and elevated liver enzymes [127].
3.6.2. Ceritinib
Ceritinib is a second generation ALK-EML4 fusion tyrosine kinase inhibitor. Ceritinib, like other second-generation TKIs was initially used in patients who progressed on first generation TKI or who could not tolerate the first generation TKI [129]. When it showed a better response in such patients, it was studied in treatment-naive patients with EML4-ALK fusion gene-positive NSCLC.
After ‘LDK 378 vs Chemotherapy in Previously Untreated Patients with ALK Rearranged NSCLC (ASCEND-4)’ trial showing its efficacy in the first line setting, FDA approved ceritinib for EML4-ALK fusion gene-positive NSCLC as a first-line therapy. ASCEND-4 is a randomized, open-label, phase III study that compared the efficacy of ceritinib vs chemotherapy as a first-line therapy in patients with EML4-ALK fusion gene-positive NSCLC. Ceritinib significantly improved the median PFS vs chemotherapy 16·6 months (95% CI 12·6-27·2) in the ceritinib group vs 8·1 months (5·8-11·1) in the chemotherapy group; HR-0·55 (95% CI 0·42-0·73; p<0·00001) [130].
Most common side effects are nausea, vomiting, diarrhea, and elevation of liver enzymes [130].
3.6.3. Alectinib
Alectinib is another second-generation ALK-EML4 fusion tyrosine kinase inhibitor, which initially showed efficacy in patients who had progressed on crizotinib [131].
The side effect profile is better than that of the first generation ALK-EML4 fusion TKI. Nausea, vomiting, diarrhea, constipation, and stomatitis are the common side effects [132, 133]. Alectinib was originally approved for patients with ALK-EML4 fusion gene-positive NSCLC who did not tolerate or who did not respond to crizotinib. However, following the results of J-ALEX and ALEX clinical trials, it is now approved as first-line therapy.
‘A Study Comparing Alectinib with Crizotinib in Treatment-Naïve Anaplastic Lymphoma Kinase-Positive Advanced Non Small Cell Lung Cancer Participants (J-ALEX)’ is a randomized, open-label, phase III clinical trial conducted in the Asian population which compared alectinib vs crizotinib in patients with ALK-EML4 fusion gene-positive patients with NSCLC who are chemotherapy naive or had received one line of chemotherapy. At the time of interim analysis, alectinib group had not reached the median PFS vs median PFS of 10·2 months (8·2-12·0) in crizotinib group [132].
These results were confirmed in the ALEX trial where median PFS was not reached for the alectinib group, HR for PFS was 0.47 (95% CI 0.34-0.65). Interestingly, when compared to crizotinib, alectinib significantly delayed time to progression to the central nervous system. The incidence of brain progression at 12 months was 9.4% in the alectinib group vs 41.4% in the crizotinib group; HR-0.16; (95% CI 0.10-0.28) [133].
3.6.4. Brigatinib
Brigatinib is another second-generation ALK-EML4 fusion TKI that is approved by the FDA for crizotinib-resistant ALK-EML4 fusion gene-positive NSCLC following the ‘A Study to Evaluate the Efficacy of Brigatinib (AP26113) In Participants With ALK-positive NSCLC Previously Treated with Crizotinib (ALTA)’ trial, a multi-center, randomized, phase II control trial showing median PFS of 9.2 months with 90 mg brigatinib (95% CI 7.4-15.6) and 12.9 months with 180 mg brigatinib (95% CI 11.1 to not reached). Nausea, vomiting, abdominal pain, constipation, and headache are the common side effects encountered [134].
3.6.5. Lorlatinib
Lorlatinib is a second generation TKI targeting ALK/ROS1 fusion protein kinase. It differs from the other ALK-ROS1 TKI by its ability to inhibit most known resistant mutations to other ALK TKIs and it crosses the blood-brain barrier. It was approved by the FDA in 2015 for the treatment of ALK-EML4 fusion gene-positive NSCLC who have progressed on one or more ALK TKIs.
The accelerated FDA approval was followed by the preliminary analysis of a phase1/II study NCT01970865 in patients with ALK-positive or ROS1-positive NSCLC with or without brain metastases who were treatment naïve or had progressed on one or more ALK TKIs. The ORR was 2% in patients who had progressed on crizotinib only, 9% in patients who had progressed on crizotinib and chemotherapy, 17% in patients who had progressed on two ALK TKIs and chemotherapy and 7% in patients who had progressed on three prior TKIs and chemotherapy [135].
The most common adverse events encountered are hypercholesterolemia (54%) and peripheral edema (37%) [135].
3.7. Bruton tyrosine kinase inhibitors (BTK)
BTK is a non-receptor tyrosine kinase of the Src family originally identified as defective in the inherited immunodeficiency disease, X linked agammaglobulinemia (XLA) [136]. Eventually, its role in B cell receptor signaling was elucidated. BTK plays an important role in B cell proliferation and survival. BTK is aberrantly expressed in many B cell malignancies including lymphoma [137]. BTK inhibitors exhibit antitumor activity both in preclinical and clinical studies involving B cell malignancies [138].
3.7.1. Ibrutinib
Ibrutinib is irreversible BTK inhibitor as it forms a covalent bond with cysteine 481 near the active site of BTK. It is highly specific owing to the fact that only nine other kinases in the human genome have a similarly placed cysteine residue [139].
Ibrutinib is currently FDA approved for the treatment of mantle cell lymphoma (MCL) (2013) and Waldenström’s macroglobulinemia (WM) (2015). In addition, ibrutinib is approved as a first-line therapy for chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) (2016) and first-line therapy for relapsed and refractory marginal zone lymphoma (MZL) (2017).
In a landmark, phase II clinical trial evaluating the safety and efficacy of ibrutinib in patients with WM who had progressed on previous treatment(s), ORR was 90.5% (95% CI 80.4-96.4) and major response rate (MRR) of 73.0% (95% CI 60.3-83.4); The median time to at least minor response was 4 weeks and partial response 8 weeks. This study led to the approval of ibrutinib in patients with relapsed and refractory WM. The previous treatments included at least one of the following: monoclonal antibody, glucocorticoid, proteasome inhibitor, nucleoside analogue, mTOR inhibitor, immunomodulator, anthracycline, ASCT and other experimental therapies [140].
An open-label sub-study of an international, multicentre, phase III trial named (iNNOVATE) evaluated the efficacy of ibrutinib in patients with WM who are refractory to rituximab. This is a descriptive study as it was not prospectively powered for statistical comparisons. Ibrutinib achieved an ORR of 90% and major response 71% in patients with rituximab-refractory WM with a median of four previous therapies [141].
In a multicenter, open-label, study named ‘A Phase III Study of Ibrutinib vs Ofatumumab in Patients With Relapsed or Refractory Chornic Lymphoid Leukemia (RESONATE-1) in patients with relapsed or refractory CLL or SLL the efficacy of ibrutinib was compared against the anti-CD20 antibody ofatumumab. The median PFS was not reached in the ibrutinib group and 8.1 months in ofatumumab group; HR for progression or death in the ibrutinib group was 0.22; (p<0.001). Ibrutinib also significantly improved the OS; HR for death was 0.43; (p=0.005). At 12 months, ibrutinib significantly increased the OSR to 90% vs 81% in the ofatumumab group as well as the ORR 42.6% in the ibrutinib group vs 4.1 % in the ofatumumab group (p<0.001) [142].
In a multi-center, ‘Open-label Phase III BTK inhibitor Ibrutinib vs Chlorambucil Patients 65 Years or Older With Treatment-naïve CLL or SLL (RESONATE-2)’ the efficacy and safety of ibrutinib were compared to chlorambucil in patients 65 years of age or older with previously untreated CLL. Ibrutinib significantly prolonged the median PFS and OS. Median PFS, not reached (ibrutinib group) vs 18.9 months (chlorambucil group) with the median follow up period of 18. 4 months; the risk of progression or death was 84% lower with ibrutinib vs chlorambucil; HR-0.16, p<0.001. The ORR also favored the ibrutinib; 86% vs 35%, p<0.001 in ibrutinib vs chlorambucil group respectively [143].
In a multi-center, open-label, phase II study that evaluated the efficacy and safety of ibrutinib in patients with MZL who were previously treated with an anti-CD20 antibody–containing regimen ORR was 48% (95% CI 35-62). With the median follow-up of 19.4 months, the median duration of response was not reached (95% CI 16.7 to not estimable), and median PFS was 14.2 months (95% CI 8.3 to not estimable) [144]. This study led to the FDA approval of ibrutinib for patients with relapsed and refractory MZL.
Common side effects reported are diarrhea, fatigue, cough, nausea, peripheral edema, cytopenia and pneumonia [143, 144].
3.7.2. Acalibrutinib
Acalibrutinib is a highly selective BTK inhibitor, which was approved by the FDA in 2017 for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. This approval was followed by an open-label, phase II study, which investigated the efficacy of acalabrutinib in patients with relapsed or refractory MCL. At the median follow up at 15.2 months, the median duration of response, median PFS, and OSR were not achieved.
The common adverse events encountered are headache, diarrhea, fatigue, and myalgia. The most common grade 3 or worse adverse events were neutropenia, and pneumonia. Treatment was discontinued in 54 (44%) patients, primarily due to progressive disease (39 [31%]) and adverse events (7 [6%]) [145].
3.8. The FMS-like tyrosine kinase 3 (FLT3) inhibitors
FLT3 is a receptor tyrosine kinase that belongs to the subclass III family [146, 147]. FLT3 is one of the most frequently mutated genes in hematologic malignancies. FLT3 mutations have been found 1–3% of patients with ALL, 5–10% of patients with myelodysplasia and 15–35% of patients with AML. Internal tandem duplication (ITD) JM domain-coding sequence of the FLT3 gene (FLT3/ITD) mutation constitutes approximately two-third of FLT3 mutations in AML patients and tyrosine kinase domain (TKD) mutations mostly point mutations in codon D835 or deletions of codon I836 constitute the remaining one third. [148–150]. Unfortunately, the first generation FLT3 kinase inhibitors did not offer significant clinical benefit as monotherapy. Research is underway to develop more potent FLT3 TKIs in the treatment of AML and to evaluate the clinical benefit when used in combination with chemotherapy.
3.8.1. Midostaurin
Midostaurin, a multi-targeted TKI, was originally developed as a protein kinase C inhibitor for the treatment of patients with solid tumors that were found to have inhibitory activity towards FLT3-like kinase in preclinical studies [151, 152].
It is FDA approved for the treatment of FLT3 mutation-positive AML patients in combination with chemotherapy, making this is as the first targeted therapy approved for the treatment of AML.
The efficacy of midostaurin was evaluated in combination with chemotherapy in patients with FTL3 mutation-positive AML in phase II clinical trial. Midostaurin significantly prolonged the OS when compared to the placebo group; HR-0.78; one-sided p=0.009. The response was consistent across the various subtype of FTL3 mutations including ITD and point mutations of TKD [153].
3.9. JAK2 kinase inhibitors
3.9.1. Ruxolitinib
JAK2 is a member of Janus kinase family and it is a non-receptor tyrosine kinase. At least 95% of patients with polycythemia vera (PV) and 50% of patients with essential thrombocytosis (ET) and myelofibrosis (MF) are found to have the gain of function mutation in the JAK2 kinase domain and this mutation drives molecular pathogenesis in these patients [154, 155]. Many trials have tested the efficacy of ruxolitinib, a JAK 1/2 inhibitor in all the three myeloproliferative disorders. Currently, ruxolitinib is FDA approved for the treatment of intermediate to high-risk MF (2011) and hydroxyurea resistant or intolerant PV (2014).
FDA approval of ruxolitinib for the treatment of MF was based on two phase III randomized control trials, Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment I (COMFORT-I) and Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment II (COMFORT-II). In the COMFORT-1 trial, the efficacy of ruxolitinib in patients with intermediate to high-risk primary MF, post-PV MF, or post-ET MF was compared against placebo with the primary endpoint being a reduction in the spleen volume of 35% more in 24 weeks. The primary endpoint was reached in 41.9% of patients in the ruxolitinib group vs 0.7% in the placebo group (p<0.001). 67.0% of the patients with a response to ruxolitinib had the response for 48 weeks or more. The total symptom score at 24 weeks improved to 50% or more in 45.9% of patients in ruxolitinib group vs 5.3% in placebo group (p<0.001) [156]. In COMFORT-II trial the efficacy of ruxolitinib in patients with intermediate to high-risk primary MF, post-PV MF, or post-ET MF was compared against the best available therapy with the primary endpoint being a reduction in the spleen volume of 35% more in 48 weeks. At week 48, the primary endpoint was achieved in 28% of the patients in the ruxolitinib group vs 0% in the group receiving the best available therapy (p<0.001). Patients in the ruxolitinib group enjoyed a better quality of life and reduction in symptoms associated with MF. Interestingly, the JAK2 mutation did not predict the response to ruxolitinib therapy [157]. COMFORT-1 trials showed a modest but statistically significant reduction in the risk of death in the ruxolitinib group when compared to the control group; HR-0.50 (95% CI 0.25-0.98 P=0.04) [156].
Randomized Study of Efficacy and Safety in Polycythemia Vera with JAK Inhibitor INCB018424 versus Best Supportive Care (RESPONSE) study compared the efficacy of ruxolitinib in patients with hydroxyurea resistant or intolerant PV against the standard therapy. The primary endpoint was both hematocrit reduction and 35% reduction in spleen volume at 32 weeks. The primary endpoint was achieved in 21% of the patients in the ruxolitinib group vs 1% in the standard-therapy group (p<0.001) [158]. The hematologic response achieved was found to be durable in the follow-up study [159]. In RESPONSE-II trial ruxolitinib was tested against best available therapy. The primary endpoint was hematocrit control in patients with PV without splenomegaly at week 28. The primary endpoint was achieved in 62% patients in ruxolitinib group vs 19% compared to the best available therapy; ORR 7·28, (95% CI 3·43-15·45 p<0·0001) [160]. In A randoMised study of best Available therapy versus JAK Inhibition in patients with high risk polycythaemia vera or essential thrombocythaemia who are resistant or intolerant to hydroxycarbamide (MAJIC-ET) trail that compared the ruxolitinib against the best available therapy in patients who are hydroxycarbamide resistant or intolerant ET. The primary outcome was the complete response at 1 year. Ruxolitinib did not achieve primary endpoint at one year, although there was some symptom improvement in a ruxolitinib group at the expense of the grade 3 and 4 anemia and thrombocytopenia [161]. Anemia, thrombocytopenia and herpes zoster infection were the common side effects reported with ruxolitinib [156–158].
3.10. Phosphoinositide 3-kinase (PI3K) inhibitors
The phosphoinositide 3-kinase (PI3K) is a superfamily of lipid kinases central to human cancer, diabetes, and aging. There are three different PI3K classes (I, II and III), as well as for the different isoforms (e.g. Class I has 4 isoforms: α, β, γ, δ) and within each class there are distinct roles for each of the PI3Ks [162]. Class I PI3K has been implicated in many cancers particularly those with pathogenic mutations. PI3K acts downstream to many growth factors and acts upstream to AKT and mTOR [163, 164]. There are several PI3K inhibitors that have been tested in various PIK3CA mutated solid cancers [165]. However, two PI3Kγ/δ inhibitors are FDA approved in non-mutated lymphomas.
3.10.1. Idelalisib
Idelalisib is a small molecule inhibitor of the delta isoform of PI3K. It is currently FDA approved for the treatment of patients with CLL who cannot tolerate chemotherapy, relapsed follicular B-cell non-Hodgkin lymphoma (NHL) and relapsed SLL.
In a multi-center, double-blind, randomized, placebo-controlled, phase III study in patients with relapsed CLL who cannot tolerate chemotherapy owing to co-morbidities, idelalisib in combination with rituximab was tested vs rituximab and placebo. The primary endpoint was median PFS. However, the study was terminated before reaching PFS because of demonstrated efficacy and safety. The median PFS was 5.5 months in the control group and was not reached in the study group. HR for progression of death in the study group 0.15; p<0.001. ORR was 81% in the study group vs 13% in the control group; ORR-29.92; p<0.001. OS at 12 months 92% vs 80% in the control group; HR for death, 0.28; p=0.02 [166]. Following this study idelalisib was FDA approved.
In a single arm, open-label phase II study in patients with relapsed, indolent NHL idelalisib treatment lead to the ORR of 57% (71 of 125 patients), with 6% meeting the criteria for a complete response. The median time to a response was 1.9 months, the median duration of response was 12.5 months, and the median PFS was 11 months [167].
The common grade IV side effects noted were diarrhea, pneumonia, dyspnea, neutropenia, elevated liver enzymes. Following this study idelalisib was approved by FDA in patients with relapsed indolent NHL.
3.10.2. Copenlalisip
Copenlalisip is a pan-class I PI3K inhibitor that was recently approved by FDA for the treatment of relapsed follicular lymphoma (FL). In a phase II study in patients with relapsed FL who have failed at least two prior systemic therapy, copenlalisip treatment resulted in the objective response rate of 59% (84 of 142 patients), median time to response was 53 days, median duration of response was 22.6 months, median PFS was 11.2 months, and median OS was not reached. Following this study, FDA approved copenlalisip for the treatment of patients with FL who have failed at least two prior systemic therapies.
The most frequent adverse events noted were transient hyperglycemia and transient hypertension, neutropenia, and pneumonia [168].
3.11. Cyclin-Dependent Kinase (CDK) 4/6 Inhibitors
CDKs tightly regulate the various phases of the eukaryotic cell cycle. Many tumorigenic signals converge on the CKD4/6-cyclin D complex in the G1 phase of the cell cycle. Tumorigenic signals lead cyclin D to form complexes with either CDK4 or CKD6 leading to phosphorylation of the Retinoblastoma (RB) gene, which leads to the release of E2F to allow transcription of genes necessary for G1 to S phase transition [169, 170]. Genotoxic events leading to DNA damage and chromosomal instability dysregulate the progression of the cell cycle from S phase to G2/M phase which is regulated by CDK1/2. Although it is conceivable that small molecule inhibitors to CDKs would offer a great opportunity in the treatment of cancer, for various reasons clinical trials proved otherwise, especially with pan-CDK inhibitors. However, CDK4/6 inhibitors are now approved in the HR-positive breast cancer [169].
3.11.1. Palbociclib
Palbociclib is a CDK4/CKD6 dual small molecule inhibitor that initially received accelerated FDA approval for the HR-positive, HER2-negative advanced or metastatic breast cancer in 2015 in combination with letrozole. However, palbociclib received FDA approval in 2017 for the same indication following an international, randomized, double-blind, placebo-controlled, clinical trial ‘Palbociclib: Ongoing Trials in the Management of Breast Cancer-2 (PALOMA-2)’ in which palbociclib plus letrozole and placebo plus letrozole was tested in postmenopausal females as a first-line therapy. The median PFS was significantly higher in the palbociclib plus letrozole group 24.8 months (95% CI 22.1 to not estimable) as compared to 14.5 months (95% CI 12.9-17.1) in the placebo–letrozole group; HR for disease progression or death, 0.58; 95% CI 0.46-0.72; p<0.001. The most common grade 3 or 4 adverse events encountered were neutropenia (66.4% of the patients in the palbociclib plus letrozole group vs 1.4% in the placebo plus letrozole group), leukopenia (24.8% vs 0%), anemia (5.4% vs 1.8%), and fatigue (1.8% vs 0.5%). Febrile neutropenia was reported in 1.8% of patients in the palbociclib plus letrozole group and in none of the patients in the placebo–letrozole group. [171]. However, unlike conventional chemotherapies, neutropenia caused by CDK4/CDK6 inhibitors are rapidly reversible due to cytostatic rather than cytotoxic effects on the bone marrow.
3.11.2. Ribociclib
Ribociclib is an another CDK4/CDK6 dual kinase inhibitor that was approved by the FDA in 2017 for the treatment of the post-menopausal women with the HR-positive, HER2-negative advanced or metastatic breast cancer following the Mammary Oncology Assessment of LEE011’s (Ribociclib’s) Efficacy and Safety (MONALEESA-2) trial. This was a randomized, placebo-controlled, phase III trial comparing the efficacy of ribociclib plus letrozole against placebo plus letrozole. At 18 months, the PFS rate was 63.0%, (95% CI 54.6-70.3) in the ribociclib group and 42.2% (95% CI 34.8- 49.5) in the placebo group. In patients with measurable disease at baseline, the ORR was 52.7% and 37.1%, respectively (p<0.001).
Common grade 3 or 4 adverse events that were reported were neutropenia (59.3% in the ribociclib group vs 0.9% in the placebo group) and leukopenia (21.0% vs 0.6%) [172].
3.11.3. Abemaciclib
Abemaciclib is another CDK4/CKD6 kinase inhibitor but more potent against CDK4. It was approved by FDA in 2017 for the treatment of the post-menopausal woman with the HR-positive, HER2-negative advanced or metastatic breast cancer in combination with fulvestrant as a frontline therapy following ‘A Study of Abemaciclib (LY2835219) Combined With Fulvestrant in Women With Hormone Receptor Positive HER2-Negative Breast Cancer (MONARCH-2)’ trial and as monotherapy in patients who had progressed on endocrine therapy or chemotherapy in the setting of metastatic disease and following ‘A phase II study of abemaciclib, in Participants with Previously Treated Breast Cancer That Has Spread (MONARCH-1’) trail.
MONARCH-2 was an international, double-blind, phase III study that analyzed the efficacy of abemaciclib plus fulvestrant who had progressed while receiving neo-adjuvant or adjuvant endocrine therapy, ≤ 12 months from the end of adjuvant endocrine therapy, or while receiving first-line endocrine therapy for metastatic disease. The combination therapy significantly prolonged the median PFS 16.4 months vs 9.3 months with fulvestrant alone; HR-0.553; 95% CI 0.449-0.681; p < .001. The common adverse events encountered in the abemaciclib vs placebo group were diarrhea (86.4% v 24.7%), neutropenia (46.0% vs 4.0%), nausea (45.1% v 22.9%), and fatigue (39.9% v 26.9%) [173].
MONARCH-1 is a trial analyzed the efficacy and safety of abamaciclib as a single agent in patients with HR-positive, HER2-negative metastatic breast cancer. At 12 months the ORR was 19.7% (95% CI 13.3-27.5) and the median PFS was 6.0 months and median OS was 17.7 months [174].
4. Expert Commentary and Five-year view
Protein kinase inhibitors have led to the paradigm shift in cancer treatments. In general, they are less toxic and in the right patient population kinase inhibitors are more potent than conventional chemotherapy. However, like conventional chemotherapy development of resistance and unwanted side effects are limitations to kinase inhibitors.
The resistance could be rendered by clonal expansion of cells that are not addicted to the particular kinase or secondary mutations in the kinases that render kinase inhibitor ineffective. Identification of mechanisms of resistance has led to the rational design of kinase inhibitors or rational use of combination therapies. For example, understanding of the role of EGFR T790M in the resistance to the EGFR TKIs in NSCLC, lead to the designing of osimertinib that particularly targeted the EGFR T790M gatekeeper mutant protein kinase [64] and identification T315I gatekeeper mutant gene as a secondary mutation responsible resistance to first and second generation Bcr-Abl kinase inhibitors lead to the designing of ponatinib that targeted the T315I mutant kinase [34]. Understanding of the role of MEK in the re-activation of MAPK pathway and the implications of MEK activation in resistance to BRAF inhibitors in melanoma lead to the combination therapy of BRAF inhibitor plus MEK inhibitor which offered better clinical benefits when compared to monotherapy [116–118].
Side effects remain a concern for kinase inhibitors. For example, FDA had to narrow the indication of ponatinib due to the risk of arterial embolism [175]. Thus, post-marketing surveillance for these medications is of utmost importance as these drugs are sometimes approved after a phase II study showing efficacy due to clinical demand.
More specific kinase inhibitors may translate to fewer side effects. For example, the Bcr-Abl kinase inhibitor bosutinib is more specific to Bcr-Abl kinase when compared to other Bcr-Abl kinase inhibitors like dasatinib or nilotinib which also inhibit c-Kit and PDGFR. Interestingly, the cardiovascular side effects attributable to PDGFR inhibition are rare in patients treated with bosutinib when compared to other Bcr-Abl kinase inhibitors [176].
Understanding of the mechanism behind a specific side effect also would help us mitigate the issue. For example, identification of paradoxical activation of MAPK pathway with BRAF inhibition in patients with melanoma lead to secondary cutaneous malignancies, helped us hypothesize the combination of BRAF and MEK inhibition would decrease the risk of the same and it turned out to be true in clinical trials testing the combination therapy [115–118]. Pharmacokinetic studies testing minimal effective dosing might also help decrease the dose-related adverse events.
Figure 4:
Chemical structure of an EGFR KI inhibitor gefitinib as adapted from National Center for Biotechnology Information.
Key Issues.
DNA sequencing has identified that 2% of human genes code for protein kinases. Discovery of oncogenes first implicated PKs in the pathogenesis of cancer. Further understanding of molecular pathogenesis of cancer has revealed many oncogenic kinases.
Advent of imatinib has revolutionized the cancer therapy and lead to the era of targeted therapy. The hope for specificity and oncogenic addiction thereby better outcome and side effect profile steered the cancer scientists and pharmaceutical companies design many PK inhibitors against various oncogenic protein kinases.
Several landmark clinical trials of protein kinase inhibitors against various cancers has led to the FDA approval of many protein kinase inhibitors for specific indications. Development of resistance, side effects and cost remain major limitations to PKIs.
Acknowledgments
Funding
This manuscript has receiving financial support from the National Cancer Institute (CCSG P30CA023074) grant awarded to the University of Arizona.
5. Abbreviations
- Abl -
Abelson
- ALK-
Anaplastic lymphoma kinase
- ATP -
Adenosine triphosphate
- Bcr-
Break point cluster
- BRAF -
Murine sarcoma viral oncogene homolog B
- BTK -
Bruton tyrosine kinase inhibitors
- CDK -
Cyclin Dependent Kinase
- CI -
Confidence Interval
- CLL -
Chronic lymphocytic leukemia
- CML -
Chronic myeloid leukemia
- CRC -
Colorectal carcinoma
- EGFR -
Epidermal growth factor receptor
- EMLK4 -
Echinoderm microtubule-associated protein-like 4
- ERK -
Extracellular signal-regulated kinases
- ET -
Essential thrombocytopenia
- FDA -
Food and Drug administration Agency
- FGFR -
Fibroblast growth factor receptor
- FLT3 -
FMS-like tyrosine kinase 3
- FLT3R -
Fms-related tyrosine kinase/Flk2/Stk-2-Receptor
- GIST -
Gastrointestinal stromal tumor
- HCC -
Hepatocellular carcinoma
- HR -
Hazard Ratio
- HR-positive -
Hormone receptor positive
- ITD -
Internal tandem duplication
- c-KIT -
proto-oncogene c-Kit or tyrosine-protein kinase Kit
- MAPK -
Mitogen-activated protein kinase
- MCL -
Mantle cell lymphoma
- MCT -
Medullary carcinoma of thyroid
- MEK –
MAPK/ERK kinase
- MET -
Tyrosine protein kinase MET
- MF-
Myelofibrosis
- MRR -
Major response rate
- mTOR-
mammalian target of rapamycin
- MZL -
Marginal zone lymphoma
- NSCLC -
Non small cell lung cancer
- OR -
Odds ratio
- ORR -
Overall response rate
- OS -
Overall survival
- PDGF -
Platelet derived growth factor
- Ph-positive -
Philadelphia chromosome positive
- PI3K -
Phosphatidylinositide 3-kinases
- PV -
Polycythemia vera
- pVHL -
von Hippel-Lindau protein
- Rb -
Retinoblastoma gene
- RCC -
Renal cell carcinoma
- RET -
Rearranged during transfection
- ROS1 -
c-ros oncogene
- SLL -
Small lymphocytic lymphoma
- TK -
Tyrosine kinase
- TKI -
Tyrosine kinase inhibitor
- TKD -
Tyrosine kinase domain
- VEGF -
Vascular endothelial growth factor
- VEGFR -
Vascular endothelial growth factor receptor
- WM -
Waldenström’s macroglobulinemia
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Annotations: (*=of importance, **= of considerable importance)
- 1.Manning G, Plowman GD, Hunter T, Sudarsanam S, Evolution of protein kinase signaling from yeast to man. Trends in Biochemical Sciences, 2002. 27: p. 514–20 [DOI] [PubMed] [Google Scholar]
- 2.Manning G, Whyte DB, Martinez R, et al. , The protein kinase complement of the human genome. Science, 2002. 298: p.1912–34 [DOI] [PubMed] [Google Scholar]; (*-provides the catalogue of protein kinase genome)
- 3.Johnson LN and Lewis RJ, Structural basis for control by phosphorylation. Chemical Reviews, 2001. 101: p. 2209–42 [DOI] [PubMed] [Google Scholar]
- 4.Stehelin D, Varmus HE, Bishop JM, et al. , DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature, 1976. 260: p. 170–3 [DOI] [PubMed] [Google Scholar]
- 5.Tsatsanis C and Spandidos DA, Oncogenic kinase signaling in human neoplasms, in Annals of the New York Academy of Sciences. 2004. p. 168–75 [DOI] [PubMed] [Google Scholar]
- 6.Blume-Jensen P and Hunter T, Oncogenic kinase signalling. Nature, 2001. 411: p. 355–65 [DOI] [PubMed] [Google Scholar]
- 7.Schram AM, Chang MT, Jonsson P, et al. , Fusions in solid tumours: Diagnostic strategies, targeted therapy, and acquired resistance. Nature Reviews Clinical Oncology, 2017. 14: p. 735–48 [DOI] [PMC free article] [PubMed] [Google Scholar]; (**-provides the understanding of the implications of multiple oncogenic kinases in carcinogenesis and cancer progression)
- 8.Geary CG, The story of chronic myeloid leukaemia. British Journal of Haematology, 2000. 110: p. 2–11 [DOI] [PubMed] [Google Scholar]
- 9.Wong S and Witte ON, The BCR-ABL story: Bench to bedside and back, in Annual Review of Immunology. 2004. p. 247–306 [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ, Talpaz M, Resta DJ., et al. , Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. New England Journal of Medicine, 2001. 344: p. 1031–7 [DOI] [PubMed] [Google Scholar]; (**Landmark article signifying the era of targetted therapy)
- 11.Kantarjian H, Sawyers C, Hochhaus A, et al. , Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. New England Journal of Medicine, 2002. 346: p. 645–52 [DOI] [PubMed] [Google Scholar]
- 12.Imatinib: New indications, but not robust evidence. Prescrire International, 2008. 17: p. 91–4 [PubMed] [Google Scholar]
- 13.O’Brien SG, Guilhot F, Larson RA, et al. , Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. New England Journal of Medicine, 2003. 348: p. 994–1004 [DOI] [PubMed] [Google Scholar]
- 14.Druker BJ, Guilhot F, O’Brien SG, et al. , Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. New England Journal of Medicine, 2006. 355: p. 2408–17 [DOI] [PubMed] [Google Scholar]
- 15.Hochhaus A, Larson RA, Guilhot F, et al. , Long-term outcomes of imatinib treatment for chronic myeloid leukemia. New England Journal of Medicine, 2017. 376: p. 917–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg E, Manley PW, Breitenstein W, et al. , Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell, 2005. 7: p. 129–41 [DOI] [PubMed] [Google Scholar]
- 17.O’Hare T, A decade of nilotinib and dasatinib: From in vitro studies to first-line tyrosine kinase inhibitors. Cancer Research, 2016. 76: p. 5911–3 [DOI] [PubMed] [Google Scholar]
- 18.Saglio G, Kim DW, Issaragrisil S, et al. , Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. New England Journal of Medicine, 2010. 362: p. 2251–9 [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian HM, Hochhaus A, Saglio G, et al. , Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. The Lancet Oncology, 2011. 12: p. 841–51 [DOI] [PubMed] [Google Scholar]
- 20.Hochhaus A, Saglio G, Hughes TP, et al. , Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia, 2016. 30: p. 1044–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giles FJ, Rea D, Rosti G, et al. , Impact of age on efficacy and toxicity of nilotinib in patients with chronic myeloid leukemia in chronic phase: ENEST1st subanalysis. Journal of Cancer Research and Clinical Oncology, 2017: p. 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortes JE, De Souza CA, Ayala M, et al. , Switching to nilotinib versus imatinib dose escalation in patients with chronic myeloid leukaemia in chronic phase with suboptimal response to imatinib (LASOR): a randomised, open-label trial. The Lancet Haematology, 2016. 3: p. e581–91 [DOI] [PubMed] [Google Scholar]
- 23.Hochhaus A, Masszi T, Giles FJ, et al. , Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia, 2017. 31: p. 1525–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah NP, et al. , Overriding imatinib resistance with a novel ABL kinase inhibitor. Science, 2004. 305: p. 399–401 [DOI] [PubMed] [Google Scholar]
- 25.Kantarjian H, Cortes J, Kim DW, et al. , Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-Month median follow-up. Blood, 2009. 113: p. 6322–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saglio G, Hochhaus A, Goh YT, et al. , Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: Efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer, 2010. 116: p. 3852–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah NP, Kantarjian HM, Kim DW, et al. , Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. Journal of Clinical Oncology, 2008. 26: p. 3204–12 [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian HM, Shah NP, Cortes JE, et al. , Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-Year follow-up from a randomized phase 3 trial (DASISION). Blood, 2012. 119: p. 1123–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golas JM, Arndt K, Etienne C, et al. , SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Research, 2003. 63: p. 375–81 [PubMed] [Google Scholar]
- 30.Kantarjian HM, Cortes JE, Kim DW, et al. , Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood, 2014. 123: p. 1309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortes JE, Kim DW, Kantarjian HM, et al. , Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Results from the BELA trial. Journal of Clinical Oncology, 2012. 30: p. 3486–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brümmendorf TH, Cortes JE, de Souza CA, et al. , Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: Results from the 24-month follow-up of the BELA trial. British Journal of Haematology, 2015. 168: p. 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. , Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: Results from the randomized BFORE trial. Journal of Clinical Oncology, 2018. 36: p. 231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou T, Commodore L, Huang WS, et al. , Structural Mechanism of the Pan-BCR-ABL Inhibitor Ponatinib (AP24534): Lessons for Overcoming Kinase Inhibitor Resistance. Chemical Biology and Drug Design, 2011. 77: p. 1–11 [DOI] [PubMed] [Google Scholar]
- 35.O’Hare T, Shakespeare WC, Zhu X, et al. , AP24534, a Pan-BCR-ABL Inhibitor for Chronic Myeloid Leukemia, Potently Inhibits the T315I Mutant and Overcomes Mutation-Based Resistance. Cancer Cell, 2009. 16: p. 401–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortes JE, Kantarjian H, Shah NP, et al. , Ponatinib in refractory Philadelphia chromosome-positive leukemias. New England Journal of Medicine, 2012. 367: p. 2075–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. , A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. New England Journal of Medicine, 2013. 369: p. 1783–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipton JH, Chuah C, Guerci-Bresler A, et al. , Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: An international, randomised, open-label, phase 3 trial. The Lancet Oncology, 2016. 17: p. 612–21 [DOI] [PubMed] [Google Scholar]
- 39.Nicolini FE, Basak GW, Kim DW, et al. , Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer, 2017. 123: p. 2875–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabriskie MS, et al. , BCR-ABL1 Compound Mutations Combining Key Kinase Domain Positions Confer Clinical Resistance to Ponatinib in Ph Chromosome-Positive Leukemia. Cancer Cell, 2014. 26: p. 428–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Pan H, and Wang Y, T315L: a novel mutation within BCR-ABL kinase domain confers resistance against ponatinib. Leukemia and Lymphoma, 2017. 58: p. 1733–5 [DOI] [PubMed] [Google Scholar]
- 42.BCR-ABL1 compound mutations drive ponatinib resistance. Cancer discovery, 2014. 4: p.13. [DOI] [PubMed] [Google Scholar]
- 43.Lynch TJ, Bell DW, Sordella R, et al. , Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. New England Journal of Medicine, 2004. 350: p. 2129–39 [DOI] [PubMed] [Google Scholar]; (**-Discovery of the mutations predicting response to EGFR kinase inhibitors bringing the EGFR kinase inhibitors back to lung cancer therapy)
- 44.Pao W and Chmielecki J, Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nature Reviews Cancer, 2010. 10: p. 760–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King CR, Kraus MH, and Aaronson SA, Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science, 1985. 229: p. 974–6 [DOI] [PubMed] [Google Scholar]
- 46.Cameron DA and Stein S, Drug insight: Intracellular inhibitors of HER2-clinical development of lapatinib in breast cancer. Nature Clinical Practice Oncology, 2008. 5: p. 512–20 [DOI] [PubMed] [Google Scholar]
- 47.Araki T, Yashima H, Shimizu K, et al. , Review of the treatment of non-small cell lung cancer with gefitinib. Clinical Medicine Insights: Oncology, 2012. 6: p. 407–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paez JG, Jänne PA, Lee JC, et al. , EGFR mutations in lung, cancer: Correlation with clinical response to gefitinib therapy. Science, 2004. 304: p. 1497–1500 [DOI] [PubMed] [Google Scholar]
- 49.Silva AP, Coelho PV, Anazetti M, et al. , Targeted therapies for the treatment of non-small-cell lung cancer: Monoclonal antibodies and biological inhibitors. Human vaccines & immunotherapeutics, 2017. 13: p. 843–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen MH, Johnson JR, Chen YF, et al. , FDA drug approval summary: Erlotinib (Tarceva®) tablets. Oncologist, 2005. 10: p. 461–6 [DOI] [PubMed] [Google Scholar]
- 51.Rosell R, Carcereny E, Gervais R,., et al. , Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. The Lancet Oncology, 2012. 13: p. 239–46 [DOI] [PubMed] [Google Scholar]
- 52.Zhou C, Wu YL, Chen G, et al. , Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. The Lancet Oncology, 2011. 12: p. 735–42 [DOI] [PubMed] [Google Scholar]
- 53.Khozin S, Blumenthal GM, Jiang X, et al. , U.S. Foodand drug administrationapproval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist, 2014. 19: p. 774–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juan O and Popat S, Treatment choice in epidermal growth factor receptor mutation-positive non-small cell lung carcinoma: Latest evidence and clinical implications. Therapeutic Advances in Medical Oncology, 2017. 9: p. 201–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore MJ, Goldstein D, Hamm J, et al. , Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology, 2007. 25: p. 1960–66 [DOI] [PubMed] [Google Scholar]
- 56.Wind S, Schnell D, Ebner T, et al. , Clinical Pharmacokinetics and Pharmacodynamics of Afatinib. Clinical Pharmacokinetics, 2017. 56: p. 235–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang JC, Hirsh V, Schuler M, et al. , Symptom control and quality of life in lux-lung 3 : A phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. Journal of Clinical Oncology, 2013. 31: p. 3342–50 [DOI] [PubMed] [Google Scholar]
- 58.Geater SL, Xu CR, Zhou C, et al. , Symptom and quality of life improvement in LUX-lung 6 : An open-label Phase III study of afatinib versus cisplatin/gemcitabine in asian patients with EGFR mutation-positive advanced non-small-cell lung cancer. Journal of Thoracic Oncology, 2015. 10: p. 883–9 [DOI] [PubMed] [Google Scholar]
- 59.Yang JCH, et al. , Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. The Lancet Oncology, 2015. 16(2): p. 141–51 [DOI] [PubMed] [Google Scholar]
- 60.Yang JC, Wu YL, Schuler M, et al. , Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: Phase III randomized LUX-Lung 5 trial. Annals of Oncology, 2016. 27: p. 417–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soria JC, Felip E, Cobo M, et al. , Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): An open-label randomised controlled phase 3 trial. The Lancet Oncology, 2015. 16: p. 897–907 [DOI] [PubMed] [Google Scholar]
- 62.Paz-Ares L, Tan EH, O’Byrne K, et al. , Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase IIb LUX-Lung 7 trial. Annals of Oncology, 2017. 28: p. 270–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ricciuti B, Baglivo S, Paglialunga L, et al. , Osimertinib in patients with advanced epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer: Rationale, evidence and place in therapy. Therapeutic Advances in Medical Oncology, 2017. 9: p. 387–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mok TS, Wu Y- L, Ahn M- J., et al. , Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer . New England Journal of Medicine , 2017. 376: p. 629–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khozin S, Weinstock C, Blumenthal GM, et al. , Osimertinib for the treatment of metastatic EGFR T790M mutation-positive non-small cell lung cancer. Clinical Cancer Research, 2017. 23: p. 2131–5 [DOI] [PubMed] [Google Scholar]
- 66.Geyer CE, Forster J, Lindquist D, et al. , Lapatinib plus capecitabine for HER2-positive advanced breast cancer. New England Journal of Medicine, 2006. 355: p. 2733–43 [DOI] [PubMed] [Google Scholar]
- 67.Schwartzberg LS, Franco SX, Florance A et al. , Lapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor-positive metastatic breast cancer. Oncologist, 2010. 15: p. 122–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin M, Holmes FA, Ejlertsen B, et al. , Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology, 2017. 18: p. 1688–1700 [DOI] [PubMed] [Google Scholar]
- 69.Shibuya M, Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. Journal of Biochemistry, 2013. 153: p. 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim KJ, Li B, Winer J, Armanini M, et al. , Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature, 1993. 362: p. 841–4 [DOI] [PubMed] [Google Scholar]
- 71.Shibuya M, VEGFR and type-V RTK activation and signaling. Cold Spring Harbor Perspectives in Biology, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iliopoulos O, Levy AP, Jiang C, et al. , Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proceedings of the National Academy of Sciences of the United States of America, 1996. 93: p. 10595–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iliopoulos O, Molecular biology of renal cell cancer and the identification of therapeutic targets. Journal of Clinical Oncology, 2006. 24: p. 5593–5600 [DOI] [PubMed] [Google Scholar]
- 74.Yang JC, Haworth L, Sherry RM, et al. , A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. New England Journal of Medicine, 2003. 349: p. 427–34 [DOI] [PMC free article] [PubMed] [Google Scholar]; (*- Origin of the era of targeted therapy in RCC)
- 75.Escudier B, Eisen T, Stadler WM, et al. , Sorafenib in advanced clear-cell renal-cell carcinoma. New England Journal of Medicine, 2007. 356: p. 125–34 [DOI] [PubMed] [Google Scholar]
- 76.Escudier B, Eisen T, Stadler WM, et al. , Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. Journal of Clinical Oncology, 2009. 27: p. 3312–8 [DOI] [PubMed] [Google Scholar]
- 77.Llovet JM, Ricci S, Mazzaferro V, et al. , Sorafenib in advanced hepatocellular carcinoma. New England Journal of Medicine, 2008. 359: p. 378–90 [DOI] [PubMed] [Google Scholar]
- 78.Cheng AL, Kang YK, Chen Z ., et al. , Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology, 2009. 10: p. 25–34 [DOI] [PubMed] [Google Scholar]
- 79.Brose MS, Nutting CM, Jarzab B, et al. , Sorafenib in radioactive iodine-refractory, locally advanced or metastatic diff erentiated thyroid cancer: A randomised, double-blind, phase 3 trial. The Lancet, 2014. 384: p. 319–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Farrell AM, Abrams TJ, Yuen HA, et al. , SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood, 2003. 101: p. 3597–3605 [DOI] [PubMed] [Google Scholar]
- 81.Faivre S, Demetri G, Sargent W, et al. , Molecular basis for sunitinib efficacy and future clinical development. Nature Reviews Drug Discovery, 2007. 6: p. 734–45 [DOI] [PubMed] [Google Scholar]
- 82.Polyzos A, Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma and various other solid tumors. Journal of Steroid Biochemistry and Molecular Biology, 2008. 108: p. 261–6 [DOI] [PubMed] [Google Scholar]
- 83.Ravaud A, Motzer RJ, Pandha HS, et al. , Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. New England Journal of Medicine, 2016. 375: p. 2246–54 [DOI] [PubMed] [Google Scholar]
- 84.Motzer RJ, Hutson TE, Tomczak P, et al. , Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New England Journal of Medicine, 2007. 356: p. 115–124 [DOI] [PubMed] [Google Scholar]
- 85.Demetri GD, van Oosterom AT, Garrett CR, et al. , Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet, 2006. 368: p. 1329–38 [DOI] [PubMed] [Google Scholar]
- 86.van Geel RMJM, Beijnen JH., et al. , Concise drug review: Pazopanib and axitinib. Oncologist, 2012. 17: p. 1081–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sternberg CN, Davis ID, Mardiak J, et al. , Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. Journal of Clinical Oncology, 2010. 28: p. 1061–8 [DOI] [PubMed] [Google Scholar]
- 88.Motzer RJ, Haas NB, Donskov F, et al. , Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. Journal of Clinical Oncology, 2017. 35: p. 3916–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Escudier B, Porta C, Bono P, et al. , Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma : PISCES study. Journal of Clinical Oncology, 2014. 32: p. 1412–8 [DOI] [PubMed] [Google Scholar]
- 90.Sonpavde G, Hutson TE, and Rini BI, Axitinib for renal cell carcinoma. Expert Opinion on Investigational Drugs, 2008. 17: p. 741–8 [DOI] [PubMed] [Google Scholar]
- 91.Rini BI, Escudier B, Tomczak P., et al. , Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. The Lancet, 2011. 378: p. 1931–9 [DOI] [PubMed] [Google Scholar]
- 92.Motzer RJ, Escudier B, Tomczak P., et al. , Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. The Lancet Oncology, 2013. 14: p. 552–62 [DOI] [PubMed] [Google Scholar]
- 93.Grülich C, Cabozantinib: A MET, RET, and VEGFR2 tyrosine kinase inhibitor, in Recent Results in Cancer Research. 2014. p. 207–14 [DOI] [PubMed] [Google Scholar]
- 94.Gebreyohannes YK, Schöffski P, Van Looy T., et al. , Cabozantinib is active against human gastrointestinal stromal tumor xenografts carrying different KIT mutations. Molecular Cancer Therapeutics, 2016. 15: p. 2845–52 [DOI] [PubMed] [Google Scholar]
- 95.Choueiri TK, Escudier B, Powles T., et al. , Cabozantinib versus everolimus in advanced renal-cell carcinoma. New England Journal of Medicine, 2015. 373: p. 1814–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choueiri TK, Escudier B, Powles T., et al. , Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. The Lancet Oncology, 2016. 17: p. 917–27 [DOI] [PubMed] [Google Scholar]
- 97.Smith M, De Bono J, Sternberg C., et al. , Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. Journal of Clinical Oncology, 2016. 34: p. 3005–13 [DOI] [PubMed] [Google Scholar]
- 98.Choueiri TK, Halabi S, Sanford BL., et al. , Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The alliance A031203 CABOSUN trial. Journal of Clinical Oncology, 2017. 35: p. 591–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elisei R, Schlumberger MJ, Müller SP., et al. , Cabozantinib in progressive medullary thyroid cancer. Journal of Clinical Oncology, 2013. 31: p. 3639–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kasi Loknath Kumar A and Huang CH, Lenvatinib mesylate. Multikinase inhibitor oncolytic. Drugs of the Future, 2014. 39: p. 113–22 [Google Scholar]
- 101.Motzer RJ, Hutson TE, Glen H., et al. , Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. The Lancet Oncology, 2015. 16: p. 1473–2 [DOI] [PubMed] [Google Scholar]
- 102.Schlumberger M, Tahara M, Wirth LJ., et al. , Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New England Journal of Medicine, 2015. 372: p. 621–30 [DOI] [PubMed] [Google Scholar]
- 103.Carlomagno F, Vitagliano D, Guida T, et al. , ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Research, 2002. 62: p. 7284–90 [PubMed] [Google Scholar]
- 104.Wells SA Jr, Robinson BG, Gagel RF., et al. , Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. Journal of Clinical Oncology, 2012. 30: p. 134–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bruix J, Qin S, Merle P., et al. , Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet, 2017. 389: p. 56–66 [DOI] [PubMed] [Google Scholar]
- 106.Grothey A, Van Cutsem E, Sobrero A., et al. , Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet, 2013. 381: p. 303–312 [DOI] [PubMed] [Google Scholar]
- 107.Demetri GD, Reichardt P, Kang YK., et al. , Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet, 2013. 381: p. 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hodis E, Watson IR, Kryukov GV., et al. , A landscape of driver mutations in melanoma. Cell, 2012. 150: p. 251–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chapman PB, Hauschild A, Robert C., et al. , Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New England Journal of Medicine, 2011. 364: p. 2507–16 [DOI] [PMC free article] [PubMed] [Google Scholar]; (*-Origin of the era of targeted therapy with the advent of vemurafenib)
- 110.McArthur GA, Chapman PB, Robert C., et al. , Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. The Lancet Oncology, 2014. 15: p. 323–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hauschild A, Grob JJ, Demidov LV., et al. , Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. The Lancet, 2012. 380: p. 358–65 [DOI] [PubMed] [Google Scholar]
- 112.Tsai J, Lee JT, Wang W., et al. , Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proceedings of the National Academy of Sciences of the United States of America, 2008. 105: p. 3041–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Friday BB and Adjei AA, Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clinical Cancer Research, 2008. 14: p. 342–6 [DOI] [PubMed] [Google Scholar]
- 114.Solit DB, Garraway LA, Pratilas CA., et al. , BRAF mutation predicts sensitivity to MEK inhibition. Nature, 2006. 439: p. 358–62 [DOI] [PMC free article] [PubMed] [Google Scholar]; (*-Identification of BRAF mutation as predictive marker for MEK inhibition)
- 115.Solit DB and Rosen N, Resistance to BRAF inhibition in melanomas. New England Journal of Medicine, 2011. 364: p. 772–4 [DOI] [PubMed] [Google Scholar]
- 116.Flaherty KT, Robert C, Hersey P., et al. , Improved survival with MEK inhibition in BRAF-mutated melanoma. New England Journal of Medicine, 2012. 367: p. 107–14 [DOI] [PubMed] [Google Scholar]
- 117.Long GV, Stroyakovskiy D, Gogas H., et al. , Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. New England Journal of Medicine, 2014. 371: p. 1877–88 [DOI] [PubMed] [Google Scholar]
- 118.Long GV, Stroyakovskiy D, Gogas H., et al. , Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. The Lancet, 2015. 386: p. 444–51 [DOI] [PubMed] [Google Scholar]
- 119.Robert C, Karaszewska B, Schachter J., et al. , Improved overall survival in melanoma with combined dabrafenib and trametinib. New England Journal of Medicine, 2015. 372: p. 30–9 [DOI] [PubMed] [Google Scholar]
- 120.Long GV, Hauschild A, Santinami M., et al. , Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. New England Journal of Medicine, 2017. 377: p. 1813–23 [DOI] [PubMed] [Google Scholar]
- 121.Larkin J, Ascierto PA, Dréno B, et al. , Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. New England Journal of Medicine, 2014. 371: p. 1867–76 [DOI] [PubMed] [Google Scholar]
- 122.Ascierto PA, McArthur GA, Dréno B., et al. , Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. The Lancet Oncology, 2016. 17: p. 1248–60 [DOI] [PubMed] [Google Scholar]
- 123.Soda M, Choi YL, Enomoto M., et al. , Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature, 2007. 448: p. 561–6 [DOI] [PubMed] [Google Scholar]; (*-Discovery of the implications of EML4-ALK fusion in NSCLC)
- 124.Takeuchi K, Soda M, Togashi Y., et al. , RET, ROS1 and ALK fusions in lung cancer. Nature Medicine, 2012. 18: p. 378–81 [DOI] [PubMed] [Google Scholar]
- 125.Jarvis LM, Crizotinib chronicles: Pfizer’s ALK inhibitor gives hope to some lung cancer patients. Chemical and Engineering News, 2010. 88): p. 20–1 [Google Scholar]
- 126.Lim SM, Kim HR, Lee JS., et al. , Open-label, multicenter, phase II Study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. Journal of Clinical Oncology, 2017. 35: p. 2613–8 [DOI] [PubMed] [Google Scholar]
- 127.Shaw AT, Kim DW, Nakagawa K., et al. , Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. New England Journal of Medicine, 2013. 368: p. 2385–94 [DOI] [PubMed] [Google Scholar]
- 128.Solomon BJ, Mok T, Kim D., et al. , First-line crizotinib versus chemotherapy in ALK-positive lung cancer. New England Journal of Medicine, 2014. 371: p. 2167–77 [DOI] [PubMed] [Google Scholar]
- 129.Shen L and Ji HF, Ceritinib in ALK-rearranged non-small-cell lung cancer. New England Journal of Medicine, 2014. 370: p. 2537. [DOI] [PubMed] [Google Scholar]
- 130.Soria JC, Tan DSW, Chiari R., et al. , First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. The Lancet, 2017. 389: p. 917–29 [DOI] [PubMed] [Google Scholar]
- 131.Shaw AT, Gandhi L, Gadgeel S, et al. , Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer : A single-group, multicentre, phase 2 trial. The Lancet Oncology, 2016. 17: p. 234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hida T, Nokihara H, Kondo M, et al. , Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. The Lancet, 2017. 390: p. 29–39 [DOI] [PubMed] [Google Scholar]
- 133.Peters S, Camidge DR, Shaw AT, et al. , Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. New England Journal of Medicine, 2017. 377: p. 829–38 [DOI] [PubMed] [Google Scholar]
- 134.Kim DW, Tiseo M, Ahn MJ, et al. , Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: A randomized, multicenter phase II trial. Journal of Clinical Oncology, 2017. 35: p. 2490–98 [DOI] [PubMed] [Google Scholar]
- 135.Solomon Benjamin J., B. TM, Felip Enriqueta, Besse Benjamin, James Leonard Philip, Clancy Jill S.,, Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). Journal of Clinical Oncology, 2016. 34: p. 9009 [Google Scholar]
- 136.Vetrie D, Vorechovský I, Sideras P, et al. , The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature, 1993. 361: p. 226–33 [DOI] [PubMed] [Google Scholar]
- 137.Katz FE, Lovering RC, Bradley LA, et al. , Expression of the X-linked agammaglobulinemia gene, btk in B-cell acute lymphoblastic leukemia. Leukemia, 1994. 8: p. 574–7 [PubMed] [Google Scholar]
- 138.Honigberg LA, Smith AM, Sirisawad M, et al. , The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America, 2010. 107: p. 13075–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pan Z, Scheerens H, Li SJ, et al. , Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem, 2007. 2: p. 58–61 [DOI] [PubMed] [Google Scholar]
- 140.Treon SP, Tripsas CK, Meid K, et al. , Ibrutinib in previously treated Waldenström’s macroglobulinemia. New England Journal of Medicine, 2015. 372: p. 1430–40 [DOI] [PubMed] [Google Scholar]
- 141.Dimopoulos MA, Trotman J, Tedeschi A, et al. , Ibrutinib for patients with rituximab-refractory Waldenström’s macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. The Lancet Oncology, 2017. 18: p. 241–50 [DOI] [PubMed] [Google Scholar]
- 142.Byrd JC, Brown JR, O’Brien S, et al. , Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. New England Journal of Medicine, 2014. 371: p. 213–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burger JA, Tedeschi A, Barr PM, et al. , Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. New England Journal of Medicine, 2015. 373: p. 2425–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Noy A, de Vos S, Thieblemont C, et al. , Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood, 2017. 129: p. 2224–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang M, Rule S, Zinzani PL, et al. , Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): A single-arm, multicentre, phase 2 trial. The Lancet, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Agnès F, Shamoon B, Dina C., et al. , Genomic structure of the downstream part of the human FLT3 gene: exon/intron structure conservation among genes encoding receptor tyrosine kinases (RTK) of subclass III. Gene, 1994. 145: p. 283–8 [DOI] [PubMed] [Google Scholar]
- 147.Abu-Duhier FM, Goodeve AC, Wilson GA, et al. , Genomic structure of human FLT3: Implications for mutational analysis [1]. British Journal of Haematology, 2001. 113: p. 1076–7 [DOI] [PubMed] [Google Scholar]
- 148.Abu-Duhier FM, Goodeve AC, Wilson GA, et al. , Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. British Journal of Haematology, 2001. 113: p. 983–8 [DOI] [PubMed] [Google Scholar]
- 149.Yamamoto Y, Kiyoi H, Nakano Y, et al. , Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood, 2001. 97: p. 2434–9 [DOI] [PubMed] [Google Scholar]
- 150.Bacher U, Haferlach C, Kern W, et al. , Prognostic relevance of FLT3-TKD mutations in AML: The combination matters an analysis of 3082 patients. Blood, 2008. 111: p. 2527–37 [DOI] [PubMed] [Google Scholar]
- 151.Propper DJ, McDonald AC, Man A, et al. , Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. Journal of Clinical Oncology, 2001. 19: p. 1485–92 [DOI] [PubMed] [Google Scholar]
- 152.Weisberg E, Boulton C, Kelly LM, et al. , Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell, 2002. 1: p. 433–43 [DOI] [PubMed] [Google Scholar]
- 153.Stone RM, Mandrekar SJ, Sanford BL, et al. , Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 Mutation. New England Journal of Medicine, 2017. 377: p. 454–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.James C, Ugo V, Le Couédic JP, A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature, 2005. 434: p. 1144–8 [DOI] [PubMed] [Google Scholar]; (*-Discovery of the implications of JAK2 mutation in polycythmia vera)
- 155.Kralovics R, Passamonti F, Buser AS, et al. , A gain-of-function mutation of JAK2 in myeloproliferative disorders. New England Journal of Medicine, 2005. 352: p. 1779–90 [DOI] [PubMed] [Google Scholar]; (*-Discovery of the implications of JAK2 mutation in myeloproliferative disorders)
- 156.Verstovsek S, Mesa RA, Gotlib J., et al. , A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. New England Journal of Medicine, 2012. 366: p. 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Harrison C, Kiladjian JJ, Al-Ali HK, et al. , JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. New England Journal of Medicine, 2012. 366: p. 787–98 [DOI] [PubMed] [Google Scholar]
- 158.Vannucchi AM, et al. , Ruxolitinib versus standard therapy for the treatment of polycythemia vera. New England Journal of Medicine, 2015. 372: p. 426–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. , Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica, 2016. 101: p. 821–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Passamonti F, Griesshammer M, Palandri F, et al. , Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. The Lancet Oncology, 2017. 18: p. 88–99 [DOI] [PubMed] [Google Scholar]
- 161.Harrison CN, Mead AJ, Panchal A, et al. , Ruxolitinib vs best available therapy for et intolerant or resistant to hydroxycarbamide. Blood, 2017. 130: p. 1889–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jean S and Kiger AA, Classes of phosphoinositide 3-kinases at a glance. Journal of Cell Science, 2014. 127: p. 923–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chalhoub N and Baker SJ, PTEN and the PI3-kinase pathway in cancer, in Annual Review of Pathology: Mechanisms of Disease. 2009. p. 127–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fruman DA and Rommel C, PI3K and cancer: Lessons, challenges and opportunities. Nature Reviews Drug Discovery, 2014. 13: p. 140–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kurtz JE and Ray-Coquard I, PI3 kinase inhibitors in the clinic: An update. Anticancer Research, 2012. 32: p. 2463–70 [PubMed] [Google Scholar]
- 166.Richard RF, Sharman JP, Coutre SE, et al. , Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. New England Journal of Medicine, 2014. 370: p. 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gopal AK, Kahl BS, de Vos S, et al. , PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. New England Journal of Medicine, 2014. 370: p. 1008–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dreyling M, Santoro A, Mollica L, et al. , Phosphatidylinositol 3-kinase inhibition by Copanlisib in relapsed or refractory indolent lymphoma. Journal of Clinical Oncology, 2017. 35: p. 3898–905 [DOI] [PubMed] [Google Scholar]
- 169.Asghar U, Witkiewicz AK, Turner NC, et al. , The history and future of targeting cyclin-dependent kinases in cancer therapy. Nature Reviews Drug Discovery, 2015. 14: p. 130–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weinberg RA, The retinoblastoma protein and cell cycle control. Cell, 1995. 81: p. 323–30 [DOI] [PubMed] [Google Scholar]
- 171.Finn RS, Martin M, Rugo HS, et al. , Palbociclib and letrozole in advanced breast cancer. New England Journal of Medicine, 2016. 375: p. 1925–36 [DOI] [PubMed] [Google Scholar]
- 172.Hortobagyi GN, Stemmer SM, Burris HA, et al. , Ribociclib as first-line therapy for HR-positive, advanced breast cancer. New England Journal of Medicine, 2016. 375: p. 1738–48 [DOI] [PubMed] [Google Scholar]
- 173.Sledge GW Jr, Toi M, Neven P, et al. , MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. Journal of Clinical Oncology, 2017. 35: p. 2875–84 [DOI] [PubMed] [Google Scholar]
- 174.Dickler MN, Tolaney SM, Rugo HS, et al. , MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, n patients with refractory HR+/HER2-metastatic breast cancer. Clinical Cancer Research, 2017. 23: p. 5218–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Valent P, Hadzijusufovic E, Hoermann G, et al. , Risk factors and mechanisms contributing to TKI-induced vascular events in patients with CML. Leukemia Research, 2017. 59: p. 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kong JH, Khoury HJ, Kim AS, et al. , The safety of Bosutinib for the treatment of chronic myeloid leukemia. Expert Opinion on Drug Safety, 2017. 16: p. 1203–09 [DOI] [PubMed] [Google Scholar]