Abstract
2-Amino-4-(3,4-(methylenedioxy)benzylamino)-6-(3-methoxyphenyl)pyrimidine (AMBMP) is a small molecule that has been previously reported to be both a Wnt agonist and a microtubule (MT) regulator. Here we report a detailed analysis of AMBMPs effects on MTs and on MT associated cellular processes including cell polarity, ciliogenesis, and cell migration. Specifically, treatment of Xenopus embryos with AMBMP leads to defects similar to the MT depolymerizing drug nocodazole, including a failure to generate or polarize cilia (depending on the timing of treatment) and a loss of the cell movements associated with radial intercalation. The dramatic effect AMBMP has on basic MT based cellular functions suggests that its usefulness as a Wnt regulator is questionable. Moreover, it may be an important new tool for experimental or pharmacological manipulation of MTs.
Introduction:
The microtubule (MT) network is an important component of the cells cytoskeleton and plays a critical role in cell division, cell polarity, cell migration, organelle trafficking and a wide array of diverse cellular functions. Additionally, MTs are essential structural components of both centrioles and cilia. As such, the regulation of MT dynamics has been an area of intense research. MTs are characterized by a feature called dynamic instability, in which they are in a constant flux between growth and catastrophe (Mitchison and Kirschner, 1984). Small molecules that facilitate either the stabilization or destabilization of MTs have been the focus of intense anti-cancer drug discovery efforts and have resulted in numerous useful therapies (Florian and Mitchison, 2016). However, the broad requirement of MTs in general cellular functions has led to numerous side effects and the peculiarities of pharmaco-kinetics has resulted in a wide range of efficacies and therapeutic usefulness. Therefore, the development and characterization of novel MT regulators continues to be a worthwhile endeavor.
The small molecule 2-Amino-4-(3,4-(methylenedioxy)benzylamino)-6-(3-methoxyphenyl) pyrimidine (AMBMP) was originally identified as a Wnt agonist due to its ability to activate a multimerized TCF/LEF luciferace reporter construct (Liu et al., 2005). Additionally, long term treatment of Xenopus embryos resulted in stunted head development and somitogenesis defects; both classic Wnt regulated processes (Liu et al., 2005). Importantly, this molecule has been listed in numerous protocols and publications as a beneficial modulator of Wnt activity during stem cell maintenance (Chen et al., 2006; Lyssiotis et al., 2011). The original publication of AMBMP has numerous citations and this small molecule has become a common tool in the Wnt field (Frey et al., 2018; Tribulo et al., 2017a; Tribulo et al., 2017b). However, the molecular target of AMBMP in regards to regulating Wnt activity remains unknown. Recently, it was published that AMBMP can bind and regulate MT dynamics (Fukuda et al., 2016). Fukuda et al. used an elegant combination of cellular assays and pathway profiling to identify AMBMP as a likely MT regulator. They provide evidence that AMBMP can bind MTs and regulate MT polymerization. Here we build off of these findings to perform a detailed analysis of the effects on AMBMP on MT growth, ciliogenesis, cell polarity and cell migration. The potent MT mediated defects observed should give pause to anyone using AMBMP as a Wnt modulator in any biological context.
Results:
AMBMP regulates MT growth
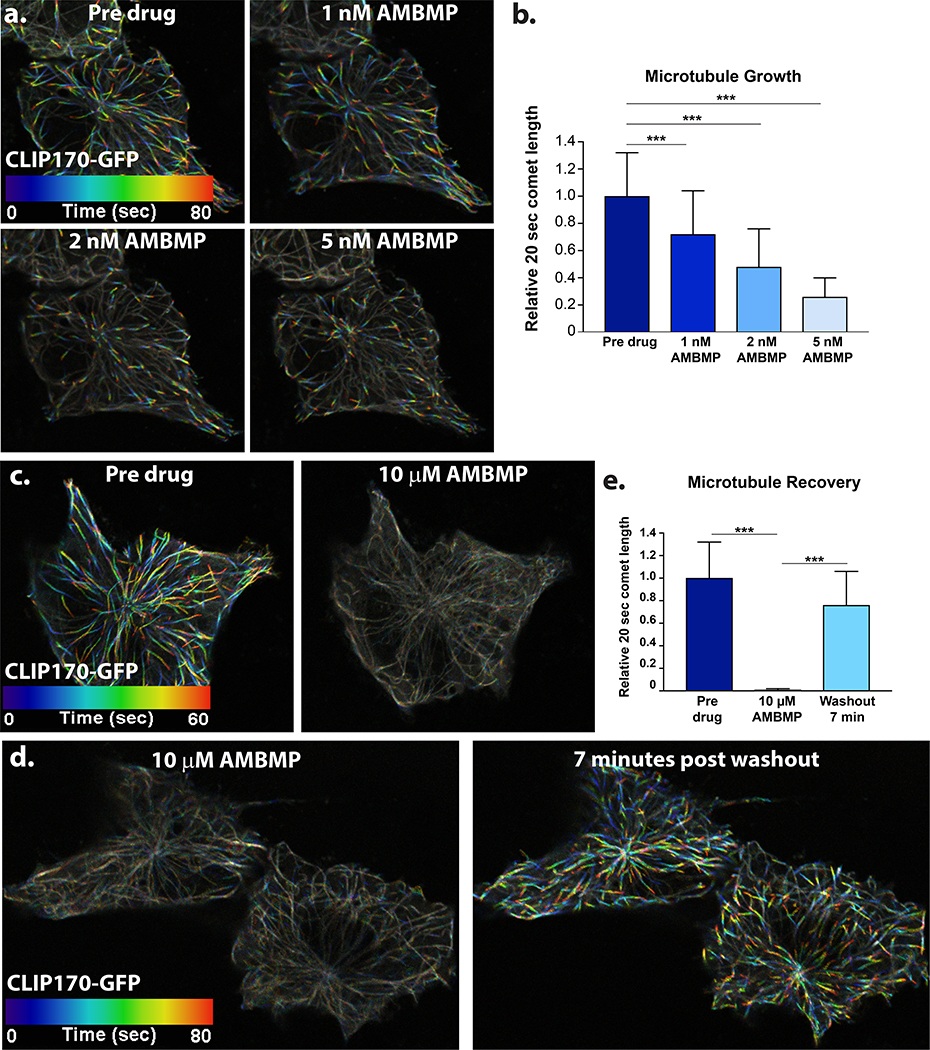
MTs are polarized structures that contain a less dynamic (−) end and very dynamic (+) end. (+) end associated proteins such as EB1 and CLIP170 track along the growing tips of MTs and have been used to measure the dynamics of MT growth (Akhmanova and Steinmetz, 2010; Mimori-Kiyosue et al., 2000; Perez et al., 1999). Tracking tips between frames from time lapse movies creates a “comet” whereby the length of the comet can be used as a proxy for MT growth. We used GFP tagged CLIP170 (CLIP170-GFP) to track MT growth and have pseudo colored the tips a different color in each frame of a movie (Figure 1, Supplemental Movie 1). Treatment of RPE1 cells with AMBMP shows a rapid dose response effect on MT growth in the nanomolar range immediately upon drug addition (Figure 1a.-b., Supplemental Movie 1). This is apparent based on both the decrease in colorimetric changes (Fig 1a) as well as a quantification of comet length (Fig 1b). Furthermore, a dose of 10μM completely abolishes all MT growth (Figure 1c. and e., Supplemental Movie 2). The rapid effect of the drug treatment (seconds) suggests that the drug’s effects are mediated via binding MTs or MT associated proteins rather than via transcriptional regulation of the Wnt pathway. To rule out the possibility that the drug was effecting binding of (+) end tracking proteins rather than MT dynamics we tested the drugs effect in Xenopus S3 cells stably transfected with GFP tagged tubulin (Potapova et al., 2006). Similar to our CLIP170-GFP data, treatment with AMBMP results in a complete loss of tubulin growth (Figure S1, Supplemental Movie 3). Importantly, washout of the drug leads to a recovery of MT growth rates indicating that the effects on MTs are temporary and reversible (Figure 1d. and e., Supplemental Movie 4).
Figure 1: AMBMP is a potent inhibitor of microtubule growth.
(a-b) Representative images of false colored CLIP170-GFP showing changes to MT growth after treatment with AMBMP (a, see Supplemental Movie 1) and quantification (b) of those changes as measured by the comet length of CLIP170-GFP in cells immediately after treatment with AMBMP at the labeled doses (n = 50–100 comets from 3 cells). (c-e) 10μM treatment with AMBMP results in a complete loss of MT growth (c and e, see Supplemental Movie 2) which recovers within 7 minutes of washout (d and e, n > 150 comets from 2 cells, see Supplemental Movie 4). *** represents a p value less than 0.0005.
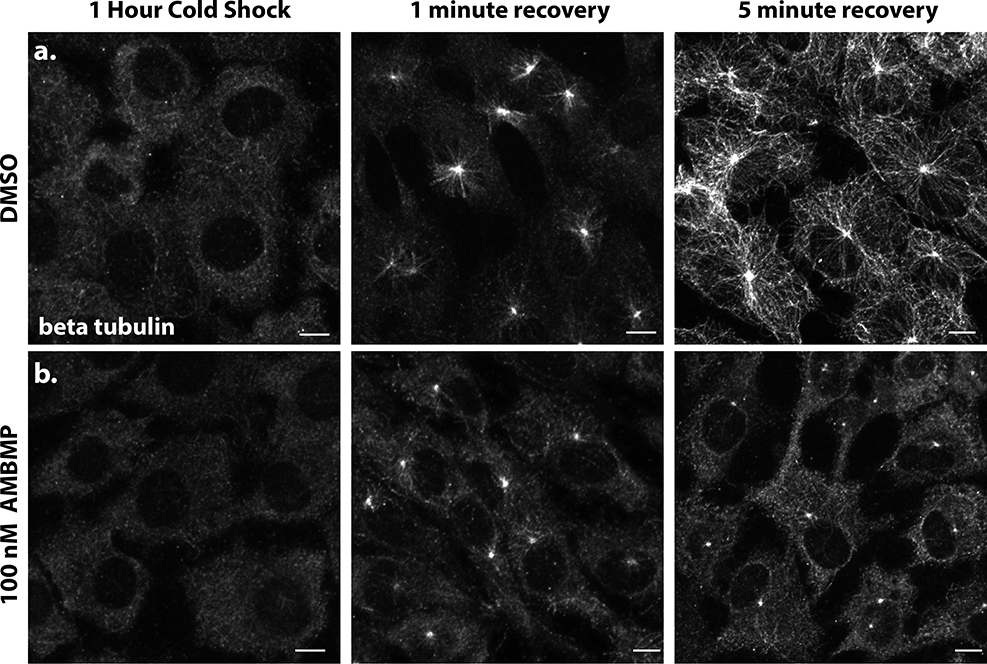
Cold shock treatment is a well-established method for depolymerizing MTs (Vorobjev and Chentsov, 1983). We evaluated MT growth after release from cold shock in both DMSO and AMBMP treated RPE1 cells. Following 1Hr of cold shock, MT staining was essentially eliminated (Figure 2a). In DMSO treated cells MT recovery can be seen as quickly as 1 minute after the release from cold treatment and by 5 minutes an elaborate MT network can be observed throughout the cell (Figure 2a). In contrast, cells treated with 100nM AMBMP showed an accumulation of tubulin at centrosomes but little growth of MT filaments even after 5 minutes (Figure 2b). These results are consistent with our CLIP170 data and suggest that AMBMP is a rapid and potent blocker of MT growth.
Figure 2: AMBMP blocks MT recovery after cold shock.
(a-b) RPE1 cells stained with an antibody to beta tubulin showing that MTs are completely lost after 1 Hr treatment at 4C and that they recover within 5 minutes in DMSO (a) but not 100 nM AMBMP (b). Scale bar equals 10 μm.
AMBMP affects ciliogenesis in Xenopus
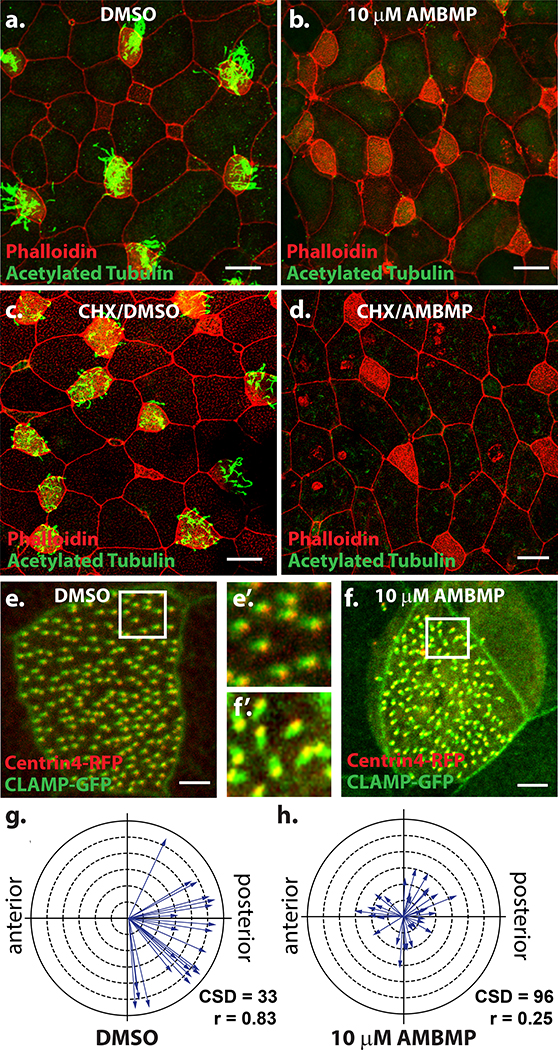
When the original analysis of AMBMP was performed on overall early Xenopus development, we noticed that embryos did not “glide” along the bottom of the dish, a feature that is driven by the multiciliated cells (MCCs) that line the embryonic epithelium. The epithelium contains hundreds of MCCs which each have approximately 150 motile cilia that work together to generate a directed fluid flow across the surface of the embryo (Werner and Mitchell, 2011). We attributed the loss of gliding to a loss of cilia driven fluid flow. To test this we treated stage 18 embryos with either DMSO or AMBMP overnight and then performed antibody staining for acetylated tubulin to mark cilia. While DMSO treated embryos were lined with cells that contained dozens of cilia (Figure 3a), embryos treated with AMBMP were completely devoid of cilia (Figure 3b). One possibility for the loss of cilia is that the MCC cell fate or ciliogenesis is regulated by Wnt signaling. Alternatively, the MT nucleation required for ciliogenesis could be affected by AMBMP. To distinguish these possibilities, we first pretreated embryos for 1Hr with cycloheximide prior to treatment with either DMSO or AMBMP. Cycloheximide blocks protein synthesis and thus if the effect of AMBMP was mediated via transcriptional regulation by Wnt signaling the pretreatment with cycloheximide would block any Wnt mediated effect on MCC cell fate specification. Interestingly, cycloheximide → DMSO treatment resulted in cells that generated cilia however, without proper protein synthesis, the cilia were often shorter or more sparse than normal (Figure 3c). In stark contrast, the cycloheximide → AMBMP still resulted in a complete loss of cilia (Figure 3d) suggesting that its effect is not mediated via Wnt based signaling. In addition to being lined with cilia, MCCs are distinguishable from neighboring cells by an elaborate network of actin that forms on their apical surface. Importantly, in cells treated with AMBMP, while there is a complete loss of cilia, the apical actin network is still readily distinguishable, further indicating that cell fate remains intact while the MT regulated ciliogenesis is disrupted (Figure 3a-d).
Figure 3: AMBMP blocks ciliogenesis and cilia polarization.
(a-b) Xenopus embryos stained with an antibody to acetylated tubulin (green) and phalloidin (red) treated with DMSO (a) or 10mM AMBMP (b) from stage 18–28 showing a complete loss of cilia in AMBMP treated embryos (b). (c-d) Similar experiment to a-b except embryos were pre-treated for 1 Hr with cycloheximide showing some cilia growth with DMSO (c) but not with AMBMP (d). (e-h) Representative images of Xenopus embryos injected with centrin4-RFP (red) and CLAMP-GFP (green) showing the loss of cilia polarity in embryos treated with 10mM AMBMP (f-f’) compared to DMSO (e-e’). (g-h) Cilia polarity is quantified with each arrow representing the data from a single cell such that the direction of the arrow represents the mean cilia orientation and the length of the arrow represents the variation. Scale bars = 25μm in a-d and 5 μm in e-f.
AMBMP affects cilia polarity
The formation of proper cilia driven fluid flow is a multistep process that requires not only the generation of motile cilia but also the coordinated polarization of cilia to orient and beat in the same direction, a process that is well characterized to require Planar Cell Polarity (PCP) signaling, hydrodynamic forces and MT dynamics (Guirao et al., 2010; Mitchell et al., 2007; Mitchell et al., 2009; Vladar et al., 2012). We previously reported that treatment of Xenopus embryos with the MT depolymerizing drug nocodazole after cilia formation, but before polarization (Stage 20–28) resulted in a complete loss of cilia polarity (Werner et al., 2011). Cilia polarity can be quantified by measuring the positon of the striated rootlet marked with CLAMP-GFP relative to the basal body (centrin-RFP) (Park et al., 2008; Werner and Mitchell, 2013). We treated Xenopus embryos during the window of cilia polarization (Stage 20–28) with 10 μm AMBMP. Similar to nocodazole, AMBMP results in a complete loss of cilia polarity. We find that mean cilia direction for each cell (represented as the direction of an arrow in Figure 3g-h) is randomized rather than polarized along the anterior-posterior axis. Furthermore, the circular standard deviation (CSD) which represents the variation in cilia polarity within each cell significantly increases from 33 in DMSO treated embryos to 96 in AMBMP treated embryos (p = 2.1E-17). This is also represented as the arrow length (r) in figure 3g-h which is calculated as 1 - circular variance (shorter arrows = larger variance). Overall these results show that AMBMP significantly disrupts the ability of cilia to properly polarize in a manner that is consistent with what has been published for other MT regulators (Kim et al., 2018; Werner et al., 2011).
AMBMP blocks in vitro and in vivo cell migration
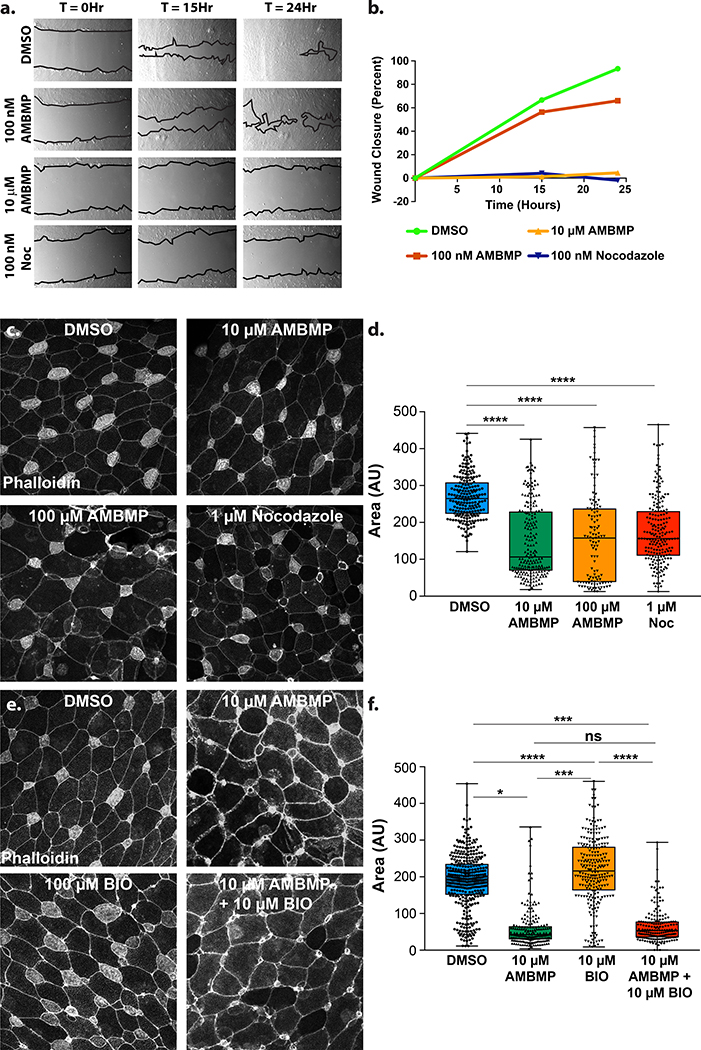
Regulation of MT dynamics is an important component of cell migration in numerous contexts (Etienne-Manneville, 2013). We performed a scratch assay on confluent RPE1 cells to compare the effects of AMBMP and nocodazole on 2 dimensional cell migration. Nocodazole treatment resulted in a striking block of cell movement at 100nM relative to a DMSO control (Figure 4a-b). While AMBMP only had a modest effect on migration at 100nM it completely blocked migration at 10μM suggesting a similar yet less potent effect on MT regulation during migration (Figure 4a-b).
Figure 4: AMBMP blocks migration.
(a-b) A representative scratch assay of RPE1 cells in the presence of DMSO, 100nM Nocodazole, 100nM and 10μM AMBMP showing a low magnification view of the scratch over time (a) with the amount of recovery quantified (b). (c-d) The completion of radial intercalation of MCCs in Xenopus embryos stained with phalloidin is observed (c) and quantified (d) by measuring the size of the apical domain in embryos treated with DMSO, 1μM nocodazole, 10 μM and 100 μM AMBMP. (e-f) Representative images (e) and quantification of MCC apical size (f) in embryos treated with DMSO, 10 μM AMBMP, 100μM BIO and AMBMP + BIO. *** represents a p value of less than 0.0005.
We have previous reported that a short stereotyped form of in vivo polarized migration termed radial intercalation requires a stabilized pool of MTs (Werner et al., 2014). Loss of MTs with 1 μM nocodazole treatment leads to a failure of MCCs to undergo intercalation (Werner et al., 2014). This phenotype can be quantified by measuring the apical size of cells as they protrude through the outer epithelia. We stained the apical actin cap of MCCs with phalloidin to identify MCCs and measured the size of the apical domain as a proxy for the completion of intercalation. Treatment of embryos with 1 μM nocodazole at stage 15, prior to intercalation, results in a failure to properly intercalate as measured by the decrease in the apical surface area of MCCs compared with DMSO (Figure 4c-d). Interestingly, when we treat Xenopus embryos with either 10 or 100 μM AMBMP we see a similar failure to intercalate (Figure 4c-d). These results suggests that similar to nocodazole, manipulating MT dynamics with AMBMP has an adverse effect on in vivo 3 dimensional migration. To further validate that the observed effects were mediated through MTs rather than Wnt signaling we performed a similar experiment using a known Wnt agonist named BIO (Sato et al., 2004). Treatment of embryos with 100 μM BIO had no effect on radial intercalation whereas a combined treatment of BIO and AMBMP gave the same phenotype as AMBMP alone (Figure 4e-f). These results suggest that the effect of AMBMP on intercalation is via its role in regulating MT dynamics rather than via activation of the Wnt pathway.
Discussion
Our results indicate that AMBMP has similar biological affects as the well characterized MT depolymerizing drug nocodazole in numerous contexts. While slightly less potent, AMBMP similarly blocks MT growth, generation of cilia polarity, and cell migration. Importantly, while there are numerous MT drugs, there appears to be a wide range of biological usefulness based on differences of pharmacokinetics (Florian and Mitchison, 2016). Thus while AMBMP may be less potent than nocodazole it could still maintain better efficacy depending on its biostability. Future in vivo work will be required to determine its biological usefulness.
AMBMP has been reported as a useful tool for maintaining Wnt activity in stem cell populations. Our results would question some of that data as it is likely that in cultures of dividing cells a potent anti-MT drug would have a wide range of Wnt independent effects. Ultimately, AMBMP has been reported to affect both MT dynamics and the Wnt signaling pathway. Are these biological effects coupled or distinct? Components of the Wnt pathway are known MT associated proteins (e.g. APC) or known MT regulators (e.g. Dvl). In fact, Wnt signaling components such as beta catenin and Axin can localize to the centrosome and facilitate MT nucleation (Fumoto et al., 2009; Huang et al., 2007). Since no Wnt based molecular target for AMBMP has been identified it is possible that the Wnt activity is mediated via changes to MT growth. Does AMBMP distinctly modulate MTs in a way that affects Wnt signaling or are the Wnt effects mediated by a secondary binding target of AMBMP? Interestingly, neither nocodazole nor other MT drugs have been reported to act as Wnt agonists. In fact, nocodazole is reported to increase levels of LATS2, a negative regulator of Wnt signaling (Li et al., 2013). Additionally, vinblastine and nocodazole treatment leads to a loss of nuclear beta catenin, and presumably Wnt signaling, in early divisions of c. elegans (Sugioka et al., 2011). Curiously, there are numerous, often contradictory, connections between Wnt signaling and the MT based cilia which could also be complicated a by loss of MT dynamics (Wallingford and Mitchell, 2011). The relationship between Wnt signaling and MT dynamics is clearly complex and likely context dependent. How various MT drugs affect this relationship remains unclear and specifically for AMBMP understanding this relationship will be important in ultimately determining its biological usefulness. Our results suggest that before AMBMP is widely used in stem cell biology, a more detailed analysis of the relationship between Wnt activity and its clear role in regulating MT dynamics is warranted.
Materials and Methods
Cell culture, plasmid transfection and cold shock treatment
Human telomerase immortalized retinal pigment epithelial (RPE-1) cells were cultured in DMEM supplemented with 10% FBS 1% penicillin-streptomycin and 2mM L-Glutamine. Cells were transfected with CLIP170-GFP using Lipofectamine 2000 (Invitrogen Inc.) according to manufacturer’s instructions and imaged 24–48 hours after transfection. For live imaging, cells were grown in 35mm microwell glass bottom dishes (MatTek Coorporation). For cold shock treatment cells were incubated at 4C for 60min and fixed at 0 minutes, 1 minute and 5 minutes with 100% ice cold methanol.
Embryo injections and drug treatments.
Xenopus experiments were performed using well established and previously described methods (Werner and Mitchell, 2013). Briefly, Xenopus embryos were generated by in vitro fertilization using standard protocols (Sive et al., 1998) approved by the Northwestern University Institutional Animal Care and Use Committee. For cilia polarity and intercalation analysis embryos were injected at either the 2 or 4 cell stage with 40–250pg mRNA or 10–20pg of plasmid DNA into all 4 blastomeres with a centriole marker (centrin4-RFP) and a rootlet marker (CLAMP-GFP) for cell polarity experiments and with tubulin promoter-GFP (DNA) for intercalation experiments. Drug treatments were carried out at concentrations and timing described in figure legends (Werner et al., 2011). Briefly, embryos were incubated in the presence of AMBMP, Nocodazole or DMSO and fixed immediately thereafter.
Immunofluorescence
Embryo staining was performed on samples fixed with 3% PFA in 80 mM K+ Pipes, pH 6.8, containing 2 mM MgCl2 and 5 mM EDTA for 2 h (Werner and Mitchell, 2013). RPE cells were either fixed with 3% PFA in 80 mM K+ Pipes, pH 6.8, containing 2 mM MgCl2 and 5 mM EDTA for 10min or 100% ice cold Methanol for 20min. Both RPE cells and embryos were blocked with 5% normal donkey serum in PBS 0.1% Triton X-100 for 1h. The following primary antibodies were used according to the manufacturer’s recommended dilutions: mouse anti–β-tubulin (7–10; Developmental Studies Hybridoma Bank) and mouse anti acetylated α-tubulin (T7451; Sigma-Aldrich) and Cy-2– or Cy-3–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.). Phalloidin 568 or 647 (Invitrogen) was used to visualize actin.
Microscopy
Microscopy was performed on a laser-scanning confocal microscope (A1R; Nikon) using a 60× oil Plan-Apo objective with a 1.4 NA. Live imaging of RPE cells was performed at 37C. Nikon Elements Software was used for all acquisition and image processing was done using either Nikon Elements or ImageJ. MT dynamics was assessed by measuring comet length. Briefly, 20 second time projections of CLIP170-GFP movies were generated and the resulting line (comet) was measured manually using Nikon Elements software. For intercalation analysis images are maximum intensity projection of Z-stacks captured every 3–5μm for 15 μm. Apical area for individual MCCs was measured using Nikon Elements software.
Supplementary Material
Acknowledgements
This work was supported by grants from the NIH-NIGMS to BJM (R01GM089970 and R01GM113922) and a pilot award from the H Foundation. RV was supported on a T32 award from NIH-NIAMS (AR060710).
References
- Akhmanova A, and Steinmetz MO 2010. Microtubule +TIPs at a glance. J Cell Sci 123:3415–3419. [DOI] [PubMed] [Google Scholar]
- Chen S, Hilcove S, and Ding S 2006. Exploring stem cell biology with small molecules. Mol Biosyst 2:18–24. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S 2013. Microtubules in cell migration. Annu Rev Cell Dev Biol. 29:471–499. [DOI] [PubMed] [Google Scholar]
- Florian S, and Mitchison TJ 2016. Anti-Microtubule Drugs. Methods Mol Biol. 1413:403–421. [DOI] [PubMed] [Google Scholar]
- Frey JL, Kim SP, Li Z, Wolfgang MJ, and Riddle RC 2018. beta-Catenin Directs Long-Chain Fatty Acid Catabolism in the Osteoblasts of Male Mice. Endocrinology. 159:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Sano O, Kazetani K, Yamamoto K, Iwata H, and Matsui J 2016. Tubulin is a molecular target of the Wnt-activating chemical probe. BMC Biochem. 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumoto K, Kadono M, Izumi N, and Kikuchi A 2009. Axin localizes to the centrosome and is involved in microtubule nucleation. EMBO Rep. 10:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, Mirzadeh Z, Cremer H, Montcouquiol M, Sawamoto K, and Spassky N 2010. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 12:341–350. [DOI] [PubMed] [Google Scholar]
- Huang P, Senga T, and Hamaguchi M 2007. A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene. 26:4357–4371. [DOI] [PubMed] [Google Scholar]
- Kim SK, Zhang S, Werner ME, Brotslaw EJ, Mitchell JW, Altabbaa MM, and Mitchell BJ 2018. CLAMP/Spef1 regulates planar cell polarity signaling and asymmetric microtubule accumulation in the Xenopus ciliated epithelia. J Cell Biol. 217:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen X, Ding X, Cheng Y, Zhao B, Lai ZC, Al Hezaimi K, Hakem R, Guan KL, and Wang CY 2013. LATS2 suppresses oncogenic Wnt signaling by disrupting beta-catenin/BCL9 interaction. Cell Rep. 5:1650–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu X, Mitchell B, Kintner C, Ding S, and Schultz PG 2005. A small-molecule agonist of the Wnt signaling pathway. Angew Chem Int Ed Engl. 44:1987–1990. [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, Lairson LL, Boitano AE, Wurdak H, Zhu S, and Schultz PG 2011. Chemical control of stem cell fate and developmental potential. Angew Chem Int Ed Engl. 50:200–242. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, and Tsukita S 2000. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol. 10:865–868. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, and Kintner C 2007. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 447:97–101. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, and Kintner C 2009. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 19:924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, and Kirschner M 1984. Dynamic instability of microtubule growth. Nature. 312:237–242. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, and Wallingford JB 2008. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 40:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Diamantopoulos GS, Stalder R, and Kreis TE 1999. CLIP-170 highlights growing microtubule ends in vivo. Cell. 96:517–527. [DOI] [PubMed] [Google Scholar]
- Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, Satinover DL, Stukenberg PT, and Gorbsky GJ 2006. The reversibility of mitotic exit in vertebrate cells. Nature. 440:954–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, and Brivanlou AH 2004. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 10:55–63. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, and Harland RM 1998. The early development of Xenopus laevis: A laboratory manual. Cold Spring Harbor Laboratories, Plainview, New York. [Google Scholar]
- Sugioka K, Mizumoto K, and Sawa H 2011. Wnt regulates spindle asymmetry to generate asymmetric nuclear beta-catenin in C. elegans. Cell. 146:942–954. [DOI] [PubMed] [Google Scholar]
- Tribulo P, Leao B, Lehloenya KC, Mingoti GZ, and Hansen PJ 2017a. Consequences of endogenous and exogenous WNT signaling for development of the preimplantation bovine embryo. Biol Reprod. 96:1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribulo P, Moss JI, Ozawa M, Jiang Z, Tian XC, and Hansen PJ 2017b. WNT regulation of embryonic development likely involves pathways independent of nuclear CTNNB1. Reproduction. 153:405–419. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, and Axelrod JD 2012. Microtubules enable the planar cell polarity of airway cilia. Curr Biol. 22:2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev IA, and Chentsov YS 1983. The dynamics of reconstitution of microtubules around the cell center after cooling. Eur J Cell Biol. 30:149–153. [PubMed] [Google Scholar]
- Wallingford JB, and Mitchell B 2011. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 25:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Hwang P, Huisman F, Taborek P, Yu CC, and Mitchell BJ 2011. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J Cell Biol. 195:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, and Mitchell BJ 2011. Understanding ciliated epithelia: The power of Xenopus. Genesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, and Mitchell BJ 2013. Using Xenopus Skin to Study Cilia Development and Function In Methods in Enzymology, Volume 525: Cilia, Part B. Vol. 525 Marshall WF, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Mitchell JW, Putzbach W, Bacon E, Kim SK, and Mitchell BJ 2014. Radial intercalation is regulated by the Par complex and the microtubule-stabilizing protein CLAMP/Spef1. J Cell Biol. 206:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.