Abstract
Drug addiction is a highly prevalent and devastating disorder with few effective treatments, resulting in enormous burdens on family and society. The cellular and behavioral effects of drugs of abuse are related to their abilities to elevate synaptic dopamine levels. Midbrain dopaminergic neurons projecting from the ventral tegmental area to the nucleus accumbens play crucial roles in substance-induced neural and behavioral plasticity. Significantly, increasing work suggests that interplay between the brain circadian system and the cellular bioenergetic machinery in these dopamine neurons plays a critical role in mediating the actions of drugs of abuse. Here, we describe recent progress in elucidating the interconnections between circadian and metabolic systems at the molecular and cellular levels and their relationships to modulation of drug reward and addiction.
Keywords: Circadian, drug abuse, reward, metabolism, NAD+
Introduction
Abuse and dependence of substances comprise some of the most globally prevalent disorders today [1]. However, our understanding of the fundamental mechanisms underlying drug abuse and dependence remain limited. The consensus is that behavioral effects of drugs of abuse are mediated predominantly by their actions in the central nervous system (CNS) through modulation of neurotransmission. Much evidence suggests that drugs’ cellular and behavioral effects are related to their ability to elevate synaptic dopamine (DA) levels [2]. Midbrain DA neurons projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) play critical roles in drug-induced neural and behavioral plasticity [3]. Most, if not all, of these drugs change patterns of VTA-NAc neurotransmission and plasticity at molecular, cellular, circuit levels [3–6]. Emerging data also suggests cellular and molecular rhythms are important regulators of drug reward, and circadian genes [7–11] have been directly implicated as modulators of the DA reward circuitry [12–16]. Additionally, disturbances in circadian regulation of the DA system alter the rewarding properties of drugs of abuse, further increasing vulnerability to substance abuse and addiction [12,17–22]. Ultimately, these drug-induced effects on the CNS circadian system are energy-demanding [12,23,24]. Yet, we know little about how neurons and other neural cell-types meet these bioenergetic demands in response to drugs of abuse. This leads to the following questions: 1) How do changes in neuronal rhythmicity and metabolism contribute to drug reward and dependence? and,2) If so, are circadian clocks directly involved in mediating these effects? Here, we focus on describing the interconnections between neuronal circadian rhythms and cellular bioenergetics, as well as their relationships to modulation of drug reward and addiction.
The CNS circadian system
In mammals, many physiological functions and behaviors are regulated by circadian rhythms. Such rhythms are coordinated by the master circadian pacemaker, the suprachiasmatic nucleus (SCN) of the anterior hypothalamus, which relays light as ‘timing’ information from the eyes to the rest of the brain and body [25]. Virtually all cells express the machinery for generating molecular rhythms, comprised of a series of transcriptional and translational feedback loops cycling with near 24-hour periods. These rhythms are driven by the circadian transcription factors CLOCK and BMAL1 that form heterodimers (CLOCK:BMAL1) [26,27]. These heterodimers are recruited to enhancer promoter elements (E-boxes) to promote gene transcription, including the circadian genes Period (Per1,2) and Cryptochrome (Cry1,2) [28,29]. PER and CRY proteins accumulate in the cytoplasm and are transported to the nucleus to inhibit their own transcription via direct interactions with CLOCK:BMAL1 [30,31]. Additional proteins modulate the phasing and amplitude of molecular clocks. Recent transcriptomic surveys suggest a range of 40–80% of genes within the mammalian genome are regulated in a rhythmic manner, depending on the assay methodology and species [32,33]. Core circadian genes, including Clock, Bmal1, Per2, and Cry2, are the most consistently rhythmic genes between tissues, indicating other clock-controlled genes (CCGs) may be tissue and/or cell-type specific [27,33,34].
Within the CNS, differential enrichment of circadian transcription factors may be responsible for regulation of circadian-dependent functions [35,36]. For example, while CLOCK is largely expressed across many brain regions, expression of the paralog NPAS2, a circadian transcription factor structurally and functionally similar to CLOCK, is primarily limited to the mammalian forebrain and striatum [19,35,37,38]. Significantly, striatal expression of NPAS2 has been implicated as a modulator of drug reward and addiction [19,39]. Recent work also suggests NPAS2 may drive circadian transcription of genes with important roles in neuronal metabolism and energy homeostasis [40–45]. These findings suggest circadian and metabolic coupling, where molecular clocks may directly regulate the expression and function of key metabolic cofactors and mitochondrial biogenesis in the brain. Furthermore, changes in cellular metabolism or redox state can feedback to modulate the function of circadian proteins [12,46–52]. Given the intimate connections between rhythms and metabolism in other highly metabolic tissues including liver and muscle [43,46,47,53,54], cellular and molecular clocks are plausible regulators of metabolism and energetics in the brain. However, the metabolic processes underlying neuronal firing and activity are poorly understood, particularly in relation to substance abuse. Recent findings demonstrating neuronal CLOCK-and NPAS2-dependent modulation of neuronal metabolism nevertheless pave the way for exploring the implications of these processes on drug reward and related behaviors.
Reciprocal Connections between CNS Molecular Clocks and Energetic Signaling
The brain consumes more oxygen and glucose than any other organ, relying mostly on the supply of energy substrates from the vasculature to fuel neurotransmission [55,56]. Adenosine triphosphate (ATP) is the primary carrier of energy and is secreted via calcium-dependent exocytosis from astrocytes [57]. Neuronal ATP is generated via several pathways including glycolysis and lactate production, as well as through mitochondrial oxidative phosphorylation and the tricarboxylic acid (TCA) cycle [58–60]. In brain as well as other metabolically-active tissues, ATP synthetic pathways are regulated by circadian-dependent environmental (e.g., feeding rhythms) and biological rhythms (e.g., molecular clocks) [61–64]. The molecular clock directly controls the transcription of metabolic cofactors including nicotinamide adenine dinucleotide (NAD+), and rate-limiting enzymes of energy substrate production [50,52,65]. Furthermore, in SCN, mitochondrial calcium rhythms are necessary for circadian rhythms of extracellular ATP [66,67]. Diurnal variation of calcium homeostasis, extracellular glutamate, and ATP release also feedback to molecular clocks to modulate gene expression rhythms [68]. Whether ATP release follows diurnal rhythms in other brain regions remains to be investigated [64]. Nevertheless, since ATP can be co-released with or facilitate the release many neurotransmitters including glutamate, GABA and DA [69–73], and this neurotransmission is regulated in a circadian manner [21,74–77], we speculate that ATP release may also be diurnally controlled in areas beyond the SCN.
Extracellular ATP modulates synaptic function and plasticity via binding and activation of several subtypes of purine receptors on pre-and postsynaptic neurons and nearby astrocytes. For example, ATP activation of presynaptic ionotropic P2X receptors (P2XR) enhances glutamatergic neurotransmission [78–80], whereas binding to the metabotropic P2YRs inhibits further release [81–83]. Several subtypes of these receptors have been linked to reward and motivation. In mice, global deletion of the P2X4R in mice leads to increased alcohol intake during both intermittent and continuous access paradigms [84]. The expression of the receptor in various brain regions, including the VTA, also negatively correlates with alcohol preference in selectively bred mice and rats [85]. In general, reduced expression of P2X4R is associated with high alcohol preference, although increased expression has been found in certain high alcohol preferring rats [86–88]. In the VTA, P2X4Rs are densely expressed at GABAergic synapses, suggesting these receptors modulate inhibitory neurotransmission [84]. These receptors also modulate dopaminergic and glutamatergic neurotransmission in mesolimbic neural circuitry [89,90]. ATP activation of P2X4Rs increased firing and release from midbrain DA neurons, leading to elevated extracellular DA in the NAc [89,91]. Presynaptic markers of DA synthesis and uptake, along with CREB-dependent pathways, may also be directly regulated by P2X4Rs [92]. At GABAergic and DAergic synapses, alcohol may inhibit the binding affinity of ATP to purine receptors, including P2X4R [93], ultimately altering neurotransmission. Other purine receptor subtypes such as P2Y1 may additionally be involved in drug reward, since blocking P2Y1Rs prevents amphetamine locomotor sensitization [94]. Given the links between ATP, purinergic receptors, and circadian pathways [95–97], these findings suggest complex, potentially reciprocal and bidirectional connections, implicating certain purine receptor subtypes as potential mediators of the impact of drugs of abuse on cellular rhythms and energetics. Purinergic signaling is also implicated in several other psychiatric disorders [98,99], where dysfunction of mitochondrial dynamics, energy homeostasis, and possibly circadian rhythms may be hallmarks of the disease.
ATP is used to synthesize another purine, the neuromodulator adenosine [99], which is also involved in drug reward and addiction. In the brain, extracellular adenosine displays robust, circadian dependent rhythms, where levels peak during the night and gradually building during the day. Adenosine is intimately involved in sleep and wakefulness, arousal, and circadian rhythms [100–105]. Adenosine activates P1 receptors (A1, A2A, A2B, and A3), which are primarily located on presynaptic terminals. Several of these receptors have been implicated in addiction [106], such as the A2AR, which is highly expressed in the striatum [107]. During withdrawal from cocaine, A2ARs are upregulated in both hippocampus and striatum of the rat [108–111], although these receptors seem to differentially modulate the behavioral effects of repeated cocaine administration. Indeed, deletion of A2ARs in striatal neurons enhanced cocaine sensitization, while deletion of the receptors in cortical and hippocampal neurons attenuated locomotor sensitization [112,113]. These receptors are involved in many other drug reward related behaviors [106].
Several mechanisms have been proposed for A2ARs to impact the behavioral response to drugs of abuse. A plausible mechanism involves the formation of heteromeric receptor complexes between A2ARs and D2Rs on GABAergic striatal neurons [114–116]. Consistent with this, activation of A2AR reduces D2R affinities for DA, enhances GABA release, and attenuates cocaine seeking behaviors [117,118], suggesting A2AR inhibits further activation of D2Rs on striatal neurons in response to cocaine. However, these receptor interactions may also be synergistic by further activating D2Rs, making such receptor associations especially relevant for drug withdrawal [119,120]. There may be therapeutic utility for targeting these receptors for the treatment of addiction, depending on the ability to further understand where and under which circumstances these specific receptor interactions are occurring [121]. Targeting these receptors may also alleviate the sleep and circadian rhythms disturbances which are common during drug withdrawal and abstinence. Furthermore, whether these circadian pathways are directly involved with these processes, including any interaction with D2R activity, remains an interesting avenue of future investigation, especially in relevant neural circuitry [122].
Circadian brain lactate production and drug-induced plasticity and relapse
Besides glucose, lactate is a major brain energy source. Astrocytes use extracellular glutamate to activate glycolysis, converting glycogen to pyruvate then lactate. Lactate is actively transported to neurons, entering the TCA cycle to produce ATP and for mitochondria to sustain oxidative phosphorylation. Together, these processes fuel neuronal metabolism to meet activity dependent demands [123]. Variations of extracellular lactate in cortical regions depend on the sleep-wake cycle, resembling biomarkers of energy homeostasis and sleep state [124,125]. Diurnal rhythms associated with neuronal activity and neurotransmission may therefore drive temporal changes in glycolysis and lactate in astrocytes.
Genes encoding proteins required for lactate production and transport are also direct transcriptional targets of circadian transcription factors. The monocarboxylate transporters of lactate between astrocytes and neurons, MCT1 and MCT2, are diurnally expressed in brain [41]. Moreover, NPAS2 drives transcription of lactate dehydrogenase A (LDHA), an enzyme that catalyzes lactate production [43]. Interestingly, expression of Mct1, Mct2 and Ldha are markedly reduced in the NAc of NPAS2-deficient mice (unpublished results), consistent with impaired lactate production and transport to neurons (Fig. 1). Similar impairments of glycolysis either via direct blockade or by preventing lactate transport through MCT1 or MCT2 knockdown reduce the acquisition and maintenance of cocaine conditioned place preference and the number of infusions during cocaine self-administration [23,24]. Intriguingly, NPAS2 knockdown in the NAc also attenuates the acquisition of cocaine conditioned place preference, further suggesting the induction of NPAS2 is important for cocaine conditioned reward[19]. Given these data, we speculate that NPAS2 may be a transcriptional regulator of energy production in the NAc via the induction of Ldha and lactate production necessary for drug-paired contextual conditioning. Repeated administration of drugs of abuse disrupts molecular clocks in the striatum, which could have consequences on neuronal energetics and homeostasis, impacting drug reward and addiction.
Figure 1.
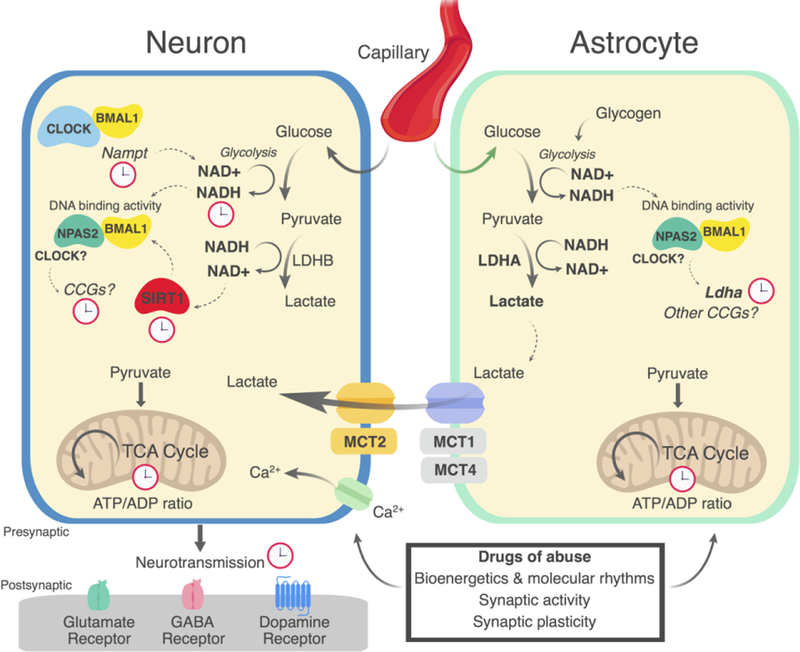
Proposed model of circadian control of astrocyte and neuronal energetic coupling. Core circadian transcription factors including BMAL1 and CLOCK or NPAS2 regulate the rate-limiting steps of NAD+ biosynthesis, primarily via the direct transcription of Nampt. The DNA binding affinity of CLOCK and NPAS2, BMAL1 dimers is controlled by the intracellular ratio of bioavailable NAD+ and NADH, where higher NADH tends to enhance the DNA binding activity of the transcriptional complex. The production of lactate through LDHA in astrocytes and LDHB in neurons is critical for elevating intracellular AD+ levels. NAD+-dependent deacetylases including SIRT1 is capable of deacetylating BMAL1 or acting on other proteins such as PGC1–03B1 (not shown) to suppress or amplify the transcription of CCGs. Some of these genes encode rate-limiting enzymes necessary for the production of lactate, a major energy substrate for neurons in response to neural stimulation. Glucose is required for glycolysis and conversion of pyruvate to lactate. Lactate is transported from astrocytes by MCT1/4 and into neurons by MCT2. Pyruvate and other energy substrates are used by mitochondria to generate ATP via oxidative phosphorylation. For example, NPAS2 has been shown to regulate the expression of Ldha. These energetic pathways may also be modulated by circadian rhythms in reward-related brain regions, gating the cellular response to drugs of abuse, potentially necessary for drug-induced synaptic and behavioral plasticity.
Circadian regulation of mitochondrial redox and drug reward
NAD+, a byproduct of lactate production, is an essential metabolic and redox cofactor crucial for the generation of ATP [126,127]. NAD+ is also important in maintaining brain energy homeostasis, calcium transport, and mitochondrial respiration [127–129]. The recycling of NAD+ requires several enzymes, including nicotinamide phosphoribosyltransferase (NAM T), the rate-limiting enzyme for the conversion of nicotinamide mononucleotide (NMN) to NAD+. In liver and brain, the CLOCK:BMAL1 complex drives the rhythmic transcription of Nampt and thus, the circadian bioavailability of NAD+ [12,52]. NAD+ and NADH availability also modulate the DNA binding activity of CLOCK and NPAS2, indicating these transcription factors are integral redox sensors [41,43,130]. Low NAD+:NADH ratios enhance DNA binding activity of NPAS2:BMAL1 and CLOCK:BMAL1 dimers, providing another level of energetic feedback to the molecular clock [43] (Fig. 1). Coupling between intracellular redox, metabolism, and circadian rhythms may be particularly important for maintaining temporal dependent changes in neuronal activity and governing the response to stimulation [131].
NAD+ also interacts with mitochondrial SIRT1, a histone and protein deacetylase, to amplify molecular rhythms in the SCN [65]. Available NAD+ enhances the deacetylase activity of SIRT1, which deacetylates BMAL1 to repress CLOCK:BMAL1-mediated transcription[49]. On the other hand, SIRT1 also deacetylates PER2 to promote its degradation and amplify CLOCK:BMAL1-mediated gene transcription [132] – actions that may directly impact the actions of drugs of abuse. Repeated cocaine or morphine administration increases expression of SIRT1 and circadian genes including Per1 and Per2 at specific diurnal phases [39,133–135]. Moreover, NAc-specific SIRT1 overexpression increases cocaine and morphine conditioned place preference [133]. Similarly, NPAS2 expression is increased in the NAc following repeated cocaine administration which may be important for the rewarding effects of cocaine [19]. Considering NPAS2:BMAL1 may be the primary driver of circadian-dependent gene transcription in the NAc, enhanced drug conditioned reward may rely on NPAS2:BMAL1-mediated transcription (Fig. 1). Thus, cocaine and other drugs of abuse may alter the phase, period, and/or amplitude of molecular rhythms in brain regions including the NAc which has downstream consequences on circadian-dependent signaling pathways.
Circadian regulation of DA biosynthesis and drug reward
The molecular clock regulates local DA synthesis, uptake, and release potentially via the direct transcriptional control of genes encoding major enzymes, transporters, and receptors involved with DA neurotransmission [19,37,136–140]. Identification of binding targets of CLOCK and NPAS2 in the striatum revealed possible novel targets for circadian regulation of DA and GABA neurotransmission in mesocorticolimbic circuitry [19,37]. Many of these targets contain E-Box and/or CRE sites in their promoters, further supporting direct transcriptional control by circadian transcription factors [19,37]. We recently extended these findings by demonstrating NAD+-and SIRT1-dependent diurnal regulation of DA synthesis and neurotransmission in the mouse VTA [12]. ranscription of the rate-limiting enzyme of DA synthesis, tyrosine hydroxylase (TH), is dynamically regulated by CREB and CLOCK at specific times of day; CREB drives transcription during the active phase (subjective night), while CLOCK recruits SIRT1 during the inactive phase (subjective day) to repress transcription. Recruitment of SIRT1 to the TH promoter during the day synchronizes with the peak of NAD+ abundance, suggesting that diurnal variation of redox state drives time-dependent activity of SIRT1 in the brain. Conversely, disruption of the molecular clock impairs SIRT1 activity and leads to increased TH expression which promotes DA synthesis. These diurnal actions positively associate with time-dependent DA cell firing in the VTA and elevated cocaine conditioned place preference. Pharmacologically increasing NAD+ availability or restoring SIRT1 activity specifically in the VTA of CLOCK-disrupted (ClockΔ19) mice reduces DA synthesis and cocaine-conditioned reward. On the other hand, driving SIRT1 activity and NAD+ in wild-type mice enhances DA synthesis and neurotransmission, as well as cocaine-conditioned reward, consistent with previous studies showing SIRT1 activation in the NAc promotes cocaine reward [12,133].
Conclusions and Future Directions
Several major pathways of energy production and utilization are under circadian control in the brain. This coupling of circadian pathways to neuronal metabolism may be critical both in maintaining homeostasis as well as optimizing brain energy production and utilization, particularly in the context of drugs of abuse. While the majority of work examining the links between metabolism and circadian regulation has been based in the periphery (e.g. liver) or brain areas such as the SCN, it is increasingly evident that these links may be applicable in other brain areas. Indeed, brain regions capable of sustaining high amplitude oscillations may employ rhythmic coupling of astrocyte-neuronal metabolism to tightly regulate activity-dependent neurotransmission [141] – a process that may be critical in mediating the behavioral effects of substances such as cocaine and morphine. We are just beginning to understand the role of astrocyte specific molecular clocks and their role in relevant neural circuits and behaviors [142,143]. Intriguingly, we have recently shown that DA neurons projecting to the NAc from the VTA can tune vesicular DA content and release in response to increases in firing [144]. These neurons concomitantly load DA vesicles with glutamate to facilitate this activity-dependent increase in DA loading and release. Recently, DA neurons from the VTA were shown to project directly to the SCN to resynchronize activity rhythms to photic phase shifting, implying alterations of dopaminergic neurotransmission to NAc may also impact the S N [145]. Further appreciation of subpopulations of midbrain dopaminergic projection neurons may help understand their possible pathway specific involvement in arousal, motivation, and reward [146].
It is also known that diurnal regulation of glutamatergic and dopaminergic neurotransmission within neural circuits related to anhedonia, motivation, and goal-directed behaviors are strongly associated with parallel time-of-day differences in behaviors of conditioned drug reward, drug-seeking and relapse. Drugs of abuse may also entrain cellular and molecular rhythms, leading to the anticipation of subsequent drug administration or drug paired-cue presentations involved in craving and relapse [147,148]. This opens the door to future work exploring whether DA/glutamate co-transmission may be a key process required for the energetic demands of drug-fueled changes in circadian rhythms and neuronal activity associated with reward and addictive behaviors.
Highlights.
Metabolism and circadian rhythms are intimately connected in brain
Mitochondrial redox and glycolysis fuel drug-induced neurotransmission
Rhythmic control of brain energetic pathways is important for drug reward
Rhythmic NAD+ biosynthesis controls dopamine neurotransmission and drug reward
Acknowledgments
Funding
US Department of Defense PRMRP Award PR141292 (ZF),John F. and Nancy. Emmerling Fund of the Pittsburgh Foundation (ZF), National Institute on Drug Abuse (NIDA) DA038654 (RWL) and DA041872 (RWL).
Acknowledgements
Figures were produced using Biorender software (biorender.io).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- 1.Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, Giovino GA, West R, Hall W, Griffiths P, et al. : Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018. [DOI] [PubMed]
- 2.Sulzer D: How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 2011, 69:628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morales M, Margolis EB: 3Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 2017, 18:73–85.* Comprehensive review highlighting the appreciation for cellular diversity in the ventral tegmental area and their roles in diverse behaviors. [DOI] [PubMed] [Google Scholar]
- 4.Badiani A, Belin D, Epstein D, Calu D, Shaham Y: Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 2011, 12:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper S, Robison AJ, Mazei-Robison MS: Reward Circuitry in Addiction. Neurotherapeutics 2017, 14:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakhoury M: 3The tail of the ventral tegmental area in behavioral processes and in the effect of psychostimulants and drugs of abuse. Prog Neuropsychopharmacol Biol Psychiatry 2018, 84:30–38. [DOI] [PubMed] [Google Scholar]
- 7.Halbout B, Perreau-Lenz S, Dixon CI, Stephens DN, Spanagel R: Per1(Brdm1) mice self-administer cocaine and reinstate cocaine-seeking behaviour following extinction. Behav Pharmacol 2011, 2:76–80. [DOI] [PubMed] [Google Scholar]
- 8.Perreau-Lenz S, Hoelters LS, Leixner S, Sanchis-Segura C, Hansson A, Bilbao A, Spanagel R: 3mPer1 promotes morphine-induced locomotor sensitization and conditioned place preference via histone deacetylase activity. Psychopharmacology (Berl) 2017, 234:1713–1724.* Interaction between the circadian protein PER1 and histone deacetylase activity in the striatum is important for morphine-induced locomotor sensitization and conditioned place preference. These effects may be specific to the sensitization and rewarding effects of opioids, and not antinociception, tolerance, and physical withdrawal from morphine. [DOI] [PubMed] [Google Scholar]
- 9.Perreau-Lenz S, Sanchis-Segura C, Leonardi-Essmann F, Schneider M, Spanagel R: Development of morphine-induced tolerance and withdrawal: involvement of the clock gene mPer2. Eur Neuropsychopharmacol 2010, 20:509–517. [DOI] [PubMed] [Google Scholar]
- 10.Perreau-Lenz S, Spanagel R: 3The effects of drugs of abuse on clock genes. Drug News Perspect 2008, 21:211–217. [DOI] [PubMed] [Google Scholar]
- 11.Perreau-Lenz S, Zghoul T, de Fonseca FR, Spanagel R, Bilbao A: 3Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addict Biol 2009, 14:253–259. [DOI] [PubMed] [Google Scholar]
- 12.Logan RW, Parekh PK, Kaplan GN, Becker-Krail DD, Williams WP 3rd, Yamaguchi S, Yoshino J, Shelton MA, Zhu X, Zhang H, et al. : 3NAD+ cellular redox and SIRT1 regulate the diurnal rhythms of tyrosine hydroxylase and conditioned cocaine reward. Mol Psychiatry 2018* Dopamine synthesis and neurotransmission from the ventral tegmental area is regulated by the molecular clock and redox modulation of metabolic cofactors. Disrupting molecular clock function leads to increased dopamine cell activity and dopamine levels in the ventral tegmental area and downstream areas, including nucleus accumbens. These changes are associated with enhanced sensitivity to the rewarding effects of cocaine. In mice with a functional molecular clock,elevatingNAD+bioavailability and SIRT1 activity in the ventral tegmental area leads to enhanced dopamine activity and promotes cocaine reward behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan RW, Williams WP 3rd, McClung CA: Circadian rhythms and addiction: mechanistic insights and future directions. Behav Neurosci 2014, 128:387–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shumay E, Fowler JS, Wang GJ, Logan J, Alia-Klein N, Goldstein RZ, Maloney T, Wong C, Volkow ND: Repeat variation in the human PER2 gene as a new genetic marker associated with cocaine addiction and brain dopamine D2 receptor availability. Transl Psychiatry 2012, 2:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, Kim KS, Dluzen DE, Lee I, Hwang O, et al. : 3Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell 2014, 157:858–868. [DOI] [PubMed] [Google Scholar]
- 16.Salaberry NL, Mateo M, Mendoza J: 3The Clock Gene Rev-Erbalpha Regulates Methamphetamine Actions on Circadian Timekeeping in the Mouse Brain. Mol Neurobiol 2017, 54:5327–5334. [DOI] [PubMed] [Google Scholar]
- 17.Andretic R, Chaney S, Hirsh J: 3Requirement of circadian genes for cocaine sensitization in Drosophila. Science 1999, 285:1066–1068. [DOI] [PubMed] [Google Scholar]
- 18.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ: 3Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci S A 2005, 102:9377–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozburn AR, Falcon E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, Arey RN, Mukherjee S, Lyons-Weiler J, Self DW, et al. : 3Direct regulation of diurnal Drd3 expression and cocaine reward by NPAS2. Biol Psychiatry 2015, 77:425–433.* Repeated cocaine administration increases NPAS2 expression in the nucleus accumbens. Knockdown of NPAS2 specifically in the nucleus accumbens reduces the cocaine conditioned reward behavior, potentially via cell-type specific expression in dopamine D1 receptor-expressing neurons. NPAS2 binds DNA and regulates a number of transcriptional targets in a diurnal manner in the nucleus accumbens. NPAS2 prefrentially binds the dopamine D3 receptor and may be involved in the regulation of cocaine reward. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parekh PK, Becker-Krail D, Sundaravelu P, Ishigaki S, Okado H, Sobue G, Huang Y, McClung CA: Altered GluA1 (Gria1) Function and Accumbal Synaptic Plasticity in the ClockDelta19 Model of Bipolar Mania. Biol Psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, Arey RN, Enwright JF 3rd, Jacobsen JP, Kumar S, et al. : 3Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry 2015, 20:1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer S, Torres-Altoro MI, Falcon E, Arey R, Marvin M, Goldberg M, Bibb JA, McClung CA: A mutation in CLOCK leads to altered dopamine receptor function. J Neurochem 2012, 123:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boury-Jamot B, Carrard A, Martin JL, Halfon O, Magistretti PJ, Boutrel B: 3Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol Psychiatry 2016, 21:1070–1076.* Infusion of an inhibitor of glycolysis activity into the basolateral amygdala acutely blocked the acquistion and maintenance of cocaine conditioned place preference behavior. The contextual pairing with cocaine required glycolytic production of lactate in this brain region. Lactate delivery to the basolateral amygdala restored contextual conditioning of cocaine pairing. Lacate facilitated cocaine-induced plasticity through an ERK-dependent signaling mechanisms involving the transcription factor Zif268, which has previously been linked to synaptic plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Xue Y, Meng S, Luo Y, Liang J, Li J, Ai S, Sun C, Shen H, Zhu W, et al. : Inhibition of Lactate Transport Erases Drug Memory and Prevents Drug Relapse. Biol Psychiatry 2016, 79:928–939.* Reducing lactate signaling specifically in the basolateral amygdala through the inhibition of glycolysis activity after retrieval of cocaine-paired contextual ‘memory’ completely prevented the exhibition of conditioned place preference to cocaine and cocaine self-administration beahviors. [DOI] [PubMed] [Google Scholar]
- 25.Mohawk JA, Green CB, Takahashi JS: 3Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 2012, 35:445–462., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partch CL, Green CB, Takahashi JS: 3Molecular architecture of the mammalian circadian clock. Trends Cell Biol 2014, 24:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi JS: 3Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 2017, 18:164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darlington TK, Lyons LC, Hardin PE, Kay SA: 3The period E-box is sufficient to drive circadian oscillation of transcription in vivo. J Biol Rhythms 2000, 15:462–471. [DOI] [PubMed] [Google Scholar]
- 29.Hardin PE: 3Transcription regulation within the circadian clock: the E-box and beyond. J Biol Rhythms 2004, 19:348–360. [DOI] [PubMed] [Google Scholar]
- 30.Gustafson CL, Partch CL: 3Emerging models for the molecular basis of mammalian circadian timing. Biochemistry 2015, 54:134–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiou YY, Yang Y, Rashid N, Ye R, Selby CP, Sancar A: 3Mammalian Period represses and de-represses transcription by displacing CLOCK-BMAL1 from promoters in a Cryptochrome-dependent manner. Proc Natl Acad Sci U S A 2016, 113:E6072–e6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, et al. : 3Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359* Massive undertaking of diurnal transcriptome analysis of more than 60 tissues and 22 brain regions. Transcriptomes were highly different among tissues and between brain regions. Estimates of ~80% of the transcriptome appear to display robust diurnal rhythms of expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB: 3A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 2014, 111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trott AJ, Menet JS: Regulation of circadian clock transcriptional output by CLOCK:BMAL1. PLoS Genet 2018, 14:e1007156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reick M, Garcia JA, Dudley C, McKnight SL: 3NPAS2: an analog of clock operative in the mammalian forebrain. Science 2001, 293:506–509. [DOI] [PubMed] [Google Scholar]
- 36.Chun LE, Woodruff ER, Morton S, Hinds LR, Spencer RL: 3Variations in Phase and Amplitude of Rhythmic Clock Gene Expression across Prefrontal Cortex, Hippocampus, Amygdala, and Hypothalamic Paraventricular and Suprachiasmatic Nuclei of Male and Female Rats. J Biol Rhythms 2015, 30:417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozburn AR, Kern J, Parekh PK, Logan RW, Liu Z, Falcon E, Becker-Krail D, Purohit K, Edgar NM, Huang Y, et al. : NPAS2 Regulation of Anxiety-Like Behavior and GABAA Receptors. Front Mol Neurosci 2017, 10:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, Reick M, Scott K, Diaz-Arrastia R, McKnight SL: 3Impaired cued and contextual memory in NPAS2-deficient mice. Science 2000, 288:2226–2230. [DOI] [PubMed] [Google Scholar]
- 39.Falcon E, Ozburn A, Mukherjee S, Roybal K, McClung CA: Differential regulation of the period genes in striatal regions following cocaine exposure. PLoS One 2013, 8:e66438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, et al. : Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 2013, 123:5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL: 3NPAS2: a gas-responsive transcription factor. Science 2002, 298:2385–2387. [DOI] [PubMed] [Google Scholar]
- 42.Kaasik K, Lee CC: 3Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 2004, 430:467–471. [DOI] [PubMed] [Google Scholar]
- 43.Rutter J, Reick M, Wu LC, McKnight SL: 3Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 2001, 293:510–514. [DOI] [PubMed] [Google Scholar]
- 44.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ: 3Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 2010, 185:5796–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshii K, Tajima F, Ishijima S, Sagami I: 3Changes in pH and NADPH regulate the DNA binding activity of neuronal PAS domain protein 2, a mammalian circadian transcription factor. Biochemistry 2015, 54:250–259. [DOI] [PubMed] [Google Scholar]
- 46.Eckel-Mahan K, Sassone-Corsi P: 3Metabolism and the circadian clock converge. Physiol Rev 2013, 93:107–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cribbet MR, Logan RW, Edwards MD, Hanlon E, Bien Peek C, Stubblefield JJ, Vasudevan S, Ritchey F, Frank E: 3Circadian rhythms and metabolism: from the brain to the gut and back again. Ann N Y Acad Sci 2016, 1385:21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aguilar-Arnal L, Katada S, Orozco-Solis R, Sassone-Corsi P: NAD(+)-SIRT1 control of H3K4 trimethylation through circadian deacetylation of MLL1. Nat Struct Mol Biol 2015, 22:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P: 3The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P: 3Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324:654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. : 3Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 2013, 342:1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. : 3Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324:651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J: 3Circadian clocks and metabolism. Handb Exp Pharmacol 2013:127–155. [DOI] [PMC free article] [PubMed]
- 54.Panda S: 3Circadian physiology of metabolism. Science 2016, 354:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris JJ, Jolivet R, Attwell D: 3Synaptic energy use and supply. Neuron 2012, 75:762–777. [DOI] [PubMed] [Google Scholar]
- 56.Belanger M, Allaman I, Magistretti PJ: Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 2011, 14:724–738. [DOI] [PubMed] [Google Scholar]
- 57.Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y: 3Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol 2014, 12:e1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez-Gambero AJ, Martinez F, Salazar K, Cifuentes M, Nualart F: 3Brain Glucose-Sensing Mechanism and Energy Homeostasis. Mol Neurobiol 2018. [DOI] [PubMed]
- 59.Nortley R, Attwell D: 3Control of brain energy supply by astrocytes. Curr Opin Neurobiol 2017, 47:80–85. [DOI] [PubMed] [Google Scholar]
- 60.Magistretti PJ, Pellerin L: 3Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci 1999, 354:1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bass J, Takahashi JS: 3Circadian integration of metabolism and energetics. Science 2010, 330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaule C: 3Circadian regulation of ATP release in astrocytes. J Neurosci 2011, 31:8342–8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ: 3Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci 2009, 30:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamazaki S, Ishida Y, Inouye S: 3Circadian rhythms of adenosine triphosphate contents in the suprachiasmatic nucleus, anterior hypothalamic area and caudate putamen of the rat--negative correlation with electrical activity. Brain Res 1994, 664:237–240. [DOI] [PubMed] [Google Scholar]
- 65.Chang HC, Guarente L: 3SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 2013, 153:1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Enoki R, Kuroda S, Ono D, Hasan MT, Ueda T, Honma S, Honma K: 3Topological specificity and hierarchical network of the circadian calcium rhythm in the suprachiasmatic nucleus. Proc Natl Acad Sci U S A 2012, 109:21498–21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noguchi T, Leise TL, Kingsbury NJ, Diemer T, Wang LL, Henson MA, Welsh DK: Calcium Circadian Rhythmicity in the Suprachiasmatic Nucleus: Cell Autonomy and Network Modulation. eNeuro 2017, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakazato R, Takarada T, Yamamoto T, Hotta S, Hinoi E, Yoneda Y: Selective upregulation of Per1 mRNA expression by ATP through activation of P2X7 purinergic receptors expressed in microglial cells. J Pharmacol Sci 2011, 116:350–361. [DOI] [PubMed] [Google Scholar]
- 69.Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC, Butt AM: 3Mechanisms of ATP-and glutamate-mediated calcium signaling in white matter astrocytes. Glia 2008, 56:734–749. [DOI] [PubMed] [Google Scholar]
- 70.Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S: 3ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem 2001, 77:664–675. [DOI] [PubMed] [Google Scholar]
- 71.Jo YH, Schlichter R: 3Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci 1999, 2:241–245. [DOI] [PubMed] [Google Scholar]
- 72.Verkhratsky A, Schousboe A, Parpura V: Glutamate and ATP: The Crossroads of Signaling and Metabolism in the Brain. Adv Neurobiol 2014, 11:1–12. [DOI] [PubMed] [Google Scholar]
- 73.Zhang YX, Yamashita H, Ohshita T, Sawamoto N, Nakamura S: 3ATP induces release of newly synthesized dopamine in the rat striatum. Neurochem Int 1996, 28:395–400. [DOI] [PubMed] [Google Scholar]
- 74.Kafka MS, Benedito MA, Blendy JA, Tokola NS: 3Circadian rhythms in neurotransmitter receptors in discrete rat brain regions. Chronobiol Int 1986, 3:91–100. [DOI] [PubMed] [Google Scholar]
- 75.Kafka MS, Wirz-Justice A, Naber D, Moore RY, Benedito MA: 3Circadian rhythms in rat brain neurotransmitter receptors. Fed Proc 1983, 42:2796–2801. [PubMed] [Google Scholar]
- 76.Castaneda TR, de Prado BM, Prieto D, Mora F: 3Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res 2004, 36:177–185. [DOI] [PubMed] [Google Scholar]
- 77.Ferris MJ, Espana RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, Jones SR: 3Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci U S A 2014, 111:E2751–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu JG, MacDermott AB: 3Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 1997, 389:749–753. [DOI] [PubMed] [Google Scholar]
- 79.Shigetomi E, Kato F: 3Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J Neurosci 2004, 24:3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakatsuka T, Tsuzuki K, Ling JX, Sonobe H, Gu JG: 3Distinct roles of P2X receptors in modulating glutamate release at different primary sensory synapses in rat spinal cord. J Neurophysiol 2003, 89:3243–3252. [DOI] [PubMed] [Google Scholar]
- 81.Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C: 3ATP inhibits glutamate synaptic release by acting at P2Y receptors in pyramidal neurons of hippocampal slices. J Pharmacol Exp Ther 2000, 293:172–179. [PubMed] [Google Scholar]
- 82.Guzman SJ, Gerevich Z: P2Y Receptors in Synaptic Transmission and Plasticity: Therapeutic Potential in Cognitive Dysfunction. Neural Plast 2016, 2016:1207393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA: Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci 2005, 25:6286–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wyatt LR, Finn DA, Khoja S, Yardley MM, Asatryan L, Alkana RL, Davies DL: Contribution of P2X4 receptors to ethanol intake in male C57BL/6 mice. Neurochem Res 2014, 39:1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franklin KM, Asatryan L, Jakowec MW, Trudell JR, Bell RL, Davies DL: P2X4 receptors (P2X4Rs) represent a novel target for the development of drugs to prevent and/or treat alcohol use disorders. Front Neurosci 2014, 8:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimpel MW, Strother WN, McClintick JN, Carr LG, Liang T, Edenberg HJ, McBride WJ: Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol 2007, 41:95–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Edenberg HJ, Lumeng L, Bell RL: 3Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacol Biochem Behav 2012, 102:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson HN, Kechris K, Bell RL, et al. : 3Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol 2009, 7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krugel U, Kittner H, Franke H, Illes P: 3Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse 2003, 47:134–142. [DOI] [PubMed] [Google Scholar]
- 90.Khakh BS: Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci 2001, 2:165–174. [DOI] [PubMed] [Google Scholar]
- 91.Choi YM, Jang JY, Jang M, Kim SH, Kang YK, Cho H, Chung S, Park MK: 3Modulation of firing activity by ATP in dopamine neurons of the rat substantia nigra pars compacta. Neuroscience 2009, 160:587–595. [DOI] [PubMed] [Google Scholar]
- 92.Khoja S, Shah V, Garcia D, Asatryan L, Jakowec MW, Davies DL: 3Role of purinergic P2X4 receptors in regulating striatal dopamine homeostasis and dependent behaviors. J Neurochem 2016, 139:134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ostrovskaya O, Asatryan L, Wyatt L, Popova M, Li K, Peoples RW, Alkana RL, Davies DL: 3Ethanol is a fast channel inhibitor of P2X4 receptors. J Pharmacol Exp Ther 2011, 337:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Franke H, Kittner H, Grosche J, Illes P: 3Enhanced P2Y1 receptor expression in the brain after sensitisation with d-amphetamine. Psychopharmacology (Berl) 2003, 167:187–194. [DOI] [PubMed] [Google Scholar]
- 95.Lommen J, Stahr A, Ingenwerth M, Ali AAH, von Gall C: 3Time-of-day-dependent expression of purinergic receptors in mouse suprachiasmatic nucleus. Cell Tissue Res 2017, 369:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Svobodova I, Bhattaracharya A, Ivetic M, Bendova Z, Zemkova H: Circadian ATP Release in Organotypic Cultures of the Rat Suprachiasmatic Nucleus Is Dependent on P2X7 and P2Y Receptors. Front Pharmacol 2018, 9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhattacharya A, Vavra V, Svobodova I, Bendova Z, Vereb G, Zemkova H: 3Potentiation of inhibitory synaptic transmission by extracellular ATP in rat suprachiasmatic nuclei. J Neurosci 2013, 33:8035–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burnstock G: Purinergic signalling: pathophysiology and therapeutic potential. Keio J Med 2013, 62:63–73. [DOI] [PubMed] [Google Scholar]
- 99.Lindberg D, Shan D, Ayers-Ringler J, Oliveros A, Benitez J, Prieto M, McCullumsmith R, Choi DS: 3Purinergic signaling and energy homeostasis in psychiatric disorders. Curr Mol Med 2015, 15:275–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Porkka-Heiskanen T, Strecker RE, McCarley RW: 3Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 2000, 99:507–517. [DOI] [PubMed] [Google Scholar]
- 101.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW: Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 1997, 276:1265–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ruby CL, O’Connor KM, Ayers-Ringler J, Choi DS: Adenosine and glutamate in neuroglial interaction: implications for circadian disorders and alcoholism. Adv Neurobiol 2014, 11:103–119. [DOI] [PubMed] [Google Scholar]
- 103.Strecker RE, Morairty S, hakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW: Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res 2000, 115:183–204. [DOI] [PubMed] [Google Scholar]
- 104.Benington JH, Heller HC: Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol 1995, 45:347–360. [DOI] [PubMed] [Google Scholar]
- 105.Saper CB, Scammell TE, Lu J: 3Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437:1257–1263. [DOI] [PubMed] [Google Scholar]
- 106.Ballesteros-Yanez I, Castillo CA, Merighi S, Gessi S: The Role of Adenosine Receptors in Psychostimulant Addiction. Front Pharmacol 2017, 8:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferre S, Diamond I, Goldberg SR, Yao L, Hourani SM, Huang ZL, Urade Y, Kitchen I: Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog Neurobiol 2007, 83:332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang SL, Han JY, Kim YB, Nam SY, Song S, Hong JT, Oh KW: Increased non-rapid eye movement sleep by cocaine withdrawal: possible involvement of A2A receptors. Arch Pharm Res 2011, 34:281–287. [DOI] [PubMed] [Google Scholar]
- 109.Marcellino D, Roberts DC, Navarro G, Filip M, Agnati L, Lluis C, Franco R, Fuxe K: Increase in A2A receptors in the nucleus accumbens after extended cocaine self-administration and its disappearance after cocaine withdrawal. Brain Res 2007, 1143:208–220. [DOI] [PubMed] [Google Scholar]
- 110.Frankowska M, Marcellino D, Adamczyk P, Filip M, Fuxe K: Effects of cocaine self-administration and extinction on D2 -like and A2A receptor recognition and D2 -like/Gi protein coupling in rat striatum. Addict Biol 2013, 18:455–466. [DOI] [PubMed] [Google Scholar]
- 111.Bailey A, Gianotti R, Ho A, Kreek MJ: Persistent upregulation of mu-opioid, but not adenosine, receptors in brains of long-term withdrawn escalating dose “binge” cocaine-treated rats. Synapse 2005, 57:160–166. [DOI] [PubMed] [Google Scholar]
- 112.Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O: The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology 2006, 31:978–987. [DOI] [PubMed] [Google Scholar]
- 113.Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, et al. : A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci 2008, 28:2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marinovic AC, Zheng B, Mitch WE, Price SR: Ubiquitin (UbC) expression in muscle cells is increased by glucocorticoids through a mechanism involving Sp1 and MEK1. J Biol Chem 2002, 277:16673–16681. [DOI] [PubMed] [Google Scholar]
- 115.Wydra K, Suder A, Borroto-Escuela DO, Filip M, Fuxe K: On the role of A(2)A and D(2) receptors in control of cocaine and food-seeking behaviors in rats. Psychopharmacology (Berl) 2015, 232:1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pinna A, Wardas J, Cristalli G, Morelli M: Adenosine A2A receptor agonists increase Fos-like immunoreactivity in mesolimbic areas. Brain Res 1997, 759:41–49. [DOI] [PubMed] [Google Scholar]
- 117.Borroto-Escuela DO, Wydra K, Pintsuk J, Narvaez M, Corrales F, Zaniewska M, Agnati LF, Franco R, Tanganelli S, Ferraro L, et al. : Understanding the Functional Plasticity in Neural Networks of the Basal Ganglia in Cocaine Use Disorder: A Role for Allosteric Receptor-Receptor Interactions in A2A-D2 Heteroreceptor Complexes. Neural Plast 2016, 2016:4827268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pintsuk J, Borroto-Escuela DO, Pomierny B, Wydra K, Zaniewska M, Filip M, Fuxe K: Cocaine self-administration differentially affects allosteric 2A-D2 receptor-receptor interactions in the striatum. Relevance for cocaine use disorder. Pharmacol Biochem Behav 2016, 144:85–91. [DOI] [PubMed] [Google Scholar]
- 119.Ferre S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN: An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des 2008, 14:1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Filip M, Zaniewska M, Frankowska M, Wydra K, Fuxe K: The importance of the adenosine A(2A) receptor-dopamine D(2) receptor interaction in drug addiction. Curr Med Chem 2012, 19:317–355. [DOI] [PubMed] [Google Scholar]
- 121.Chen JF, Eltzschig HK, Fredholm BB: Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov 2013, 12:265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Freyberg Z, McCarthy MJ: Dopamine D2 receptors and the circadian clock reciprocally mediate antipsychotic drug-induced metabolic disturbances. NPJ Schizophr 2017, 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yellen G: Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol 2018* Review highlighting the role of astrocytes in neuronal energy supply. Also addresses other major mechanisms of energy generation, supply, and utilization in the brain. [DOI] [PMC free article] [PubMed]
- 124.Kalinchuk AV, Urrila AS, Alanko L, Heiskanen S, Wigren HK, Suomela M, Stenberg D, Porkka-Heiskanen T: Local energy depletion in the basal forebrain increases sleep. Eur J Neurosci 2003, 17:863–869. [DOI] [PubMed] [Google Scholar]
- 125.Rempe MJ, Wisor JP: Cerebral lactate dynamics across sleep/wake cycles. Front Comput Neurosci 2014, 8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hertz L, Chen Y: Integration between Glycolysis and Glutamate-Glutamine Cycle Flux May Explain Preferential Glycolytic Increase during Brain Activation, Requiring Glutamate. Front Integr Neurosci 2017, 11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Canto C, Menzies KJ, Auwerx J: NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab 2015, 22:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Requardt RP, Hirrlinger PG, Wilhelm F, Winkler U, Besser S, Hirrlinger J: Ca(2)(+) signals of astrocytes are modulated by the NAD(+)/NADH redox state. J Neurochem 2012, 120:1014–1025. [DOI] [PubMed] [Google Scholar]
- 129.Requardt RP, Wilhelm F, Rillich J, Winkler U, Hirrlinger J: The biphasic NAD(P)H fluorescence response of astrocytes to dopamine reflects the metabolic actions of oxidative phosphorylation and glycolysis. J Neurochem 2010, 115:483–492. [DOI] [PubMed] [Google Scholar]
- 130.Putker M, O’Neill JS: 3Reciprocal Control of the Circadian Clock and Cellular Redox State -a Critical Appraisal. Mol Cells 2016, 39:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP, Sweedler JV, Cox CL, Gillette MU: 3Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 2012, 337:839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U: SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134:317–328. [DOI] [PubMed] [Google Scholar]
- 133.Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, Renthal W, Neve R, Liu X, Shao N, et al. : Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci 2013, 33:16088–16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferguson D, Shao N, Heller E, Feng J, Neve R, Kim HD, Call T, Magazu S, Shen L, Nestler EJ: 3SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J Neurosci 2015, 35:3100–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, et al. : Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron 2009, 62:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Parekh PK, McClung CA: Circadian Mechanisms Underlying Reward-Related Neurophysiology and Synaptic Plasticity. Front Psychiatry 2015, 6:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arey RN, Enwright JF 3rd, Spencer SM, Falcon E, Ozburn AR, Ghose S, Tamminga C, McClung CA: An important role for cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors. Mol Psychiatry 2014, 19:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, Arey RN, Enwright JF, Jacobsen JP, Kumar S, et al. : 3Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry 2015, 20:1479–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, et al. : 3Regulation of monoamine oxidase by circadian-clock components implies clock influence on mood. Curr Biol 2008, 18:678–683. [DOI] [PubMed] [Google Scholar]
- 140.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, et al. :3Mania-likebehavior induced by disruption of CLOCK. Proc Natl Acad Sci U S 2007, 104:6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH: 3Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 2017, 93:1420–1435 e1425.* Metabolic activity of cells is robustly rhythmic in the SCN where neurons and astrocytes are active in complete antiphase —neurons are active during the day, while astrocytes are active during the night. Astrocytes inhibit the activity of SCN neurons by modulating extracellular glutamate levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barca-Mayo O, Pons-spinal M, Follert P, Armirotti A, Berdondini L, De Pietri Tonelli D: Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun 2017, 8:14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, et al. : 3The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 2005, 11:35–42. [DOI] [PubMed] [Google Scholar]
- 144.guilar JI, Dunn M, Mingote S, Karam CS, Farino ZJ, Sonders MS, Choi SJ, Grygoruk A, Zhang Y, Cela C, et al. : 3Neuronal Depolarization Drives Increased Dopamine Synaptic Vesicle Loading via VGLUT. Neuron 2017, 95:1074–1088.e1077.* Alterations in pH (i.e., hyperacidification) are critical for increasing synaptic vesicle packaging of dopamine by cellular depolarization and depend on the vesicular glutamate transporter. VGLUT2 facilitates dopamine neurotransmission by driving dopamine vesicle packaging and release. A subset of neurons express VGLUT2 and dopamine in the mouse midbrain, suggesting these neurons use co-transmission of glutamate and dopamine to modulate projection targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grippo RM, Purohit AM, Zhang Q, Zweifel LS, Guler AD: 3Direct Midbrain Dopamine Input to the Suprachiasmatic Nucleus Accelerates Circadian Entrainment. Curr Biol 2017, 27:2465–2475 e2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Poulin JF, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, Chan CS, Dombeck DA, Deisseroth K, Awatramani R: 3Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci 2018. [DOI] [PMC free article] [PubMed]
- 147.Kosobud AE, Gillman AG, Leffel JK 2nd, Pecoraro NC, Rebec GV, Timberlake W: Drugs of abuse can entrain circadian rhythms. ScientificWorldJournal 2007, 7:203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.DePoy LM, McClung CA, Logan RW: Neural Mechanisms of Circadian Regulation of Natural and Drug Reward. Neural Plast 2017, 2017:5720842. [DOI] [PMC free article] [PubMed] [Google Scholar]


