Abstract
Cervical human papillomavirus (HPV) infection is highly transmissible. Although there are many studies on HPV infection in general population of women globally, little attention has been paid to female sex workers (FSWs) in Asia. In this study, we used a meta-analytic approach to systematically analyse the literature to elucidate the prevalence and genotype distribution of cervical HPV infection among FSWs in Asia. Fourteen eligible studies were identified in five databases, and data including 4198 FSWs from nine Asian countries were aggregated. Crude estimates of cervical HPV prevalence among FSWs in this region ranged from 12.8% to 84.8%. FSWs had a nearly 10-fold risk of HPV infection than the general population of women. Stratified analysis showed that HPV prevalence was higher in East Asia than other subregions and in younger FSWs than older FSWs. HPV genotype distribution was statistically different between East Asia and South-east Asia. In East Asia, the most prevalent genotypes were HPV 16 (23.9%), 18 (11.0%), 58 (9.4%), 56 (6.3%) and 52 (5.3%), while they were HPV 52 (12.9%), 16 (8.5%), 58 (5.2%), 18 (5.0%) and 66 (4.9%) in South-east Asia. HPV 31, 33 and 35 were less frequently found in both subregions. HPV infection was substantial among FSWs in some Asian countries. More studies are necessary to illustrate the overall picture of HPV infection in this region.
Keywords: Asia, female sex workers, genotype, meta-analysis
Introduction
Cervical cancer is the second most common malignant neoplasm in women worldwide.1 Globally, it accounts for 9.8% of all cancers in females, with an estimated incidence of 493 243 cases and an estimated mortality of 273 505 cases each year.1,2 With a relatively high prevalence of cervical cancer but a low screening rate, women in Asia bear a significant share of the global cervical cancer burden, contributing to 51.6% of cervical cancer cases and 50.3% of cervical cancer deaths.3
Persistent cervical infection with oncogenic human papillomavirus (HPV) has been established as the necessary cause of cervical cancer, in virtually 100% of cases.2,4–6 At present, over 100 HPV genotypes have been described and at least 50 of these genotypes are known to infect the female genital tract.7–9 Using epidemiologic classification, genital HPV genotypes are subdivided into three groups: low-risk types that cause genital warts; high-risk types that are frequently associated with cervical intraepithelial neoplasia and other anogenital malignancies; and unclassified types, whose oncogenicity is yet undetermined.10
Cervical HPV infection is highly transmissible and the risk of infection rises with increased sexual activity.11,12 Because they have more concurrent sexual partners, female sex workers (FSWs) are believed to be at particularly high risk for HPV infection and transmission.12 In this systematic review, we used a meta-analytic approach to systematically analyse the literature to elucidate the prevalence and genotype distribution of cervical HPV infection among FSWs in Asia.
Methods
Search strategy
This comprehensive literature search was conducted by two independent investigators in PubMed, Embase, Academic Source Premier, Google Scholar and China National Knowledge Information to identify potential published papers from January 1993 to October 2010 without language restriction. Search terms included ‘human papillomavirus’ or ‘HPV’, combined with ‘female sex workers’, ‘commercial sex workers’, or ‘prostitutes’. References cited in the retrieved papers were also evaluated for inclusion. The search was done in four stages (identification, screening, eligibility and inclusion) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.13,14
Eligibility criteria and quality assessment
Quality assessment was also performed independently by two investigators. If a study met the following criteria, it would be qualified and included: (1) studies of prevalence and/or genotype distribution of cervical HPV infection; (2) studies of FSWs from any country in Asia; (3) studies with a clear description of cervical cells collection method; and (4) studies with a clear description of method to detect HPV DNA. Any disagreements were resolved by consensus or contact with the authors of original studies. Reviews were excluded, and studies with the largest sample size were selected if the data or data subsets were published in more than one paper.
Data extraction
For each included study, the following information was extracted: (1) study country; (2) publication year; (3) sample size; (4) mean age of FSWs; (5) cervical cells collection method; (6) HPV DNA testing method; and (7) overall cervical HPV prevalence. When available, these data were also extracted: (1) primers used to detect HPV positive samples; (2) concurrent infection with multiple genotypes; (3) number of cervical abnormalities detected using a cytology or histology testing method; (4) HPV genotype-specific prevalence; and (5) HPV prevalence among women in the general population (only from two case-control studies).
Data on HPV genotype distribution from one study conducted in the Philippines was obtained via personal communication with the corresponding author. Genotype-specific prevalence was presented for the 19 most common HPV genotypes previously identified in the general population of women, namely HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 70, 73, 82, 6 and 11.15,16
Statistical analyses
In this present study, HPV prevalence was defined as the proportion of FSWs that had positive results of HPV DNA among all cases that were tested for HPV DNA, regardless of genotype. Concurrent infection with multiple genotypes was separated into constituent genotypes, thus genotype-specific prevalence represented data from both single and multiple infections. Subregions of Asia included East Asia (China, Japan and Korea), South-east Asia (Thailand, Vietnam, Singapore, Indonesia and the Philippines), and South Asia (India).
Comprehensive meta-analysis software (CMA, version 2.0, Biostat Inc., Englewod, NJ, USA) was used to analyse the abstracted data. Publication bias was assessed using the Begg rank correlation test (P < 0.05 indicating statistical significance).17 The Q test (P < 0.10 indicating statistical significance) and I2 value (ranging between 0% and 100%, with a lower value indicating less heterogeneity) were calculated to measure between-studies heterogeneity.18 Stratified analysis was used to calculate the adjusted cervical HPV prevalence according to different variables: subregion; mean age of FSWs; cervical cells collection method; HPV DNA testing method; and primer set. The random-effects model was applied to the case-control studies to estimate the combined odds ratio (OR) of cervical HPV prevalence. In total, it took us 3 months to complete the data analyses.
Results
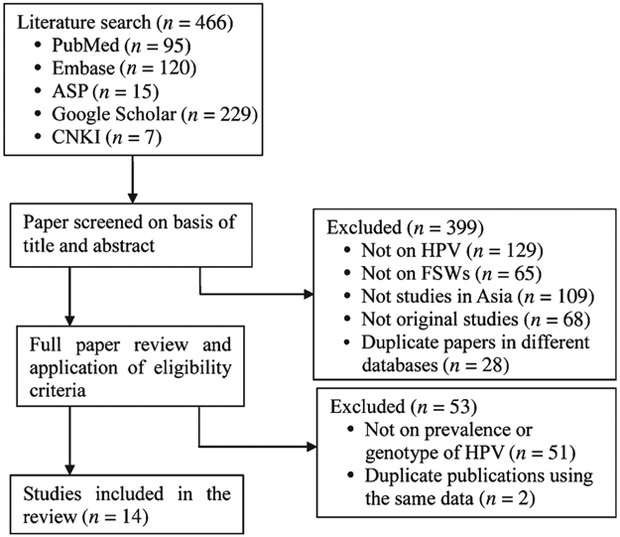
Initially, 466 potentially relevant papers were identified in all the databases. Then 399 papers were excluded based on title and abstract screening. In the full paper review stage, 53 papers were excluded according to the eligibility criteria. Finally, 14 studies with a total of 4198 FSWs from nine Asian countries were included in the analysis (Fig. 1).19–32 No additional studies were identified in the citations of all the retrieved papers. The publication bias was not statistically significant (Begg rank correlation test, P > 0.05).
Fig. 1.
Flow diagram of search strategy for published studies. ASP, Academic Source Premier; CNKI, China National Knowledge Information; FSW, female sex workers; HPV, human papillomavirus.
Of the 14 studies, six were from East Asia,19–24 six were from South-east Asia,25–30 and two were from South Asia.31,32 All of the studies were published from 2000 to 2009, except one was published in 1993.19 Sample sizes ranged from 27 to 546. Seven studies completed cervical cell cytology or histology testing and presented the number of cervical abnormalities, but did not show the detailed genotype distribution in each grade of abnormal lesion.21,22,25,28,30–32 Two studies compared the cervical HPV prevalence between FSWs and women in the general population.22,26 Five studies discussed concurrent HPV infection with multiple genotypes and provided detailed breakdowns of genotype-specific distribution.23, 24, 27, 28, 30The studies are summarised in Table 1.
Table 1. Summary of the 14 included studies with a total of 4198 female sex workers from nine countries in AsiaA.
HC, hybrid capture assay; HPV, human papillomavirus; PCR, polymerase chain reaction
| Country and publication year |
Sample size |
Mean age |
Cervical cells collection method |
DNA testing assay |
Primer | Abnormality in
histology/ cytologyB % (No.) |
Cervical HPV prevalence (%) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 16 | 18 | 31 | 33 | 35 | 39 | 45 | 51 | 52 | 56 | 58 | 59 | 66 | 68 | 70 | 73 | 82 | 6 | 11 | Multiple infections |
|||||||
| East Asia | |||||||||||||||||||||||||||
| China, 199319 | 196 | 34.0 | swab | HC | 12.8 | ||||||||||||||||||||||
| China, 200420 | 130 | 22.6 | swab | PCR | 43.8 | 23.8 | 12.3 | ||||||||||||||||||||
| China, 200621 | 309 | 34.6 | cytobrush | HC | 16.8 (52) | 46.9 | |||||||||||||||||||||
| Japan, 200022 | 546 | 28.9 | swab | HC | 7.1 (39) | 56.2 | |||||||||||||||||||||
| Korea, 200323 | 417 | cytobrush | PCR | MY09/11 | 46.5 | 15.1 | 4.7 | 1.9 | 2.5 | 6.0 | 3.8 | 3.5 | 5.7 | 5.0 | 4.1 | 5.0 | 3.1 | 1.9 | 3.5 | 1.6 | 0.3 | 24.5 | |||||
| Korea, 200824 | 188 | 24.0 | swab | Nested-PCR | MY09/11, GP5+/6+ | 83.5 | 36.1 | 20.9 | 4.8 | 5.6 | 2.4 | 2.4 | 4.0 | 4.0 | 4.8 | 9.6 | 16.9 | 0.8 | 0.8 | 4.0 | 4.8 | 7.2 | 23.4 | ||||
| South-east Asia | |||||||||||||||||||||||||||
| Thailand, 200125 | 251 | 24.0 | swab | PCR | MY09/11 | 5.2 (13) | 47.0 | 13.9 | 6.0 | 2.4 | |||||||||||||||||
| Thailand, 200626 | 524 | 26.8 | self-admin.tampon | PCR | MY09/11 | 22.9 | |||||||||||||||||||||
| Vietnam, 200827 | 282 | 25.0 | cytobrush | PCR | PGMY09/11 | 84.8 | 10.3 | 6.0 | 0.7 | 2.1 | 0.7 | 3.9 | 1.4 | 6.4 | 17.7 | 3.5 | 6.7 | 4.6 | 3.2 | 3.2 | 3.5 | 1.8 | 0.7 | 2.5 | 4.3 | 31.2 | |
| Singapore, 200128 | 187 | 35.0 | cytobrush | PCR | PVCOU/D | 4.8 (9) | 14.4 | 4.3 | 2.7 | 1.1 | 0.5 | 1.6 | 0.5 | 3.7 | 2.7 | 3.7 | |||||||||||
| Indonesia, 200329 | 543 | 25.3 | swab | PCR | 38.5 | ||||||||||||||||||||||
| Philippines, 200930 | 369 | 24.5 | cytobrush | PCR | GP5+/6+ | 15.2 (56) | 57.2 | 6.8 | 3.3 | 3.0 | 1.1 | 0.8 | 2.7 | 5.7 | 2.4 | 9.2 | 4.3 | 4.6 | 5.1 | 7.0 | 1.1 | 0.8 | 0.5 | 2.2 | 2.7 | 0.8 | 25.5 |
| South Asia | |||||||||||||||||||||||||||
| India, 200131 | 27 | 27.5 | swab | HC | 22.2 (6) | 63.0 | |||||||||||||||||||||
| India, 200832 | 229 | 30.0 | cytobrush | PCR | 3.1 (7) | 25.3 | 17.0 | 14.3 | |||||||||||||||||||
The cell was blank if the original study did not give the respective information.
Some studies detected cervical lesions using histology or cytology testing method. Abnormalities in histology were cervical intraepithelial neoplasia 1, 2 and 3; abnormalities in cytology were low-grade squamous intraepithelial lesion and high-grade squamous intraepithelial lesion.
Cervical HPV prevalence
There was substantial heterogeneity among the 14 studies (P for Q test <0.001; I2 = 97.6%). The crude overall HPV prevalence of each study ranged from 12.8% to 84.8%. The prevalence of concurrent infection with multiple genotypes was around 25%, except one study in Singapore was 3.7% (Table 1).
As shown in Table 2, the adjusted cervical HPV prevalence was calculated according to different subgroup variables (P for Q test <0.1; I2 > 75%). The prevalence was slightly different across subregions, from 47.8% in East Asia to 42.1% in South Asia. Young FSWs were particularly vulnerable to HPV infection; the prevalences were 59.3% and 53.7% in the age groups of less than 25 and 25–29 years old, respectively, but sharply declined in the age group of more than 30 years old (22.9%). The cervical swab was less sensitive in detecting HPV infection than the cytobrush (43.2% v. 53.2%), and the self-administration tampon only detected a 22.9% prevalence of HPV infection. Polymerase chain reaction (PCR) was slightly better than hybrid capture (HC) assay in detecting HPV DNA (46.4% v. 41.7%). The modified primer GPMY09/11 was much more sensitive than MY09/11 (84.4% v. 50.6%), and the prevalence was 57.2% when GP5+/6+ was used as the primer.
Table 2.
Stratified analysis of cervical human papillomavirus infection among 4198 female sex workers
| Subgroup variableA | Crude prevalence (%) |
Adjusted prevalence | |
|---|---|---|---|
| % | 95% Confidence intervals |
||
| Subregion | |||
| East Asia | 49.6 | 47.8 | 33.7–62.3 |
| South-east Asia | 42.9 | 43.5 | 26.4–62.4 |
| South Asia | 29.3 | 42.1 | 13.1–77.9 |
| Mean age (years)B | |||
| <25 | 57.9 | 59.3 | 43.0–73.8 |
| 25–29 | 46.4 | 53.7 | 32.9–73.2 |
| ≥30 | 27.7 | 22.9 | 11.0–41.5 |
| Cervical cells collection method | |||
| Swab | 44.3 | 43.2 | 29.4–58.1 |
| Cytobrush | 52.7 | 53.2 | 36.3–69.4 |
| Self-administered tampon | 22.9 | 22.9 | 19.5–26.7 |
| HPV DNA testing method | |||
| Polymerase chain reaction | 44.6 | 46.4 | 33.3–60.0 |
| Hybrid capture assay | 45.8 | 41.7 | 24.0–61.8 |
| Primer setB | |||
| GP5+/6+ | 57.2 | 57.2 | 52.1–62.1 |
| MY09/11 | 42.7 | 50.6 | 28.9–72.1 |
| GPMY09/11 | 84.8 | 84.8 | 80.1–88.5 |
All the subgroup variables were statistically significant for cervical human papillomavirus prevalence according to Q test for between-studies heterogeneity (P for Q test <0.1; I2 > 75%).
Some studies did not provide the information.
Analysis of the two case–control studies with the random-effects model revealed that HPV prevalence among FSWs was significantly higher than that among women in the general population, with an OR of 9.4 (95% confidence interval (CI): 4.0–21.9, P < 0.001).
HPV genotype distribution
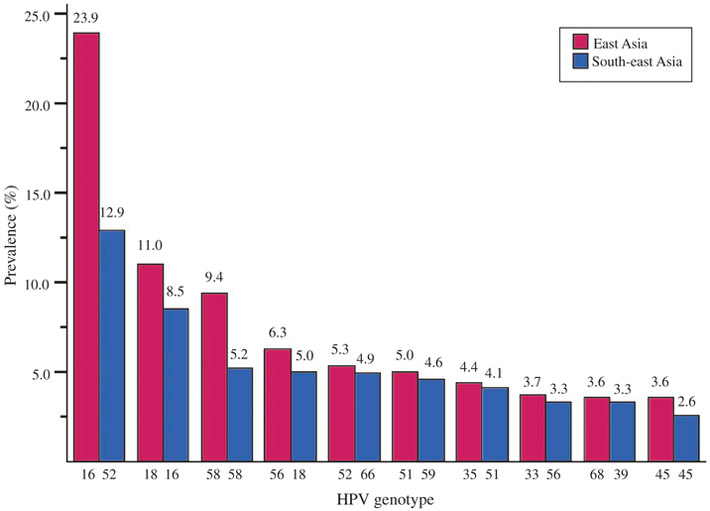
As shown in Fig. 2, based on the studies that reported detailed breakdowns of HPV genotype-specific distribution, the 10 most common high-risk HPV genotypes among FSWs in East Asia were HPV 16, 18, 58, 56, 52, 51, 35, 33, 68 and 45 in descending order, and HPV 16 and 18 accounted for 34.9% HPV prevalence among all of the screened FSWs. In contrast, the cervical HPV genotype distribution was statistically different in South-east Asia (χ2 = 63.1, P < 0.001). The top 10 high-risk genotypes were HPV 52, 16, 58, 18, 66, 59, 51, 56, 39 and 45 in descending order, and HPV 16 and 18 only contributed to 13.5% HPV prevalence in South-east Asia. Overall, HPV 16 was among the most prevalent genotypes, ranking first in East Asia (23.9%) and second in South-east Asia (8.5%). Notably, the most prevalent genotype in South-east Asia, HPV 52 (12.9%), was far less common in East Asia, coming in at fifth (5.3%). HPV 6 and 11, the most prevalent low-risk genotypes worldwide,33 were not among the top 10 genotypes among FSWs in either East Asia or South-east Asia (2.8% and 2.5% for HPV 6 in East and South-east Asia respectively, 1.8% and 2.0% for HPV 11 in East and South-east Asia respectively).
Fig. 2.
Prevalence of 10 most common high-risk human papillomavirus (HPV) genotypes by subregions.
Discussion
Compared with a large number of studies among women in the general population, the shortage of epidemiological HPV studies specific to FSWs suggests that such research is not yet emphasised in many Asian countries. This may be due to a lack of awareness, lack of prioritisation, or lack of resources for HPV research as compared with HIV, other infectious diseases or other oncogenic diseases among FSWs.
The large variation of overall cervical HPV prevalence of each study was different from the studies among women in the general population in Asia, which revealed less heterogeneous prevalence.34 The included studies reporting more than 80% HPV prevalence may have resulted from recruiting FSWs in lower age groups,24,27 and using more sensitive methods to detect infection.27 Moreover, as mentioned in the original studies, 14% HSV-2 and 2% HIV co-infection were found in the two studies, respectively,24,27 which could increase the susceptibility of HPV.35 In those included studies that showed a HPV prevalence of lower than 30%, some included FSWs were more than 30 years old; 19, 28, 32 some used self-administration tampon to collect cervical cells;26 and some used HC assay to detect HPV DNA.19
Previous meta-analysis on cervical HPV prevalence among women in the general population in Asia indicated that HPV prevalence was no more than 9.0% in the normal cervical cytology group.36,37 HPV prevalence was 67%, 78% and 86% in the low-grade squamous intraepithelial lesion group (LSIL), high-grade squamous intraepithelial lesion group, and cervical cancer group, respectively.15,16 Although none of the included studies in the present review provided HPV prevalence stratified by cytology classification, we could infer from our study that Asian FSWs may have a high prevalence of cervical LSIL, which could progress to cervical cancer.38
Despite having only two studies that compared FSWs to women in the general population, the high risk for cervical HPV infection among FSWs was consistent and statistically significant. Although a near 10-fold increased risk should be interpreted with some caution due to the few number of studies, this dramatic finding highlights the urgent need for more research and prevention in this area.
Although, in absolute terms, cervical HPV infection is more prevalent in FSWs, the distribution of the most common genotypes is the same as that in the general population of women. Previous studies among women in the general population in Asia reported that for high-risk genotypes, HPV 16 was the most prevalent and HPV 18 the second-most prevalent.2,3 In addition, among high-risk genotypes, HPV 31, 33 and 35 were less frequently found,39 and HPV 52 and 58 were relatively common.16 This review found a similar genotype distribution among Asian FSWs.
At present, HPV prevention utilises bivalent and quadrivalent prophylactic vaccines targeting oncogenic HPV genotypes and has been shown to be safe and highly effective.40,41 Results from our meta-analysis indicated that, vaccination against HPV 16 and 18 had the potential to prevent 34.9% and 13.5% of the prevalence of high-risk genotypes among all the screened FSWs in East Asia and South-east Asia, respectively. To more effectively prevent infection, HPV 52 and 58 could be considered as additional vaccine targets in the future. For East Asia and South-east Asia, it would, respectively, prevent 49.6% and 31.6% of cervical HPV prevalence in the screened FSWs who received prophylactic vaccine before exposure to high-risk sex behaviours. These proportions might be higher if considering cross-protection against other high-risk HPV genotype infections.
To our knowledge, this is the first meta-analysis of prevalence and genotype distribution of cervical HPV infection among FSWs in Asia. With a relatively large sample size and description of genotype-specific prevalence, the findings have utility in informing future research that will contribute to formulating prevention strategies for FSWs and designing more effective vaccines. However, because of relatively fewer studies compared with the studies among the general population of women, the present study does have some limitations. First, the significant heterogeneity of some factors, such as co-infection with other sexually transmissible infections and different frequency of condom use, inhibited the calculation of the overall pooled prevalence. Second, because HC assay could not detect individual genotypes, studies using this method could not contribute to the analysis of HPV genotype distribution. Third, although some original studies provided the number of FSWs with cytology or histology abnormality in cervical HPV screening, we were unable to obtain information about HPV prevalence and genotype distribution by different grades of pre-cancerous lesions. Finally, no detailed data of HPV genotype distribution in South Asia and no information from Central, West and North Asia limited any attempt to make generalisations for the whole Asian continent.
Conclusions
In summary, more studies on cervical HPV prevalence and genotype distribution are necessary to illustrate the overall picture of HPV infection in this high-risk population in Asia. Gathering data that includes HPV genotype-specific prevalence by different cytology and histology grade can aid future analysis to prevent cervical cancer among FSWs efficiently. Discrepancies in HPV prevalence among FSWs should be further investigated using collaborative inter-regional study designs in the subregions of Asia where data are still lacking.
Acknowledgements
This work was supported by grants from the Mega Project of China National Science Research for the 11th Five-Year Plan (2008ZX10001–005) and the Fogarty International Clinical Research Scholars Program (R24 TW007988).
Footnotes
Conflicts of interest
None declared.
References
- 1.Ferlay J, Bray F, Pisani P. Globocan 2002: cancer incidence, mortality and prevalence worldwide IARC CancerBase, No. 5. Version 2.0 Lyon: IARC Press; 2004. Available online at: http://www-dep.iarc.fr/ [accessed February 2011]. [Google Scholar]
- 2.WHO/ICO Information Centre on HPV and Cervical Cancer. HPV and cervical cancer in the world: 2007 Report. Vaccine 2007; 25: C1–C26. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Louie KS, Clifford G. Burden and trends of type-specific human papillomavirus infections and related diseases in the Asia Pacific region. Vaccine 2008; 26: M1–16. doi: 10.1016/j.vaccine.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55: 244–65. doi: 10.1136/jcp.55.4.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol 2005; 6: 204. doi: 10.1016/S1470-2045(05)70086-3 [DOI] [PubMed] [Google Scholar]
- 6.Lehoux M, D’Abramo CM, Archambault J. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public Health Genomics. 2009; 12: 268–80. doi: 10.1159/000214918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004; 324: 17–27. doi: 10.1016/j.virol.2004.03.033 [DOI] [PubMed] [Google Scholar]
- 8.de Villiers EM, Whitley C, Gunst K. Identification of new papillomavirus types. Methods Mol Med 2005; 119: 1–13. [DOI] [PubMed] [Google Scholar]
- 9.Burk RD, Chen Z, Van Doorslaer K. Human papillomaviruses: genetic basis of carcinogenicity. Public Health Genomics. 2009; 12: 281–90. doi: 10.1159/000214919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348: 518–27. doi: 10.1056/NEJMoa021641 [DOI] [PubMed] [Google Scholar]
- 11.Bogaards JA, Xiridou M, Coupe VM, Meijer CJ, Wallinga J, Berkhof J. Model-based estimation of viral transmissibility and infection-induced resistance from the age-dependent prevalence of infection for 14 high-risk types ofhuman papillomavirus. Am J Epidemiol 2010; 171: 817–25. doi: 10.1093/aje/kwp466 [DOI] [PubMed] [Google Scholar]
- 12.Javanbakht M, Gorbach PM, Amani B, Walker S, Cranston RD, Datta SD, et al. Concurrency, sex partner risk, and high-risk human papillomavirus infection among African American, Asian, and Hispanic women. Sex Transm Dis 2010; 37: 68–74. doi: 10.1097/OLQ.0b013e3181bcd3e7 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14: 1157–64. doi: 10.1158/1055-9965.EPI-04-0812 [DOI] [PubMed] [Google Scholar]
- 16.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121: 621–32. doi: 10.1002/ijc.22527 [DOI] [PubMed] [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, editors. Publication bias. In: Introduction to Meta-analysis, 1st edition. Chichester: Wiley; 2009; 277–91. [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60.doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngan HY, Collins RJ, Wong KY, Cheung A, Lai CF, Liu YT. Cervical human papilloma virus infection of women attending social hygiene clinics in Hong Kong. Int J Gynaecol Obstet 1993; 41: 75–9. doi: 10.1016/0020-7292(93)90157-R [DOI] [PubMed] [Google Scholar]
- 20.Yang F, Li YS, Zhou H, Wu ZH. Detection of sexually transmitted infections among detained female commercial sex workers by fluorescent quantitative PCR assay in Shenzhen. Chin J Lepr Skin Dis 2004; 20: 334–5. [Google Scholar]
- 21.Jia HQ. Epidemiological investigation of HPV prevalence and incidence rates of cervical cancer and CIN on entertainment female sex workers. Available online at: http://www.lunwenchina.net.cn/buypaper/fkx/50997.html [accessed October 2010].
- 22.Ishi K, Suzuki F, Saito A, Kubota T. Prevalence of human papillomavirus infection and its correlation with cervical lesions in commercial-sex workers in Japan. J Obstet Gynaecol Res 2000; 26: 253–7. doi: 10.1111/j.1447-0756.2000.tb01318.x [DOI] [PubMed] [Google Scholar]
- 23.Choi BS, Kim O, Park MS, Kim KS, Jeong JK, Lee JS. Genital human papillomavirus genotyping by HPV oligonucleotide microarray in Korean commercial sex workers. J Med Virol 2003; 71: 440–5. doi: 10.1002/jmv.10498 [DOI] [PubMed] [Google Scholar]
- 24.Yun H, Park J, Choi I, Kee M, Choi B, Kim S. Prevalence of human papillomavirus and herpes simplex virus type 2 infection in Korean commercial sex workers. J Microbiol Biotechnol 2008; 18: 350–4. [PubMed] [Google Scholar]
- 25.Thomas DB, Ray RM, Kuypers J, Kiviat N, Koetsawang A, Ashley RL, et al. Human papillomaviruses and cervical cancer in Bangkok. III. The role of husbands and commercial sex workers. Am J Epidemiol 2001; 153: 740–8. doi: 10.1093/aje/153.8.740 [DOI] [PubMed] [Google Scholar]
- 26.Chandeying V, Garland SM, Tabrizi SN. Prevalence and typing of human papilloma virus (HPV) among female sex workers and outpatient women in southern Thailand. Sex Health 2006; 3: 11–4. doi: 10.1071/SH05019 [DOI] [PubMed] [Google Scholar]
- 27.Hernandez BY, Vu Nguyen T. Cervical human papillomavirus infection among female sex workers in southern Vietnam. Infect Agent Cancer 2008; 3: 7. doi: 10.1186/1750-9378-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan R, Khoo L, Ho TH, Koh CF, Lee IW, Yam KL, et al. A comparative study of cervical cytology, colposcopy and PCR for HPV in female sex workers in Singapore. Int J STD AIDS 2001; 12: 159–63. doi: 10.1258/0956462011916956 [DOI] [PubMed] [Google Scholar]
- 29.Ford K, Reed BD, Wirawan DN, Muliawan P, Sutarga M, Gregoire L. The Bali STD/AIDS study: human papillomavirus infection among female sex workers. Int J STD AIDS 2003; 14: 681–7. doi: 10.1258/095646203322387947 [DOI] [PubMed] [Google Scholar]
- 30.Miyashita M, Agdamag DM, Sasagawa T, Matsushita K, Salud LM, Salud CO, et al. High-risk HPV types in lesions of the uterine cervix of female commercial sex workers in the Philippines. J Med Virol 2009; 81: 545–51. doi: 10.1002/jmv.21416 [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee R, Mukhopadhyay D, Murmu N, Jana S. Prevalence of human papillomavirus infection among prostitutes in Calcutta. J Environ Pathol Toxicol Oncol 2001; 20: 113–7. [PubMed] [Google Scholar]
- 32.Sarkar K, Bhattacharya S, Bhattacharyya S, Chatterjee S, Mallick AH, Chakraborti S, et al. Oncogenic human papilloma virus and cervical pre-cancerous lesions in brothel-based sex workers in India. J Infect Public Health. 2008; 1: 121–8. doi: 10.1016/j.jiph.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 33.Cox JT. Epidemiology and natural history of HPV. J Fam Pract 2006; (Suppl): 3–9. [PubMed] [Google Scholar]
- 34.Bao YP, Li N, Smith JS, Qiao YL. Human papillomavirus type distribution in women from Asia: a meta-analysis. Int J Gynecol Cancer 2008; 18: 71–9. doi: 10.1111/j.1525-1438.2007.00959.x [DOI] [PubMed] [Google Scholar]
- 35.Marais DJ, Carrara H, Ramjee G, Kay P, Williamson AL. HIV-1 seroconversion promotes rapid changes in cervical human papillomavirus (HPV) prevalence and HPV-16 antibodies in female sex workers. J Med Virol 2009; 81: 203–10. doi: 10.1002/jmv.21343 [DOI] [PubMed] [Google Scholar]
- 36.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007; 7: 453–9. doi: 10.1016/S1473-3099(07)70158-5 [DOI] [PubMed] [Google Scholar]
- 37.Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005; 366: 991–8. doi: 10.1016/S0140-6736(05)67069-9 [DOI] [PubMed] [Google Scholar]
- 38.Ponten J, Guo Z. Precancer of the human cervix. Cancer Surv 1998; 32: 201–29. [PubMed] [Google Scholar]
- 39.Garland SM, Cuzick J, Domingo EJ, Goldie SJ, Kim YT, Konno R, et al. Recommendations for cervical cancer prevention in Asia Pacific. Vaccine 2008; 26: M89–98. doi: 10.1016/j.vaccine.2008.06.020 [DOI] [PubMed] [Google Scholar]
- 40.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367: 1247–55. doi: 10.1016/S0140-6736(06)68439-0 [DOI] [PubMed] [Google Scholar]
- 41.Future II study group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356: 1915–27. doi: 10.1056/NEJMoa061741 [DOI] [PubMed] [Google Scholar]