Supplemental Digital Content is available in the text.
Keywords: uterine vascular resistance, smoking, air pollution, nitrogen dioxide, nitrogen oxide, resistance index
Abstract
Background:
Prenatal exposure to air pollution and smoking increases the risk of pregnancy complications and adverse birth outcomes, but pathophysiologic mechanisms are still debated. Few studies to date have examined the influence of air pollution on uterine vascular resistance, and no studies have examined the independent impact of these exposures. We aimed to assess the impact of prenatal exposure to traffic-related air pollution and smoking on uterine vascular resistance.
Methods:
Our study included 566 pregnant women recruited between 1993 and 1996 in Los Angeles who completed visits at three gestational ages. Information on smoking was collected, and uterine vascular resistance was measured at each visit by Doppler ultrasound. We calculated three resistance indices: the resistance index, the pulsatility index, and the systolic/diastolic ratio. We estimated exposure to NO2 at the home address of the mother using a land use regression model and to nitrogen oxides using CALINE4 air dispersion modeling. We used generalized linear mixed models to estimate the effects of air pollution and smoking on uterine vascular resistance indices.
Results:
Land use regression–derived NO2 and CALINE4-derived nitrogen oxides exposure increased the risk of high uterine artery resistance in late pregnancy. Smoking during pregnancy also increased the risk of higher uterine resistance and contributed to bilateral notching in mid-pregnancy.
Conclusion:
Our results suggest that uterine vascular resistance is a mechanism underlying the association between smoking and air pollution and adverse birth outcomes.
What this study adds
Though there is convincing evidence from epidemiological studies that air pollution exposure is associated with negative pregnancy outcomes, the physiologic mechanisms underlying these relations are largely unknown. Our study suggests that increased resistance to blood flow in the uterine arteries during pregnancy may be one such mechanism. This finding could help expand our understanding of the potentially causal relation between air pollution exposure and adverse pregnancy outcomes. Furthermore, smoking during pregnancy appears to have a similar detrimental impact on blood flow in the uterine arteries affecting pregnancy earlier in gestation, highlighting the importance of considering both the effects of smoking and air pollution on pregnancy.
Introduction
Maternal exposure to air pollution during pregnancy has been linked to several adverse birth outcomes, including low birth weight and preterm and small for gestational age births.1 Studies have also found an increased risk of gestational hypertension and preeclampsia with prenatal air pollution exposure.2,3 Maternal hypertensive disorders and intrauterine growth restriction are hypothesized to share common pathways as both are characterized by abnormal placental implantation and subsequent inadequate uteroplacental blood flow.4,5 Smoking during pregnancy has also been consistently associated with a number of adverse birth outcomes (fetal growth restriction, preterm birth, stillbirth) and pregnancy complications (placental abruption, placenta previa, spontaneous abortions, ectopic pregnancies).6 Interestingly, smoking has been found to reduce risk of preeclampsia, though the mechanisms for this are not well understood.6,7 Thus, further studies that aim to examine the impact of smoking on placental development and function are needed.
Doppler ultrasound has long been used to assess uterine resistance to blood flow and to register the presence of “notching” in the uterine arteries. In nonpregnant women and in early pregnancy, blood flow in the uterine arteries typically has a high systolic and low diastolic flow profile, with the presence of an early diastolic “notch” seen on Doppler ultrasound. As normal pregnancy progresses, uterine resistance to blood flow decreases and the notch disappears around 18–24 weeks of gestation. Two commonly used blood flow resistance indices are the pulsatility index (PI; peak systolic flow minus end diastolic flow divided by mean flow) and the resistance index (RI; peak systolic flow minus end diastolic flow divided by peak systolic flow), with higher values denoting a lower diastolic flow.8,9 High uterine flow resistance and notching have clinical relevance in mid-to-late pregnancy as they have been shown to be predictive of a range of pregnancy complications and adverse fetal outcomes, most notably preeclampsia and intrauterine growth restriction.8,10–13
It is plausible that air pollution contributes to impaired uterine vascular resistance as studies have shown that air pollution upregulates endothelin, a vasoconstrictor, and increases plasma viscosity, though no studies have examined this specifically in pregnant women.14,15 Furthermore, animal studies have also shown that air pollution can cause changes in placental morphology that could contribute to decreased uteroplacental blood flow.16 Studies on prenatal air pollution and uterine vascular resistance are limited, with only two studies having examined this to date.14,17 One study in the Netherlands found an association between NO2 exposure and third-trimester uterine bilateral notching, but no associations between PM10 and NO2 exposure and uterine artery resistance in the second and third trimester. The other study, conducted in Brazil, examined uterine artery resistance in relation to NO2 and O3 exposure and found no associations.
The impact of smoking during pregnancy on uterine vascular resistance has been more frequently studied, with studies finding some support for an increased vascular resistance in the uterine arteries in response to maternal smoking, but the evidence is inconclusive.18–22 It is plausible that smoking results in reduced blood flow as smoking has been shown to cause structural changes in the placenta that decrease vascularization, and nicotine has been shown to have vasoconstrictive effects on the uterine artery.23–25
The purpose of this study is to elucidate how air pollution and active smoking may affect uterine vascular resistance measured via ultrasound examinations in early, mid, and late pregnancy in a multiethnic sample of pregnant women living in Los Angeles in the mid-1990s.
Methods
Study population
Our study population was drawn from the Behavior in Pregnancy Study, which enrolled 688 ethnically and socioeconomically diverse women from private practices and prenatal clinics between 1993 and 1996 in Los Angeles, California. Briefly, this prospective study recruited healthy women aged 18 years or older, less than 20 weeks pregnant, and intending to deliver at the study hospital, Cedars-Sinai Medical Center, and followed them to delivery. A comprehensive questionnaire was administered at three gestational ages: visit 1 (18–20 weeks’ gestation), visit 2 (28–30 weeks’ gestation), and visit 3 (35–37 weeks’ gestation). Detailed demographic information, maternal residence address, and pregnancy history were obtained at baseline (visit 1) while information on medical conditions or maternal behaviors including smoking status was collected at each visit. From among 688 mothers, 639 gave birth to a live infant and 578 completed one or more study visits. Mothers with a twin pregnancy (n = 4) and stillbirths (n = 2) and infants with birth weight <500 g (n = 5) or gestational age >308 days (n = 1) were excluded, leaving 566 women for our analyses. Of these 566 women, 478 completed all three visits, 66 completed two visits, and 22 completed one visit. This study has been approved by the UCLA Institutional Review Board of Human Subjects.
Uterine vascular resistance
At each visit, real-time Doppler velocimetry was conducted to measure uterine vascular blood flow using an ATL, HDI 3000 Ultrasound machine (Philips Medical System, the Netherlands). Doppler measurements were performed on the left and right proximal uterine artery at each visit. Measurements on each uterine artery were obtained to the point near the cross-over with the external iliac artery. For each waveform, the peak systolic (S) and end diastolic (D) velocities were measured three times, and the mean value of these three measurements were calculated. We calculated three related flow indices: the PI, the RI, and the systolic/diastolic (S/D) ratio.26,27 We found no difference between left and right uterine artery resistance indices; thus, we averaged values for both sides (left and right uterine artery RI) at each visit for each participant as previously done in this cohort.12 The presence of uterine notching was also assessed at each visit.
Traffic-related air pollution exposure
Exposures to traffic-related air pollutants were assessed at participants’ residential address reported at baseline (visit 1). Addresses were geocoded using three methods including (1) geocoded to the parcel level using the TeleAtlas Address Point database (n = 404); (2) geocoded using address interpolation via the TeleAtlas EZ Locate geocoding service (n = 117); and (3) geocoded using Google Earth (n = 38), equivalent to highest quality matching using EZ Locate). Seven addresses could not be mapped, resulting in missing air pollution assignments.
CALINE4 nitrogen oxides exposure estimates
We used the CALINE4 line dispersion model,28,29 which is a Gaussian dispersion model which uses information on meteorology, roadway geometry, and traffic emissions to estimate local exposure in each pregnancy period to traffic-derived nitrogen oxides (NOx). Our exposure estimates were limited to roadways within 5 km of participants’ residences. The CALINE4 model implemented in this population has been extensively described previously.30 Briefly, model inputs included traffic count data from Tele Atlas/Geographic Data Technology, hourly wind speed, and direction data from 20 routine ambient air quality stations in the study region for the study time period, and vehicle fleet average emission factors based on the California Air Resource Board’s emission factors (EMFAC2007) vehicle emissions model (CARB2007).
Land use regression exposures
Individual exposures to nitrogen oxides NO, nitrogen dioxide (NO2), and NOx were estimated at residential locations from land use regression (LUR) model surfaces. The method of creating LUR surfaces has been previously described in detail.31 Briefly, LUR surfaces for NO, NO2, and NOx measures were based on 2-week average Ogawa passive diffusion sampler measures of NO2 and NOx collected in September 2006 and February 2007 at 181 locations (total of 196 samplers) throughout LA County. The final LUR regression models included the predictors: traffic volumes, truck routes and road network, land use data, coordinates of the sampling sites, and satellite-derived soil brightness. The models explained 81%, 86%, and 85% of the variance in calculated NO (NOx minus NO2) and measured NO2 and NOx concentrations, respectively. LUR NO, NO2, and NOx are necessarily highly correlated; thus, we used only NO2 as an indicator of traffic- related air pollution for our main analysis. We present results for LUR NOx in Supplementary Table 1; http://links.lww.com/EE/A12. Our LUR models best approximate annual average concentrations, thus providing spatial but not temporal contrasts. We did not attempt to temporally adjust LUR estimates for pregnancy periods due to the small number of women (n = 102) who lived close (5 km) to an ambient monitoring station which could provide information on temporal variability. Temporal adjustment using exposure estimates obtained miles away from a home relies on the unvalidated assumption that ambient monitors can adequately capture the local variation in air pollution, which is likely not true, especially for traffic-related air pollutants.32 Thus, our LUR exposures are best interpreted as spatial, long-term (annual average) exposure contrasts instead of pregnancy period–specific exposures. Our CALINE4 and LUR models have been previously found to be associated with pregnancy and birth outcomes in previous studies in LA county.30,33–35 Since our CALINE4 and LUR models are modeling a mixture of traffic-related indicators, NO2 and NOx should be interpreted as proxies for the traffic-related pollution mixture.
Smoking exposure
At visit 1, women were asked whether (1) they ever smoked cigarettes and (2) they smoked cigarettes in the 3 months before pregnancy or at any time during their pregnancy. At each subsequent study visit, they were asked whether they smoked since their last study visit. Based on these questions, we created a time-varying maternal smoking history variable in which we classified women as smokers, former smokers, or never smokers. At visit 1, women were classified as smokers if they reported smoking in the 3 months prior to pregnancy or at any time during their pregnancy. At visit 2, women were classified as smokers if they reported smoking at visit 1 or between visit 1 and 2. At visit 3, women were classified as smokers if they reported smoking at visit 1, visit 2, or between visit 2 and 3. Women were categorized as former smokers if they reported having ever smoked cigarettes at baseline and reported no smoking in the 3 months prior to pregnancy and at any visit during pregnancy. Never smokers were women who reported no smoking at any time prior to or during pregnancy. Thus, our questionnaire did not allow us to differentiate between women who had smoked regularly at some point in time prior to pregnancy from women who had only ever tried cigarettes briefly. Additionally, due to the way the baseline question on smoking during pregnancy was phrased, we could not examine smoking in the 3 months before pregnancy separately from smoking during pregnancy.
Statistical analysis
We used generalized linear mixed models with a random intercept for study participant. We fit linear mixed effects models using the SAS mixed procedure to estimate the effects of air pollution on uterine vascular resistance indices in standard deviation (SD) values (RI/SD of RI) at each study visit. We fit separate models for the effects of NOx in natural-log scaled values per interquartile range (IQR) and NO2 per IQR, adjusting for smoking. We also implemented the SAS glimmix procedure to fit separate logistic mixed effects models for the impact of NOx in natural-log scaled values per IQR and NO2 per IQR, adjusting for smoking, on an RI above the 90th percentile. We examined uterine vascular resistance values above the 90th percentile because they have been shown to be predictive of preeclampsia and intrauterine growth restriction.36,37 Employing logistic mixed effects models, we also examined the influence of NOx and NO2 on the presence of notching in the uterine artery. To assess smoking as an independent risk factor for uterine vascular resistance and notching, we examined smoking (smoker status at each study visit vs. never smoker, former smoker vs. never smoker) using linear mixed effects models for RI values in SD and logistic mixed effects models for RI values above the 90th percentile and presence of uterine notching, adjusting for NO2 and NOx. Based on our review of the literature and directed acyclic graphs, we adjusted for the following continuous (maternal age at delivery, prepregnancy body mass index [BMI], parity) and categorical covariates (maternal race [white vs. nonwhite], maternal education [<12, 12, >12] infant sex, marital status [single, separated, divorced, widowed vs. married], prenatal care payment [government vs private]) in all our models.21,30,38 Time-fixed predictors had the same value across study visits (maternal age at delivery, prepregnancy BMI, parity, maternal race, maternal education, infant sex, marital status, prenatal care payment, NO2) while time-varying predictors had distinct values for each study visit (smoking, NOx). We included interaction terms between study visit and each of our main exposures (NO2, NOx, and smoking) to allow for estimation of their effects in each period.
We conducted sensitivity analyses for the LUR NO2 and CALINE NOx associations by conducting separate analyses in which we limited our sample to nonobese women, women with no uterine notching, women without infections during pregnancy, and nulliparous women to assess whether our results remain consistent. Nulliparous women, obese women, or women with infections during pregnancy may be more likely to have pregnancy complications, and notching may be more associated with adverse pregnancy outcomes than uterine vascular resistance indices.21,39–43 We also examined associations for LUR NO2 and CALINE NOx among subgroups of smokers: never smokers, former smokers, and smokers. We conducted analyses stratified by race/ethnicity to address potential variation by race/ethnicity for the groups for which we had sufficient sample size (White, Hispanic, and African American). All analyses were done using SAS 9.4 software (Cary, NC).
RESULTS
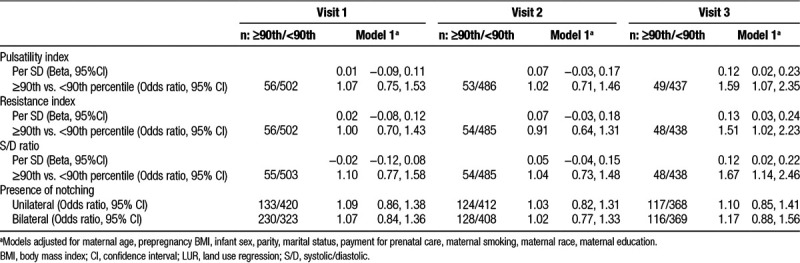
Demographic characteristics of our study population are reported in Table 1. Table 2 displays mean and standard deviation values for the uterine vascular resistance indices and the proportion of women with uterine notching at each study visit. The estimates for the association between LUR-derived NO2 per IQR and the uterine PI, RI, and S/D ratio and notching are shown in Table 3. LUR NO2 increased the uterine PI, RI, and S/D ratio at the third visit (β = 0.12 SD, 95% confidence interval [CI]: 0.02, 0.23; β = 0.13 SD, 95% CI: 0.03, 0.24; β = 0.12 SD, 95% CI: 0.02, 0.22 per IQR, respectively). LUR NO2 was also associated with an increased risk of uterine PI, RI, and S/D ratio values above the 90th percentile at the third exam (odds ratio [OR] = 1.59, 95% CI: 1.07, 2.35; OR = 1.51, 95% CI: 1.02, 2.23; OR = 1.67, 95% CI: 1.14, 2.46, respectively).
Table 1.
Baseline characteristics of pregnant women residing in Los Angeles (n = 566)

Table 2.
Characteristics of uterine vascular resistance at each visit
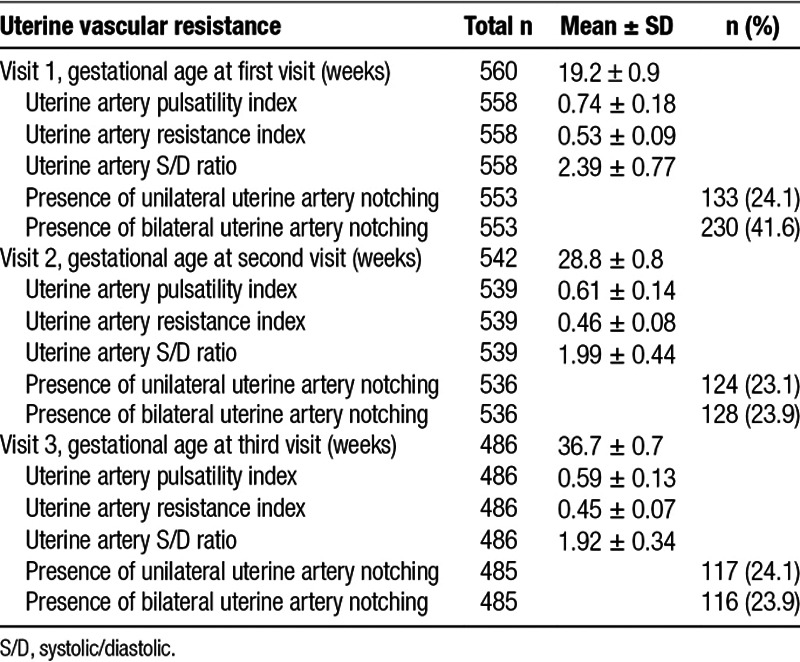
Table 3.
Impact of LUR NO2 (per IQR) on uterine artery resistance
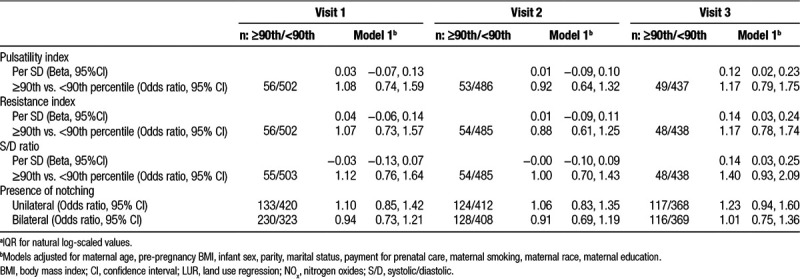
Estimates for the association between CALINE4 log-transformed NOx per IQR and the uterine PI, RI, and S/D ratio and notching are shown in Table 4. NOx was associated with increased uterine PI, RI, and S/D ratio at the third visit (β = 0.12 SD, 95% CI: 0.02, 0.23; β = 0.14 SD, 95% CI: 0.03, 0.24; β = 0.14 SD, 95% CI: 0.03, 0.25 per IQR, respectively]. Uterine PI, RI, and S/D ratio values above the 90th percentile at the third exam were also elevated particularly for the S/D ratio (OR = 1.40, 95% CI: 0.93, 2.09), but all confidence intervals crossed the null.
Table 4.
Impact of CALINE4 NOxa on uterine artery resistance
When assessing the impact of LUR-derived NOx on uterine artery resistance, we observed results that were consistent with our LUR-derived NO2 exposures (Supplementary Table 1; http://links.lww.com/EE/A12).
In sensitivity analyses, associations with LUR-derived NO2 and CALINE NOx at the third exam for uterine vascular resistance indices among nonobese women, nulliparous women, women with no uterine notching, and women with no infections during pregnancy were similar to those for the total population (data not shown). When stratifying by smoking status, at the third exam, LUR NO2 increased uterine vascular resistance indices among never smokers and bilateral notching odds among current smokers (OR = 4.16, 95% CI: 1.73, 10.02). Among never smokers, NO2 also appeared to increase uterine vascular resistance indices at the first exam though confidence intervals included the null (Supplementary Table 2; http://links.lww.com/EE/A12). CALINE NOx increased uterine vascular resistance indices in the third exam among never smokers as well as for current smokers in the third exam, particularly for the S/D ratio (β = 0.30, 95% CI: 0.01, 0.59; Supplementary Table 3; http://links.lww.com/EE/A12). NOx exposure in the third exam also increased the odds of bilateral notching among current smokers (OR = 3.92, 95% CI: 1.29, 7.59; Supplementary Table 3; http://links.lww.com/EE/A12).
When stratified by race/ethnicity, Whites and Hispanics showed patterns similar to our overall sample, with increased uterine vascular resistance with LUR NO2 exposure at the third exam and CALINE NOx exposure at the third exam (Supplementary Table 4; http://links.lww.com/EE/A12 and Supplementary Table 5; http://links.lww.com/EE/A12). However, RI estimates for African American women were largely null, but NO2 exposure marginally increased the risk of unilateral uterine notching (OR = 1.73, 95% CI: 0.98, 3.06; Supplementary Table 4; http://links.lww.com/EE/A12).
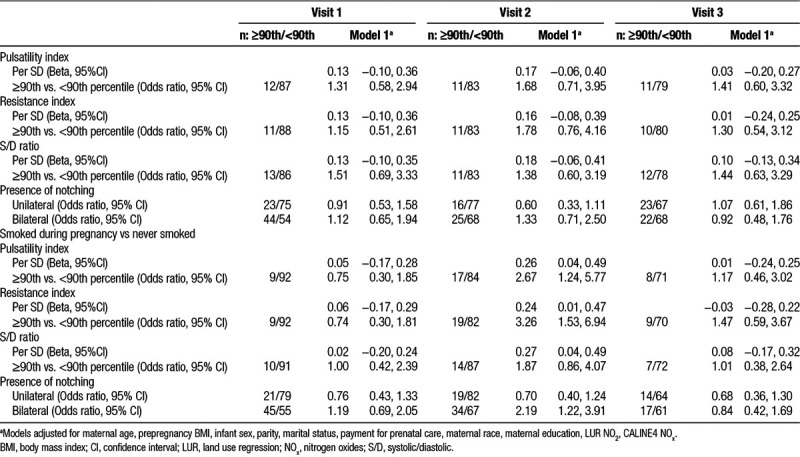
When examining the impact of smoking on uterine vascular resistance while adjusting for LUR NO2 and CALINE4 NOx exposure, we found that being a former smoker elevated resistance values across all three periods, but all confidence intervals crossed the null. Smoking during pregnancy was associated with increased uterine PI, RI, and S/D index values in SD and having resistance values above the 90th percentile at the second exam, particularly for the PI and RI. Smoking during pregnancy also increased the risk of uterine bilateral notching at the second exam (OR = 2.19, 95% CI: 1.22, 3.91; Table 5).
Table 5.
Impact of smoking on uterine artery resistance former smoker vs never smoked
Discussion
We found that traffic-related air pollution exposure derived from our LUR and CALINE4 models increased the risk of high uterine artery resistance in late pregnancy. Our results did not change when we restricted to nonobese women, nulliparous women or those without uterine notching and infections during pregnancy, and never smokers. Interestingly, among former and current smokers, at the third exam, LUR NO2 exposure was not associated with the uterine vascular resistance indices, although we found an increased risk of uterine artery bilateral notching with air pollution among smokers. In contrast, CALINE4 NOx exposure was associated with an increased risk of bilateral notching in late pregnancy among smokers, as well as high uterine vascular resistance. We did not find any associations for air pollution and uterine vascular resistance among our subgroup of African American women at the third exam, except for an increased risk of unilateral notching with LUR NO2 exposure in the third exam. Smoking during pregnancy, on the other hand, increased the risk of uterine vascular resistance, as well as uterine bilateral notching earlier in gestation, that is, at the time of the second exam.
Only two previous studies have examined the impact of air pollution on uterine artery resistance, and they did not report any associations with NO2 exposure and high uterine vascular resistance in the third trimester but found an association between NO2 exposure and bilateral notching.14,17 One potential explanation for the null findings for NO2 exposure in other studies is that these studies conducted the ultrasound exams early in the third trimester (mean or median of 31 weeks). The consequences of air pollution may not manifest until the late third trimester since our estimates for the second exam in the earlier part of the third trimester (28–30 weeks) were largely null. Additionally, air pollution exposures throughout pregnancy may have a cumulative impact on blood flow resistance, and these might be most pronounced and notable toward the end of pregnancy. There is no consensus on which period during pregnancy is most susceptible to the effects of air pollution, but the evidence in the literature suggests that effect sizes are slightly stronger for the first and third trimester.44 Plausible mechanisms that may underlie the association between air pollution and uterine vascular resistance include alterations in blood viscosity, endothelial function, inflammation, or hypertension.15 A recent study showed that NO2 exposure in pregnant women decreased vascularization indices in the first trimester, further supporting that NO2 may contribute to diminished placental vascularization.45 Studies have shown that increased uterine artery vascular resistance indices throughout pregnancy and notching, even among low-risk women, are strong predictors of adverse pregnancy outcomes, particularly for bilateral uterine notching.12,21,46–49 We were unable to assess the impact of exposures in the first trimester as our first study visit occurred between 18 and 20 weeks of gestation; however, it is the presence of elevated resistance indices and notching after 18–24 weeks of gestation that are most strongly indicative of adverse pregnancy events.50,51
The inconsistent findings for NO2 and the resistance indices in former/current smokers and African American women compelled us to investigate the resistance patterns and birth outcomes in these subgroups in more detail. Specifically, we hypothesized that air pollution associations in these women might be harder to detect if they either already have much higher uterine vascular resistance due to other risk factors and/or are at higher risk of fetal loss. Several studies have shown that smoking increases uterine blood flow and notching.18,20–22 Additionally, smokers are more likely to have an increased risk of fetal loss52 and preterm birth.6,12 In our study, 60 of the 566 mothers gave birth preterm. We observed that the number of former smokers decreased over follow-up from 99 to 90 former smokers by visit 3, with eight of these nine infants being born preterm. For those who smoked during pregnancy, the number of smokers decreased from 101 to 79 by visit 3, and more than half of those who dropped out were preterm births (13 of the 22). Thus, smoking may be a competing risk factor whereby loss to follow-up of fetuses impacted by smoking may account for us not having been able to estimate air pollution influences on uterine blood flow at the third visit since women at highest risk were not pregnant anymore and thus could not complete the third visit. Additionally, this may also at least partially explain why we did not see any association of the resistance indices with air pollution in late pregnancy for African American women; they were at greatest risk of preterm delivery with 34 out of 238 African American women (14%) delivering preterm.6,12 Early fetal loss or miscarriage could also be impacting our results. Since smoking has been associated with an increased risk of uterine vascular resistance and miscarriage, and our study includes only live born children, this could induce collider-stratification bias which would negatively confound the association between NO2 exposure and uterine vascular resistance.53 By conditioning on smoking, we attempted to address this potential bias, though the possibility of residual confounding remains since smoking was self-reported and we did not have information on smoking intensity.
For smoking, we confirmed previous findings of an increased risk of uterine vascular resistance indices and notching for women who actively smoked during pregnancy.18,20,22 These associations were largely seen in mid-pregnancy. For former smokers, we found elevated resistance indices in all exams, but our findings were inconclusive due to wide confidence intervals. No other studies to our knowledge have compared the effects of being a former smoker versus current smoker on uterine vascular resistance. Smoking is a plausible risk factor for high uterine artery resistance as it has been shown to alter placental morphology, with studies showing a reduction in the size of placental villous capillaries for mothers who smoked during pregnancy.25,54 We might expect former smokers to have a less risky health profile than women who continued smoking during pregnancy as various studies have found that former smokers are more likely to be primiparous, privately insured and college educated; yet they may also be more prone to pregnancy complications due to increases in weight and blood pressure after smoking cessation.55,56 This is confirmed in our sample as former smokers were more likely to be older, White, nulliparous, and have higher education and socioeconomic status; however, they did not differ with respect to prepregnancy BMI or weight gain during pregnancy. A study that examined misclassification of self-reported smoking with cotinine measurements found that 24% of active smokers were misclassified as quitters because they inaccurately reported that they had quit or relapsed by mid-pregnancy. Furthermore, women who were misclassified as quitters were more likely to report that they quit during rather than before pregnancy.57 In our study, we classified smoking exposure as any smoking prior to when their uterine vascular resistance was assessed, thereby considering the long-term impact of smoking on pregnancy. We did not have information on secondhand smoking exposure and thus were unable to assess its potential effect.
There were several strengths and limitations of our study. Since our LUR spatial pollution surfaces were based on data collected about a decade after the uterine vascular resistance measures were obtained, we relied on the assumption that on average the spatial relations between high and low traffic pollution areas remained stable. Studies conducted in the Netherlands, Italy, Canada, and Great Britain have found that spatial contrasts in NO2 remain relatively stable over time, demonstrating good agreement between LUR estimates derived up to 12 years apart, though performance may suffer slightly in cases where air pollution is decreasing over time.58–61 If our assumption is incorrect, then this would likely introduce nondifferential exposure misclassification. Another limitation of our study is that our LUR exposures cannot be interpreted as pregnancy-specific exposures but are more reflective of annual-average exposures. However, our CALINE4 NOx exposure estimates, which provided a strong temporal match with our outcomes in each pregnancy period, showed consistent results with our LUR NO2 results, with higher uterine vascular resistance in the third exam, though estimates for resistance values above the 90th percentile were attenuated. Our two air pollution models serve to supplement each other as the CALINE4 model measures more local traffic-related air pollution sources while the LUR model includes a larger regional source impact as well.
Furthermore, since we relied on address reported at enrollment into the study to generate the pollution measures, this could have resulted in nondifferential misclassification if women moved during pregnancy, most likely for any exposure received in later pregnancy. A review of residential mobility rates during pregnancy found that overall mobility rates were 9%–32% in the United States and abroad from the 1980s to 2000s, but most were short distance moves (<10 km).62 Also, we did not have information on time activity to account for personal exposures at work and away from residences during pregnancy, which may introduce further exposure misclassification. If women spent more time at home in late pregnancy, as has been previously observed,63 this would reduce exposure misclassification due to time activity in the third period and also potentially explain the stronger associations observed in this period.
Some associations observed may be due to chance due to small sample sizes in our subgroup analyses; however, the associations between NO2 and NOx exposures on uterine vascular resistance indices at the third exam were very robust in all sensitivity analyses. Some strengths of this study include the ethnically and socioeconomically diverse sample for which we had detailed covariate information. Additionally, few studies to date have examined these measures of uterine vascular resistance in relation to air pollution. Of these studies, this is the first to also consider the impact of smoking on these indices and especially the resultant greater loss to follow-up during pregnancy via preterm delivery in smokers.
In conclusion, we found that exposure to traffic-related air pollution increased uterine artery resistance in late pregnancy while active smoking increased resistance in mid-pregnancy and possibly contributed to preterm deliveries. Our results suggest that uterine artery resistance may be a pathophysiologic mechanism explaining the adverse pregnancy and birth outcomes previously associated with air pollution. Nevertheless, further studies of susceptible time periods and mechanisms underlying these associations are warranted. Additionally, attention to the impact of smoking when assessing the effect of air pollution on uterine artery resistance has been lacking to date, and further investigation is warranted as smoking appears to be an independent and possibly competing risk factor for higher uterine vascular resistance and adverse pregnancy outcome.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH; R03ES017314 to B.R.), and the California Tobacco-Related Disease Research program, Award 340657 to J.E.H. and Cornelius Hopper Diversity Award Supplement (grant No. 24RT-033H) to Z.A.C. C.J.H. received support from NIH RO1 HD047609; Miriam Jacobs Chair. C.J. received funding from the NIH U01 HD087221. P.C.L. is supported in part by the Taiwan Ministry of Science and Technology (NSC106-2314-B-227-010); Taipei City Hospital (grant No. 10601-62-051).
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
The data and the computer code are not available for replication because the data are not publicly available.
Supplementary Material
Footnotes
Published online 21 June 2018
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Shah PS, Balkhair T. Air pollution and birth outcomes: A systematic review. Environ Int. 2011;37(2):498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen M, Halldorsson TI, Olsen SF, Hjortebjerg D, Ketzel M, Grandström C, Raaschou-Nielsen O, Sørensen M. Impact of road traffic pollution on pre-eclampsia and pregnancy-induced hypertensive disorders. Epidemiology. 2017;28(1):99–106. doi: 10.1097/EDE.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen M, Stayner L, Slama R, Sorensen M, Figueras F, Nieuwenhuijsen MJ, Raaschou-Nielsen O, Dadvand P. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. 2014;64(3):494–500. doi: 10.1161/HYPERTENSIONAHA.114.03545. [DOI] [PubMed] [Google Scholar]
- 4.Khong TY, Wolf FD, Robertson WB, I B. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small-for-gestational age infants. Obstet Gynecol Survey. 1987;42(8):503–505. [Google Scholar]
- 5.McParland P, Pearce JM. Review article: Doppler blood flow in pregnancy. Placenta. 1988;9(4):427–450. doi: 10.1016/0143-4004(88)90055-0. [DOI] [PubMed] [Google Scholar]
- 6.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Res. 2004;6(April):125–140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 7.England L. Smoking and risk of preeclampsia: a systematic review. Front Biosci. 2007;12(1):2471. doi: 10.2741/2248. [DOI] [PubMed] [Google Scholar]
- 8.Cnossen JS, Morris RK, ter Riet G, Mol BWJ, van der Post JAM, Coomarasamy A, Zwinderman AH, Robson SC, Bindels PJE, Kleijnen J, Khan KS. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. Can Med Assoc J. 2008;178(6):701–711. doi: 10.1503/cmaj.070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jauniaux E, Jurkovic D, Campbell S. In vivo investigations of the anatomy and the physiology of early human placental circulations. Ultrasound Obstet Gynecol. 1991;1(6):435–445. doi: 10.1046/j.1469-0705.1991.01060435.x. [DOI] [PubMed] [Google Scholar]
- 10.Harrington K, Cooper D, Lees C, Hecher K, Campbell S. Doppler ultralsound of the uterine arteries: the importance of bilateral notching in the prediction of pre-eclampsia, placental abruption or delivery of a small-for-gestational-age baby. Ultrasound Obstet Gynecol. 1996;7(3):182. doi: 10.1046/j.1469-0705.1996.07030182.x. [DOI] [PubMed] [Google Scholar]
- 11.Harrington K, Fayyad A, Thakur V, Aquilina J. The value of uterine artery Doppler in the prediction of uteroplacental complications in multiparous women. Ultrasound Obstet Gynecol. 2004;23(1):50–55. doi: 10.1002/uog.932. [DOI] [PubMed] [Google Scholar]
- 12.Misra VK, Hobel CJ, Sing CF. Placental blood flow and the risk of preterm delivery. Placenta. 2009;30(7):619–624. doi: 10.1016/j.placenta.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Severi FM, Bocchi C, Visentin A, Falco P, Cobellis L, Florio P, Zagonari S, Pilu G. Uterine and fetal cerebral Doppler predict the outcome of third-trimester small-for-gestational age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2002;19(3):225–228. doi: 10.1046/j.1469-0705.2002.00652.x. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho MA, Bernardes LS, Hettfleisch K, Pastro LD, Vieira SE, Saldiva SR, Saldiva PH, Francisco RP. Associations of maternal personal exposure to air pollution on fetal weight and fetoplacental Doppler: A prospective cohort study. Reprod Toxicol. 2016;62:9–17. doi: 10.1016/j.reprotox.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, Glinianaia S, Hoggatt KJ, Kannan S, Hurley F, Kalinka J, Šrám R, Brauer M, Wilhelm M, Heinrich J, Ritz B. Meeting report: atmospheric pollution and human reproduction. Environ Health Persp. 2008;116(6):791–798. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veras MM, Damaceno-Rodrigues NR, Caldini EG, Maciel Ribeiro AA, Mayhew TM, Saldiva PH, Dolhnikoff M. Particulate urban air pollution affects the functional morphology of mouse placenta1. Biol Reprod. 2008;79(3):578–584. doi: 10.1095/biolreprod.108.069591. [DOI] [PubMed] [Google Scholar]
- 17.van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PY, Russcher H, Lindemans J, Miedema HM, Steegers EA, Jaddoe VW. Air pollution exposure and markers of placental growth and function: the generation R study. Environ Health Persp. 2012;120(12):1753–1759. doi: 10.1289/ehp.1204918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albuquerque CA, Smith KR, Johnson C, Chao R, Harding R. Influence of maternal tobacco smoking during pregnancy on uterine, umbilical and fetal cerebral artery blood flows. Early Human Dev. 2004;80(1):31–42. doi: 10.1016/j.earlhumdev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Alptekin H, Işik H, Alptekin N, Kayhan F, Efe D, Cengiz T, Gök E. A prospective comparative study to assess the effect of maternal smoking at 37 weeks on Doppler flow velocity waveforms as well as foetal birth weight and placental weight. J Obstet Gynaecol. 2016;37(2):1–5. doi: 10.1080/01443615.2016.1217506. [DOI] [PubMed] [Google Scholar]
- 20.Machado deB J, Filho PVM, Petersen GO, Chatkin JM. Quantitative effects of tobacco smoking exposure on the maternal-fetal circulation. BMC Pregnancy Childbirth. 2011;11(1):24–24. doi: 10.1186/1471-2393-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaillard R, Arends LR, Steegers EAP, Hofman A, Jaddoe VWV. Second- and third-trimester placental hemodynamics and the risks of pregnancy complications. Am J Epidemiol. 2013;177(8):743–754. doi: 10.1093/aje/kws296. [DOI] [PubMed] [Google Scholar]
- 22.Geelhoed JJM, el Marroun H, Verburg BO, van Osch-Gevers L, Hofman A, Huizink AC, Moll HA, Verhulst FC, Helbing WA, Steegers EA, Jaddoe VW. Maternal smoking during pregnancy, fetal arterial resistance adaptations and cardiovascular function in childhood. BJOG. 2011;118(6):755–762. doi: 10.1111/j.1471-0528.2011.02900.x. [DOI] [PubMed] [Google Scholar]
- 23.Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Human Dev. 2007;83(11):699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20(2):115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 25.Larsen LG, Clausen HV, Jønsson L. Stereologic examination of placentas from mothers who smoke during pregnancy. Am J Obstet Gynecol. 2002;186(3):531–537. doi: 10.1067/mob.2002.120481. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RS, Trudinger BJ, Cook CM. A comparison of Doppler ultrasound waveform indices in the umbilical artery—II. Indices derived from the mean velocity and first moment waveforms. Ultrasound Med Biol. 1986;12(11):845–854. doi: 10.1016/0301-5629(86)90002-5. [DOI] [PubMed] [Google Scholar]
- 27.Thompson RS, Trudinger BJ, Cook CM. Doppler ultrasound waveform indices: A/B ratio, pulsatility index and Pourcelot ratio. BJOG. 1988;95(6):581–588. doi: 10.1111/j.1471-0528.1988.tb09487.x. [DOI] [PubMed] [Google Scholar]
- 28.Benson P. Report No.FHWA/CA/TL-84/15. Sacramento, CA: Prepared by the California Department of Transportation; 1989. CALINE4 – A Dispersion Model for Predicting Air Pollutant Concentrations Near Roadways. [Google Scholar]
- 29.Chen H, Bai S, Eisinger D, Niemeier D, Claggett M. Predicting near-road PM2.5Concentrations. Transport Res Record J Transport Res Board. 2009;2123:26–37. [Google Scholar]
- 30.Ritz B, Qiu J, Lee P-C, Lurmann F, Penfold B, Erin Weiss R, McConnell R, Arora C, Hobel C, Wilhelm M. Prenatal air pollution exposure and ultrasound measures of fetal growth in Los Angeles, California. Environ Res. 2014;130:7–13. doi: 10.1016/j.envres.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su JG, Jerrett M, Beckerman B, Wilhelm M, Ghosh JK, Ritz B. Predicting traffic-related air pollution in Los Angeles using a distance decay regression selection strategy. Environ Res. 2009;109(6):657–670. doi: 10.1016/j.envres.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozkaynak H, Baxter LK, Dionisio KL, Burke J. Air pollution exposure prediction approaches used in air pollution epidemiology studies. J Expo Sci Environ Epidemiol. 2013;23(6):566–72. doi: 10.1038/jes.2013.15. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh JKC, Wilhelm M, Su J, Goldberg D, Cockburn M, Jerrett M, Ritz B. Assessing the influence of traffic-related air pollution on risk of term low birth weight on the basis of land-use-based regression models and measures of air toxics. Am J Epidemiol. 2012;175(12):1262–1274. doi: 10.1093/aje/kwr469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and term low birth weight in Los Angeles County, California. Environ Health Persp. 2011;120(1):132–138. doi: 10.1289/ehp.1103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Wilhelm M, Chung J, Ritz B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ Res. 2011;111(5):685–692. doi: 10.1016/j.envres.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan FY, Pun TC, Khoo J, Lee CP, Lam YH. Pregnancy screening by uterine artery doppler velocimetry – which criterion performs best? Obstet Gynecol. 1995;85(4):596–602. doi: 10.1016/0029-7844(95)00006-D. [DOI] [PubMed] [Google Scholar]
- 37.North RA, Ferrier C, Long D, Townend K, Kinciad-Smith P. Uterine artery Doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth. Obstet Gynecol. 1994;83(3):378–386. [PubMed] [Google Scholar]
- 38.Tayyar A, Guerra L, Wright A, Wright D, Nicolaides KH. Uterine artery pulsatility index in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound Obstet Gynecol. 2015;45(6):689–697. doi: 10.1002/uog.14789. [DOI] [PubMed] [Google Scholar]
- 39.Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction (Cambridge, England) 2013;146(5):R151–R162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91(3):436–440. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prefumo F, Bhide A, Sairam S, Penna L, Hollis B, Thilaganathan B. Effect of parity on second-trimester uterine artery Doppler flow velocity and waveforms. Ultrasound Obstet Gynecol. 2004;23(1):46–49. doi: 10.1002/uog.908. [DOI] [PubMed] [Google Scholar]
- 42.Prior T, Mullins E, Bennett P, Kumar S. Influence of parity on fetal hemodynamics and amniotic fluid volume at term. Ultrasound Obstet Gynecol. 2014;44(6):688–92. doi: 10.1002/uog.13332. [DOI] [PubMed] [Google Scholar]
- 43.Seravalli V, Block-Abraham DM, Turan OM, Doyle LE, Blitzer MG, Baschat AA. Second-trimester prediction of delivery of a small-for-gestational-age neonate: Integrating sequential Doppler information, fetal biometry, and maternal characteristics. Prenatal Diagn. 2014;34(11):1037–1043. doi: 10.1002/pd.4418. [DOI] [PubMed] [Google Scholar]
- 44.Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102(2):182–190. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hettfleisch K, Bernardes LS, Carvalho MA, Pastro LD, Vieira SE, Saldiva SR, Saldiva P, Francisco RP. Short-term exposure to urban air pollution and influences on placental vascularization indexes. Environ Health Persp. 2016;125(4):753–759. doi: 10.1289/EHP300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arrue M, Garcia M, Rodriguez-Bengoa MT, Landa JM, Urbieta L, Maiztegui M, Salgueiro L, Belar M, Trecet JC, Lekuona A. Do low-risk nulliparous women with abnormal uterine artery Doppler in the third trimester have poorer perinatal outcomes? A longitudinal prospective study on uterine artery Doppler in low-risk nulliparous women and correlation with pregnancy outcomes. J Mater Fetal Neonatal Med. 2016;7058(November):1–4. doi: 10.1080/14767058.2016.1190822. [DOI] [PubMed] [Google Scholar]
- 47.Jamal A, Abbasalizadeh F, Vafaei H, Marsoosi V, Eslamian L. Multicenter screening for adverse pregnancy outcomes by uterine artery doppler in the second and third trimester of pregnancy. Medical Ultrasonogr. 2013;15(2):95–100. doi: 10.11152/mu.2013.2066.152.aj1fa2. [DOI] [PubMed] [Google Scholar]
- 48.Papageorghiou AT, Yu CKH, Cicero S, Bower S, Nicolaides KH. Second-trimester uterine artery Doppler screening in unselected populations: a review. J Mater Fetal Neonatal Med. 2002;12(2):78–88. doi: 10.1080/jmf.12.2.78.88. [DOI] [PubMed] [Google Scholar]
- 49.Prefumo F, Guven M, Ganapathy R, Thilaganathan B. The longitudinal variation in uterine artery blood flow pattern in relation to birth weight. Obstet Gynecol. 2004;103(4):764–768. doi: 10.1097/01.AOG.0000118310.51730.2d. [DOI] [PubMed] [Google Scholar]
- 50.Maulik D, Mundy D, Heitmann E, Maulik D. Umbilical artery Doppler in the assessment of fetal growth restriction. Clin Perinatol. 2011;38(1):65–82. doi: 10.1016/j.clp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Park YW, Cho JS, Kim HS, Kim JS, Song CH. The clinical implications of early diastolic notch in third trimester Doppler waveform analysis of the uterine artery. J Ultrasound Med. 1996;15(1):47–51. [PubMed] [Google Scholar]
- 52.Pineles BL, Park E, Samet JM. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2014;179(7):807–823. doi: 10.1093/aje/kwt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol. 2015;44(1):345–354. doi: 10.1093/ije/dyu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rath G, Dhuria R, Salhan S, Jain AK. Morphology and morphometric analysis of stromal capillaries in full term human placental villi of smokig mothers: an electron microscope study. Clin Ter. 2011;162:301–305. [PubMed] [Google Scholar]
- 55.Colman GJ, Joyce T. Trends in smoking before, during, and after pregnancy in ten states. Am J Prev Med. 2003;24(1):29–35. doi: 10.1016/s0749-3797(02)00574-3. [DOI] [PubMed] [Google Scholar]
- 56.Janzon E, Hedblad B, Berglund G, Engstrom G. Changes in blood pressure and body weight following smoking cessation in women. J Int Med. 2004;255(2):266–272. doi: 10.1046/j.1365-2796.2003.01293.x. [DOI] [PubMed] [Google Scholar]
- 57.England L, Grauman A, Qian C, Wilkins D, Schisterman E, Yu K, Levine R. Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine Tobacco Res. 2007;9(10):1005–1013. doi: 10.1080/14622200701491255. [DOI] [PubMed] [Google Scholar]
- 58.Cesaroni G, Porta D, Badaloni C, Stafoggia M, Eeftens M, Meliefste K, Forastiere F. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health. 2012;11:48. doi: 10.1186/1476-069X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G. Stability of measured and modelled spatial contrasts in NO2 over time. Occup Environ Med. 2011;68(10):765–770. doi: 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- 60.Gulliver J, de Hoogh K, Hoek G, Vienneau D, Fecht D, Hansell A. Back-extrapolated and year-specific NO2 land use regression models for Great Britain - Do they yield different exposure assessment? Environ Int. 2016;92–93(2):202–209. doi: 10.1016/j.envint.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 61.Wang R, Henderson SB, Sbihi H, Allen RW, Brauer M. Temporal stability of land use regression models for traffic-related air pollution. Atmos Environ. 2013;64(January):312–319. [Google Scholar]
- 62.Bell ML, Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Exposure Sci Environ Epidemiol. 2012;22(5):429–438. doi: 10.1038/jes.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nethery E, Brauer M, Janssen P. Time–activity patterns of pregnant women and changes during the course of pregnancy. J Exposure Sci Environ Epidemiol. 2009;19(3):317–324. doi: 10.1038/jes.2008.24. [DOI] [PubMed] [Google Scholar]


