Summary:
Adoptive cell transfer of tumor-infiltrating lymphocytes (TILs) can mediate objective tumor regression in 49% to 72% of patients with many long-term durable responses. To undergo treatment a patient must have (1) a resectable tumor from which (2) TIL can be generated that (3) exhibit tumor-specific reactivity. From July 2002 to July 2007, 787 tumors from 402 patients were processed for possible use in the generation of TIL, leading to the eventual treatment of 107 patients (27%). Viable TILs were generated in 376 patients (94%), and active, specific TILs were identified in 269 patients (67%). Patient demographics and tumor characteristics were analyzed for possible prognostic factors for growth and activity. Gastrointestinal-derived TIL grew less frequently, whereas lymph node and lung-derived TIL exhibited specific activity more often. TIL that grew and exhibited specific reactivity were from tumors that were larger in diameter and digests that had a higher percentage of lymphocytes. Despite these considerations, active, specific TIL could be generated from almost any site of metastasis. As more centers begin exploring the use of adoptive transfer with TIL, this compendium may provide a framework for therapeutic decision making and future investigation.
Keywords: metastatic melanoma, tumor-infiltrating lymphocytes, adoptive cell transfer
In 2009, patients with metastatic melanoma have few curative options. Standard dacarbazine chemotherapy can induce short-term objective responses but has little to no effect on overall survival.1 Immunotherapy with high-dose interleukin-2 (IL-2) can mediate long-term survival but in small percentages of patients.2 Combination biochemotherapy is administered frequently and can also result in modest objective responses, but with no improvement on overall survival compared with chemotherapy alone.3 Preliminary results using ipilimumab (anti-CTLA4 antibody) to block an inhibitory receptor on lymphocytes indicate that durable responses can also be seen in some patients. Thus, the only consistent modality able to achieve durable complete tumor regression is immunotherapy. The presumed goal of vaccinations, cytokine treatments, and antibody therapies such as ipilimumab is to stimulate an endogenous antitumor immune response of sufficient magnitude and intensity to cause the rejection of tumor. In some patients with melanoma, a population of suitable antitumor lymphocytes seems to exist naturally, particularly within the lymphocytes-infiltrating tumors (tumor-infiltrating lymphocytes; TILs). A more direct approach to achieving sufficient reactivity to effect tumor rejection is to activate and expand an autologous population of cells containing tumor-reactive lymphocytes in vitro and transfer it to the properly prepared recipient in a process known as adoptive cellular therapy (ACT). When this has been done using TIL in patients with metastatic melanoma, objective tumor regression rates of 49% to 72% (complete and partial responses assessed by RECIST criteria) have been seen, with some patients achieving complete and durable responses.4,5
Although ACT with TIL has delivered promising results in phase 1 and 2 trials at the Surgery Branch, NCI, it is not currently possible to treat every patient with metastatic melanoma with this strategy for the following reasons: lack of an available tumor for surgical harvest, inability to isolate and grow viable TIL from a harvested tumor, or the inability to show robust, specific effector function of isolated TIL. Other investigative protocols have evolved in an effort to address these limitations. Use of genetic engineering to create antigen-specific effector T cells from peripheral blood lymphocytes may be an alternative for those patients without tumors amenable to surgical resection or those from whose tumors viable TIL cannot be grown.6–12 As the detection of tumor-specific effector function can be hampered by a lack of suitable tumor targets (either autologous tumor or HLA-matched allogeneic melanoma lines), a clinical effort is underway using minimally cultured TIL in which the specific effector activity is unknown, providing a theoretical alternative for those patients from whose tumor TIL can be successfully grown but would not necessarily exhibit in vitro effector function.13
This study is an effort to describe a 5-year series of surgical metastasectomy and tumor processing for generation of TIL, focusing on patient-specific success rates in the generation of viable TIL (“growth”) and exhibition of specific effector function (generically referred to as “activity”) and possible association with patient-specific and tumor-specific prognostic factors. It will also briefly discuss treatment patterns at the Surgery Branch, NCI and explore clinical management questions that are frequently encountered in adoptive cell transfer strategies.
MATERIALS AND METHODS
Tumor Acquisition
All tumors were resected as part of Institutional Review Board-approved Surgery Branch, NCI protocols and were numbered consecutively upon arrival in the TIL laboratory. The period of analysis (July 2002 to July 2007) encompassed tumors #2050 to 2845. If multiple specimens were harvested from a patient in 1 surgical setting (eg, a subcutaneous nodule resection followed by a lung metastasectomy), each specimen was identified separately (ie, 2051 to 1, 2051 to 2). Tumors were excluded from analysis for the following reasons: nonmelanoma histology, prospectively identified as “research only,” tumor sent entirely to pathology, tumor resected specifically for alternative protocols, or tumors frozen immediately after processing without any attempt to culture.
Data Acquisition
Patient characteristics (HLA, sex, date of birth, etc.) were obtained from official medical records documentation following Institutional Review Board-approved collection methods. Preoperative history and physical examinations were used to determine the date of the most recent administration of any prior treatment for each surgical procedure. Separate analyses of prior treatments were performed for treatments received within 3 months of tumor resection and at any time before surgery. When specific dates were not provided, a mid-month value was assigned, providing accuracy to within 15 days. There was no differentiation between treatment received in an adjuvant setting versus metastatic setting. Chemotherapy was defined as therapeutic agents with known activity against melanoma, and specifically did not include cytoxan and fludarabine used in preparative regimens for adoptive cell transfer. Vaccine therapy included peptide administration, recombinant viruses, autologous tumor, dendritic cell administration, and other forms of experimental vaccines. IL-2 and interferon-α (IFN-α) therapy was defined to include high-dose, low-dose, or combination therapy. The various components of biochemotherapy were counted individually (ie, a patient who was treated with vincristine, IFN-α, and IL-2 in June 2005 would be assigned a value of June 15, 2005 for chemotherapy, IFN, and IL-2). Gross tumor descriptions (site of harvest, longest diameter) were taken from TIL laboratory documentation, operative reports, or surgical pathology reports. When documenting site of harvest, any subcutaneous nodule, regardless of location, was defined as “subcutaneous.” Similarly, all lymph nodes were grouped together (axilla, inguinal, intra-abdominal, etc). Gastrointestinal tumors were limited to those arising from stomach, small bowel, colon, and rectum. “Other visceral” included metastases on adrenal, spleen, pancreas, uterus, and ovaries. “Other” included breast and intramuscular metastases.
Tumor Processing
All tumors were processed by the TIL laboratory to generate cultures from tumor fragments and/or digests as previously described.14 In brief, a tumor was manually dissected from attached normal tissues and areas of frank necrosis. Small (2 to 3 mm) fragments were placed into individual wells of a 24-well plate with exactly 8 fragments per culture initiated for the majority of tumors. An additional portion underwent overnight digestion with collagenase, hyaluronidase, and DNAse. If a single tumor digestion included 2 or more pooled tumors from the same surgical setting (eg, 2716 to 1 from a lymph node and 2716 to 3 from an intraluminal jejunal lesion), it was counted as 1 unique digest for overall results but was counted individually for site-specific subset analysis (eg, above example was included in both the lymph node and gastrointestinal subsets). Tumor digestions were analyzed for their percentage of lymphocytes and total number of lymphocytes by light microscopy using size criteria and standard trypan blue exclusion. Typically 4 wells of tumor digest were initiated for each tumor.
Outcome Measurements
Tumors were considered positive for growth if a fragment or enzymatic digestion yielded viable TIL in sufficient quantities to perform functional assays or to cryopreserve for future testing. The definition of positive growth was at least 5 × 106 TIL after initial culture in IL-2 alone (for the majority of samples more than 20 × 106 lymphocytes were obtained). This minimum number allows 1 subsequent expansion using anti-CD3 antibody and IL2 to produce over 1010 TILs for infusion in nearly all patients. Determination of specific activity was based on enzyme-linked immunosorbent assay measuring IFN-γ release in overnight coculture against 1 of more of the following: HLA-A–matched tumor cell lines, autologous tumor cell lines, or autologous fresh tumor. Standard tumors lines included but were not limited to HLA-A2, A3+ lines (526, 624) and HLA-A1, A24+ lines (888, 938).15 Tumors were considered positive if at least 1 fragment or digest well released greater than 400 pg/mL of IFN-γ and was greater than or equal to twice background values. In a tumor in which 4 or more fragments or digest wells were tested, it could also be considered positive if greater than 25% of the tested cultures released greater than 200 pg/mL of IFN-γ and greater than or equal to twice the relevant background values. Although somewhat arbitrary, these criteria were chosen based on experience whereby cultures meeting these specifications would consistently show tumor recognition in repeated assays where lesser values proved unreliable. IFN-γ release from TIL alone, or after coculture with HLA-A mismatched cell lines or cryopreserved allogeneic tumor cells were all considered background values. An example is shown in Table 1. Relevant background values are italicized, and values greater than 200 pg/ml and twice background are in bold. When analyzing fresh tumor targets, only HLA-A mismatched fresh tumors were used for background. Fragments (F3 to F8) and digests (W1 to W4) of tumor 2188 were deemed (−) to shared antigen, but (+) to fresh tumor. The digest had a single value over 400 pg/mL, in which the fragments had 3 of 6 tested fragments greater than 200 pg/mL. This algorithm for activity was applied retrospectively to a historical database of tumor coculture results. On occasion, the algorithm produced a positive result when a tumor had been prospectively deemed insufficiently reactive at the time of initial testing; this was labeled an erroneous exclusion.
TABLE 1.
An Example of Coculture Results to Determine Specific Activity
| Targets | ||||||||
|---|---|---|---|---|---|---|---|---|
| Interferon-γ (pg/ml) > Indicates off-scale Effector | Tissue Culture Lines A1, A24 |
Tissue Culture Lines A2, A3 |
Tissue Culture Autologous-2183 Shared Antigen-2188 | Fresh Tumor Autologous-2188 Shared Antigen-2183 | Fresh Tumor (A1, A3) Mismatch-2188 Shared Antigen-2183 | |||
| None | 888 | 938 | 526 | 624 | 2183 | 2188 | 2088 | |
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| MART reactive (+) control | 6 | 6 | 16 | 6900 | > 31,870 | 8940 | 26 | 53 |
| Gp100 reactive (+) control | 0 | 0 | 0 | 2880 | 2570 | 6490 | 1 | 0 |
| (−) control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2188 (A2, A11) fragments | ||||||||
| F3 | 0 | 9 | 14 | 238 | 69 | 33 | 310 | 55 |
| F4 | 0 | 10 | 0 | 0 | 0 | 0 | 111 | 0 |
| F5 | 0 | 0 | 0 | 199 | 183 | 231 | 50 | 16 |
| F6 | 18 | 37 | 9 | 0 | 5 | 3 | 171 | 33 |
| F7 | 37 | 486 | 5 | 33 | 60 | 37 | 294 | 98 |
| F8 | 26 | 394 | 28 | 252 | 159 | 522 | 291 | 100 |
| 2188 (A2, A11) digest | ||||||||
| W1 | 25 | 13 | 18 | 16 | 35 | 9 | 518 | 284 |
| W2 | 32 | 12 | 15 | 9 | 8 | 8 | 114 | 31 |
| W3 | 0 | 0 | 2 | 70 | 0 | 103 | 372 | 51 |
| W4 | 10 | 0 | 0 | 172 | 0 | 0 | 500 | 191 |
| 2183 (A1, A2) fragments | ||||||||
| F2 | 11 | 87 | 30 | > 2301 | 2740 | 1470 | 78 | 83 |
| F3 | 92 | 326 | 52 | 5950 | 14,820 | 5040 | 176 | 198 |
| F4 | 1527 | 275 | 222 | 590 | 233 | 565 | 716 | 1138 |
| F5 | 157 | 41 | 50 | 130 | 84 | 1300 | 126 | 147 |
| F7 | 1734 | 435 | 306 | 653 | 469 | 672 | > 2776 | > 3076 |
| F8 | 111 | 44 | 32 | 139 | 91 | 194 | 128 | 115 |
Bold values indicate tumor recognition as defined in Methods.
Statistical Analysis
Fisher exact test was used to determine associations between dichotomous parameters and the positive or negative tumor result (growth or activity). Comparisons between positive and negative results for continuous parameters were done using a Wilcoxon rank sum test. All P values are 2-tailed and have not been adjusted for multiple comparisons. Logistic regression analysis was performed to model for TIL growth and activity, simultaneously including all factors with P≤0.15 on univariate analysis. The final logistic model was developed by a backward elimination procedure.
RESULTS
Viable TILs were grown in almost all patients undergoing surgical metastasectomy, and active TILs were identified in the majority of patients. In the 5-year period from July 2002 to July 2007, 1080 tumors were registered by the TIL laboratory, the majority of which were melanoma metastases (n=982). Some of these specimens were prospectively identified as tissue inappropriate for processing: 130 were “research only” specimens (eg, a biopsy of a regressing lesion in a responding patient), 21 specimens were submitted to the surgical pathology department without processing (eg, a clinical need to determine presence or absence of bowel perforation), and 6 tumors were resected specifically for non-TIL protocols. In addition, surgical metastasectomy rendered 29 patients free of clinically evident disease and those 38 tumors were frozen without processing.
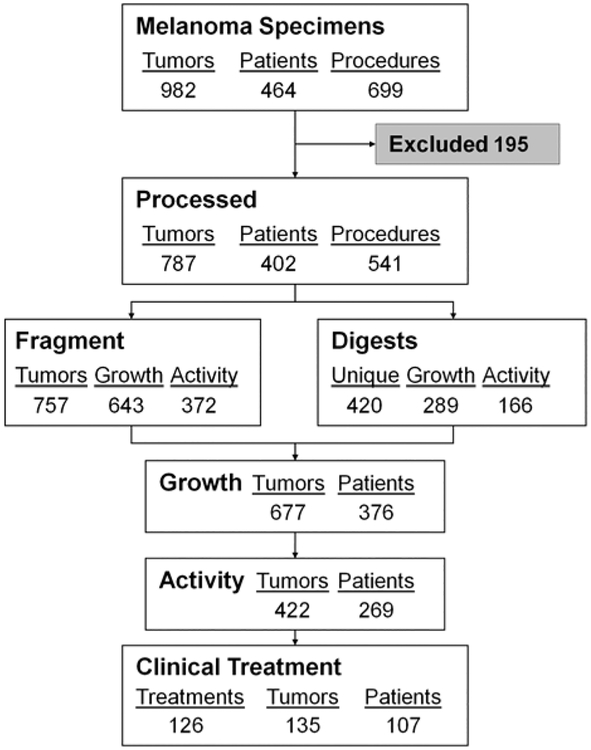
After exclusion of these specimens, 787 tumors remained in the TIL laboratory database for this analysis. These tumors were harvested from 402 unique patients who underwent 541 surgical procedures (Table 2). TILs were successfully grown from 85% of tumors set up as fragments and 75% of enzymatic digests, with 50% and 44%, respectively, showing specific activity (of total attempts at culture and excluding those never tested) (Table 3). The combination of fragment and digest processing led to generation of viable TIL from 376 patients (94%) and active TIL in 269 (67%) of the 402 patients, leading to the treatment of 107 patients on various adoptive cell transfer protocols within the Surgery Branch, NCI (Fig. 1).
TABLE 2.
Tumor Characteristics and Patient Demographics
| Tumor | Percentage of Total | Patient | Percentage of Total | |
|---|---|---|---|---|
| Total | 787 | 402 | ||
| Patient demographics | ||||
| Male | 251 | 62 | ||
| HLA-A201 | 286 | 71 | ||
| Age* mean (range) | 48 (17–77) | |||
| No. tumors/patient† | ||||
| Mean | 2 | |||
| Median | 1 | |||
| Range | 1–15 | |||
| Tumor characteristics | ||||
| Male | 484 | 62 | ||
| HLA-A201 | 549 | 70 | ||
| First harvest | 534 | 68 | ||
| Diameter (cm)‡ | ||||
| Mean | 3.65 | |||
| Median | 3.0 | |||
| Range | 0.3–27 | |||
| Site of harvest | ||||
| Subcutaneous | 323 | 41 | 167 | 42 |
| Lymph node | 229 | 29 | 173 | 43 |
| Liver | 35 | 4 | 23 | 6 |
| Lung | 71 | 9 | 54 | 13 |
| Brain | 14 | 2 | 13 | 3 |
| GI | 40 | 5 | 29 | 7 |
| Bone | 3 | 0.4 | 3 | 1 |
| Other visceral | 47 | 6 | 36 | 9 |
| Other | 25 | 3 | 23 | 6 |
| Prior therapy | ||||
| Chemotherapy | 273 | 34 | 132 | 32 |
| IFN-α | 367 | 46 | 173 | 43 |
| IL-2 | 522 | 65 | 257 | 63 |
| Vaccine | 344 | 43 | 165 | 41 |
| Anti-CTLA-4 | 97 | 12 | 56 | 14 |
| ACT | 124 | 15 | 57 | 14 |
| None | 78 | 10 | 44 | 11 |
ACT indicates adoptive cell transfer with a cytoxan/fludarabine preparative regimen; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; GI, gastrointestinal; IFNa, interferon alpha 2b; IL-2, interleukin-2.
Age at first resection.
Excludes 19 patients whose resection history preceded July 2002.
Excludes 7 specimens without documented size.
TABLE 3.
Tumor Processing Results for Fragment-derived and Digest-derived Cultures
| Fragment | Digest | |||
|---|---|---|---|---|
| No. set up | 771 | 420 | ||
| Frozen | 14 | 34 | ||
| Culture attempted | 757 | 386 | ||
| Growth (+) | 642 | (85%)* | 289 | (75%)* |
| Frozen without testing | 18 | 7 | ||
| Tested in coculture | 624 | 282 | ||
| vs. autologous cell line | 69/113 | (61%) | 30/51 | (59%) |
| vs. autologous fresh tumor | 178/406 | (44%) | 106/216 | (49%) |
| vs. shared antigen cell line | 271/586 | (46%) | 94/364 | (36%) |
| (+) for specific reactivity | 372 | (50%)† | 166 | (44%)† |
| Autologous reactivity only | 101 | (27%) | 72 | (43%) |
| Shared antigen reactivity only | 143 | (38%) | 39 | (23%) |
| Both | 128 | (34%) | 55 | (33%) |
Percent growth positive of cultures attempted.
Percent active of cultures attempted, excluding those frozen without testing.
FIGURE 1.
Surgical metastasectomy within the Surgery Branch, NCI from July 2002 to 2007. Every tumor was recorded and exclusions criteria were applied as described. Each remaining specimen was processed for fragment-derived cultures, digest-derived cultures, or both. A tumor was considered positive for growth if at least 1 fragment-derived or digest-derived culture met criteria (fragment 313, digest 35, both 329). Similarly, a tumor was considered active based on interferon-γ enzyme-linked immunosorbent assay as described (fragment 210, digest 50, both 162). Of the 107 patients treated, 63 were treated with cells derived from fragments only, 10 from digests only, and 34 from a mixture of digest-derived and fragment-derived tumor infiltrating lymphocyte.
Analysis of Factors Associated With Growth or Activity of TIL
Although many parameters were explored, only a limited number of factors were ultimately associated with either growth or activity of TIL (Table 4). Sex and age were not associated with likelihood of growth or activity. HLAA2 status was not associated with growth, but A201-positive tumors were more likely to exhibit activity. Analyses of prior therapies were done for prior treatments within 3 months of TIL harvest or at any time before harvest. No specific prior therapy within 3 months was significantly detrimental to TIL outgrowth or reactivity, perhaps due to small numbers. Therefore, we only present data for treatments given any time before harvest. Although treatment naive tumors seemed to generate TIL more frequently, and vaccine therapy seemed to hinder growth, neither retained significance in a logistic regression analysis. Prior ACT using cyclophosphamide and fludarabine had a small negative effect on the likelihood of subsequently growing TIL on a per-tumor basis and it was more difficult to generate viable TIL from gastrointestinal metastases, whereas tumors from lung and lymph nodes metastases grew active TIL more frequently (Table 4).
TABLE 4.
Likelihood of Growth and Activity for Tumor and Patient Prognostic Factors
| Growth | Activity | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | % (+) |
P | Yes | No | % (+) |
P | |
| All tumors | 677 | 110 | 86 | 422 | 356 | 54 | ||
| Sex | ||||||||
| Male | 415 | 69 | 86 | 0.83 | 264 | 213 | 55 | 0.46 |
| Female | 262 | 41 | 86 | 158 | 143 | 52 | ||
| Age (mean) | 44.7 | 44.5 | 0.77 | 45.2 | 44.0 | 0.16 | ||
| HLA | ||||||||
| A201 | 477 | 72 | 87 | 0.38 | 311 | 233 | 57 | 0.0149 |
| Other | 200 | 38 | 84 | 111 | 123 | 47 | ||
| Prior therapy | ||||||||
| Chemo-therapy | 227 | 46 | 83 | 0.11 | 134 | 135 | 50 | 0.08 |
| IFN-α | 314 | 53 | 86 | 0.76 | 182 | 181 | 50 | 0.04 |
| IL-2 | 447 | 75 | 86 | 0.74 | 282 | 235 | 55 | 0.82 |
| Vaccine | 283 | 61 | 82 | 0.009 | 171 | 169 | 50 | 0.06 |
| αCTLA-4 | 83 | 14 | 86 | 0.88 | 45 | 49 | 48 | 0.19 |
| ACT | 97 | 27 | 78 | 0.01 | 65 | 59 | 52 | 0.69 |
| None | 73 | 5 | 94 | 0.04 | 41 | 33 | 55 | 0.9 |
| Site of harvest | ||||||||
| Subcutaneous | 271 | 52 | 84 | 0.17 | 160 | 162 | 50 | 0.034 |
| Lymph node | 211 | 18 | 92 | 0.0014 | 137 | 89 | 61 | 0.026 |
| Liver | 30 | 5 | 86 | 1.0 | 21 | 12 | 64 | 0.29 |
| Lung | 63 | 8 | 89 | 0.59 | 46 | 25 | 65 | 0.0796 |
| Brain | 9 | 5 | 64 | 0.03 | 7 | 7 | 50 | 0.79 |
| GI | 28 | 12 | 70 | 0.0076 | 19 | 21 | 48 | 0.42 |
| Bone | 2 | 1 | 67 | 0.36 | 0 | 3 | 0 | 0.095 |
| Other visceral | 40 | 7 | 85 | 0.83 | 23 | 23 | 50 | 0.65 |
| Other | 23 | 2 | 92 | 0.56 | 9 | 14 | 39 | 0.20 |
ACT indicates adoptive cell transfer; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; GI, gastrointestinal; IFNα, interferon alpha; IL-2, interleukin-2.
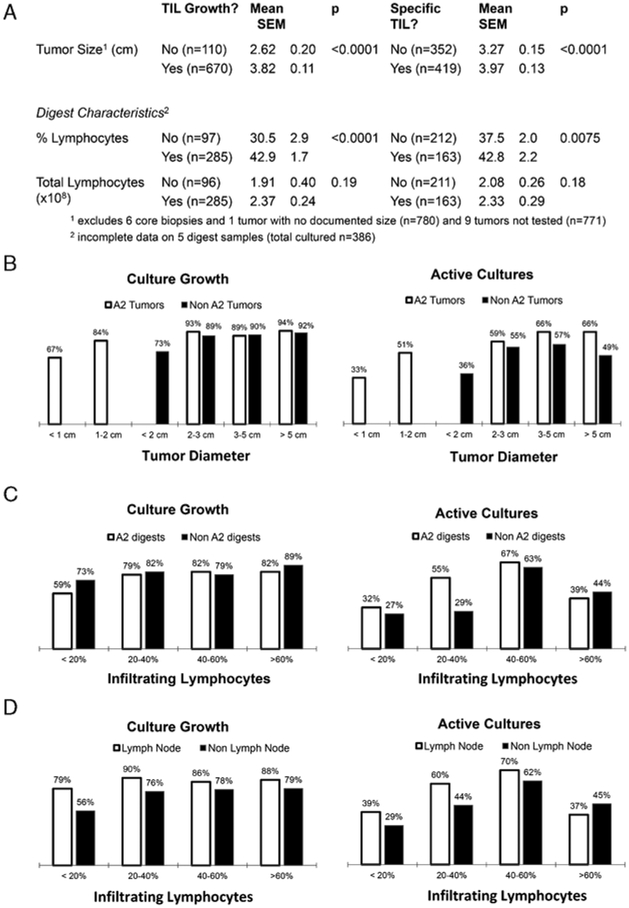
Physical properties of the tumor specimens and subsequent processing were analyzed for possible associations. Specimens that yielded viable TILs were larger than those that did not. The group of active tumors also had a larger diameter than those from which active TILs were not identified (Figs. 2A, B). One hypothesis was that larger tumors often provide enough reagent to generate autologous targets and by enlarging the target array, tumors were more likely to have a positive result. However, after eliminating tumors deemed active solely on the basis of autologous reactivity, the active tumors (n=303, mean diameter 3.88 cm) were still larger than nonreactive tumors (n=361, mean diameter 3.28 cm, P = 0.0001). Traditional “cutoff” values for suitability for resection were 1.0 cm for HLA-A2+ patients and 2.0 cm for HLA-A2− patients. Although these values remained reasonable, there were tumors below these threshold values that yielded TIL eventually used for treatment. Separate logistic regression models were developed to try to determine if a set of parameters could be used jointly to identify TIL which would grow or become active. Four parameters exhibiting some association with TIL growth (P<0.15) remained in the final model with a P-value of less than 0.05 when jointly considered: any prior chemotherapy, any prior cell treatment, gastrointestinal location, and diameter. The best combination of sensitivity and specificity that could be achieved was 72.4% and 61.8%, respectively, indicating only a modest ability to correctly classify a given tumor as to its likelihood of growing TIL.
FIGURE 2.
Analysis of tumor size and digest characteristics on tumor infiltrating lymphocyte (TIL) growth and tumor reactivity. A, Tumors that generated viable and tumor-reactive TIL had longer mean diameters than those tumors that either did not generate TIL or generated nonreactive TIL. B, The likelihood of successful TIL growth (left) and tumor recognition (right) is shown as a function of the HLA-A2 allele and tumor size. C, Success rates for growth (left) and antitumor reactivity (right) segregated by percentage of infiltrating lymphocytes and presence of the HLA-A2 allele. D, Success rates for growth (left) and antitumor reactivity (right) as a function of lymphocyte infiltration in digests derived from nodal and non-nodal tissue.
With similar criteria, 5 parameters were included in a logistic regression model for tumor recognition by TIL: any prior vaccine, HLA A201, lymph node or lung location, and diameter. In this model, the best overall combination of sensitivity and specificity was 67.5% and 49.1%, respectively, again insufficient to correctly classify tumors according to whether they would produce active TIL.
Active Digests Had a Higher Percentage of Lymphocytes
Tumor digests that yielded viable and active TIL had higher percentages of lymphocytes than those that did not (Fig. 2A). It is interesting to note that, although digests primarily composed of lymphocytes continued to grow well, those with greater than 60% lymphocytes were less likely to be active (Fig. 2C). One hypothesis was that those digests were composed primarily of metastases harvested from lymph nodes leading to a mixed population of TIL and normal lymphocytes. However, when lymph node-derived and nonlymph node-derived subsets were analyzed, the trend remained (Fig. 2D).
Active TIL Can Be Generated After an Unsuccessful First Attempt
The realities of ACT as a treatment modality often led to surgical management decisions, particularly when a first attempt at generating active TIL was not successful. The database was analyzed to assess the utility of multiple attempts and intra-patient concordance of results. Three hundred and eighty-three patients had 534 tumors resected at a first attempt to generate TIL. In 171 patients, TIL was not generated for the following reasons: no growth (39 patients), negative coculture (111 patients), and subjective failure (31 patients). In 52 patients, subsequent harvests were performed solely for the purpose of generating TIL. This specifically excluded any patient undergoing an operative procedure for clinically necessary reasons, therapeutic or palliative. In 34 patients, active TILs were generated and 13 were eventually treated. These values (65% active, 25% treated) were not different than the global results (67% active, 27% treated). Looking at all patients (n=103) with 2 or more harvests to determine concordance of results, there was no clear pattern. Fifty-one patients exhibited concordant activity (all positive n=34, all negative n=17) and 52 patients exhibited discordant activity, with 28 patients generating active TIL after previously negative attempts.
Active TIL Can Be Generated in Patients Who Have Previously Received a Non-myeloablative Preparative Chemotherapy Regimen
The use of various ACT protocols have generated a group of patients who have undergone lymphodepleting regimens and concerns have been raised about the ability to find and cultivate TIL from tumors that have been exposed to this chemotherapy. One hundred and twenty-four tumors were resected from 57 patients after a lymphodepleting regimen. Viable TILs were cultivated from 78% of these tumors versus 87% of tumors not previously treated with a lymphodepleting regimen (P = 0.01). Nevertheless, on a per-patient basis, the success of growing TIL in these patients was still 86% with 67% of patients producing tumor-reactive TIL.
Decline of performance status or development of intracranial metastases were the most common clinical reasons patients with active TILs were not treated. Active TIL was generated in 269 patients, however, only 107 (40%) of those patients were treated. Thorough review of the medical charts of the 162 patients not treated was performed to assign technical or clinical explanations (Table 5). Fifteen patients have active TIL cryopreserved and available for treatment, but are alive with no evidence of progressive disease. Thirty-six patients were no longer eligible for treatment due to protocol-specific exclusions arising after surgery. Experimental clinical trials to evaluate unproven treatment efficacy necessitate the elimination of concurrent comorbidities of unknown risk, including endobronchial lesions that could lead to obstructive pneumonia or the hemorrhagic risk of untreated intracranial metastases. Documented ongoing infections and elevated liver enzymes were also common exclusion criteria. Twenty-six patients experienced a rapid progression of their disease accompanied by a decline in performance status that rendered them ineligible for treatment.
TABLE 5.
Reasons for Nontreatment in 162 Patients With Active TIL
| Clinical Reasons | Technical Reasons | ||
|---|---|---|---|
| No evidence of progressive disease | 15 | Lost activity on further testing (thaw, tREP, COA) | 21 |
| Patient elected to return to home MD | 4 | Erroneous exclusion | 29 |
| Alternate protocol assignment | 19 | Treated with earlier TIL | 3 |
| Protocol-specific exclusion (including new brain metastasis) | 36 (20) | Lost to follow-up | 9 |
| Progressive disease with decline in performance status | 26 |
COA indicates certificate of analysis; TIL, tumor infiltrating lymphocyte; tREP, test rapid expansion protocol.
The technical reasons listed include 2 artifacts from the design of this retrospective study. In retrospectively applying an algorithm to determine reactivity, there were TIL cultures that were “erroneously excluded” from use by a subjective judgment of insufficient activity, made at the time of culture. In addition, 9 patients were lost to follow-up because they had resections for therapeutic intent, but TILs were incidentally cultured and they returned to the care of their home physicians. These types of failures to treat should be eliminated in a prospective protocol with a predesignated algorithm for TIL administration.
DISCUSSION
Here, we report a descriptive analysis of a 5-year database of tumor metastasectomy for the generation of TIL. Adoptive cell transfer for the treatment of patients with metastatic melanoma was developed and continues to be refined at the Surgery Branch, NCI. Efforts to expand the ACT experience beyond the phase 2 experience in the Surgery Branch required an intimate evaluation of the methods and protocols that eventually lead to a successful patient treatment. Dudley et al have reported on the characteristics of treatment regimens, physical properties and in vitro function of the transferred cells, and eventual patient outcomes. This report represents an effort to describe the first step in the generation of a cell-based therapy, that is, surgical resection.
Although certain differences did prove to be statistically significant, it is important to remember that viable TILs were grown in almost every patient and from every attempted site of resection. Bigger tumors did generate active TIL more frequently, but there were TIL from small tumors that contributed to patient treatments. No analysis of any measurable factor delineated cutoff values with sufficient sensitivity or specificity. The Surgery Branch has adopted guidelines for resection of 1 cm for HLA-A201 patients and 2 cm for all others. The establishment of those criteria makes analysis of the subset of smaller tumors impossible due to inherent selection bias.
This analysis supports multiple attempts to generate TIL, even in patients who have received lymphodepleting chemotherapy. Success rates for growth and activity are not different when compared with the global result.
A major concern is the frequency of patients undergoing TIL harvests which did not eventuate in therapy. Many of the reasons patients with active TIL did not receive treatment are inherent problems when caring for patients with advanced metastatic melanoma. The disease can move quickly and can be debilitating. The infectious and hemorrhagic risks associated with ACT also preclude many healthy-appearing individuals from obtaining treatment. By better defining an objective algorithm for administering a TIL culture and following more rigorous treatment planning, it is hoped that fewer patient treatment opportunities will be lost and this is a priority in current protocols. Perhaps dispensing with the assay for tumor reactivity (acknowledging our imperfect ability to detect all tumor recognition) and administering a younger population of TIL with less delay might also result in a net benefit with more patients reaching treatment.13
ACT with TIL remains the most effective treatment for patients with metastatic melanoma, though critics are quick to point to the small percentage of patients that eventually reach treatment. This database was not sufficient to perform a retrospective analysis of TIL ACT on an intent-to-treat basis. It includes patients for whom palliative, sometimes emergent surgery was necessary and though the cells were processed in the slim hope that the patient would be a candidate for ACT, the clinical course of the patient could be predicted. Conversely, it includes patients for whom metastasectomy was rendering them free, or nearly free, of disease, and tumors were processed based on a high likelihood of recurrence that has yet to occur. ACT with TIL can and should continue to be tested, within and outside the Surgery Branch, and this analysis may provide a framework for discussing surgical options for patients with metastatic melanoma.
ACKNOWLEDGMENTS
The authors thank the TIL laboratory technicians and Ellen Douglas, Joyce Landry, and Jessica Yingling for assistance with data collection and entry.
Funded by NIH Intramural Research.
Footnotes
All authors have declared there are no financial conflicts of interest with regard to this work.
REFERENCES
- 1.Balch CM, Atkins MB, Sober AJ. Cutaneous melanoma In: DeVita VT Jr, Helman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:1754–1808. [Google Scholar]
- 2.Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes MS, Yu YYL, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Zheng Z, Robbins PF, et al. Primary human lymphocytes transduced with NY-ESO-1 antigen specific TR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roskowski JJ, Lyons GE, Kast WM, et al. Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65:1570–1576. [DOI] [PubMed] [Google Scholar]
- 9.Engles B, Noesnner E, Frankenberger B, et al. Redirecting human T lymphocytes toward renal cell carcinoma specificity by retroviral transfer of T cell receptor genes. Hum Gene Ther. 2005;16:799–810. [DOI] [PubMed] [Google Scholar]
- 10.Cohen CJ, Zheng A, Bray R, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson LA, Heemskerk B, Powell D, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177: 6548–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran KQ, Zhou J, Durflinger KH, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31: 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley ME, Wunderlich JR, Shelton TE, et al. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003; 26:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]