The worldwide human obesity epidemic has provoked a great deal of experimentation to understand the biological controls of energy expenditure and food intake, two processes that together determine energy balance. Because food intake relies on feeding behavior that is determined by the brain, studies have focused on how the central nervous system receives and behaviorally responds to signals of metabolic status. On page 1293 of this issue, Lagerlӧf et al. (1) report that a glycosylation enzyme serves as a neuronal nutrient sensor that is critical in the control of food intake and body weight.
Feeding behavior responds to metabolic need. Thus, food deprivation stimulates feeding, whereas signals arising from the biological consequences of food ingestion impinge on the central nervous system and lead to meal termination. These signals include sensorineural events generated by the gustatory, olfactory, and gastrointestinal properties of foods, neuroendocrine signals stimulated by food ingestion, and signals generated by nutrients themselves (2).
Nutrient sensing is critical for cellular viability in general, but in the central nervous system, it may serve a larger role in the control of whole-body energy balance by modulating food intake (3). Lagerlӧf et al. have identified a new role for O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) as a nutrient sensor in brain neurons for controlling food intake and body weight. OGT catalyzes the post-translational modification of proteins by O-GlcNAc (a monosaccharide derivative of glucose) and is regulated by nutrient access. Acute, inducible decreases in the expression of OGT in the adult mouse brain, under the control of the neuronal marker α-calcium/calmodulin-dependent protein kinase type II (αCAMKII), rapidly produced pronounced overeating followed by massive weight gain and adiposity gain, with no gain in lean mass. Preventing this hyperphagia by pair-feeding animals to the levels of control subjects prevented the development of obesity, demonstrating that the hyperphagia produced by the absence of OGT expression is sufflcient for the obese phenotype. The observed hyperphagia is characterized by both increased meal size and meal duration, with no change in meal frequency, supporting the interpretation that OGT signaling in brain neurons is critical for satiation.
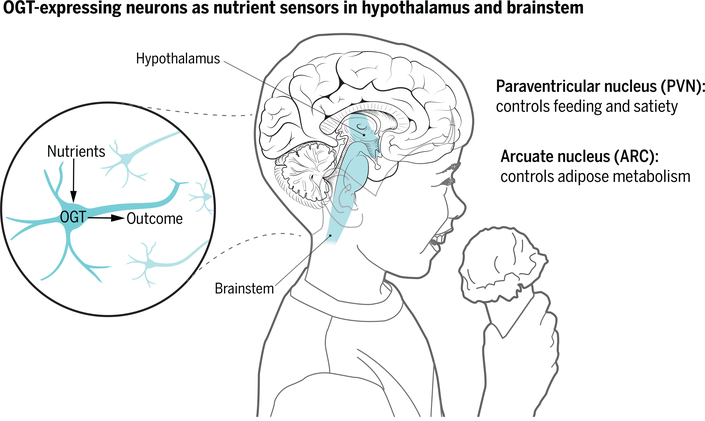
The effect of reduced OGT expression was most pronounced in neurons of the hypothalamic paraventricular nucleus (PVN), a region implicated in controlling food intake and body weight (see the figure). An increase in blood glucose concentration after feeding increased the expression of OGT and the transcription factor c-Fos (a marker of neuronal activation) in PVN αCAMKII neurons, whereas reduced OGT expression blocked c-Fos activation and decreased excitatory synaptic input in these neurons. Deletion of the gene encoding OGT specifically in αCAMKII-expressing PVN neurons had the same effects as reducing expression in all αCAMKII-expressing neurons in the brain, and was accompanied by massive body weight gain and hyperphagia characterized by increased meal size. These findings show that a decrease in OGT action in PVN αCAMKII neurons is sufflcient to mediate hyperphagia and obesity. Conversely, optogenetic stimulation of PVN αCAMKII neurons in mice acutely reduced food intake (smaller meals were consumed).
Heterogeneous function.
In the mouse, OGT in hypothalamic paraventricular nucleus (PVN) α-CAMKII neurons acts as a nutrient sensor by controlling overeating, whereas OGT in hypothalamic arcuate (ARC) agouti-related protein (AgRP) neurons controls the metabolic activity of adipose tissue. These OGT-expressing neuronal regions also exist in the human brain.
These are potent and neuroanatomically specific effects of OGT function on body weight. However, the localization of OGT expression in feeding-related neurons is not limited to the PVN. Multiple hypothalamic and brainstem regions implicated in the control of food intake express OGT, including the hypothalamic arcuate (ARC), ventromedial, dorsomedial, and lateral hypothalamic nuclei, as well as the brainstem nucleus of the solitary tract and the parabrachial nucleus (1, 2). Indeed, the nutritional regulation of OGT function is heterogeneous across neuroanatomically and neurochemically distinct neuronal populations (1, 4). Feeding and postfeeding blood glucose concentrations increased the amount of O-GlcNAc in PVN αCAMKII neurons, whereas fasting reduced the presence of this monosaccharide. By contrast, in ARC neurons expressing the orexigenic peptide agouti-related protein (AgRP), fasting increases OGT expression and the amount of O-GlcNAc in the cell (4). How fasting induces two opposite effects on neuronal OGT expression and activity remains unclear; the fasting-induced orexigenic gut peptide ghrelin increases O-GlcNAc amounts in AgRP neurons (4), but ghrelin action in PVN αCAMKII neurons has not been examined.
The metabolic consequences of reduced neuronal OGT for energy balance are also heterogeneous and appear to depend on the neurochemical and neuroanatomical phenotypes of the OGT-expressing neurons. Whereas adult-onset decreases in OGT expression in PVN αCAMKII neurons drives hyperphagia and obesity, constitutive reduction of OGT expression in ARC AgRP neurons leads to thermogenically effective browning of white fat, without observable changes in food intake or body weight, and protects against the development of obesity and glucose intolerance (4). Whether OGT action in PVN αCAMKII neurons is an important target in animal models of obesity and glucose tolerance remains to be determined.
Intriguingly, a decrease in OGT expression in ARC AgRP neurons or in PVN αCAMKII neurons reduces neuronal excitability, albeit through apparently distinct mechanisms. Reduced OGT in AgRP neurons also reduces the spontaneous action potential rate by blocking O-GlcNAcylation of inwardly rectifying Kv channels, whereas a decrease of OGT expression in PVN αCAMKII neurons reduces their miniature excitatory postsynaptic current frequency. Whether OGT modulates each of these two ionic determinants of neuronal excitability in both ARC AgRP neurons and PVN αCAMKII neurons is not known.
The identification of nutritionally regulated OGT expression in multiple neuron populations that control feeding sets the stage for elucidating how the consequences of OGT nutrient sensing are coordinated to determine both acute energy availability and long-term energy balance. Cerebrospinal fluid concentrations of multiple nutrients rise rapidly after meal ingestion and have simultaneous access to multiple brain nutrient-sensitive sites. For example, the amount of the essential branched-chain amino acid L-leucine in cerebrospinal fluid rapidly increases during the ingestion of a high-protein meal and activates both brainstem and hypothalamic sites (5). Local leucine administration to either of these sites in isolation rapidly decreases food intake through a selective reduction in meal size. Although these distinct effects appear to be redundant, they underscore the need for a more integrative experimental approach to understanding the role of neuronal nutrient sensors in the control of food intake, where meal-related nutrient stimuli have simultaneous access to multiple, neuroanatomically distributed nutrient-sensing sites. ■
ACKNOWLEDGMENTS
G.J.S. is supported by NIH grants DK 105441, DK 026687 and DK 020541.
REFERENCES AND NOTES
- 1.Lagerlöf O et al. Science 351, 1293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz GJ, Zeltser LM, Annu. Rev. Nutr 33, 1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran TH, Forum Nutr. 63, 94 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Ruan HB et al. , Cell 159, 306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blouet C et al. , J. Neurosci 29, 8302 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]