Abstract
Background and Objectives:
The purpose of this investigation was to determine the effect of hospital volume on treatment decisions, treatment results, and overall patient survival in extremity soft tissue sarcoma.
Methods:
The National Cancer Database was used to identify patients ≥18 years of age with non-metastatic soft tissue sarcoma of the extremity treated with surgery. Patients in high- and low-volume centers were matched by propensity score and placed into two equal comparative groups of 2,437 patients each.
Results:
Chemotherapy was used at a higher rate in high-volume centers (22% vs. 17%, p<0.001) and external beam radiation usage was similar (55% vs. 52%, p=0.108). There was a lower incidence of positive margins in high-volume centers (12% vs 17%, p<0.001). There was no significant difference in the rates of limb salvage surgery or readmissions at high-volume hospitals compared to low-volume. In a multivariate Cox proportional hazards model, low-volume facilities demonstrated diminished overall survival at all time points (hazard ratio at 5 years = 1.24, 95% CI 1.10-1.39).
Conclusions:
Treatment at high-volume hospitals was associated with fewer positive margins and increased overall survival at 2, 5, and 10 years. Continued efforts should focus on optimizing the balance between patient access to specialty care and experience of the treating center.
Keywords: sarcoma, hospital volume, survival, treatment, National Cancer Database
Introduction
Soft tissue sarcomas (STS) are a rare malignancy of mesenchymal origin with more than 70 subtypes[1]. The American Cancer Society projected that in 2017 the United States there would be 12,390 new cases and 4,990 deaths related to soft tissue sarcoma[2]. The treatment of STS varies greatly depending on the sarcoma location, size, and histology. Typical first line intervention for non-metastatic extremity sarcoma is complete surgical excision, with consideration of external beam radiation to minimize local recurrence in high-risk scenarios, such as close margins and high-grade tumors. Some centers routinely offer neoadjuvant chemotherapy or other systemic treatments with high-risk soft tissue sarcoma, either as standard practice or through a clinical trial. Determination of the optimal treatment can be complex and ideally requires a team of dedicated specialists, including surgeons, medical oncologists, radiation oncologists, pathologists, and radiologists. The need for multidisciplinary, specialized care suggests that this condition may benefit from regionalized treatment at dedicated centers. However, consolidation of treatment at high-volume centers may result in impediments to access and potentially subject sarcoma patients to additional difficulties surrounding diagnosis and treatment.
Previous investigations have found that increased hospital volumes were associated with more favorable outcomes in various forms of cancer [3–5] and surgical procedures [6–8]. We used the National Cancer Database (NCDB) to further investigate the influence of facility volume on treatment decisions and oncologic outcomes in soft tissue sarcoma. The NCDB is a robust database that captures more than 70% of all cancers reported in the United States, and is sponsored jointly by the American College of Surgeons and the American Cancer Society. It is viewed as an important tool for questions involving cancer treatment in the US[9]. The NCDB has previously been utilized for investigations in sarcoma[10–12] and the effect of facility volume on some cancer outcomes[13–15], but not the effect of facility volume in sarcoma.
Our goal was to use the NCDB to create a propensity score matched analysis comparing low-volume and high-volume facilities. Our primary outcome of interest was overall survival at 2, 5, and 10 years, with secondary outcomes of treatment decisions (use of radiation or chemotherapy, type of surgery) and treatment outcomes (positive margins, 30-day readmissions).
Materials and Methods
We queried the NCDB from 1998 to 2012 to capture all patients 18 years of age and older with a diagnostically confirmed STS in the upper or lower extremity treated with surgical excision. We excluded any subjects with metastatic disease, unknown staging information, or unknown vital status. Any patients with an additional malignancy, other than the known STS, were excluded to reduce the possibility of recording death or adverse events from an unrelated condition. Similarly, any patients with missing data for any of our variables of interest were removed from the analysis. We obtained patient age, sex, race, insurance status, distance to treating facility, tumor grade, tumor size, and tumor site directly from the database. We included undifferentiated pleomorphic sarcoma, fibrosarcoma, liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor (MPNST), and their subtypes. We did not include well-differentiated liposarcoma (atypical lipomatous tumors) or dermatofibrosarcoma protuberans due to their indolent course and low risk to metastasize. We used two socioeconomic measures, ZIP code based median household income and percentage of population with a high school degree, to create a composite socioeconomic (SES) summary variable, as has been done in previous reports[16,17].
Our primary outcome of interest was overall survival after treatment for sarcoma, measured at 2-, 5-, and 10-years post treatment. Institutions that participate in the NCDB are required to submit survival data annually, which is reported as all-cause survival. Further investigation into cause of death, and therefore cause-specific survival, is not possible. We also investigated secondary outcomes of treatment decisions (use of radiation or chemotherapy and type of surgery) and treatment results (surgical margins and 30-day readmissions). Radiation and chemotherapy are only recorded in the database when used in the first course of treatment. Margins classified as microscopic (e.g. an R1 resection) and macroscopic (e.g. an R2 resection) were both considered “positive.”
Statistical Analysis
A propensity score analysis, consisting of matching, stratification, and logistic regression of clinically important predictor variables, is a powerful tool for reducing selection bias in observational studies by mimicking aspects of randomization[18]. Prior investigations have utilized a similar technique to study the volume-outcome relationship in health care[13,19]. In this study, propensity scores were used to match patients on demographic (age, sex, race, SES composite, insurance) and tumor (histology, grade, size, site) variables to ensure that patients treated in low-volume centers shared clinical characteristics with those managed in high-volume facilities. We combined histology into five groups – fibrous (including undifferentiated pleomorphic sarcoma, malignant fibrous histiocytoma, myxofibrosarcoma, and other fibrosarcoma subtypes), lipomatous (including myxoid liposarcoma and other liposarcoma subtypes), leiomyosarcoma and its subtypes, synovial sarcoma, and malignant peripheral nerve sheath tumor. These groups were used for propensity matching and for the multivariate comparisons. This process was successful at selecting an equal number of subjects with comparable baseline patient and tumor characteristics (Table 1).
Table 1.
A comparison of the baseline characteristics between high- and low-volume facilities prior to and after propensity score matching
| Variable | Low Volume – Unmatched | Low Volume - Matched | High Volume | p-value |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | Unmatched/matched | |
| Age | ||||
| <60 | 2808 (52) | 1370 (56) | 1350 (55) | 0.021 / 0.564 |
| ≥60 | 2629 (48) | 1067 (44) | 1087 (45) | |
| Sex | ||||
| Male | 2842 (52) | 1311 (54) | 1296 (53) | 0.455 / 0.667 |
| Female | 2595 (48) | 1126 (46) | 1141 (47) | |
| Race | ||||
| White | 4606 (85) | 2147 (88) | 2103 (86) | 0.026 / 0.168 |
| Black | 596 (11) | 226 (9) | 259 (11) | |
| Other | 235 (4) | 64 (3) | 75 (3) | |
| Histology | ||||
| Fibrous | 2994(55) | 1372(56) | 1368(56) | <0.001/0.487 |
| Lipomatous | 1202(22) | 545(22) | 522(21) | |
| Leiomyosarcoma | 870(16) | 319(13) | 311(13) | |
| Synovial sarcoma | 268(5) | 154(7) | 179(7) | |
| MPNST+ | 103(2) | 47(2) | 57(2) | |
| Grade | ||||
| Low grade | 2238 (41) | 820 (34) | 814 (33) | <0.001 / 0.856 |
| High grade | 3199 (59) | 1617 (66) | 1623 (67) | |
| Size | ||||
| ≤ 5 cm | 2141 (39) | 821 (34) | 804 (33) | <0.001 / 0.875 |
| 5-10 cm | 1739 (32) | 799 (33) | 808 (33) | |
| >10 cm | 1557 (29) | 817 (33) | 825 (34) | |
| Site | ||||
| Upper Extremity | 1365 (25) | 515 (21) | 544 (22) | 0.008 / 0.314 |
| Lower Extremity | 4072 (75) | 1922 (79) | 1893 (78) | |
| SES* | ||||
| <4 | 922 (17) | 408 (17) | 409 (17) | 0.039 / 0.670 |
| 4-5 | 1267 (23) | 602 (25) | 638 (26) | |
| 6-7 | 1549 (29) | 693 (28) | 679 (28) | |
| 8 | 1699 (31) | 734 (30) | 711 (29) | |
| Insurance | ||||
| Not insured | 303 (6) | 68 (3) | 79 (3) | <0.001 / 0.302 |
| Private | 2761 (51) | 1405 (58) | 1377 (57) | |
| Medicaid | 339 (6) | 98 (4) | 114 (5) | |
| Medicare | 1886 (35) | 813 (33) | 793 (33) | |
| Other government | 45 (1) | 29 (1) | 40 (2) | |
| Unknown | 103 (2) | 24 (1) | 34 (1) |
MPNST – malignant peripheral nerve sheath tumor,
SES – socioeconomic status composite score
Bivariate methods (chi square and t-tests) were performed to determine univariate measures of association for distance to treating facility, surgery type, surgical margins, and 30-day readmissions. Next, a Kaplan-Meier survival analysis with a log-rank test was used to determine the effect of hospital volume on patient survival over 10 years. Finally, a multivariate Cox proportional hazards model was performed at 2, 5, and 10 years controlling for hospital volume, patient demographics, tumor characteristics, and treatment factors. All statistical analyses were performed using SAS (version 9.3; SAS Institute, Cary, NC).
This investigation was determined to be exempt from Institutional Review Board review as it included only secondary, deidentified data. The NCDB performs a review of the project proposal prior to release of the data set. The views expressed by the authors do not necessarily reflect those of the American College of Surgeons or the American Cancer Society.
Results
The initial study population consisted of 7,874 cases of STS that fit our criteria. To define treating facilities as either high- or low-volume, we investigated each center’s annual volume of STS patients from 1998 to 2012. Annual case volume, as opposed to total case volume in the database, was a more representative metric because not all hospitals contributed patients for all the years of our investigation. Out of a total of 1,200 facilities that treated at least one extremity STS in one year, we classified those with an average annual sarcoma volume of 10 or more (22 facilities, 2%) as high-volume, and those that treated less than 10 (1,178 facilities, 98%) as low-volume. A total of 2,437 patients (31% of cohort) received treatment at high-volume centers and 5,437 patients (69% of cohort) were managed at low-volume centers. The high-volume centers treated a mean of 14.6 patients annually (standard deviation 6.5, median 12.3 [range 10.1-39.4]) and the low volume centers treated a mean of 1.5 patients annually (standard deviation 1.0, median 1.2 [range 1.0-8.5]) (p<0.001). After matching, the 2,437 high-volume patients had a mean follow-up of 50.7 months (standard deviation 35.8), and the 2,437 low-volume patients had a mean follow-up of 51.2 months (standard deviation 36.5).
The mean distance traveled to receive treatment at a high-volume center was substantially farther than low-volume hospitals (84 miles vs. 39 miles, p <0.001) (Table 2). There was no clear difference in the frequency of 30-day readmissions (7% vs. 7%, p =0.804) or limb salvage surgeries (92% vs. 92%, p =0.667) in low- or high-volume centers. High-volume centers revealed greater use of chemotherapy (22% vs. 17%, p<0.001), a similar use of external beam radiation (55% vs. 52%, p =0.108), and fewer positive surgical margins (12% vs. 17%, p < 0.001).
Table 2.
Univariate comparisons of treatment factors for high- and low-volume centers
| Variable | Low Volume | High Volume | P-value |
|---|---|---|---|
| Mean miles to treating center (St. Dev.) | 38.9 (143.5) | 83.7 (196.8) | <0.001 |
| Surgery Type (%) | |||
| Limb Salvage | 2254 (92) | 2246 (92) | 0.667 |
| Amputation | 183 (8) | 191 (8) | |
| Radiation Treatment (%) | |||
| None | 1164 (48) | 1108 (45) | 0.108 |
| External Beam | 1273 (52) | 1329 (55) | |
| Chemotherapy (%) | |||
| No | 2028 (83) | 1902 (78) | <0.001 |
| Yes | 409 (17) | 535 (22) | |
| Margins (%) | |||
| Negative | 2034 (83) | 2144 (88) | <0.001 |
| Positive | 403 (17) | 293 (12) | |
| 30-day Readmission (%) | |||
| No | 1861 (93) | 1922 (93) | |
| Yes | 139 (7) | 148 (7) | 0.804 |
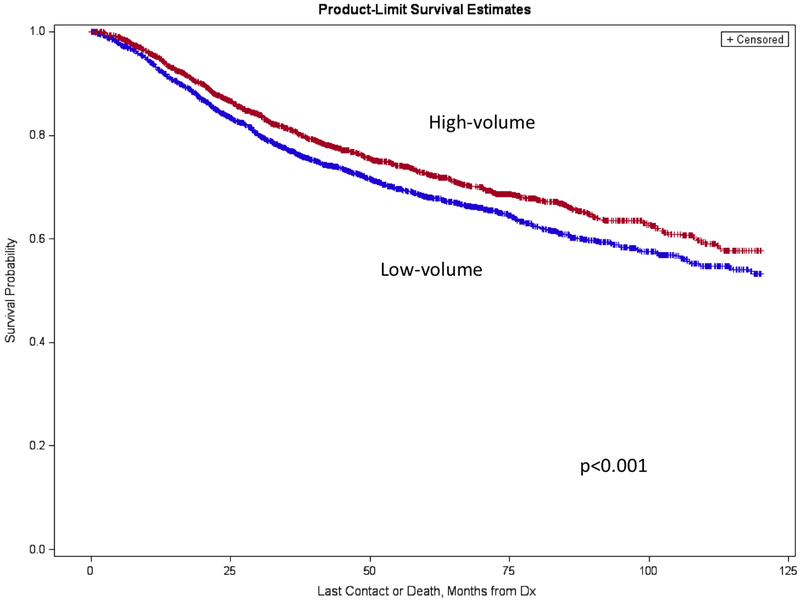
High-volume centers demonstrated a favorable overall survival at 2, 5, and 10 years after treatment compared to low-volume hospitals (Table 3). The differences were apparent by 2 years and maintained 10 years after diagnosis (Figure 1).
Table 3.
Univariate comparison of 2-, 5-, and 10-year survival between low and high volume centers
| 2-year (95%CI) | 5-year (95% CI) | 10-year (95% CI) | |
|---|---|---|---|
| Low-Volume | 84.0 (82.5-85.5) | 68.1 (66.0-70.2) | 53.3 (50.0-56.6) |
| High-Volume | 87.0 (85.6-88.4) | 72.7 (70.6-74.7) | 57.6 (54.1-61.1) |
| p-value | 0.003 | 0.001 | 0.001 |
Figure 1.
Kaplan-Meier analysis displaying 10-year survival curves in high- and low-volume centers.
A multivariate Cox proportional hazards model identified several independent prognostic factors for mortality in extremity STS (Table 4). In decreasing magnitude, risk factors for increased mortality at 5 years included size >10 cm (HR = 3.71, 95% CI 3.12-4.42), high-grade (HR = 3.17, 95% CI 2.63-3.81), size 5-10 cm (HR = 1.96, 95% CI 1.65-2.34), age ≥60 years (HR = 1.52, 95% CI 1.27-1.81), Medicare insurance (HR = 1.49, 95% CI 1.26-1.77), positive margins (HR = 1.29, 95% CI 1.10-1.51), composite SES score <4 (HR = 1.25, 95% CI 1.05-1.50), treatment at a low-volume facility (HR = 1.24, 95% CI 1.10-1.39), and male sex (HR = 1.17, 95% CI 1.04-1.32). Survival benefits were seen in lipomatous tumors (HR = 0.55, 95% CI 0.46-0.66) and in patients treated with radiation (HR = 0.69, 95% CI 0.61-0.78). A similar benefit was not observed in patients treated with chemotherapy (HR = 0.98, 95% CI 0.84-1.13).
Table 4.
Multivariate Cox Proportional Hazard ratios (HR) and 95% Confidence Intervals (CI) for mortality at 2, 5, and 10 years
| Variable | 2 years | 5 years | 10 years | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age | ||||||
| <60 | Ref | Ref | Ref | |||
| ≥60 | 1.47 | 1.16-1.86 | 1.52 | 1.27-1.81 | 1.57 | 1.34-1.85 |
| Sex | ||||||
| Female | Ref | Ref | Ref | |||
| Male | 1.15 | 0.98-1.34 | 1.17 | 1.04-1.32 | 1.18 | 1.06-1.31 |
| Race | ||||||
| White | Ref | Ref | Ref | |||
| Black | 1.22 | 0.96-1.56 | 1.14 | 0.94-1.38 | 1.13 | 0.94-1.34 |
| Other | 0.81 | 0.45-1.44 | 0.81 | 0.54-1.23 | 0.80 | 0.53-1.19 |
| Histology | ||||||
| Fibrous | Ref | Ref | Ref | |||
| Lipomatous | 0.49 | 0.38-0.64 | 0.55 | 0.46-0.66 | 0.59 | 0.50-0.70 |
| Leiomyosarcoma | 0.81 | 0.62-1.05 | 0.94 | 0.78-1.14 | 1.08 | 0.90-1.13 |
| Synovial sarcoma | 0.56 | 0.38-0.85 | 0.93 | 0.73-1.20 | 1.04 | 0.82-1.30 |
| MPNST+ | 1.09 | 0.68-1.75 | 1.10 | 0.76-1.59 | 1.26 | 0.90-1.75 |
| Grade | ||||||
| Low grade | Ref | Ref | Ref | |||
| High grade | 6.38 | 4.59-8.89 | 3.17 | 2.63-3.81 | 2.61 | 2.23-3.06 |
| Size | ||||||
| ≤5 cm | Ref | Ref | Ref | |||
| 5-10 cm | 2.55 | 1.96-3.31 | 1.96 | 1.65-2.34 | 1.85 | 1.58-2.16 |
| >10 cm | 5.23 | 4.05-6.76 | 3.71 | 3.12-4.42 | 3.31 | 2.82-3.87 |
| Site | ||||||
| Upper Extremity | Ref | Ref | Ref | |||
| Lower Extremity | 1.08 | 0.87-1.33 | 1.05 | 0.90-1.22 | 1.06 | 0.91-1.22 |
| SES* | ||||||
| 8 | Ref | Ref | Ref | |||
| 6-7 | 1.03 | 0.83-1.28 | 1.06 | 0.90-1.24 | 1.11 | 0.96-1.28 |
| 4-5 | 1.21 | 0.97-1.50 | 1.17 | 0.99-1.37 | 1.18 | 1.02-1.37 |
| <4 | 1.19 | 0.93-1.51 | 1.25 | 1.05-1.50 | 1.25 | 1.06-1.47 |
| Insurance | ||||||
| Private insurance | Ref | Ref | Ref | |||
| Not insured | 1.43 | 0.91-2.23 | 1.20 | 0.84-1.72 | 1.25 | 0.90-1.75 |
| Medicaid | 1.16 | 0.79-1.72 | 1.22 | 0.92-1.62 | 1.27 | 0.97-1.66 |
| Medicare | 1.54 | 1.22-1.94 | 1.49 | 1.26-1.77 | 1.57 | 1.34-1.84 |
| Other government | 1.19 | 0.59-2.42 | 1.34 | 0.80-2.24 | 1.34 | 0.81-2.20 |
| Unknown | 2.56 | 1.39-4.70 | 1.77 | 1.04-3.02 | 1.92 | 1.20-3.07 |
| Margins | ||||||
| Negative | Ref | Ref | Ref | |||
| Positive | 1.50 | 1.23-1.82 | 1.29 | 1.10-1.51 | 1.22 | 1.06-1.42 |
| Radiation | ||||||
| No | Ref | Ref | Ref | |||
| External beam | 0.57 | 0.49-0.67 | 0.69 | 0.61-0.78 | 0.73 | 0.65-0.82 |
| Chemotherapy | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 0.91 | 0.75-1.11 | 0.98 | 0.84-1.13 | 0.94 | 0.81-1.07 |
| Volume | ||||||
| High | Ref | Ref | Ref | |||
| Low | 1.26 | 1.10-1.47 | 1.24 | 1.10-1.39 | 1.22 | 1.10-1.36 |
MPNST – malignant peripheral nerve sheath tumor
SES = socioeconomic composite score
Discussion
Soft tissue sarcoma is a family of neoplasia that reflects many salient considerations in the debate between healthcare access and regionalization. From one perspective, decentralizing treatment into many local lower-volume centers would provide a convenient outlet for expeditious patient evaluation and may reduce logistical burdens, diagnostic delays, or social challenges with treatment or recovery. Alternatively, STS is a condition that traditionally requires a team of specialized experts, and consolidating treatment into a small number of higher-volume tertiary care centers may demonstrate improved adherence to practice guidelines and result in better and more predicable outcomes. Our goal was to investigate a propensity score matched cohort of patients treated at high- and low-volume facilities to determine if there were clear differences in treatment decisions, treatment results, and overall survival. We determined that high-volume centers demonstrated a higher utilization of chemotherapy, fewer positive margins, and improved overall survival. There are several findings that warrant further discussion.
Many reports have discussed the effect of hospital volume on various surgical procedures and medical conditions, the vast majority reporting superior outcomes in higher volume centers[20–24]. In sarcoma specifically, there are a number of reports discussing retroperitoneal soft tissue sarcoma[25–28], but few focused on extremity soft tissue sarcoma. In patients with extremity STS, as reported by Guttierez et al, there was a higher rate of limb salvage and radiation utilization in high-volume centers, but the authors did not see a clear difference in 5- or 10-year survival[29]. A recent report from the Netherlands demonstrated improved adherence to treatment guidelines at high-volume facilities, but also did not show a difference in 5-year survival in high- and low-volume centers[30]. Ogura et al found that high-volume centers experienced fewer postoperative complications and in-patient mortality than low-volume centers, but did not comment on overall survival[31].
Our findings suggest increased utilization of chemotherapy, and a similar utilization of radiation, at high-volume centers. It is not clear from the data set whether the increased use of adjuvant measures are appropriate or excessive, but this may reflect increased access to novel therapeutics through clinical trials at high-volume facilities. Contrary to Guttierez et al, we did not find that the amputation rates differed between high- and low-volume centers. This could possibly be explained by a more modern investigative cohort, as our period of interest was 1-2 decades later than the previous study and at a time when limb salvage surgery is ubiquitous and the standard-of-care for extremity STS.
We found a lower rate of positive margins and improved survival at high-volume facilities. These are important findings as, in most cases, sarcoma excision is performed with the goal of negative margins, and a positive margin may reflect a failure of planning or execution. Similarly, prolonged survival is the primary goal of cancer treatment, and is the most meaningful metric when comparing the outcomes of two groups. Contrary to prior reports, we found a clear difference in survival that was apparent by 2 years post treatment and maintained to 10 years. We may have been able to detect this difference given our use of propensity score matching, as lower-grade and smaller tumors are overrepresented at low-volume centers and have an inherently favorable prognosis[29]. The relationship of survival to facility volume was apparent using a risk-adjusted cohort in a multivariate model for which other patient, tumor, and treatment factors were accounted. Thus, our data clearly demonstrate that facility volume is an independent risk factor for diminished survival in extremity STS. This result is not surprising given similar volume-based conclusions in pancreatic and esophageal cancers, both rare conditions requiring a team of experts for optimal management[20]. However, the ultimate implications of these findings, specifically regarding the delivery of specialty care, are interesting and complex.
A prior report by Halm et al noted that higher-volume institutions experienced fewer inpatient deaths than lower-volume institutions in a number of conditions, but with substantial variation in the magnitude, and implied clinical significance, of the survival benefit[32]. For instance, entities such as pancreatic and esophageal cancers demonstrated clear superiority at high-volume centers[20], likely due to the rarity and complexity of diagnosis and management, while more common and straightforward procedures such as colon cancer showed a difference of much less clinical significance[33]. Soft tissue sarcoma compares more favorably to the magnitude of differences seen in rare malignancies requiring multidisciplinary management, and treatment of STS in a high-volume center demonstrated a clear long-term survival benefit, not simply differences in short-term adverse events or treatment decisions.
Recent efforts to improve survival in soft tissue sarcoma have generally ended disappointingly with little deviation from the results of standard treatment[34,35]. Outcome improvement in sarcoma has traditionally centered on optimizing surgical techniques, introducing adjuvant treatments, and manufacturing new medical therapies. Interestingly, it was the use of radiation, and not chemotherapy, that resulted in a survival benefit in our multivariate analysis, further highlighting the need for effective systemic treatment. While there is no question that better options for recalcitrant sarcoma, in particular metastatic disease, are needed through clinical trials and other research efforts to improve survival in many patients, the results of the current study should not be ignored. Specifically, our data imply that changes in the delivery of care, in addition to the care itself, may make a tangible impact on patient survival. The solutions are not obvious or easy, and there are many potential barriers including long travel distances, insurance coverage, time off of work, lack of social support, financial hardship, and other logistical concerns that may diminish the feasibility of evaluation and treatment of every patient in a high-volume center. At the very least, increased awareness of differences in outcomes dependent on facility volume by providers and informed patients may allow for increased referrals to hospitals best equipped to manage the myriad complexities of soft tissue sarcoma. In addition, focusing resources on increasing access to high-volume centers, such as transportation support, local housing during treatment, telemedicine evaluations for surveillance, and outreach clinics, may prove an effective intervention to optimize appropriate referrals and outcomes within the limitations of the current treatment modalities and health systems.
There are several limitations to this investigation. First, our analysis was centered on hospital volume, not surgeon volume. Prior reports have shown mixed correlations between hospital and surgeon volume and outcomes[8,13,36,37]; we believe that facility volume is a more important metric in sarcoma as it is a condition that requires multidisciplinary care and optimal treatment is dependent on more than individual surgical prowess. Next, the NCDB reports overall survival only and some of our patients may have died from causes unrelated to their underlying sarcoma. However, this would have been an equal risk in both cohorts and we have no reason to believe that death from other causes would be dramatically different between the groups. The cut-off of 10 STS annually that we chose to distinguish between high- and low-volume centers is supported by prior reports[30], but is relatively arbitrary and underestimates the total number of sarcomas treated by excluding bone, metastatic, recurrent, retroperitoneal, uterine, and head and neck sarcoma. We believe this approach is justified, as our goal was not to determine an optimal number of sarcomas for a center to treat, but rather to investigate general differences in treatment and outcome as a function of relative facility volume. Future work should determine if there are a minimum number of cases of STS that a facility should treat to attain optimal outcomes. Finally, a common limitation of most large data sources is the lack of information given the specifics of individual patient presentations and treatment decision-making. In particular, “chemotherapy” is recorded generally, and the specific drugs and dosages are unknown. It is possible that a specific systemic intervention did demonstrate a survival benefit that we were not able to detect given the limitations of the database. Providers and patients alike continue to be in dire need of novel and effective systemic treatments in sarcoma.
Our results support the conclusion that high-volume centers provide superior outcomes, in the form of fewer positive margins and improved survival, in patients with soft tissue sarcoma. Treating providers and patients alike should be aware that, although high-volume centers may be at greater distance and present increased logistical challenges, management by a team of experienced specialists could provide real benefits in long-term overall survival. Future efforts should focus on strategies to improve access to regional facilities with infrastructure and experience in sarcoma treatment.
Synopsis:
We used the National Cancer Database to create a propensity-matched cohort of low- and high-volume facilities treating extremity soft tissue sarcoma. We found that high-volume facilities demonstrated a lower rate of positive margins and a higher overall survival at 2, 5, and 10 years. This information will be helpful in future discussions regarding regionalization and access in extremity soft tissue sarcoma.
Footnotes
Work performed at the University of Iowa, Iowa City, Iowa
References
- 1.Hui JY: Epidemiology and Etiology of Sarcomas. Surg Clin North Am 2016;96:901–914. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Facts and Figures 2017. In “American Cancer Society.” 2017. [Google Scholar]
- 3.Cheung MC, Koniaris LG, Perez EA, et al. : Impact of hospital volume on surgical outcome for head and neck cancer. Ann Surg Oncol 2009;16:1001–1009. [DOI] [PubMed] [Google Scholar]
- 4.Begg CB, Cramer LD, Hoskins WJ, Brennan MF: Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 5.Bach PB, Cramer LD, Schrag D, et al. : The influence of hospital volume on survival after resection for lung cancer. N Engl J Med 2001;345:181–188. [DOI] [PubMed] [Google Scholar]
- 6.Soohoo NF, Zingmond DS, Lieberman JR, Ko CY: Primary total knee arthroplasty in California 1991 to 2001: does hospital volume affect outcomes? J Arthroplasty 2006;21:199–205. [DOI] [PubMed] [Google Scholar]
- 7.Doro C, Dimick J, Wainess R, et al. : Hospital volume and inpatient mortality outcomes of total hip arthroplasty in the United States. J Arthroplasty 2006;21:10–16. [DOI] [PubMed] [Google Scholar]
- 8.Manley M, Ong K, Lau E, Kurtz SM: Total knee arthroplasty survivorship in the United States Medicare population: effect of hospital and surgeon procedure volume. J Arthroplasty 2009;24:1061–1067. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Stewart AK, Winchester DP, Ko CY: The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kneisl JS, Coleman MM, Raut CP: Outcomes in the management of adult soft tissue sarcomas. J Surg Oncol 2014;110:527–538. [DOI] [PubMed] [Google Scholar]
- 11.Corey RM, Swett K, Ward WG: Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer Med 2014;3:1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarides AL, Eward WC, Speicher PJ, et al. : The Use of Radiation Therapy in Well-Differentiated Soft Tissue Sarcoma of the Extremities: An NCDB Review. Sarcoma 2015;2015:186581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waingankar N, Mallin K, Smaldone M, et al. : Assessing the relative influence of hospital and surgeon volume on short-term mortality after radical cystectomy. BJU Int 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman BC, Paniccia A, Hosokawa PW, et al. : Impact of Facility Type and Surgical Volume on 10-Year Survival in Patients Undergoing Hepatic Resection for Hepatocellular Carcinoma. J Am Coll Surg 2017;224:362–372. [DOI] [PubMed] [Google Scholar]
- 15.Stokes W, Amini A, Maroni PD, et al. : Patterns of care and survival outcomes for adolescent and young adult patients with testicular seminoma in the United States: A National Cancer Database analysis. J Pediatr Urol 2017. [DOI] [PubMed] [Google Scholar]
- 16.Du XL, Fang S, Coker AL, et al. : Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer 2006;106:1276–1285. [DOI] [PubMed] [Google Scholar]
- 17.Miller BJ, Gao Y, Duchman KR: Does surgery or radiation provide the best overall survival in Ewing’s sarcoma? A review of the National Cancer Data Base. J Surg Oncol 2017. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC: An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YW, Mahal BA, Muralidhar V, et al. : Association Between Treatment at a High-Volume Facility and Improved Survival for Radiation-Treated Men With High-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2016;94:683–690. [DOI] [PubMed] [Google Scholar]
- 20.Speicher PJ, Englum BR, Ganapathi AM, et al. : Traveling to a High-volume Center is Associated With Improved Survival for Patients With Esophageal Cancer. Ann Surg 2017;265:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munasinghe A, Markar SR, Mamidanna R, et al. : Is It Time to Centralize High-risk Cancer Care in the United States? Comparison of Outcomes of Esophagectomy Between England and the United States. Ann Surg 2015;262:79–85. [DOI] [PubMed] [Google Scholar]
- 22.La Torre M, Nigri G, Ferrari L, et al. : Hospital volume, margin status, and long-term survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2012;78:225–229. [PubMed] [Google Scholar]
- 23.Luft HS, Bunker JP, Enthoven AC: Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364–1369. [DOI] [PubMed] [Google Scholar]
- 24.Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB: Hospital volume and operative mortality in the modern era. Ann Surg 2014;260:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurice MJ, Yih JM, Ammori JB, Abouassaly R: Predictors of surgical quality for retroperitoneal sarcoma: Volume matters. J Surg Oncol 2017. [DOI] [PubMed] [Google Scholar]
- 26.Bonvalot S, Miceli R, Berselli M, et al. : Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol 2010;17:1507–1514. [DOI] [PubMed] [Google Scholar]
- 27.van Dalen T, Hennipman A, Van Coevorden F, et al. : Evaluation of a clinically applicable post-surgical classification system for primary retroperitoneal soft-tissue sarcoma. Ann Surg Oncol 2004;11:483–490. [DOI] [PubMed] [Google Scholar]
- 28.Bonvalot S, Rivoire M, Castaing M, et al. : Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol 2009;27:31–37. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez JC, Perez EA, Moffat FL, et al. : Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg 2007;245:952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoekstra HJ, Haas RLM, Verhoef C, et al. : Adherence to Guidelines for Adult (Non-GIST) Soft Tissue Sarcoma in the Netherlands: A Plea for Dedicated Sarcoma Centers. Ann Surg Oncol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogura K, Yasunaga H, Horiguchi H, et al. : Impact of hospital volume on postoperative complications and in-hospital mortality after musculoskeletal tumor surgery: analysis of a national administrative database. J Bone Joint Surg Am 2013;95:1684–1691. [DOI] [PubMed] [Google Scholar]
- 32.Halm EA, Lee C, Chassin MR: Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 2002;137:511–520. [DOI] [PubMed] [Google Scholar]
- 33.Bos AC, van Erning FN, Elferink MA, et al. : No Difference in Overall Survival Between Hospital Volumes for Patients With Colorectal Cancer in The Netherlands. Dis Colon Rectum 2016;59:943–952. [DOI] [PubMed] [Google Scholar]
- 34.Ratan R, Patel SR: Chemotherapy for soft tissue sarcoma. Cancer 2016;122:2952–2960. [DOI] [PubMed] [Google Scholar]
- 35.Comandone A, Petrelli F, Boglione A, Barni S: Salvage Therapy in Advanced Adult Soft Tissue Sarcoma: A Systematic Review and Meta-Analysis of Randomized Trials. Oncologist 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harmon JW, Tang DG, Gordon TA, et al. : Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 1999;230:404–411; discussion 411–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karanicolas PJ, Dubois L, Colquhoun PH, et al. : The more the better?: the impact of surgeon and hospital volume on in-hospital mortality following colorectal resection. Ann Surg 2009;249:954–959. [DOI] [PubMed] [Google Scholar]