Abstract
Background
The transmission of visceral nociception can be inhibited by electroacupuncture (EA) at the spinal level. However, relationships between current intensity and EA-induced analgesia are still lacking. This study compares the effects of different intensities of EA at local acupoints and heterotopic acupoints on nociceptive responses of spinal wide dynamic range (WDR) neurons induced by noxious colorectal distension (CRD).
Materials and methods
Experiments were conducted on 40 Sprague Dawley rats anesthetized with 10% urethane. Discharges of WDR neurons in the L1–L3 segments of the dorsal horn of the spinal cord were recorded extracellularly by glass micropipettes. Different intensities of EA (0.5, 1, 2, 4, 6, and 8 mA, 0.5 ms, 2 Hz) were applied to contralateral “Zusanli” (ST 36) or “Neiguan” (PC 6), with either the same or different segmental innervation of the colon.
Results
In local acupoints, the increased discharges of WDR neurons evoked by CRD were significantly inhibited by EA at 0.5–8 mA. A positive relationship between current intensity and the inhibiting rate was observed within 0.5–4 mA, but the inhibiting rate reached a plateau when EA exceeded 4 mA. In heterotopic acupoints, the increased discharges of WDR neurons evoked by CRD were significantly inhibited by EA at 2–8 mA. A positive relationship between current intensity and the inhibiting rate was observed within 2–6 mA. Further increase in the current beyond 6 mA also resulted in a plateau effect.
Conclusion
Within a certain range, the nociceptive responses of dorsal horn neurons induced by CRD could be inhibited by EA in an intensity-dependent manner.
Keywords: electroacupuncture, current intensity, wide dynamic range, colorectal distension
Introduction
Visceral pain is one of the most common forms of pain caused by stress or injury arising from the internal organs.1,2 Electroacupuncture (EA) has been widely used for analgesia by passing electrical current to acupoints via acupuncture needles connected to an electrical stimulator. Accumulating evidence suggests that EA is an efficacious treatment for alleviating acute and chronic visceral pain.3–5 Interestingly, such an antinociceptive effect was not seen in sham EA without electrical stimulation, even if sham EA is applied to the same acupoints as used in the real EA group.6 Consequently, we speculate that current intensity is important for effective analgesia. In fact, many human and animal studies have shown that EA with different intensities can excite different types of peripheral afferent fibers and produce different extents of analgesia.7–9 However, a comprehensive understanding of the relationship between current intensity and EA-induced analgesia on visceral pain is still unknown.
Electrophysiological studies can directly examine the mechanism of EA-induced analgesia. It is generally accepted that EA achieves analgesic effects on visceral pain via somato-visceral interactions on convergent neurons at different levels of the central nervous system.10 The wide dynamic range (WDR) neurons of the spinal dorsal horn could play an important role in the management of visceral nociception.11 These neurons receive a convergence of inputs from the external environment (the skin) and the internal milieu (the viscera, muscles, etc.).12 We have previously confirmed that WDR neurons could be activated by EA and manual acupuncture in normal rats.13 Interestingly, in rats with noxious colorectal distension (CRD), the nociceptive response of WDR neurons could be inhibited by manual acupuncuture.14 These observations indicate the involvement of WDR neurons in the antinociceptive effects of EA on visceral pain. It is worth noting that WDR neurons can encode the strength of mechanical stimuli in a certain range. The discharges of WDR neurons linearly increase during graded electrical or thermal stimuli.15,16 As such, it is evident that these neurons may respond to graded intensities of EA.
Therefore in this study, we observe the responses of WDR neurons to different intensities of EA in rats with noxious CRD stimulation and we aim to explain the relationships between current intensity and the effects of EA on inhibiting nociceptive visceral afferents.
Materials and methods
Animals
Experiments were performed on 40 male Sprague Dawley rats weighing between 220 and 280 g that were purchased from the Laboratory Animal Center of China Academy of Military Medical Sciences (production license number: SCXK-(Military)-2012-0004; Beijing, China). After arrival, the rats were given food and water ad libitum and habituated to the experimental environment for at least 1 week. All animals were housed in standard conditions at an ambient temperature of 22°C±0.5°C and under artificial 12 hours light/dark cycle. All manipulations and procedures had been approved by Animal Ethics Committee of China Academy of Chinese Medical Science (no. 20160218) and were conducted according to the Guideline on the Humane Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of the People’s Republic of China in 2006.
Noxious visceral stimulus
Previous studies have shown that CRD stimuli with an intensity of >40 mmHg were recognized as noxious visceral stim uli.17 In this experiment, 60 mmHg CRD was administered as noxious visceral stimuli. In brief, a 6 cm balloon was gently inserted into the descending colon and rectum through the anus. During the recording sessions, the balloon was inflated with air, and the intracolonic pressure was monitored using a pressure transducer. The pressure of CRD stimulation applied to rats was 60 mmHg, lasting 50 seconds. To prevent possible sensitization triggered by frequent stimulation of colorectum, the interval between two bouts of CRD stimulation was at least 10 minutes.
EA stimulation
A pair of noninsulated needles (0.22 mm×25 mm, Beijing Zhongyan Taihe Medical Instrument Co. Ltd., Beijing, China) were inserted into acupoint ST 36 (on the anterolateral side of the hind limb near the anterior crest of the tibia below the knee under the tibialis anterior muscle) or PC 6 (on the medial of forelimb and 3 mm above the wrist) ~0.3 cm apart from each other. After insertion, acupoints were stimulated with different intensities of EA via the inserted needles connected to an electrical stimulator (88–102 G; Nihon Kohden, Tokyo, Japan). During a 30-second EA session, intensities of 0.5, 1, 2, 4, 6, and 8 mA were applied in a random order. The duration and frequency of electrical stimulation were set at 0.5 ms and 2 Hz, respectively.
Extracellular recording
After 12 hours of fasting, unit discharges of WDR neurons in anesthetized rats were recorded. Following intraperitoneal injection of 100 µg of atropine sulfate, the rats were deeply anesthetized with 10% urethane (1.0–1.2 g/kg) and were then artificially ventilated through an endotracheal tube. Body temperature was maintained at 37.5°C±0.5°C using a feedback-controlled heating blanket.
A laminectomy was performed at the lumbar spinal to expose the L1–L3 segments of the spinal cord, and the corresponding vertebrae were fixed in a rigid frame. Unitary extracellular recordings were made using glass micropipettes (8–12 MΩ) filled with a mixture of 2% Pontamine Sky Blue dye and 0.1 M of natrium asceticism. According to the three-dimensional coordinates of WDR neurons, 0.5–1.5 mm lateral to the midline and 500–1500 µm beneath the surface, micropipettes were then inserted into the right-hand side of the spinal cord. Neuronal discharges were routed to a window discriminator and displayed on an oscilloscope. Outputs of the window discriminator and amplifier were fed into a data collection system (PowerLab) and a personal computer-based data acquisition system (Chart 5.0) to compile histograms or watermark files.
Experimental procedure
After finding a neuron with stable discharges, noxious (pinch) and innocuous (brush) skin stimuli were used to identify the targeted neurons. As reported in our previous study,13 WDR neurons were excited by both the noxious and innocuous stimulation applied to their skin receptive fields. However, WDR neurons gave no response to stimulation applied to non-receptive fields. When a WDR neuron was identified, the responses of WDR neurons to pinch and brush skin stimuli were recorded, and the skin receptive fields of WDR neurons were mapped.
Second, the responses of WDR neurons to CRD stimulation were recorded. The responses of WDR neurons to different intensities of EA stimulation without CRD were then recorded.
Third, the responses of WDR neurons to different intensities of EA stimulation during CRD were recorded. A standard conditioned recording procedure was administered for 60 seconds: 1) The background discharge was recorded for 5 seconds. 2) 60 mmHg CRD stimulation was applied to rats for 50 seconds. In the first 10 seconds, the response of neuronal discharges to a single CRD stimulation was observed. 3) During CRD, different intensities of EA were applied at the contralateral ST 36 or PC 6 for 30 seconds. The effects of EA on CRD-induced neuronal discharges were observed. 4) After stopping EA, CRD stimulation was continued for 10 seconds. 5) After stopping CRD, the recovery of neuronal discharge was recorded for 5 seconds.
After single-unit recordings, the recording sites were marked by electrophoretic deposition of Pontamine Sky Blue and checked by H&E coloration. Locations of the recording sites were then determined using the rat brain atlas.
Data collection and statistical analysis
The average frequency of neuronal discharges (spikes/s) and the inhibitory percentage of EA on CRD-induced neuronal discharges were calculated and analyzed by PowerLab, Chart 5.0, and SPSS 13.0. All data are presented as mean ± standard error of the mean (SEM). Paired t-test analyses were used for statistical analysis. P<0.05 was deemed statistically significant.
Results
General characteristics of WDR neurons in the dorsal horn of the spinal cord
A total of 132 neurons were recorded in the L1–L3 segments of the dorsal horn of the spinal cord, among which 115 neurons were identified as WDR neurons. The receptive field of these neurons was distributed in the ipsilateral caudal parts of the body, including the hip region, tail root, hind limb, and hind paw (Figure 1A). The activity of WDR neurons was excited by both innocuous (brush) and noxious (pinch) stimulation of their peripheral receptive field (Figure 1B). Examination of the rat spinal slices verified that the majority of recording sites were located in laminae IV and V, and a few in laminae I and VI of the gray matter (Figure 1C). These sites were marked by electrophoresis with the Pontamine Sky Blue dye at the end of the experiment (Figure 1D).
Figure 1.

General features of WDR neurons.
Notes: (A) The peripheral receptive field of WDR neurons located in the L1– L3 spinal cord. (B) The response of a WDR neuron to non-noxious (brush) and noxious (pinch) stimulus. (C) The location of identified WDR neurons in the spinal cord. (D) A representative example showing the location of the WDR neuron marked by Pontamine Sky Blue.
Abbreviations: BG, background; WDR, wide dynamic range.
The response of WDR neurons to 60 mmHg CRD stimulation
If neurons were identified as WDR neurons, their response to CRD stimulation was tested. Among 115 WDR neurons, 106 (92.17%) neurons were excited by CRD, 3 (2.61%) were inhibited, and 6 (5.22%) showed no response to CRD. We observed the reaction of 10 WDR neurons to 60 mmHg CRD stimulation. An example of the activating reactions induced by CRD is shown in Figure 2A. After CRD, the average discharge frequency of WDR neurons increased from 2.83±0.32 spikes/s of background to 9.96±1.20 spikes/s (P<0.05), with an increasing rate of 259.94%±26.59% (Figure 2B). These data indicate that 60 mmHg CRD stimulation could significantly activate WDR neurons and could be recognized as visceral nociception.
Figure 2.

Responses of WDR neurons to 60 mmHg CRD stimulation.
Notes: (A) Representative example showing the activity of a WDR neuron evoked by 60 mmHg CRD stimulation. Top row shows the original unit discharges and bottom row shows the histograms. (B) The cumulative result shows that the discharge of WDR neurons increased significantly after CRD compared to BG. ***P<0.05 compared with BG.
Abbreviations: BG, background; CRD, colorectal distention; WDR, wide dynamic range.
The response of WDR neurons to graded intensities of EA stimulation without CRD
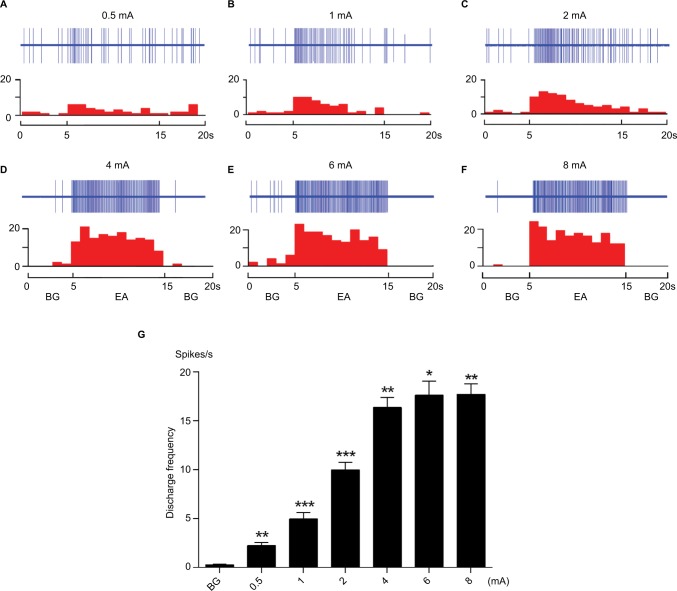
In this section of the experiment, we examined the response of WDR neurons to graded intensities of EA stimulation without CRD. As shown in Figure 3, the activity of WDR neurons was significantly enhanced by EA stimulation applied at ipsilateral ST 36, which was located in the receptive field of these neurons. Examples of neuronal discharges evoked by 0.5–8 mA EA stimulation are shown in Figure 3A–F. The cumulative results (n=8) showed that the discharges of WDR neurons evoked by EA increased as the intensity of EA increased within the range 0.5–4 mA. Further increases in current intensity beyond 4 mA resulted in a saturation effect of this neuron (Figure 3G).
Figure 3.
The responses of WDR neurons to graded intensity of EA stimulation at ipsilateral ST 36.
Notes: (A–F) Example showing the activity of a WDR neuron evoked by 0.5–8 mA EA stimulation. The top row shows original unit discharges and the bottom row represents this information in a histogram. (G) The cumulative result shows that the discharge of WDR neurons was significantly increased after EA. *P<0.05, **P<0.01, ***P<0.001 compared with BG.
Abbreviations: BG, background; EA, electroacupuncture, WDR, wide dynamic range.
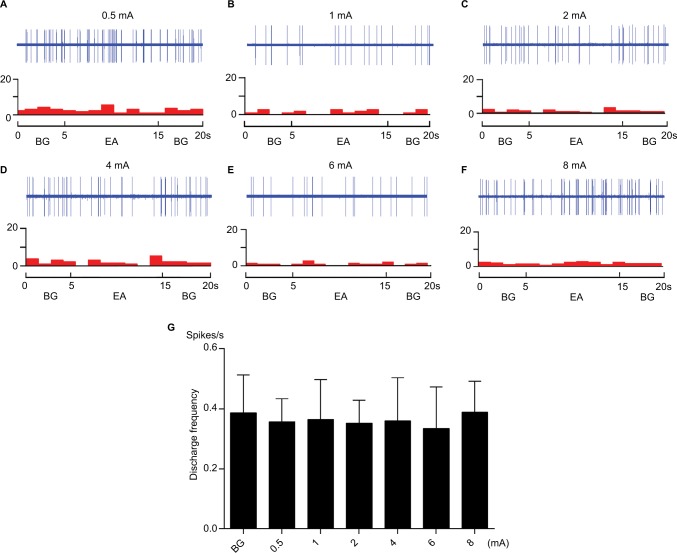
However, when EA stimulation was applied at contralateral ST 36 and PC 6, which were located in the nonreceptive field of WDR neurons, EA had no effect on the activity of WDR neurons (Figures 4 and 5). This is consistent with our previous study that WDR neurons only receive the inputs of EA from acupoints located in their skin receptive fields in the normal state.18
Figure 4.
The responses of WDR neurons to graded intensity of EA stimulation at contralateral ST 36.
Notes: (A–F) Example of neuronal response to EA stimulation at contralateral ST 36. The top row shows original unit discharges and the bottom row shows this information in a histogram. (G) The cumulative result shows that the discharge frequency of WDR neurons was not changed after EA.
Abbreviations: BG, background; EA, electroacupuncture; WDR, wide dynamic range.
Figure 5.
The responses of WDR neurons to graded intensity of EA stimulation at contralateral PC 6.
Notes: (A–F) Example of neuronal response to EA stimulation at contralateral PC 6. The top row shows original unit discharges and the bottom row shows this information in a histogram. (G) The cumulative result shows that the discharge frequency of WDR neurons was not changed after EA.
Abbreviations: BG, background; EA, electroacupuncture; WDR, wide dynamic range.
The response of WDR neurons to different intensities of EA at contralateral ST 36 during CRD
According to our previous studies,18 during CRD-induced acute visceral pain conditions, the nociceptive responses of WDR neurons could be inhibited by EA applied at acupoints located in their nonreceptive fields. However, EA at acupoints located in the receptive fields of WDR neurons could affect the nociceptive responses of these neurons. To explore the dose– effect relationship of EA on the nociceptive response of WDR neurons, EA stimulation was applied at contralateral ST 36.
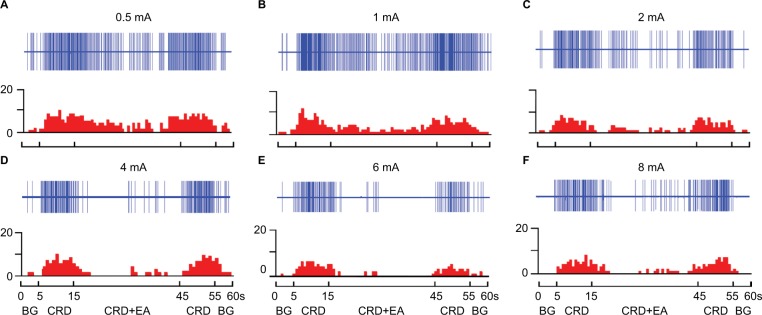
As illustrated in Figures 6 and 7, EA at 0.5 and 1 mA produced a slight but significant inhibition. After 0.5 mA of EA, the average discharge frequency of WDR neurons decreased from 8.59±0.41 spikes/s of CRD to 7.30±0.43 spikes/s (P<0.05, n=8), with an inhibiting percentage of 15.36%±1.86%. After 1 mA of EA, the neuronal discharges decreased from 8.47±0.63 spikes/s of CRD to 6.30±0.63 spikes/s (P<0.05, n=8), with an inhibiting percentage of 26.39%±3.04%.
Figure 6.
Examples of the response of WDR neurons to different intensities of EA at ST 36 during CRD.
Notes: EA at 0.5 mA (A), 1 mA (B), 2 mA (C), 4 mA (D), 6 mA (E), and 8 mA (F) was applied to ST 36 during CRD. A decrease in the discharges of WDR neurons during the CRD+EA sequence was observed. Top row shows the original unit discharges and bottom row shows the histograms.
Abbreviations: BG, background; CRD, colorectal distention; EA, electroacupuncture; WDR, wide dynamic range.
Figure 7.

The effects of different intensities of EA at ST 36 on CRD-induced discharges of WDR neurons.
Notes: (A) A histogram shows that the noxious discharges of WDR neurons induced by CRD can be inhibited by EA in the range 0.5–8 mA. Data consist of the average spikes per second (mean ± SEM). *P<0.05, **P<0.01, ***P<0.001, compared with CRD. (B) A stimulus–response curve shows that the inhibiting percentage increased as the intensity of EA increased with 0.5–4 mA.
Abbreviations: CRD, colorectal distention; EA, electroacupuncture; SEM, standard error of the mean; WDR, wide dynamic range.
EA at 2 mA produced a moderate inhibition, with the neuronal discharges decreasing from 8.87±0.98 spikes/s of CRD to 4.45±0.70 spikes/s, with an inhibiting percentage of 50.10%±5.53% (P<0.01, n=8). EA at 4 mA produced a strong inhibition, with neuronal discharges decreasing from 8.26±0.84 spikes/s of CRD to 3.01±0.48 spikes/s, with an inhibiting percentage of 64.64%±2.72% (P<0.001, n=8).
When the intensity of EA was further increased to 6 and 8 mA, the inhibiting effect of EA on nociceptive response of WDR neurons reached a plateau. After 6 mA of EA, the neuronal discharges decreased from 8.14±0.75 spikes/s of CRD to 2.67±0.18 spikes/s, with an inhibiting percentage of 66.37%±2.13% (P<0.001, n=8). After 8 mA of EA, the neuronal discharges decreased from 8.06±0.40 spikes/s of CRD to 2.77±0.35 spikes/s, with an inhibiting percentage of 65.91%±3.09% (P<0.001, n=8).
Response of WDR neurons to different intensities of EA at contralateral PC 6 during CRD
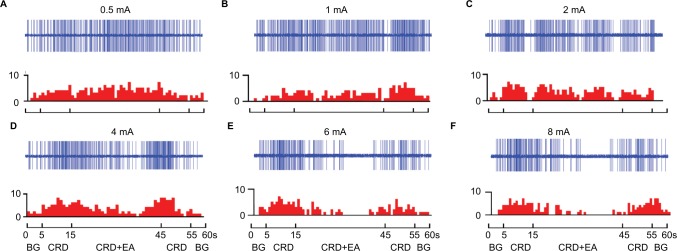
It is well known that the antinociceptive effects of EA treatment are different when EA is applied to acupoints with the same or different segmental innervation with the pain organs.7–9 For these experiments, EA stimulation was applied at contralateral PC 6, which innervates different segments of rectum and colon, to study the dose–effect relationship of EA at heterotopic acupoints on visceral nociception. As illustrated in Figures 8 and 9, the discharges of WDR neurons evoked by CRD were inhibited by 2–8 mA EA stimulation of PC 6.
Figure 8.
Examples of the response of WDR neurons to different intensities of EA at PC 6 during CRD.
Notes: EA at 0.5 mA (A), 1 mA (B), 2 mA (C), 4 mA (D), 6 mA (E), and 8 mA (F) was applied to PC 6 during CRD. A decrease in the discharges of WDR neurons during the CRD+ EA sequence was observed within 2–8 mA. Top rows show the original unit discharges and bottom rows show the histograms.
Abbreviations: BG, background; CRD, colorectal distention; EA, electroacupuncture.
Figure 9.

The effects of different intensities of EA at PC 6 on CRD-induced discharges of WDR neurons.
Notes: (A) A histogram shows that the noxious discharges of WDR neurons induced by CRD can be inhibited by EA in the range 2–8 mA. Data consist of the average spikes per second (mean ± SEM). ***P<0.001, compared with CRD. (B) A stimulus–response curve shows that the inhibiting percentage increased as the intensity of EA increased with 2–6 mA.
Abbreviations: CRD, colorectal distention; EA, electroacupuncture; SEM, standard error of the mean; WDR, wide dynamic range.
The cumulative results showed that EA with an intensity of 0.5 and 1 mA had no significant suppressive effect on CRD-induced noxious discharges of WRD neurons. Furthermore, 2 mA of EA produced a slight but significant inhibition, with the average discharge frequency of WDR neurons decreasing from 9.18±0.27 spikes/s of CRD to 7.30±0.45 spikes/s, and an inhibiting percentage of 19.45%±4.14% (P<0.001, n=8).
Additionally, 4 mA of EA produced a moderate inhibition, with neuronal discharges decreasing from 8.78±0.47 spikes/s of CRD to 5.51±0.28 spikes/s, and an inhibiting percentage of 36.39%±3.70% (P<0.001, n=8). Application of 6 mA of EA produced a strong inhibition, with neuronal discharges decreasing from 8.52±0.50 spikes/s of CRD to 3.77±0.38 spikes/s, with an inhibiting percentage of 56.12%±4.01% (P<0.05, n=8).
Interestingly, when the intensity of EA was further increased to 8 mA, the inhibiting effect of EA on nociceptive response of WDR neurons also reached a plateau. After 8 mA of EA, the neuronal discharges decreased from 8.20±0.49 spikes/s of CRD to 3.74±0.39 spikes/s and had an inhibiting percentage of 54.70%±3.69% (P<0.001, n=8).
Discussion
Since the early 1950s, treatment with EA has been widely used for analgesia in clinical practice. According to the traditional Chinese medicine theory and modern acupuncture researches, the analgesic effect of EA treatment can be specific and extensive. EA stimulation of acupoints located in the same segmental innervation of the pain area (local acupoints) can elicit segmental analgesia, whereas stimulation of acupoints away from the pain area (heterotopic acupoints) can elicit extrasegmental analgesia.8,9 We, therefore, sought to elucidate the relationship between current intensity and EA-induced local and extensive analgesia on visceral pain. This study compares the effects of different intensities of EA at local acupoints and heterotopic acupoints on the nociceptive responses of spinal WDR neurons induced by noxious CRD stimulation. The results of the present study demonstrated that the increased discharges of spinal WDR neurons evoked by CRD were inhibited by EA at both local and heterotopic acupoints, but the intensity of EA necessary to trigger segmental inhibition and extrasegmental inhibition is different. We also saw an increase in the inhibiting rate to graded intensity of EA. However, when EA exceeded a certain range, both the segmental inhibition and extrasegmental inhibition induced by EA reached a plateau. These results indicate that, within a certain range, the transmission of visceral nociception could be inhibited by EA in an intensity-dependent manner.
Intensity of EA to trigger segmental inhibition and extrasegmental inhibition on visceral nociception is different
After different intensities of EA were applied to rats, increased discharges of WDR neurons induced by CRD were significantly decreased, indicating afferent impulses from visceral nociception could be inhibited by EA. However, the intensity of EA for segmental and extrasegmental analgesia is different. We found that the threshold of EA at ST 36 to trigger inhibition of the noxious response of WDR neurons was lower than that of EA at PC 6. In ST 36, EA at 0.5 and 1 mA was effective for segmental inhibition, whereas the inhibition induced by EA at PC6 occurred only when the intensity of EA increased to 2 mA. Based on earlier neurophysiological studies, electrical current in the range 0.25–0.5 mA was found to be effective for Aδ-fiber activation and 1–2 mA for C-fibers activation.19 As a result, it is evident that, on visceral pain, EA-induced segmental analgesia is mediated by both A- and C-fibers, and extrasegmental analgesia is mediated only by C-fibers. Our results are consistent with previous studies on somatic pain that show that EA with a high enough intensity to excite Aβ-fibers is capable of producing local analgesia, but excitation of Aδ- and some C-fibers induces a more potent and extensive analgesia.7,20,21
ST 36 has a unique antinociceptive effect on visceral nociception induced by EA
In this study, the inhibiting percentage of ST 36 and PC 6 on the nociceptive response of WDR neurons induced by EA was calculated. We found that EA at ST 36 produced a higher inhibiting percentage compared with EA at PC 6 with the same intensity. ST 36 is a lower confluent acupoint of the meridians of the stomach. It is innervated by the same segment of the rectum and colon and involved in the treatment of gastrointestinal diseases.14,18,22 The lumbar spinal WDR neurons receive ipsilateral ST36 afferent input.23 In addition, ST36 is the most commonly used acupoint for analgesia in the clinic. It has been confirmed by many behavioral experiments that EA and acupuncture stimulation applied at acupoints ST 36 exert positive effects on rats with visceral hyperalgesia.24–29 These findings give evidence of the unique antinociceptive effect of ST 36 on visceral nociception.
Segmental inhibition of visceral nociception induced by low-intensity EA involved in gate control theory
Low-intensity EA applied at ST 36 produced a slight but significant inhibition on the nociceptive responses of WDR neurons. The segmental analgesia induced by low-intensity EA can be explained by the gate control theory, as proposed by Ronald Melzak and Patrick Wall in 1965.30 These authors opined that activation of myelinated low-threshold afferent fibers can decrease the activity in ascending nociceptive pathways that is generated by small-diameter afferents. As such, the transmission of visceral nociceptive signals can be depressed by local low-intensity stimulation. Moreover, other non-noxious stimulation, such as brushing sensitized skin, can also depress pain via gate control fibers.31,32
The core of gate control theory is the segmental modulation of nociception. Our results indicate that the inhibiting effects of low-intensity EA for visceral nociception are localized to the segment of the stimulated acupoints. Similarly, previous studies have shown that the nociceptive reflex evoked by stimulating the sural nerve can be depressed by EA below the threshold of Aδ-fiber activation applied at the ipsilateral local acupoints. However, heterotopic EA below the threshold of Aδ-fiber activation was totally ineffective in inducing analgesia.8,9 In addition, the analgesic effects of local acupoints disappeared when Aβ-fiber function was blocked by snake venom.8 Consequently, low-intensity EA stimulation applied at local acupoints can inhibit nociceptive inputs at the spinal level, in which gate control may be involved and low-threshold Aβ-fibers are found to be crucial.
Extrasegmental inhibition on visceral nociception induced by high intensity of EA involved in diffuse noxious inhibitory control
When the intensity of EA increased to the noxious range (2 mA), the nociceptive responses of WDR neurons could be inhibited by EA at not only ST 36 but also PC 6. In other words, the extensive antinociceptive effects of EA on visceral nociception were observed on heterotopic acupoint PC 6. PC 6 is located within the medial forelimb and 3 mm above the wrist and with different segmental innervation of the colon. It has been reported that acupuncture transcutaneous electrical stimulation of PC 6 is effective in rectal discomfort during barostat-induced rectal distension in human.33 In addition, EA of PC 6 can suppress CRD-induced increases in mean arterial pressure, heart rate, and heart rate variability, thereby suggesting the beneficial effects of EA in relieving visceral pain and mediating autonomic nervous system function.34 Furthermore, EA at PC 6 can improve the symptoms of colonic motility in irritable bowel syndrome rats.35
Our results indicate that high-intensity EA could produce a more potent and extensive analgesia. This is in agreement with observations obtained on humans and animals, which show that EA stimulation within the noxious range can produce extrasegmental analgesia.9,36 In addition to the use of heterotopic EA stimulation for analgesia, a notable experiment revealed that heterotopic nociceptive conditioning stimulation applied to a given body location is also effective in reducing the perception of pain and response elicited by noxious test stimuli delivered at a remote body location.37–39
Mechanisms underlying extrasegmental analgesia induced by heterotopic noxious stimulation involve diffuse noxious inhibitory controls (DNICs). DNIC refers to the observation that some neurons in the dorsal horn of the spinal cord and the trigeminal system are strongly inhibited when a nociceptive stimulus is applied to any part of the body. This is distinct from their excitatory receptive fields.40,41 Recently, researchers have hypothesized that DNIC is involved in the analgesic mechanism of acupuncture and moxibustion, as DNIC shares many properties with heterotopic EA-induced analgesia.42 First, DNIC was triggered only by the activation of Aδ- and/or C-fibers, whereas non-noxious stimuli were completely ineffective.43,44 Similarly, extrasegmental analgesia can only be induced by EA exceeding the threshold of Aδ- and C-fiber activation. In contrast, the analgesic effects of heterotopic acupoints decreased when the function of C-fibers was blocked by capsaicin pretreatment.8 Second, DNIC is a form of supraspinal descending endogenous analgesia, and the neuronal substrate for DNIC must involve supraspinal structures.45,46 Interestingly, we have previously observed that manual acupuncture could inhibit the nociceptive response of WDR neurons, but this effect was abolished by a blockade of the central descending pathway.14 This indicates that the effects of acupuncture on visceral nociception may not only be modulated by WDR neurons, but by many others too in the supraspinal center. Taken together, these data provide direct evidence for the involvement of DNIC in the antinociception of heterotopic EA stimulation, suggesting that EA at the intensity of Aδ- or/and C-fiber activation can trigger DNIC effects and produce extrasegmental analgesia.
Correlation between stimuli intensity and DNIC effects
Within the noxious range, different intensities of stimulation can trigger graded DNIC effects. Our results indicate that the nociceptive responses of WDR neurons could be inhibited by EA at both local acupoints and heterotopic acupoints when the intensity of EA increased to 2 mA. This demonstrates an obvious DNIC effect. Moreover, there was a positive correlation between current intensity and the degree of inhibition. The reaction characteristics of WDR neurons to EA closely match that of convergent neurons in the nucleus caudalis, recorded under similar experimental conditions.47
The wind-up phenomenon can illustrate the reaction characteristics of WDR neurons to EA. It has previously been reported that many convergent neurons can encode the intensity of mechanical stimuli, especially within the noxious range.15,48,49 Wind-up is defined as a frequency-dependent facilitation to the response of convergent neurons evoked by repetitive stimulation of afferent C-fibers.50–52 After receiving repetitive stimulation of C-fibers, the plasticity of the neurons changes and the excitability increases. However, when the noxious inputs exceed a certain range, increased excitation within the brain cannot be elicited by noxious inputs.53 The effective frequencies to elicit wind-up are in the range 0.5–2.0 Hz.54,55 The frequency of EA used in the present study is 2 Hz. In this condition, the excitability of the WDR neurons can be elicited by strong intensity EA stimulation. As such, within a certain range, the nociceptive response of WDR neurons was inhibited in an intensity-dependent fashion by EA stimulation.
However, it must be noted that if the electrical current is strong enough to excite C-fibers, EA treatment will inevitably cause unbearable pain in clinical practice. The present results show that when the intensity of EA exceeded 4–6 mA, the inhibiting effects of EA on visceral nociception reached a plateau. In fact, plateau saturation to the response of convergent neurons to the graded intensity of mechanical stimulation has already been reported.48 The plateau effect on visceral nociception may be due to the saturation of the neuronal response to EA stimulation.
Limitations
One limitation of this study is that only 2.0 Hz EA was administered to rats, knowing that EA with lower or higher frequency may influence its analgesic effect. Thus, further studies are needed to explore the intensity-related effect of EA on visceral pain under different frequencies in order to provide an optimal combination of intensity and frequency for visceral pain.
Conclusion
This study defined the relationship between current intensity and the effects of EA on visceral nociception. We demonstrated that visceral nociceptive afferents could be inhibited by EA at both local acupoints and heterotopic acupoints. At local acupoints, a positive relationship between current intensity and the extent of EA-induced analgesia was observed within 0.5–4 mA. In heterotopic acupoints, a positive relationship between current intensity and the extent of EA-induced analgesia was observed within 2–6 mA. However, further increases in the current beyond 4–6 mA resulted in both the segmental and extrasegmental analgesia induced by EA reaching a plateau. These data indicate that, within a certain range, the transmission of visceral nociception could be inhibited by EA in an intensity-dependent manner. Therefore, regulation of current intensity to optimal range may promote the antinociceptive effects of EA.
Acknowledgments
This work was funded by the Fundamental Research Funds for the Central Public Welfare Research Institutes (nos. ZZZD16001, ZZ10-006 and GH2017-07); China Postdoctoral Science Foundation (2017M612472); National Natural Science Foundation of China (nos. 81403475, 81400977, 81473780); Joint Sino-German Research Project (GZ1236); China Scholarship Council (201709920086), Deutscher Akademischer Austauschdienst (91658555); and Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme (2016).
Footnotes
Author contributions
LY, LL, and QQ performed the experiments; BZ and PR designed the experiments; LY and YZ analyzed the data; LY, WW, YY, and KL co-wrote the manuscript; All authors discussed the results and reviewed the final manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Meerveld BG, Johnson AC. Mechanisms of stress-induced visceral pain. J Neurogastroenterol Motil. 2018;24(1):7–18. doi: 10.5056/jnm17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceuleers H, Van Spaendonk H, Hanning N, et al. Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: the role of proteases. World J Gastroenterol. 2016;22(47):10275–10286. doi: 10.3748/wjg.v22.i47.10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu HR, Fang XY, Wu HG, et al. Effects of electroacupuncture on corticotropin-releasing hormone in rats with chronic visceral hypersensitivity. World J Gastroenterol. 2015;21(23):7181–7190. doi: 10.3748/wjg.v21.i23.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macpherson H, Tilbrook H, Agbedjro D, Buckley H, Hewitt C, Frost C. Acupuncture for irritable bowel syndrome: 2-year follow-up of a randomised controlled trial. Acupunct Med. 2017;35(1):17–23. doi: 10.1136/acupmed-2015-010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Zhang Y, Qi D, Li W. Downregulation of the spinal NMDA receptor NR2B subunit during electro-acupuncture relief of chronic visceral hyperalgesia. J Physiol Sci. 2017;67(1):197–206. doi: 10.1007/s12576-016-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian SL, Wang XY, Ding GH. Repeated electro-acupuncture attenuates chronic visceral hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat irritable bowel syndrome model. Life Sci. 2008;83(9–10):356–363. doi: 10.1016/j.lfs.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Xin J, Su Y, Yang Z, et al. Distinct roles of ASIC3 and TRPV1 receptors in electroacupuncture-induced segmental and systemic analgesia. Front Med. 2016;10(4):465–472. doi: 10.1007/s11684-016-0482-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhu B, Xu WD, Rong PJ, Ben H, Gao XY. A C-fiber reflex inhibition induced by electroacupuncture with different intensities applied at homotopic and heterotopic acupoints in rats selectively destructive effects on myelinated and unmyelinated afferent fibers. Brain Res. 2004;1011(2):228–237. doi: 10.1016/j.brainres.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Xu WD, Zhu B, Rong PJ, Bei H, Gao XY, Li YQ. The pain-relieving effects induced by electroacupuncture with different intensities at homotopic and heterotopic acupoints in humans. Am J Chin Med. 2003;31(5):791–802. doi: 10.1142/S0192415X03001478. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wang W, Li D, et al. The beneficial effect of electro-acupuncture given at PC6 (Neiguan-point) by the increase in cardiac transient outward K+ current channel which depends on the gene and protein expressions in artificially induced myocardial ischemia rats. Acupunct Electrother Res. 2014;39(3–4):259–273. doi: 10.3727/036012914x14109544776132. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Gong K, Zhou W, et al. Involvement of subtypes γ and ε of protein kinase C in colon pain induced by formalin injection. Neurosignals. 2011;19(3):142–150. doi: 10.1159/000328311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Rev. 2002;40(1–3):29–44. doi: 10.1016/s0165-0173(02)00186-8. [DOI] [PubMed] [Google Scholar]
- 13.Yu LL, Li L, Rong PJ, et al. Changes in responses of neurons in spinal and medullary subnucleus reticularis dorsalis to acupoint stimulation in rats with visceral hyperalgesia. Evid Based Complement Alternat Med. 2014;2014:768634. doi: 10.1155/2014/768634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rong PJ, Zhu B, Huang QF, Gao XY, Ben H, Li YH. Acupuncture inhibition on neuronal activity of spinal dorsal horn induced by noxious colorectal distention in rat. World J Gastroenterol. 2005;11(7):1011–1017. doi: 10.3748/wjg.v11.i7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouhassira D, Gall O, Chitour D, Le Bars D. Dorsal horn convergent neurones: negative feedback triggered by spatial summation of nociceptive afferents. Pain. 1995;62(2):195–200. doi: 10.1016/0304-3959(94)00270-O. [DOI] [PubMed] [Google Scholar]
- 16.Ferrington DG, Sorkin LS, Willis WD., Jr Responses of spinothalamic tract cells in the superficial dorsal horn of the primate lumbar spinal cord. J Physiol. 1987;388:681–703. doi: 10.1113/jphysiol.1987.sp016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41(2):167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 18.Rong PJ, Zhu B, Huang QF, Gao XY, Ben H, Li YH. Zhēn cì yìzhì zhí jiécháng shānghài xìng kuòzhāng yǐnqǐ de dà shǔ jǐsuǐ bèi jiǎo shénjīng yuán fǎnyìng [Acupuncture inhibiting responses of spinal dorsal horn neurons induced by noxious dilation rectum and colon] Zhongguo Zhen Jiu. 2005;25(9):645–650. Chinese. [PubMed] [Google Scholar]
- 19.Bouhassira D, Le Bars D, Villanueva L. Heterotopic activation of A delta and C fibres triggers inhibition of trigeminal and spinal convergent neurones in the rat. J Physiol. 1987;389(1):301–317. doi: 10.1113/jphysiol.1987.sp016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine JD, Gormley J, Fields HL. Observations on the analgesic effects of needle puncture (acupuncture) Pain. 1976;2(2):149–159. [PubMed] [Google Scholar]
- 21.Gw L. Characteristics of afferent fiber innervation on acupuncture points zusanli. Am J Physiol. 1983;245(4):R606–R612. doi: 10.1152/ajpregu.1983.245.4.R606. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, Ding S, Wu F, et al. Strong manual acupuncture manipulation could better inhibit spike frequency of the dorsal horn neurons in rats with acute visceral nociception. Evid Based Complement Alternat Med. 2015;2015(5):1–9. doi: 10.1155/2015/675437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng Z. Zú sānlǐ xué yǔ sānchā shénjīng hé jiān shénjīng liánxì de diàn shēnglǐxué yánjiū [The functional connection among the “zusanli”-spinal dorsal horn neurons-trigeminal sensory nucleus of rats] Zhen Ci Yan Jiu. 1995;20(3):29–32. Chinese. [PubMed] [Google Scholar]
- 24.Db Q, Zhang SH, Zhang YH, Wu SQ, Li WM. A rat model for studying electroacupuncture analgesia on acute visceral hyperalgesia. Exp Anim. 2018;67(1):51–61. doi: 10.1538/expanim.17-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi D, Wu S, Zhang Y, Li W. Electroacupuncture analgesia with different frequencies is mediated via different opioid pathways in acute visceral hyperalgesia rats. Life Sci. 2016;160:64–71. doi: 10.1016/j.lfs.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Yu WC, Huang GY, Zhang MM, Wang W. Effect of connexin 43 knockout on acupuncture-induced down-regulation of c-fos expression in spinal dorsal horn in visceral pain mice. Zhen Ci Yan Jiu. 2008;33(3):179–182. [PubMed] [Google Scholar]
- 27.Zhou YY, Wanner NJ, Xiao Y, et al. Electroacupuncture alleviates stress-induced visceral hypersensitivity through an opioid system in rats. World J Gastroenterol. 2012;18(48):7201–7211. doi: 10.3748/wjg.v18.i48.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jc W, Ziea ET, Lao L, et al. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16(3):306–314. doi: 10.5056/jnm.2010.16.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin YP, Peng Y, Yi SX, Tang S. Bùtóng pínlǜ diàn zhēn de xiàoguǒguānyú P wùzhí hé b-nèi fēi tài de biǎodá wèi zhàng tòng yǐnqǐ de dà shǔ xià qiūnǎo [Effect of different frequency electroacupuncture on the expression of substance P and beta-endorphin in the hypothalamus in rats with gastric distension-induced pain] Zhen Ci Yan Jiu. 2009;34(4):252–257. Chinese. [PubMed] [Google Scholar]
- 30.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–978. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 31.Löken LS, Duff EP, Tracey I. Low-threshold mechanoreceptors play a frequency-dependent dual role in subjective ratings of mechanical allodynia. J Neurophysiol. 2017;118(6):3360–3369. doi: 10.1152/jn.00977.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi T. Mechanism of acupuncture on neuromodulation in the gut—a review. Neuromodulation. 2011;14(1):8–12. doi: 10.1111/j.1525-1403.2010.00295.x. [DOI] [PubMed] [Google Scholar]
- 33.Leung WW, Jones AY, Ss N, Wong CY, Lee JF. Acupuncture transcutaneous electrical nerve stimulation reduces discomfort associated with barostat-induced rectal distension: a randomized-controlled study. World J Gastroenterol. 2012;19(3):381–388. doi: 10.3748/wjg.v19.i3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen SP, Gao YH, Xc Y, Liu JL. Diàn zhēn bùtóng jīng xué duì zhí-jiécháng kuòzhāng dà shǔ xiěyā jí zìzhǔ shénjīng xìtǒng de yǐngxiǎng [Effects of electroacupuncture of different acupoints on changes of blood pressure and autonomic nerve system after colorectal distension in rats] Zhen Ci Yan Jiu. 2010;35(5):335–341. Chinese. [PubMed] [Google Scholar]
- 35.Wang S, Guo MW, Gao YS, et al. Diàn zhēn “nèi guān”“tiān shū” xué duì cháng yì jī zònghé zhēng dà shǔ jiécháng dònglì jí jiécháng D 2 shòu tǐ de yǐngxiǎng [Effect of electroacupuncture at “Neiguan” (PC 6) and “Tianshu” (ST 25) for colonic motility and D 2 receptor in irritable bowel syndrome rats] Zhen Ci Yan Jiu. 2018;43(1):25–29. doi: 10.13702/j.1000-0607.170416. Chinese. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Fu W, Yi W, et al. Extrasegmental analgesia of heterotopic electroacu-puncture stimulation on visceral pain rats. Brain Res. 2011;1373:160–171. doi: 10.1016/j.brainres.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Meléndez-Gallardo J, Eblen-Zajjur A. Noxious mechanical heterotopic stimulation induces inhibition of the spinal dorsal horn neuronal network: analysis of spinal somatosensory-evoked potentials. Neurol Sci. 2016;37(9):1491–1497. doi: 10.1007/s10072-016-2613-y. [DOI] [PubMed] [Google Scholar]
- 38.Torta DM, Churyukanov MV, Plaghki L, Mouraux A. The effect of heterotopic noxious conditioning stimulation on Aδ-, C- and Aβ-fibre brain responses in humans. Eur J Neurosci. 2015;42(9):2707–2715. doi: 10.1111/ejn.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oono Y, Fujii K, Motohashi K, Umino M. Diffuse noxious inhibitory controls triggered by heterotopic CO2 laser conditioning stimulation decreased the SEP amplitudes induced by electrical tooth stimulation with different intensity at an equally inhibitory rate. Pain. 2008;136(3):356–365. doi: 10.1016/j.pain.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Sprenger C, May A, Büchel C. Pain contra pain : the concept of DNIC. Schmerz. 2010;24(6):569–574. doi: 10.1007/s00482-010-0985-0. [DOI] [PubMed] [Google Scholar]
- 41.Le Bars D, Villanueva L, Bouhassira D, Willer JC. Diffuse noxious inhibitory controls (DNIC) in animals and in man. Patol Fiziol Eksp Ter. 1992;4:55–65. [PubMed] [Google Scholar]
- 42.Murase K, Kawakita K. Diffuse noxious inhibitory controls in anti-nociception produced by acupuncture and moxibustion on trigeminal caudalis neurons in rats. Jpn J Physiol. 2000;50(1):133–140. doi: 10.2170/jjphysiol.50.133. [DOI] [PubMed] [Google Scholar]
- 43.Bao H, Zhou Z, Yu Y, Han J. C Xiānwéi bùshì diàn zhēn zhèn tòng de zhǔyào chuán rù xiānwéi, ér shì mísàn xìng shānghài xìng yìzhì kòng-zhì de zhǔyào xiānwéi [C fiber is not necessary in electroacupuncture analgesia, but necessary in diffuse noxious inhibitory controls (DNIC)] Zhen Ci Yan Jiu. 1991;16(2):120–124. Chinese. [PubMed] [Google Scholar]
- 44.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6(3):283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 45.Moont R, Pud D, Sprecher E, Sharvit G, Yarnitsky D. ‘Pain inhibits pain’ mechanisms: is pain modulation simply due to distraction? Pain. 2010;150(1):113–120. doi: 10.1016/j.pain.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979;6(3):305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- 47.Villanueva L, Le Bars D. The encoding of thermal stimuli applied to the tail of the rat by lowering the excitability of trigeminal convergent neurones. Brain Res. 1985;330(2):245–251. doi: 10.1016/0006-8993(85)90683-3. [DOI] [PubMed] [Google Scholar]
- 48.Villanueva L, Bing Z, Bouhassira D, Le Bars D. Encoding of electrical, thermal, and mechanical noxious stimuli by subnucleus reticularis dorsalis neurons in the rat medulla. J Neurophysiol. 1989;61(2):391–402. doi: 10.1152/jn.1989.61.2.391. [DOI] [PubMed] [Google Scholar]
- 49.Gall O, Villanueva L, Bouhassira D, Le Bars D. Spatial encoding properties of subnucleus reticularis dorsalis neurons in the rat medulla. Brain Res. 2000;873(1):131–134. doi: 10.1016/s0006-8993(00)02524-5. [DOI] [PubMed] [Google Scholar]
- 50.Uhl I, Krumova EK, Regeniter S, et al. Association between wind-up ratio and central serotonergic function in healthy subjects and depressed patients. Neurosci Lett. 2011;504(2):176–180. doi: 10.1016/j.neulet.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 51.Traub RJ. Spinal modulation of the induction of central sensitization. Brain Res. 1997;778(1):34–42. doi: 10.1016/s0006-8993(97)00946-3. [DOI] [PubMed] [Google Scholar]
- 52.Bosma RL, Ameli Mojarad E, Leung L, Pukall C, Staud R, Stroman PW. Neural correlates of temporal summation of second pain in the human brainstem and spinal cord. Hum Brain Mapp. 2015;36(12):5038–5050. doi: 10.1002/hbm.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douglass DK, Carstens E, Watkins LR. Spatial summation in human thermal pain perception: comparison within and between dermatomes. Pain. 1992;50(2):197–202. doi: 10.1016/0304-3959(92)90161-4. [DOI] [PubMed] [Google Scholar]
- 54.Herrero JF, Laird JM, López-García JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61(2):169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79(1):75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]