Abstract
Purpose
The present study was attempted to compare the differences in gray matter volume (GMV) between the acute eye pain (EP) patients and the healthy controls (HCs) using voxel-based morphometry (VBM), and to explore the relationship with clinical features and behavioral performance.
Methods
A total of 24 patients (17 males, 7 females) with acute EP and 24 (17 males, 7 females) age-, sex-, and education-matched HCs were recruited from the Ophthalmology Department of the First Affiliated Hospital of Nanchang University. Functional magnetic resonance imaging (fMRI) scans were conducted in all subjects. We analyzed the original three-dimensional (3D) T1 brain images by VBM and compared the GMV values with the HCs. The acute EP patients can be distinguished from the HCs by receiver operating characteristic (ROC) curve.
Results
Compared with HCs, the acute EP patients had significantly lower GMV values in the brain regions of the left cerebellum posterior lobe, the left limbic lobe, the right insula, the left insula, the left thalamus, the left caudate, and the right cuneus. In addition, the WMV values of the whole brain in acute EP patients decreased slightly.
Conclusions
These results demonstrated that the acute EP patients showed an abnormal reduction in GMV in some brain regions, which might provide valuable information for further exploration of underlying neural mechanisms. These abnormal brain regions may reflect the functional disorders of acute EP patients in somatosensory, motor, cognitive functions, and so on.
Translational Relevance
The VBM study provides a diagnostic method for identifying the cause of acute EP, additionally, a novel direction was presented for further exploration of underlying neural mechanisms of acute EP.
Keywords: eye pain, voxel-based morphometry, gray matter volume
Introduction
Eye pain (EP) is a common symptom of many eye diseases. There are many diseases known to cause eye pain, such as infectious keratitis, dry eye, scleritis, iridocyclitis,1 and so on. The cornea is supplied by the ophthalmic branch of the trigeminal nerve and is one of the most densely innervated structures of the body. It is very sensitive to external stimuli due to the rich corneal nerve fibers. Keratitis or corneal ulcer will lead to severe ocular pain with symptoms, such as photophobia, tears, and headache.2 The prevalence of infectious keratitis was 0.192% and the prevalence of viral, bacterial, and fungal keratitis was 0.11%, 0.075%, and 0.007% according to a cross-sectional study from China.3
Corneal nerve damage triggers the peripheral and central trigeminal sensory network then causes corneal pain.4 It has been confirmed in a study that corneal pain activated the trigemino-parabrachial pathway.5 Several lines of evidence indicated that corneal pain might be associated with activation of the primary somatosensory cortex. In addition, corneal pain could lead to neuronal activation in the spinal trigeminal nucleus, insular cortex, and anterior cingulate cortex.6,7 Studies have demonstrated that EP is accompanied by mental disorders. For instance, dry eye (DE) patients with EP suffer from insomnia and depression.8–10 In addition to eye symptoms, a large number of EP patients complain of headache. Although the main causes of EP can be easily diagnosed by simple examination techniques, sometimes the etiology is not so apparent that may turn to neuroimaging diagnosis.11,12 This provides direction and idea for us to explore the underlying pathophysiology of EP and carry out the complex differential diagnosis.
Functional magnetic resonance imaging (fMRI) has been successfully applied to evaluate brain activity changes in different pain-related diseases.13–15 And several fMRI studies have demonstrated that synchronous neuronal activity may have a crucial role in neurophysiological activity.16,17 In our previous studies, we have used amplitude of low-frequency fluctuation (ALFF) method and regional homogeneity (ReHo) method to explore brain activity alterations in acute EP patients.18,19 Although the findings showed neuronal morphologic alterations in the EP patients, the understanding of anatomic neuromechanism of acute EP still remains unknown.
Voxel-based morphometry (VBM) is a commonly used tool for studying patterns of brain change in development or disease and neuroanatomic correlates of subject characteristics.20 As a novel MRI technology, VBM has been broadly used in eye diseases in recent years, which provides a powerful tool for clarifying the pathophysiological mechanism and monitoring the course of diseases. The VBM method has already been used in diagnosis, treatment, and prognosis evaluation in many eye diseases, such as amblyopia, strabismus, glaucoma, optic neuritis, and so on.21–24 In particular, the VBM method has achieved good results in exploring the neural mechanisms of nervous and mental diseases, such as Alzheimer's disease and schizophrenia.25,26 To the best of our knowledge, the current study is the first to detect the differences of GMV between EP patients and healthy controls (HCs) and to explore their clinical manifestations, which provide a significant basis for the study of the pathogenesis and treatment of EP.
Materials and Methods
Subjects
This study included 24 patients with acute EP (17 males, 7 females) from the Ophthalmology Department of the First Affiliated Hospital of Nanchang University. The inclusion criteria on patients with acute EP included (1) acute EP patients with keratitis or corneal ulcer of the eyes, and (2) bilateral eyes without any other ocular diseases (amblyopia, strabismus, cataracts, optic neuritis, glaucoma, retinal degeneration, etc.). The exclusion criteria for EP patients were as follows: (1) chronic EP symptoms for a long time (>3 weeks); (2) EP due to glaucoma or ocular trauma; (3) EP with serious related complications (orbital cellulitis, ocular perforation, endophthalmitis, or other serious conditions); (4) EP patient with associated pain, such as a headache in other parts of the body; and (5) cardiovascular diseases, such as heart disease or hypertension. Twenty-four HCs (17 males and 7 females) with age-, sex-, and education status–matched subjects in the EP group participated in our study. All the HCs met the following criteria: (1) no ocular diseases (strabismus, amblyopia, dry eye, cataracts, glaucoma, etc.); (2) naked eye corrected visual acuity >1.0; (3) ability to be scanned with an MRI (no cardiac pacemaker or implanted metal devices); and (4) no psychiatric disorders (bipolar disorder, depression, etc.).
The research was approved by the medical ethics of the First Affiliated Hospital of Nanchang University. And the research methods followed the Declaration of Helsinki and were in line with the principles of medical ethics. All participants volunteered to participate and understand the purpose, methods, and potential risks and signed informed consent.
MRI Parameters
Three-Tesla MR scanner (Trio; Siemens, Munich, Germany) was applied to perform the MRI scanning. We acquired the functional images using a spoiled gradient–recalled echo sequence with the following parameters: repetition time = 1900 ms, echo time = 2.26 ms, flip angle = 9°, thickness = 1.0 mm, field of view = 250 × 250 mm, gap = 0 mm, inplane resolution = 256 × 256, and we obtained 176 images. Finally, 240 functional volumes (repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, slice thickness = 4.0 mm, field of view = 220 × 220 mm, gap = 1.2 mm, inplane resolution = 64 × 64, 29 axial slices) were successfully obtained. The information and MRI parameters are shown in Table 1.
Table 1.
Information About MRI Parameters
| Date Acquisition |
Brain Volume Sequence |
|
| Scan parameters | Repetition time (TR)/echo time (TE) | 1900/2.26 ms |
| Thickness/gap | 1/0 mm | |
| Matrix | 256 × 256 | |
| Field of view (FOV) | 250 × 250 mm | |
| Flip angle | 9° |
VBM Analysis
Incomplete functional data were eliminated by using MRIcro software (www.MRIcro.com). We processed the structural images with the voxel-based morphometry toolbox (VBM8; http://dbm.neuro.uni-jena.de/vbm8/) implemented in Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk), which was running on MATLAB 7.9.0 (R2009b; The MathWorks, Natick, MA). VBM, features as semiautomate and unbiased, is a whole-brain technique for characterizing regional cerebral differences in structural MRIs.17 On the basis of VBM8, the brains were segmented into white matter, gray matter, and cerebrospinal fluid using the default estimation options (60-mm cut off for estimating the Gaussian smoothness of bias in image intensity; International Consortium for Brain Mapping [ICBM] European template for initial affine transformation). By the high-dimensional Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) approach implemented in VBM8, we normalized spatial into the Montreal Neurological Institute (MNI) standard space. White and gray matter templates were produced by DARTEL. And we applied the generated template for all volunteered participants' standardized white matter and gray matter. Then smoothing the modulated volumes through a 6-mm full-width-at-half-maximum (FWHM) Gaussian kernel. Finally, presenting the modulated, normalized, and smoothed images to group-level analyses.
Statistical Analysis
To investigate the group differences in GMV and WMV between acute EP groups and the HC, we performed a general linear model (GLM) analysis with the SPM8 (http://www.fil.ion.ucl.ac.uk/spm) toolkit. Setting the significance level at P less than 0.05, and we used Gaussian random field (GRF) theory correction for multiple comparisons, minimum z greater than 2.3. To generate a color drawing, statistically significant voxels were superimposed on the standardization of 3-dimensional magnetization prepared rapid-acquisition gradient echo sequences (3DT1WI). Selecting 20 neighboring voxels to analyze the atrophy GMV in patients with acute EP. The mean GMV values in the different brain regions between the two groups were analyzed by receiver operating characteristic (ROC) curves.
Brain Behavior
Based on the VBM measuring results, distinct brain regions were divided into various regions of interests (ROIs) by using REST software (version, 1.8; www.resting-fmri.Sourceforge.net). We regarded the average of GMV value over all voxels as the mean GMV value of each ROI. At last, we applied correlation analysis to investigate the relationship between the mean GMV value in different brain regions in the RD group and the clinical manifestations. P less than 0.05 was deemed to indicate a statistically significant difference.
Clinical Data Analysis
All the clinical data of the patients with EP were gathered, comprising the duration of EP disease, visual analogue scale (VAS), and best-corrected visual acuity (VA). The clinical and demographic variables between EP and HC groups were compared by using SPSS20.0 software (SPSS, Chicago, IL) with independent sample t-test and P less than 0.05 was deemed to designate a statistically significant difference.
Results
Demographics and Visual Measurements
There were no significant differences in age (P = 0.926) or weight (P = 0.623) between EP group and HCs. The duration of EP expressed as mean ± standard deviation was 6.08 ± 2.12 days. The Best-corrected VA of two groups are listed in Table 2 in detail.
Table 2.
Demographics and Clinical Measurements by Groups
| Condition |
EP |
HC |
t |
P Value |
| Male/female | 17/7 | 17/7 | N/A | >0.99 |
| Age, yr | 39.85 ± 5.79 | 40.31 ± 6.12 | −0.079 | 0.926 |
| Weight, kg | 68.68 ± 6.89 | 67.53 ± 5.24 | −0.364 | 0.623 |
| Handedness | 24R | 24R | N/A | >0.99 |
| Duration of EP, d | 6.67 ± 2.63 | N/A | N/A | N/A |
| Best-corrected VA-right eye | 0.25 ± 0.01 | 1.01 ± 0.22 | −0.413 | 0.001 |
| Best-corrected Va-left eye | 0.18 ± 0.02 | 1.02 ± 0.21 | −0.435 | 0.001 |
N/A, not applicable.
P < 0.05 independent t-tests comparing two groups.
VBM Differences
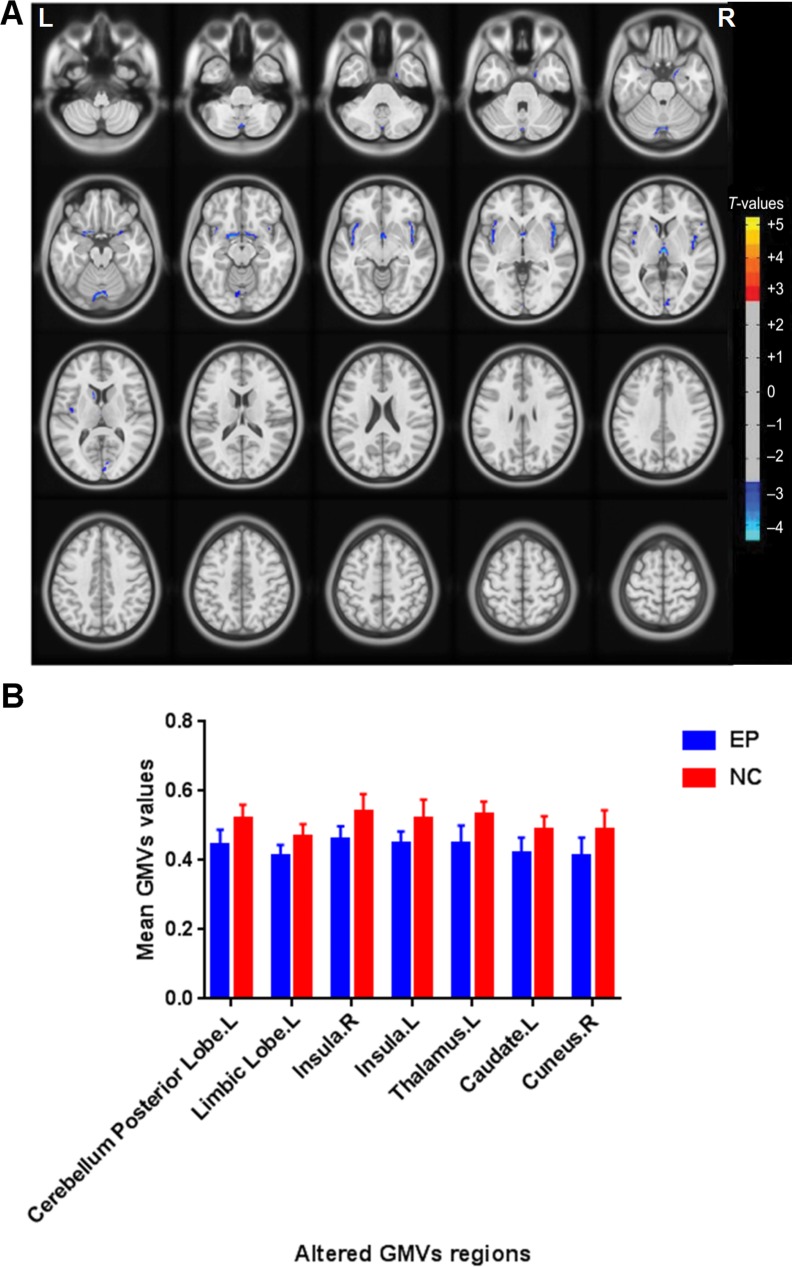
Compared with the HCs group, the EP group had significantly lower GMV in the brain regions of the left cerebellum posterior lobe, the left limbic lobe, the right insula, the left insula, the left thalamus, the left caudate, and the right cuneus (Fig. 1 [blue] and Table 3). Additionally, we observed that patients with acute EP had both decreased the mean of GMV values and WMV values (Table 4).
Figure 1.
Regional GMV decrease in patients with acute EP compared with HCs. (A) Significantly decreased areas were observed in the left cerebellum posterior lobe, the left limbic lobe, the right insula, the left insula, the left thalamus, the left caudate, and the right cuneus. The blue areas indicate lower GMV brain regions. The significance level was set at P < 0.05, (GRF) theory corrected (z > 2.3). The mean GMV values between the EPs and HCs group (B).
Table 3.
GMV Differences Between EP Group and HCs Group
| Condition |
Brain Regions |
MNI Coordinates |
Cluster Size |
Peak t Value |
||
| X |
Y |
Z |
||||
| EP < NC | Cerebellum posterior lobe, L | −7.5 | −81 | −19.5 | 296 | −7.2225 |
| EP < NC | Limbic lobe, L | −16.5 | 3 | −15 | 245 | −8.0419 |
| EP < NC | Insula, R | 43.5 | 7.5 | −3 | 562 | −6.8695 |
| EP < NC | Insula, L | −42 | −1.5 | −3 | 460 | −5.8375 |
| EP < NC | Thalamus, L | −1.5 | −18 | 3 | 185 | −9.7415 |
| EP < NC | Caudate, L | −9 | 13.5 | 7.5 | 94 | −6.2255 |
| EP < NC | Cuneus, R | 9 | −84 | 9 | 112 | −5.1334 |
The statistical threshold was set at the voxel level with P < 0.05 for multiple comparisons using GRF theory (z > 2.3, P < 0.001, cluster > 80 voxels, AlphaSim corrected).
Table 4.
Comparison of Whole Brain Volume Between EP Group and HC Group
| GMV (ML) |
WMV (ML) |
|
| EPs group | 619.60 ± 53.43 | 460.89 ± 60.50 |
| HCs group | 634.62 ± 50.17 | 472.09 ± 38.83 |
| t | −0.917 | −0.707 |
| P | 0.365 | 0.484 |
ROC Curve
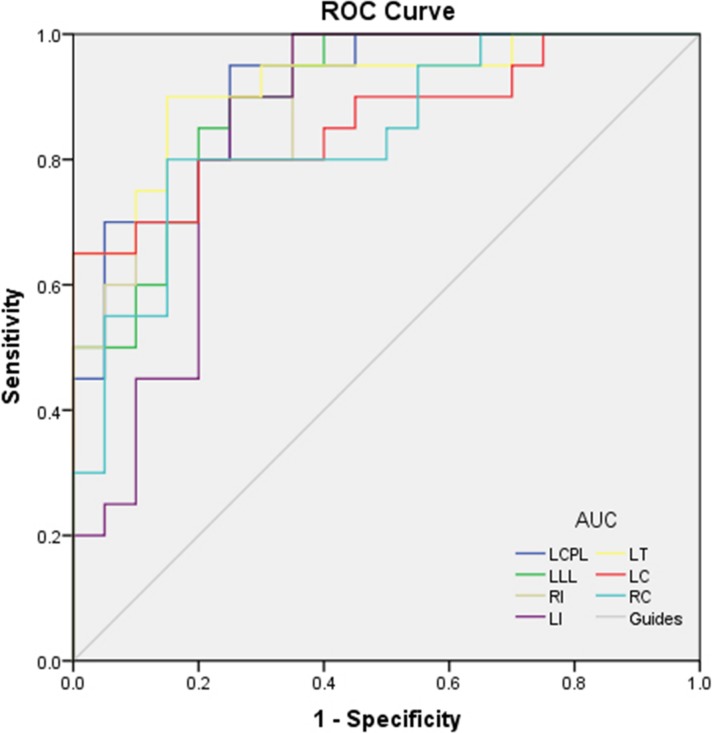
It was assumed that the differences of GMV values between the two groups might be helpful as diagnostic markers. We used ROC curve to analyze the mean GMV values of different brain regions. The areas under the curve (AUC) for GMV values were as follows: the AUC were 0.908 (P < 0.001) for left cerebellum posterior lobe, left limbic lobe 0.895 (P < 0.001), right insula 0.895 (P < 0.001), left insula 0.848 (P < 0.001), left thalamus 0.918 (P < 0.001), left caudate 0.860 (P < 0.001), and right cuneus 0.838 (P < 0.001) (Fig. 2).
Figure 2.
ROC curve analysis of the GMV values for altered brain regions. The AUCs were 0.908 (P < 0.001; 95% CI: 0.819–0.996) for LCPL, LLL 0.895 (P < 0.001; 95% CI: 0.800–0.990), RI 0.895 (P < 0.001; 95% CI: 0.801–0.989), LI 0.848 (P < 0.001; 95% CI: 0.721–0.974), LT 0.918 (P < 0.001; 95% CI: 0.830–1.000), LC 0.860 (P < 0.001; 95% CI: 0.743–0.977), and RC 0.838 (P < 0.001; 95% CI: 0.713–0.962). LCPL, left cerebellum posterior lobe; LLL, left limbic lobe; RI, right insula; LI, left insula; LT, left thalamus; LC, left caudate; RC, right cuneus.
Correlation Analysis
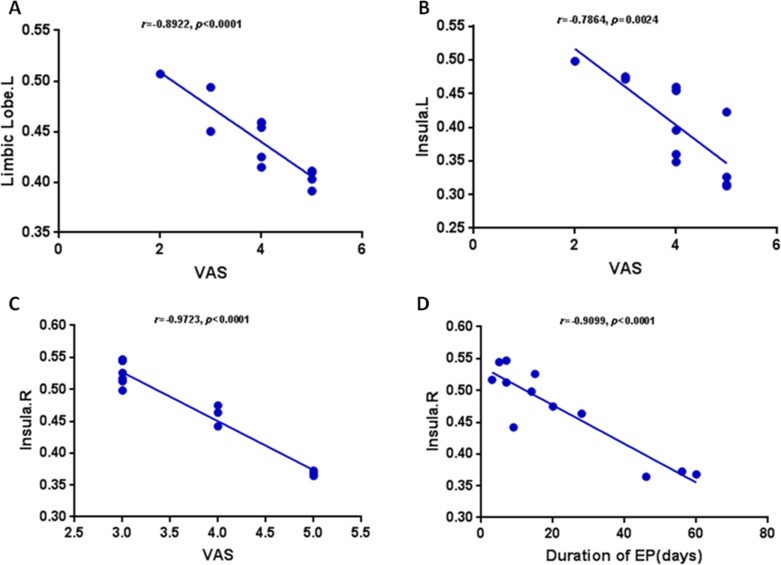
In the acute EP group, the value of VAS in acute EP showed a negative correlation with the GMV value of the left limbic lobe (r = −0.8922, P < 0.0001). The value of VAS in acute EP showed a negative correlation with the GMV value of the left insula (r = −0.7864, P = 0.0024) and the right insula (r = −0.9723, P < 0.0001). The duration of EP showed a negative correlation with the GMV value of the right isula (r = −0.9099, P < 0.0001). The details are presented in Figure 4.
Figure 4.
Correlation between the mean GMV values of the differences and the behavioral performances. The value of VAS in acute EP showed a negative correlation with the GMV value of the left limbic lobe (r = −0.8922, P < 0.0001) (A). The value of VAS in acute EP showed a negative correlation with the GMV value of the left insula (r = −0.7864, P = 0.0024) (B). The value of VAS in acute EP showed a negative correlation with the GMV value of the right insula (r = −0.9723, P < 0.0001) (C). The duration of EP showed a negative correlation with the GMV value of the right isula (r = −0.9099, P < 0.0001) (D).
Discussion
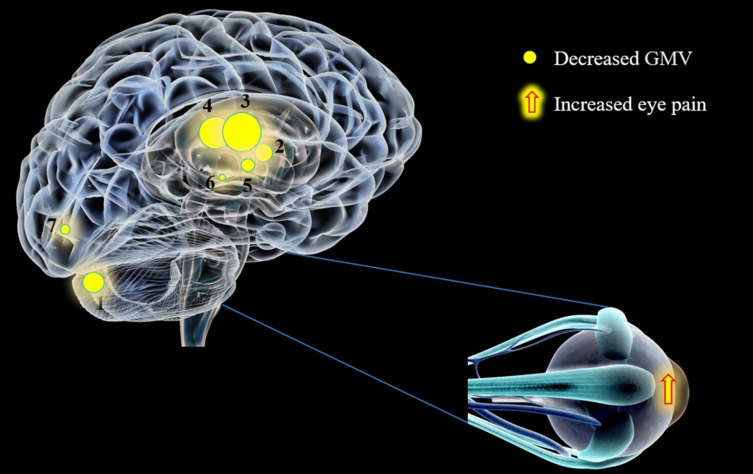
The value and novelty of our study was to address, for the first time in the VBM method, changes in GMV and WMV in acute EP patients. The results demonstrated that both GMV and WMV reduced in EP patients compared with HCs. Moreover, we discovered markedly decreased GMV in the left cerebellum posterior lobe, the left limbic lobe, the right insula, the left insula, the left thalamus, the left caudate, and the right cuneus with the increased eye pain (Fig. 3).
Figure 3.
The GMV values of altered brain regions in the EP group. Compared with the HCs, the GMV of the following regions were decreased to the following various extents: (1) cerebellum posterior lobe, L (t = −7.2225); (2) limbic lobe, L (t = −8.0419); (3) insula, R (t = −6.8695); (4) insula, L (t = −5.8375); (5) thalamus, L ( t = −9.7415); (6) caudate, L (t = −6.2255); and (7) cuneus, R (t = −5.1334) in EP patients. The sizes of the spots denote the degree of quantitative changes. L, left; R, right.
As mentioned in the previous paragraph, acute eye pain is a common symptom of many eye diseases, such as infectious keratitis, dry eye, scleritis, iridocyclitis, glaucoma, and so on.1 Except for a clear etiology leading to eye pain, there is still some unexplained eye pain even eye pain without visual symptoms.27 In addition to investigate of the primary cause, recent studies have not been limited to ophthalmic diseases, but have explored the relationship between eye pain and systemic diseases, including pain in other parts of body, mental illness, and psychiatric disease.28,29 We have investigated neural activity changes between eye pain patients using resting-state fMRI in our previous studies.18,19 In this study, we used VBM method to evaluate GMV and WMV changes in acute eye patients and to explore possible underlying mechanisms or causes of eye pain.
The cerebellum posterior lobe is mainly related to cognitive function and participates in executive function and working memory.30 In addition to the above functions, recent researches have turned to exploring the role of the cerebellum in perceptual processes, emotion, and affective disorders.31–33 In this study, we showed that the left cerebellum posterior lobe was atrophied in patients with acute EP. Although we cannot prove that EP patients will be accompanied by visual impairment, we can put forward a hypothesis that may occur in the course of EP patients with visual impairment and even cognitive disorder. It has been proven that the cerebellum is a transit point for the emotional pathways of the limbic system, mainly managing the expression of negative emotions.34 Therefore, some scholars have explored the volume of the cerebellum in patients with depression, the results indicated that the cerebellum volume of patients with depression reduced, the ALFF value and the ReHo value of the cerebellum posterior lobe decreased.35,36 These results are in accordance with the reduction of the GMV value in the cerebellum posterior lobe in acute EP patients discovered in our study.
The limbic lobe (limbic gyrus) consists of the cingulate gyrus, the parahippocampal gyrus, and the callosal area.37 The cingulate cortex in the default mode network (DMN) converge information from other brain regions to the center. Previous studies suggest that the anterior gyrus is associated with behaviors, cognitive processes, and pain responses.38,39 The posterior gyrus is involved in cognition, memory, and related to some diseases, such as Alzheimer's disease.40,41 As a core part of the limbic system, the parahippocampal gyrus plays an indispensable role in visual memory and emotions.42 Clinical researches have indicated that pain-related diseases are involved in activation of the limbic system.43,44 In our study, the acute EP patients showed lower GMV values compared with the HCs, which indicated that the dysfunction of the limbic lobe. It is plausible that the dysfunction of the limbic lobe reflects negative emotions that occur in EP patients. Furthermore, we found that the VAS in patients with acute EP correlated negatively with the GMV value of the left limbic lobe (r = −0.8922, P < 0.0001), which indicated that the more severe the eye pain, the lower the value of GMV. This is consistent with our previous speculation that the limbic lobe is associated with negative emotions.
The human insula is hidden in the depth of the cerebral hemisphere by the overlying frontal, parietal, and temporal lobes. However, the function of insula still remains uncertain. Except for memory and visceral sensation, several update reviews have indicated that the insula is also associated with mood, addiction, cognition, and pain regulation.45–47 There are nerve fibers in connection between the insula and prefrontal lobe, anterior cingulate, and amygdala. The prefrontal lobe is involved in pain regulation, and atrophy of its gray matter may lead to somatic pain.48 Besides, the “u” type fiber between anterior cingulate and anterior insula is associated with algogenesis.49 Hatton et al.50 investigated the entire brain structural phase magnetic resonance in patients with generalized anxiety disorder (GAD) and found that the GMV of insula was significantly lower than the HC group. Some fMRI studies indicated that primary insomnia (PI) patients have abnormal neurons intrinsic brain activity in various brain regions, including insula.51,52 As we mentioned above, DE patients with EP suffer from insomnia and depression8–10; thus, we can consider mental disorders in EP patients together with the insula changes. In this experiment, GMV values of the left insula and the right insula in EP patients both decreased.
Accordingly, we suspect the decrease of GMV in the two parts of insula may be the pathological mechanism of pain and mental disorders. We found that the VAS in patients with acute EP correlated negatively with the GMV value of the left insula (r = −0.7864, P = 0.0024) and right insula (r = −0.9723, P < 0.0001). It has been mentioned that the insula is associated with pain regulation, the decreased GMV value of the insula may reflect the severity of acute EP. Furthermore, the duration of acute EP correlated negatively with the GMV value of the right insula (r = −0.9099, P < 0.0001), and we assume that the longer the duration of EP, the more serious of the EP. The decreased GMV value of the insula may reflect the existence of some mental disorders, such as anxiety, insomnia, and depression,50–52 which can explain the possibility of mental disorders in patients with acute eye pain. In addition, we found that some brain regions have no correlation with VAS or the duration; thus, we speculate that the values are also affected by vision.
The thalamus is the largest part of the diencephalon and is composed of many functional nuclei.53 The function of the thalamus includes coordinating the development and expression of movement, emotional drive, planning, and cognition in targeted behaviors. Based on the date from several studies, it is possible that some neuropsychiatric disorders, such as insomnia and chronic fatigue syndrome, are related to abnormalities of the thalamus.54,55 The GMV of the thalamus in EP patients in our study reduced and we speculated that mental disorders in EP patients might be relative to the reduction of the left thalamus.
The caudate nucleus is a part of basal ganglia and is related to motor tasks and may contribute to pain as well as changes in cognition and behavior in patients with migraine.56 The acute EP patients in our study showed lower GMV values in the left caudate, which indicates caudate dysfunction.
As a part of visual path, the cuneus is associated with spatial location and it is an essential role in visual processing, and it interacts with the primary visual cortex V1 to encode visual information to the extrastriate cortices.57,58 In our study, EP patients decreased the GMV values in the brain regions of the right cuneus, we speculated that EP may lead to dysfunction of the right cuneus.
There are still several shortcomings in our experiments. First, the reliability of the results may be affected by the limited number of subjects. Second, the causes of EP are multifaceted, and making an accurate assessment of various types of EP is impossible. Last, the natural difference of the selected subjects' brain structure may also influence the experiment.
Conclusion
As discussed above, we have elucidated that the acute EP patients showed decreased GMV values in some brain regions, including some somatosensory regions, and an overall reduced WMV value. In addition to atypical pain, the acute EP patients might suffer from mental disorders. These findings provide valuable information for exploring the neural mechanisms of acute EP.
Acknowledgments
Supported by grants from the National Natural Science Foundation of China (No: 81660158, 81160118, 81400372); Natural Science Key Project of Jiangxi Province (No: 20161ACB21017); Youth Science Foundation of Jiangxi Province (No: 20151BAB215016); Technology and Science Foundation of Jiangxi Province (No: 20151BBG70223); and Health Development Planning Commission Science Foundation of Jiangxi Province (No: 20175116).
Disclosure: D.-Y. Lan, None; P.-W. Zhu, None; Y. He, None; Q.-H. Xu, None; T. Su, None; B. Li, None; W.-Q. Shi, None; Q. Lin, None; Y.-C. Yang, None; Q. Yuan, None; J.-W. Fang, None; Q.-H. Li, None; Y. Shao, None
References
- 1.Lee AG, Al-Zubidi N, Beaver HA, Brazis PW. An update on eye pain for the neurologist. Neurol Clin. 2014;32:489–505. doi: 10.1016/j.ncl.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal P, Borsook D, Moulton EA. Oculofacial pain: corneal nerve damage leading to pain beyond the eye. Invest Ophthalmol Vis Sci. 2016;57:5285–5287. doi: 10.1167/iovs.16-20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song X, Xie L, Tan X, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS One. 2014;9:e113843. doi: 10.1371/journal.pone.0113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2015;100:128–134. doi: 10.1136/bjophthalmol-2014-306280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aicher SA, Hegarty DM, Hermes SM. Corneal pain activates a trigemino-parabrachial pathway in rats. Brain Res. 2014;1550:18–26. doi: 10.1016/j.brainres.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moulton EA, Becerra L, Rosenthal P, Borsook D. An approach to localizing corneal pain representation in human primary somatosensory cortex. PLoS One. 2012;7:e44643. doi: 10.1371/journal.pone.0044643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang Y, Zhou W, Wang P, et al. Alkali burn induced corneal spontaneous pain and activated neuropathic pain matrix in the central nervous system in mice. Cornea. 2017;36:1408–1414. doi: 10.1097/ICO.0000000000001336. [DOI] [PubMed] [Google Scholar]
- 8.Galor A, Seiden BE, Park JJ, et al. The association of dry eye symptom severity and comorbid insomnia in US veterans. Eye Contact Lens. 2018;44(Suppl 1):S118–S124. doi: 10.1097/ICL.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labbé A, Wang YX, Jie Y, Baudouin C, Jonas JB, Xu L. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol. 2013;97:1399–1403. doi: 10.1136/bjophthalmol-2013-303838. [DOI] [PubMed] [Google Scholar]
- 10.Szakáts I, Sebestyén M, Németh J. The role of health anxiety and depressive symptoms in dry eye disease. Curr Eye Res. 2016;41:1044. doi: 10.3109/02713683.2015.1088955. [DOI] [PubMed] [Google Scholar]
- 11.Lee AG, Al-Zubidi N, Beaver HA, Brazis PW. An update on eye pain for the neurologist. Neurol Clin. 2014;32:489–505. doi: 10.1016/j.ncl.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Szatmáry G. Neuroimaging in the diagnostic evaluation of eye pain. Curr Pain Headache Rep. 2016;20:52. doi: 10.1007/s11916-016-0582-8. [DOI] [PubMed] [Google Scholar]
- 13.Goksan S, Hartley C, Emery F, et al. fMRI reveals neural activity overlap between adult and infant pain. eLife. 2015;4 doi: 10.7554/eLife.06356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JE, Neil C, Jarred Y, Sean M. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLoS One. 2011;6:e24124. doi: 10.1371/journal.pone.0024124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jutzeler CR, Curt A, Kramer JLK. Relationship between chronic pain and brain reorganization after deafferentation: a systematic review of functional MRI findings. Neuroimage Clin. 2015;9:599–606. doi: 10.1016/j.nicl.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Li Y, An D, et al. Altered regional activity and inter-regional functional connectivity in psychogenic non-epileptic seizures. Sci Rep. 2015;5:11635. doi: 10.1038/srep11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amemiya S, Takahashi K, Mima T, et al. Reversible alterations of the neuronal activity in spontaneous intracranial hypotension. Cephalalgia. 2015;36:162–171. doi: 10.1177/0333102415585085. [DOI] [PubMed] [Google Scholar]
- 18.Pan ZM, Li HJ, Bao J, et al. Altered intrinsic brain activities in patients with acute eye pain using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:251–257. doi: 10.2147/NDT.S150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang LY, Li HJ, Huang X, et al. Assessment of synchronous neural activities revealed by regional homogeneity in individuals with acute eye pain: a resting-state functional magnetic resonance imaging study. J Pain Res 201; 11:843–850. doi: 10.2147/JPR.S156634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 21.Barnes GR, Li X, Thompson B, Singh KD, Dumoulin SO, Hess RF. Decreased gray matter concentration in the lateral geniculate nuclei in human amblyopes. Invest Ophthalmol Vis Sci. 2010;51:1432–1438. doi: 10.1167/iovs.09-3931. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang J, Lu Y, Xin H, et al. The atrophy of white and gray matter volume in patients with comitant strabismus: evidence from a voxel-based morphometry study. Mol Med Rep. 2017;16:3276–3282. doi: 10.3892/mmr.2017.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Cai P, Shi L, et al. Voxel-based morphometry of the visual-related cortex in primary open angle glaucoma. Curr Eye Res. 2012;37:794–802. doi: 10.3109/02713683.2012.683506. [DOI] [PubMed] [Google Scholar]
- 24.Xin H, Qiang Z, Hu PH, et al. White and gray matter volume changes and correlation with visual evoked potential in patients with optic neuritis: a voxel-based morphometry study. Med Sci Monit. 2016;22:1115–1123. doi: 10.12659/MSM.897837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ting WK, Fischer CE, Millikin CP, et al. Grey matter atrophy in mild cognitive impairment / early Alzheimer disease associated with delusions: a voxel-based morphometry study. Curr Alzheimer Res. 2015;12:165–172. doi: 10.2174/1567205012666150204130456. [DOI] [PubMed] [Google Scholar]
- 26.Van dVJ, Gromann PM, Swart M, et al. Grey matter, an endophenotype for schizophrenia? A voxel-based morphometry study in siblings of patients with schizophrenia. J Psychiatry Neurosci. 2015;40:207–213. doi: 10.1503/jpn.140064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans RW, Foroozan R. Eye pain without visual symptoms. Headache. 2010;47:1206–1209. doi: 10.1111/j.1526-4610.2007.00888.x. [DOI] [PubMed] [Google Scholar]
- 28.Crane AM, Levitt RC, Felix ER, et al. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol. 2016;101:227–231. doi: 10.1136/bjophthalmol-2015-308214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galor A, Small L, Feuer W, et al. The relationship between ocular itch, ocular pain, and dry eye symptoms (An American Ophthalmological Society Thesis) Trans Am Ophthalmol Soc. 2018;115:T5. [PMC free article] [PubMed] [Google Scholar]
- 30.Koziol LF, Budding D, Andreasen N, et al. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 2014;13(1):151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schutter DJLG. A cerebellar framework for predictive coding and homeostatic regulation in depressive disorder. Cerebellum. 2016;15:30–33. doi: 10.1007/s12311-015-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumann O, Borra RJ, Bower JM, et al. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14:197–220. doi: 10.1007/s12311-014-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adamaszek M, D'Agata F, Ferrucci R, et al. Consensus paper: cerebellum and emotion. Cerebellum. 2016;16:1–25. doi: 10.1007/s12311-016-0815-8. [DOI] [PubMed] [Google Scholar]
- 34.Groenewold NA, Opmeer EM, De JP, et al. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Lee HY, Tae WS, Yoon HK, et al. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: an optimized voxel-based morphometry study. J Affect Disord. 2011;133:128–136. doi: 10.1016/j.jad.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Guo WB, Liu F, Xue ZM, et al. Abnormal neural activities in first-episode, treatment-naïve, short-illness-duration, and treatment-response patients with major depressive disorder: a resting-state fMRI study. J Affect Disord. 2011;135:326–331. doi: 10.1016/j.jad.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 37.Rajmohan V, Mohandas E. The limbic system. Indian J Psychiatry. 2007;49:132–139. doi: 10.4103/0019-5545.33264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apps MAJ, Rushworth MFS, Chang SWC. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron. 2016;90:692–707. doi: 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzabazis, Alexander, Yeomans, et al. Shaped magnetic field pulses by multi-coil repetitive transcranial;magnetic stimulation (rTMS) differentially modulate anterior cingulate;cortex responses and pain in volunteers and fibromyalgia patients. Mol Pain. 2013;10:16. doi: 10.1186/1744-8069-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irish M, Halena S, Kamminga J, et al. Scene construction impairments in Alzheimer's disease - a unique role for the posterior cingulate cortex. Cortex. 2015;73:10–23. doi: 10.1016/j.cortex.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Catani M, Dell'Acqua F, Thiebaut dSM. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37:1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149:222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baliki MN, Chang PC, Baria AT, Centeno MV, Apkarian AV. Resting-sate functional reorganization of the rat limbic system following neuropathic injury. Sci Rep. 2013;4:6186. doi: 10.1038/srep06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasquoine PG. Contributions of the insula to cognition and emotion. Neuropsychol Rev. 2014;24:77–87. doi: 10.1007/s11065-014-9246-9. [DOI] [PubMed] [Google Scholar]
- 46.Droutman Read, Stephen J, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci. 2015;19:414–420. doi: 10.1016/j.tics.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp. 2012;34:109–149. doi: 10.1002/hbm.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogdanov VB, Viganò A, Noirhomme Q, et al. Cerebral responses and role of the prefrontal cortex in conditioned pain modulation: an fMRI study in healthy subjects. Behav Brain Res. 2015;281:187–198. doi: 10.1016/j.bbr.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cauda F, Costa T, Torta DME, et al. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatton SN, Lagopoulos J, Hermens DF, Naismith SL, Bennett MR, Hickie IB. Correlating anterior insula gray matter volume changes in young people with clinical and neurocognitive outcomes: an MRI study. BMC Psychiatry. 2012;12:45. doi: 10.1186/1471-244X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang T, Yan J, Li S, et al. Increased insular connectivity with emotional regions in primary insomnia patients: a resting-state fMRI study. Eur Radiol. 2017;27:3703–7309. doi: 10.1007/s00330-016-4680-0. [DOI] [PubMed] [Google Scholar]
- 52.Nie X, Peng DC, Li HJ, et al. Frequency-dependent alterations in amplitude of low-frequency fluctuations in primary insomnia: resting-state fMRI study. Chinese Journal of Medical Imaging Technology. 2016;32:204–208. [Google Scholar]
- 53.Mastropasqua C, Bozzali M, Spanò B, Koch G, Cercignani M. Functional anatomy of the thalamus as a model of integrated structural and functional connectivity of the human brain in vivo. Brain Topogr. 2015;28(4):548–558. doi: 10.1007/s10548-014-0422-2. [DOI] [PubMed] [Google Scholar]
- 54.Kang JM, Joo SW, Son YD, et al. Low white-matter integrity between the left thalamus and inferior frontal gyrus in patients with insomnia disorder. J Psychiatry Neurosci. 2018;43:170195. doi: 10.1503/jpn.170195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng FX, Lu ZM, Zhou MM, et al. Aberrant whole-brain functional connectivity in children with chronic fatigue syndrome. Chinese Journal of Medical Imaging Technology. 2014;30:366–374. [Google Scholar]
- 56.Seghatoleslam M, Ghadiri MK, Ghaffarian N, Speckmann EJ, Gorji A. Cortical spreading depression modulates the caudate nucleus activity. Neuroscience. 2014;267:83–90. doi: 10.1016/j.neuroscience.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 57.Wyble B, Swan G. Mapping the spatiotemporal dynamics of interference between two visual targets. Atten Percept Psychophys. 2015;77:2231–2243. doi: 10.3758/s13414-015-0938-x. [DOI] [PubMed] [Google Scholar]
- 58.Gerardmercier F, Carelli PV, Pananceau M, Troncoso XG, Frégnac Y. Synaptic correlates of low-level perception in V1. J Neurosci. 2016;36(14):3925–3942. doi: 10.1523/JNEUROSCI.4492-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]