Abstract
The nonspecific divalent cation channel TRPM7 (transient receptor potential-melastatin-like 7) is involved in many Ca2+ and Mg2+-dependent cellular processes, including survival, proliferation and migration. TRPM7 expression predicts metastasis and recurrence in breast cancer and several other cancers. In cultured cells, it can induce an invasive phenotype by promoting Ca2+-mediated epithelial-mesenchymal transition. We previously showed that in neuroblastoma cells that overexpress TRPM7 moderately, stimulation with Ca2+-mobilizing agonists leads to a characteristic sustained influx of Ca2+. Here we report that sustained influx through TRPM7 is abruptly abrogated by elevating intracellular levels of cyclic adenosine monophosphate (cAMP). Using pharmacological inhibitors and overexpression studies we show that this blockage is mediated by the cAMP effector Protein Kinase A (PKA). Mutational analysis demonstrates that the Serine residue S1269, which is present proximal to the coiled-coil domain within the protein c-terminus, is responsible for sensitivity to cAMP.
Introduction
TRPM7 is a ubiquitously expressed channel-kinase that regulates ion levels, affects gene expression and phosphorylates several target proteins. This versatile protein is involved in many cellular processes including cell survival, proliferation and migration [1, 2]. TRPM7 is also involved in other clinically relevant pathological processes, such as anoxic neuronal death [3, 4] and cardiac pathology [5, 6].
The involvement of TRPM7 in cancer development is increasingly recognized. Breast cancer patients with a high TRPM7 expression have a poor prognosis [7–9] and TRPM7 single nucleotide polymorphisms (SNPs) are associated with breast cancer [10]. Ca2+ signals mediated by TRPM7 are thought to facilitate metastasis by inducing the epithelial-mesenchymal transition (EMT). This gives rise to a more motile and aggressive phenotype. Therefore, TRPM7 may be considered a prometastatic protein and an important player in Ca2+ driven dissemination of cancer [11].
The TRPM7 protein shows 6 trans-membrane domains, a pore-forming domain, a coiled-coil domain and an α-kinase domain. TRPM7 forms a tetrameric channel present at the plasma membrane that is permeable to divalent ions including Ca2+, Mg2+, and Zn2+ [12]. A role in intracellular vesicles, in particular on Zn2+ storage vesicles, has also been demonstrated recently [13]. The TRPM7 kinase domain phosphorylates several target proteins, including the myosin heavy chain, and thereby affects cell adhesion and migration [14, 15]. The α-kinase can also be cleaved off and translocate to the nucleus where it regulates gene function epigenetically [16, 17].
We focused on the role of TRPM7 at the plasma membrane as a Ca2+ entry pathway. Extracellular Ca2+ levels are about 4 orders of magnitude higher than intracellular levels, and this steep gradient enables Ca2+ to fulfill its important role as intracellular messenger involved in a wide variety of cellular processes, including polarization, adhesion, and migration. TRPM7 is also involved in setting the basal Ca2+ concentration as its expression and knockdown affect levels of cytosolic Ca2+ [14, 18] and calcium “sparks” or “flickers”, respectively [19–21]. These short lived local Ca2+ peaks are thought to coordinate the direction of cell migration.
The mechanism of TRPM7 channel activation is not yet fully elucidated. TRPM7 activity is influenced by many cellular and environmental cues. PIP2 hydrolysis has been reported to close TRPM7 channels [22] but on the other hand, we and others found that agonist-induced triggering of phospholipase C (PLC) may activate the channel instead [18, 23–25]. In addition, Mg2+ levels, nucleotide concentration, cAMP levels, pH and reactive oxygen species (ROS) all have been reported to affect TRPM7 channel gating, for reviews see Yee et al. 2014 and Visser et al. 2014 [1, 2]. Note that some of these proposed mechanisms have been heavily debated, perhaps because in different studies, different readout methods were used to quantify TRPM7 activity. For example, in whole-cell patch clamping studies, Mg2+-free pipette solutions have been used to evoke large outward rectifying TRPM7 currents that are easily quantified [22, 26]. We and others noted that the effects of cell signaling on TRPM7 currents as detected in whole-cell patch clamping do not always mirror the effects of these signals on TRPM7-dependent cell-biological read-outs, including migration, adhesion and the regulation of Ca2+ levels. We therefore have been applying non-invasive techniques to study TRPM7 activity. Using fluorescent monitoring of intracellular Ca2+ levels we reported that in TRPM7-overexpressing mouse neuroblastoma cells (N1E-115/TRPM7), addition of PLC-activating agonists causes a sustained Ca2+ influx that is not observed in N1E-115 control cells [14, 18]. This result contrasts with the inhibitory action of PLC activation as detected in whole-cell patch clamp studies [22] and we therefore addressed this issue in detail. Importantly, activation of TRPM7 by PLC-coupled agonists was confirmed in perforated-patch clamp experiments [18, 27, 28], in other cell lines [25], and it is in line with reported biological effects downstream of TRPM7 which appear to be enhanced, rather than inhibited, by PLC-activating stimuli [14, 19, 23, 24, 29]. It is important to note that as yet, it is unclear by what mechanism exactly BK causes TRPM7 opening. Nevertheless, Ca2+ fluorometry offers a convenient and highly sensitive readout to study TRPM7 activation at the plasma membrane and avoids problems caused by internal perfusion.
Using the N1E-115 neuroblastoma system, we here describe that the TRPM7-mediated sustained Ca2+ influx is abrogated upon elevation of cAMP levels. This effect is PKA dependent and involves serine residue S1269 in the carboxyl terminus of TRPM7. Our results reveal a hitherto unrecognized control mechanism for TRPM7 and emphasize the complex cellular regulation of this versatile protein.
Materials and methods
Cell culture and transfection
Mouse neuroblastoma cells overexpressing TRPM7-WT (N1E-115/TRPM7) have been previously described [14]. The S1224A and S1269A mutations were introduced in wild type TRPM7 cDNA in a pTracer vector, using the Phusion Site-Directed Mutagenesis Kit and primers, both from Life Technologies (Waltham, MA, USA). Primers: S1269A-forward: TCACACGAGAATTGGCTATTTCCAAACACT, S1269A-reverse: AGTGTTTGGAAATAGCCAATTCTCGTGTGA, S1224A-forward: CTACATAAAAAGAGCATTACAGTCTTTAGA and S1224A-reverse: TCTAATGATTGTAATGATCTTTTTATGTAG. Constructs were checked by sequencing and inserted as an XhoI-Not1 fragment into a LZRS-neomycin resistant retroviral vector and introduced in the parental N1E-115 cells. Cells were selected for neomycin resistance and adhesive properties. Retroviral transduction resulted in stable and moderate TRPM7-S1269A overexpression to levels comparable to those of WT TRPM7 in N1E-115/TRPM7-WT cells. Total mRNA was extracted using the GeneJET RNA Purification Kit (Thermo Fisher) according to manufactures protocol and cDNA was synthesized using SuperScript II rtPCR enzyme (Thermo Fisher). PCR was performed using SYBR-Green (Takara) using the following primers: Fw TAGCCTTTAGCCACTGGACC and Rv GCATCTTCTCCTAGATTGGCAG. Expression levels in N1E-115 wild type cells were set to 1. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% Fetal Calf Serum (FCS). Penicillin and streptomycin added at 100 μg/ml each were from Gibco, Life Technologies (Waltham, MA, USA). Cells were seeded on 24-mm glass-coverslips in 6-well plates. Transient transfections were done using 1 μg DNA and 3 μg Polyethylenimine (PEI; Polysciences Inc. Warrington, PA, USA) per well.
Materials and constructs
The PKA regulatory and catalytic subunits were transiently expressed [30]. Fluorometry was done using Förster/Fluorescence Resonance Energy Transfer (FRET) based biosensors for cytosolic Ca2+ and cAMP. For Ca2+ the troponin C-based sensor, Twitch-2B was used [31]. Additionally, for cAMP the Epac-based sensors Epac-SH187 and Epac-SH189 were used [32]. The compounds bradykinin, forskolin, 3-isobutyl-1-methylxanthine (IBMX) and ionomycin were from Calbiochem-Novabiochem Corp. (La Jolla, USA). Prostaglandin E1 (PGE1) was from Sigma-Aldrich (Zwijndrecht, The Netherlands). The PKA inhibitor H-89 was obtained from (Biolog, Bremen, Germany). The Epac-selective cAMP analogue 007-AM (8-pCPT-2-O-Me-cAMP-AM) was from Cayman Chemical (Michigan, USA). CaCl2 salt was from Merck (Darmstadt, Germany). Fluorescent Ca2+ indicators Oregon Green 488 BAPTA-1-AM and Fura Red-AM were from Invitrogen, Life Technologies (Waltham, MA, USA).
Fluorometric Ca2+ and cAMP measurements
Fluorometry was done on an inverted Nikon widefield microscope. A 63x oil immersion lens was used, in combination with a 34°C stage heater. The microscopy medium was kept at a pH of 7.2, using HBS (HEPES buffered saline). This buffer contained 10 mM glucose, 2 mM CaCl2, 5 mM KCl, 140 mM NaCl, 1 mM MgCl2 and 10 mM HEPES. The excitation of the Cyan Fluorescent Protein (CFP) was set at 425 nm. CFP and Yellow Fluorescent Protein (YFP) emissions were measured simultaneously using band-pass filters at 470±20 and 530±25 nm respectively. Ca2+ measurements were done using FRET biosensors and chemical dyes. The Ca2+ FRET sensor Twitch-2B was used. Also, the Ca2+ dyes Oregon Green 488 BAPTA-1-AM and Fura Red-AM were used. No quantitative differences could be detected between these methods, with respect to the TRPM7 Ca2+ signals. Before the experiments the FRET ratio was set at 1.0 and a baseline was recorded. After the Ca2+ experiments a calibration was done adding ionomycin (10 μM) and a high dose of Ca2+ (CaCl2, 10 mM).
Fluorescence Lifetime Imaging (FLIM) experiments
Fluorescence Lifetime Imaging experiments were carried out using a Leica TCS-SP8 FALCON confocal FLIM microscope and our Epac-SH189 FRET/FLIM cAMP sensor. In brief, in this sensor, the acceptor moiety was replaced with a dark (i.e. non-emitting) YFP variant [32]. Thus, the sensor is not suited for ratiometric detection, but FRET changes are apparent as a characteristic shortening of the donor lifetime. Unlike ratiometry, FLIM measurements are inherently quantitative, allowing easy comparison of cAMP levels between cells.
FLIM was also used for simultaneous sensing of cAMP and Ca2+ levels in our cells. Use of the dark acceptor sensor preserves the long-wavelength part of the spectrum for use of a second, independent indicator. We tested a range of red-shifted Ca2+ dyes but none of them had the necessary combination of high dynamic signal, good loading and high affinity. Therefore we reverted to loading Oregon-Green BAPTA-1 (permeable AM ester at 7 μg/ml) into N1E-115 cells transiently transfected with Epac-SH189. As the emission of Oregon-Green largely overlaps with that of the FRET donor, we used line-by-line sequential excitation imaging with a 440 nm diode laser (which excites CFP and to a much lesser extent Oregon Green) and the 488 nm line of the argon laser. Laser intensities and detector sensitivity were adjusted so as to optimally retrieve both signals. Experiments in which Ca2+ and cAMP signals could not be discriminated reliably were rejected in the analysis.
Statistics
Each figure is representative of experiments on at least three different days. For clarity, a representative Ca2+ trace is shown. The Ca2+ peaks were normalized 0 to 1, using feature scaling with the following formula: Xi 0 to 1 = (Xi-Xmin)/(Xmax-Xmin). Where relevant, the respective traces are complemented with quantification, based on the indicated number of Ca2+ traces. Statistical significance was determined using a two-tailed unpaired Student's t-test. The following convention was used to indicate statistical significance: P ≤ 0.05 = *, P ≤ 0.01 = **, and P ≤ 0.001 = ***. Error bars indicate the Standard Error of the Mean (SEM) for the indicated number of measurements.
PKA consensus site prediction
On three different prediction web servers for PKA phosphorylation sites, S1224 and S1269 were indicated as PKA consensus sites. These servers were: NetPhos3.1 [33], pkaPS [34] and GPS 2.0 [35]
Results and discussion
TRPM7-mediated sustained Ca2+ influx terminates upon cAMP elevation
In N1E-115 control cells addition of bradykinin (BK) evokes a single, brief Ca2+ spike that terminates after approximately one minute. We and others previously showed that moderate overexpression of TRPM7 changes the kinetics of the BK response in that the initial transient response is followed by a more sustained phase of elevated Ca2+ levels that lasts several minutes (Fig 1A) [14, 18, 25]. We also showed that the initial transient response is due to IP3-mediated release of Ca2+ from internal stores, whereas the sustained Ca2+ influx phase is strictly dependent on TRPM7 channels functioning at the plasma membrane. Furthermore, sustained Ca2+ influx depends on the presence of a Ca2+ gradient across the membrane and it is blocked by TRPM7 inhibitors including La3+, 2-APB, SKF96365 and Waixenicin-A [18, 21]. These results also showed that analysis of calcium levels by ratiometric fluorometry of N1E-115/TRPM7 cells presents a robust and very sensitive readout of TRPM7 activity in intact cells.
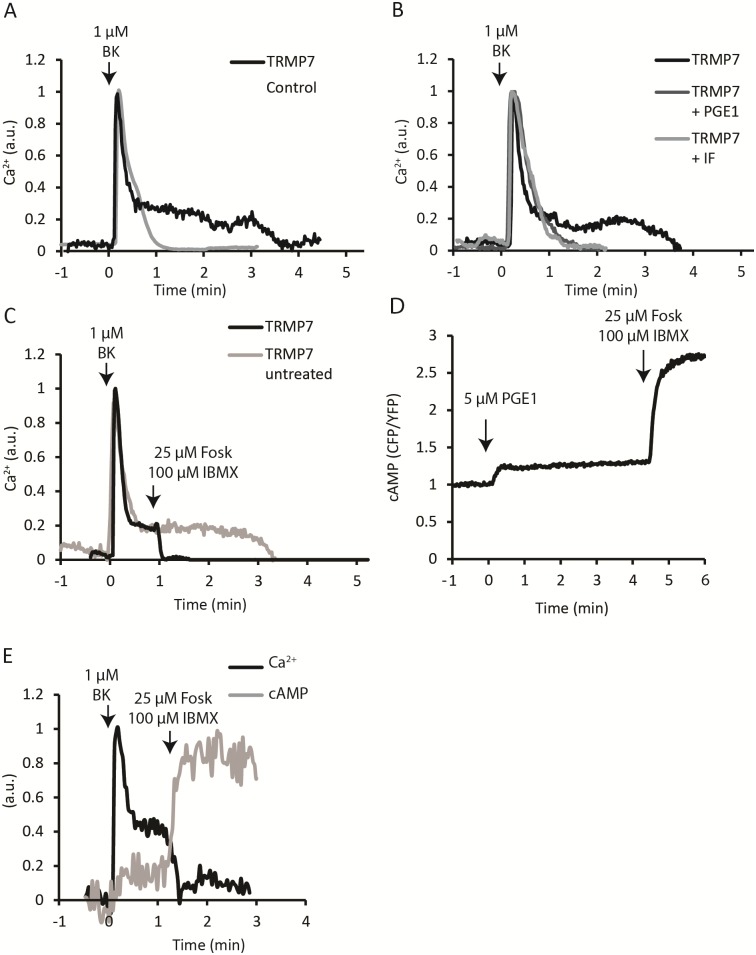
Fig 1. TRPM7-mediated sustained Ca2+ influx is abrogated by cAMP elevation.
(A) Stimulation with bradykinin triggers a sustained influx of extracellular Ca2+ in cells overexpressing TRPM7 (black), lasting for several minutes. Control cells (grey) show only a single brief Ca2+ transient upon bradykinin stimulation that typically lasts approximately one minute. Calcium levels are expressed as arbitrary units (a.u.). (B) Pretreatment with PGE1 (dark grey) or IBMX/forskolin (lighter grey) prevents the sustained influx in TRPM7-WT overexpressing cells. (C) IBMX/forskolin stimulation abruptly terminates the TRPM7-mediated sustained Ca2+ influx (arrow). (D) Using the Epac-based FRET biosensor it was verified that both PGE1 and IBMX/forskolin treatment elevate cytosolic cAMP concentrations. (E) Simultaneous recording of cAMP using FLIM/FRET and Ca2+ with Oregon-Green BAPTA-1 demonstrates tight temporal relationship between cAMP elevation and termination of Ca2+ influx.
When testing a panel of GPCR agonists for induction of sustained Ca2+ influx, we unexpectedly observed that pretreatment with prostaglandin E1 (PGE1, 5 μM) prevented the sustained influx following stimulation with BK. While untreated N1E-115/TRPM7 cells show a sustained BK response that lasted for several minutes, in cells pretreated with PGE1 the cytosolic Ca2+ returned to baseline within 1–2 minutes (Fig 1B).
The calcium-dependent fluorescence levels were quantified at 2 minutes post stimulation with BK. Compared to wild-type N1E-115 cells, in untreated TRPM7 cells Ca2+-dependent fluorescence remained significantly elevated (15% +/- 10%; N = 7; p = 0,006). By contrast, in PGE1 pretreated TRPM7 cells Ca2+-dependent fluorescence had returned to near-baseline values after 2 minutes (2% +/- 1%; N = 12; p = 0.265). We next set out to investigate the responsible mechanism. PGE1 fails to activate PLC in these cells [18] but it is well-known to stimulate production of the second messenger cAMP in various cell types. To determine whether a rise in cAMP levels may be involved in inhibition of TRPM7-mediated Ca2+ influx, we pretreated cells with forskolin (25 μM) and IBMX (100 μM), which elevates cAMP levels by activating adenylate cyclase and blocking phosphodiesterase, respectively. Indeed, similar to PGE1 pretreatment, in cells pretreated with IBMX + forskolin (IF) the Ca2+ returned to baseline within 1–2 minutes (Fig 1B). At 2 minutes post BK addition, Ca2+-dependent fluorescence had decreased to 4% +/- 3% (N = 24; p = 0.052) in IF-pretreated cells.
Strikingly, when added during the sustained phase of calcium entry, addition of IBMX/forskolin abruptly terminated TRPM7-mediated Ca2+ influx (Fig 1C). Using our Epac-based FRET biosensor [32] we confirmed that treatment with PGE1 and IF raises cytosolic cAMP levels in N115 cells, as expected (Fig 1D). Thus, elevation of cAMP levels abrogates sustained Ca2+ influx in N1E-115/TRPM7 cells. To examine the temporal relationship between agonist-induced termination of Ca2+ influx and cAMP production in more detail we set out to detect Ca2+ and cAMP simultaneously in single cells. This proved less straight-forward than anticipated due to spectral overlap of the cAMP sensor with suitable Ca2+ dyes (Materials and methods). For these studies, we therefore had to revert to detection of cAMP using our novel dedicated fluorescence lifetime sensor [32] and Fluorescence Lifetime Imaging (FLIM). This FLIM sensor features dark (i.e., non-emitting) acceptors which allowed simultaneous detection of cAMP and the emission of Oregon-Green BAPTA-1 (see Materials and methods). Residual spectral overlap made it hard to quantitatively unmix contributions of both signals (Fig 1E, S1 File), but in 5 experiments, counting in total 23 cells, we observed that inhibition of TRPM7-mediated Ca2+ influx followed within 5–20 s after addition of IBMX/forskolin. As these experiments are quite demanding, we focused on side-by-side comparisons of each of those signals in the remainder of our studies.
Involvement of protein kinase A
To determine which cAMP effector protein is involved, we used specific activators and inhibitors for PKA and Epac (Exchange Protein directly Activated by cAMP), the two most prominent targets downstream of cAMP. Initially, we tested for Epac involvement, using the Epac-selective cAMP analogue 007-AM (8-pCPT-2-O-Me-cAMP-AM) [36]. Pretreatment of cells with 1 μM 007-AM had no effect on basal calcium levels and also did not affect the BK-induced sustained influx (Fig 2A). 007-AM did cause rapid FRET changes in our Epac-based biosensor [32], indicating that it readily permeates the membrane and activates the Epac protein (Fig 2A). These experiments exclude a role for Epac in the cAMP-induced termination of Ca2+ influx. In contrast, the PKA inhibitor H-89 (10 μM) [37] completely prevented the IBMX/forskolin-induced termination of sustained Ca2+ influx (Fig 2B) in 13 out of 13 experiments (p < 0.001). These data indicate that PKA may play an important role in the termination of Ca2+ influx through TRPM7.
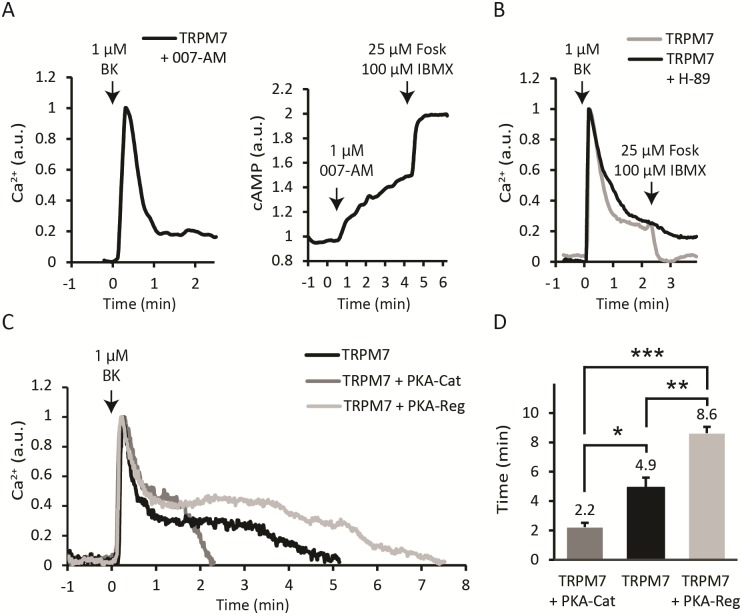
Fig 2. Involvement of PKA in the abrogation of Ca2+ influx.
(A) Left panel, pretreatment with the Epac-selective cAMP analogue 007-AM (1 μM, 5 minutes) did not affect the sustained influx in 12/12 cells, p<0.001. Right panel, 007-AM activates the Epac FRET biosensor, verifying its biological activity in 30/30 cells, p<0.001. (B) Pretreatment with PKA inhibitor H-89 (10 μM, 5 minutes) largely blocked sensitivity of Ca2+ influx to cAMP, as addition of IBMX/forskolin during the sustained phase was without effect. (C) Overexpressing either PKA-Cat or PKA-Reg significantly affected the duration of the sustained Ca2+ influx. The left panel shows that the sustained Ca2+ influx of TRPM7 alone (black) lasted approximately 5 minutes. Overexpressing the catalytic subunit (dark grey) reduced the duration of the influx to approximately 2 minutes. Conversely, overexpressing the regulatory subunit (light grey) prolonged the influx to approximately 8 minutes. Representative traces from a single experiment are shown; data are quantified in the right panel. *, p < 0.05; **, p<0.01; ***, p<0.001.
At rest, PKA is a tetramer of two identical catalytic kinase (Cat) subunits and two regulatory subunits (Reg) that inhibit activity of the kinases. cAMP binding to the regulatory subunits causes the complex to dissociate, releasing the catalytic subunits from inhibition and allowing them to phosphorylate consensus sequences in a variety of cellular proteins. To further investigate the link between PKA activation and termination of Ca2+ influx through TRPM7 we overexpressed PKA subunits individually. It may be expected that overexpression of the catalytic subunit increases PKA activity and therefore reduces influx, whereas overexpression of the regulatory subunit would prevent kinase activity. Note that for these experiments, PKA subunits were overexpressed transiently, as it is difficult to achieve stable overexpression of either catalytic or regulatory subunits of PKA due to its growth-regulatory effects. For these series of experiments, a genetically encoded Ca2+ FRET sensor was cotransfected to serve both as a transfection marker and for Ca2+ readout. Indeed, overexpression of either PKA-Cat or PKA-Reg significantly affected the duration of the sustained Ca2+ influx. The sustained phase in control N1E-115/TRPM7 cells lasted on average 294 seconds (N = 5 experiments with 2–3 cells each). Overexpressing the catalytic subunit reduced the influx length to 132 seconds (P = 0.012; N = 4 experiments). Conversely, overexpressing the regulatory subunit elongated the influx to 516 seconds (P = 0.008, N = 3 experiments) (Fig 2C). Taken together, these data strongly indicate that PKA is the effector that mediates termination of the sustained Ca2+ influx following elevation of cAMP.
A single point mutation, S1269A, renders TRPM7 resistant against cAMP-mediated termination of Ca2+ influx
In an attempt to identify possible PKA phosphorylation sites that may mediate cAMP sensitivity of Ca2+ influx, we revisited a set of serine point mutants that we had previously prepared for a study into the possible function of the coiled-coil region in TRPM7. Three serines within this set are known to be phosphorylated, namely S1224, S1255 and S1269 [38]. PKA phosphorylates serine (and to a lesser extent threonine) residues of target proteins at PKA consensus sites which consist of arginine residues at positions -3 and often also at -2, and a hydrophobic residue at +1 (RrXSϕ) [39]. Two of the mutants, S1224 and S1269 (see Materials and methods) conformed to the PKA consensus signature and consequently we focused on those two for further analysis.
N1E-115 cells were retrovirally induced to express either TRPM7-S1224A or TRPM7-S1269A, mutants which cannot be phosphorylated at the respective residues. Proper transduction was checked by qPCR (Fig 3E). Ca2+ fluorometry showed that cells expressing TRPM7-S1224A failed to display the characteristic sustained influx of Ca2+ seen in TRPM7-WT overexpressing cells when challenged with BK (Fig 3C). This may be because S1224A mutants do not localize at the plasma membrane properly, or alternatively, the channel may be defective in activation. In contrast, TRPM7-S1269A expressing cells displayed a prominent sustained Ca2+ influx following stimulation with BK. This indicates that the channel was expressed, that it is at the plasma membrane and that it is functional. Strikingly, addition of forskolin and IBMX to those cells failed to terminate the sustained response evoked by stimulation with BK. This implies that S1269A mutant channels have lost their sensitivity for regulation by cAMP (Fig 3). As a control, FLIM measurements showed that basal cAMP levels were very similar in these cells, ruling out that expression of the constructs differentially affected cAMP (Fig 3D) so as to affect mutant channel activity. Thus we conclude that PKA-dependent phosphorylation of S1269 must be a key determinant of agonist-induced Ca2+ influx through TRPM7 in these cells.
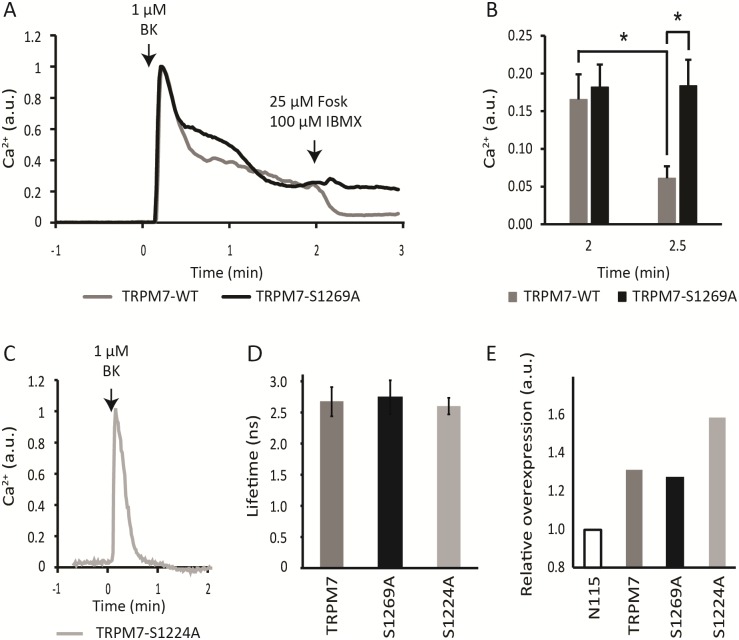
Fig 3. The S1269A phosphorylation-dead mutant is insensitive to cAMP-mediated termination of Ca2+ influx.
(A) Representative traces of Ca2+ responses in TRPM7-S1269A mutant (black) and TRPM7-WT (grey) expressing cells. Note that in the S1269A mutant sustained influx fails to terminate after being challenged with IBMX/forskolin (arrow). (B) Quantification of Ca2+ levels before (left bars, taken 2 min after addition of BK) and 30 s after (right bars) addition of IBMX/forskolin and expressed as average + SD of N = 7 TRPM7-WT (gray) and N = 10 TRPM7-S1269A (black) cells. IBMX/forskolin were added at 2 minutes post BK addition. In TRPM7-WT expressing cells but not in S1269A mutant expressing cells cAMP caused a significant drop in calcium levels (P = 0.016). The difference between TRPM7-WT and TRPM7-S1269A expressing cells following addition of IBMX/forskolin is also significant (P = 0.013). (C) Ca2+ response following stimulation with BK in cells transfected with TRPM7-S1224A shows absence of sustained influx. Similar results were obtained in 3 experiments of 3–4 cells each. (D) Fluorescence Lifetimes of Epac-SH189 in N1E-115 cells expressing wtTRPM7, S1269A and S1224A demonstrate that basal cAMP levels do not differ among these cell lines. (E) Transduction was checked by qPCR. A moderate 1.3x overexpression of S1269A was seen, as is common for N1E-115-TRPM7 cells. S1224 had a 1.6x overexpression. Note that although moderate, the overexpression levels observed are in line with literature reports [14, 40].
Concluding remarks
We found that in cells that overexpress TRPM7, bradykinin stimulation results in a sustained influx of extracellular Ca2+ that is sensitive to elevated intracellular cAMP levels. Using pharmacological inhibitors (H-89 and 007-AM) and overexpression of PKA subunits we showed that this effect is mediated by the cAMP effector protein kinase A. We also identified S1269, which is proximal to the coiled-coil region of TRPM7, as a key residue mediating this response. Together, these findings reveal a novel level of complexity in the regulation of TRPM7.
S1269 is found within a PKA consensus phosphorylation site (RELSI) present approximately 15 residues C-terminal from the coiled-coil domain. Three independent online prediction engines identified this site as possible PKA phosphorylation site, but we note that it lacks the preferential (although not obligatory) arginine at position -2 within the consensus motive (RrXSφ, [39]). This raises the formal possibility that another kinase downstream of PKA is responsible for S1269 phosphorylation. Using mass-spectrometric analysis of tryptic peptides, we attempted to directly demonstrate phosphorylation of TRPM7 in cells transiently overexpressing PKA catalytic subunits, but failed to detect phosphorylation of S1269 or other residues. However, such experiments are challenging and well outside our expertise and therefore we may have simply missed phosphorylation. Kim et al. [38] reported that S1269 is subject to phosphorylation but no information on the responsible kinase was included in that study. In a recent study by Cai et al. [41] S1269 was also found to be phosphorylated. Interestingly, these authors also showed phosphorylation in a kinase-defective mutant of TRPM7, indicating phosphorylation by an (unidentified) external kinase.
Is TRPM7 involved in store-depletion induced calcium entry? Superficially, the prolonged Ca2+ influx following BK addition is reminiscent to Icrac (Ca2+-Release Activated Ca2+ current, also called Store-Operated Calcium Entry, SOCE), and indeed, TRPM7 had initially been proposed as a candidate for Icrac. The notion was subsequently rejected in literature [42]. In addition, we were never able to induce store-operated Ca2+ entry in either N1E-115 cells or in N1E-115/TRPM7 cells [18] and in our cells, TRPM7 is sensitive to the specific inhibitor Waixenicin-A [21] whereas Icrac isn’t [43]. However, in a recent spin to this discussion, Faouzi and colleagues [44] reported that while TRPM7 is not identical to the SOCE channel, SOCE appeared to be controlled by the TRPM7 kinase domain in DT40 B lymphocytes. Nevertheless, in N1E-115 cells the phenotype of kinase deficient TRPM7 does not differ from wtTRPM7 [14, 27] and therefore we can reject this possibility in our cells.
Although TRPM7 is sensitive to many external agents, including receptor agonists, pH, ROS and even mechanical stress, the exact mechanisms by which channel gating is regulated are still not fully elucidated. The extensive cytosolic C-terminus of TRPM7 houses phosphorylation sites for several kinases, domains that mediate interaction with PIP2, a caspase cleavage site and an α-kinase domain that is important for interaction with PLC [1, 2]. Moreover, the protein is heavily autophosphorylated and interacts with several cytoskeletal proteins that, in turn, may convey signals to the channel [40]. It remains to be addressed how S1269 affects agonist-induced Ca2+ influx through TRPM7. In TRPM8, Tsuruda et al. showed that the C-terminal domain containing the coiled coil is important for tetramer formation [45]. Before embarking on these studies, we had hypothesized that addition of a negatively charged phosphate close to the coiled-coil region could potentially affect channel tetramerization, leading to its inactivation. The second PKA consensus site studied here, S1224, is present within the coiled-coil region, it is target for phosphorylation [38] albeit not in kinase-deficient TRPM7 expressing cells [41]. We showed that expression of S1224A mutant constructs failed to produce channels that mediate discernable Ca2+ influx. Nevertheless, more recently a convincing study showed that, at least in Zebrafish, truncated TRPM7 mutants lacking the coiled coil domain form functional channel oligomers [46]. Since our studies did not produce direct experimental support for a role of S1269 phosphorylation in channel oligomerization, alternative hypotheses for the strong phenotype of S1269A have to be considered. Conceivably, cAMP-dependent S1269 phosphorylation could induce an inhibitory conformational change in the protein and/or otherwise affect channel conductive properties. However, given that expression of the S1269A mutant phenocopies WT TRPM7 expression in inducing sustained Ca2+ influx, we can exclude altered expression or deficient routing to the plasma membrane.
A further complicating factor is that in our study (mutant) proteins are overexpressed in a normal TRPM7-wildtype background. This is because (in line with reported literature) we failed to achieve full TRPM7 knockout in our cells, and only slight overexpression of TRPM7 was tolerated [14]. Up- and downregulation of mRNA levels in TRPM7-dependent malignancies is also typically very modest [47], implicating tight regulation of TRPM7 levels. Consequently, in our study most tetramers of mutant channels can be expected to contain one or more wt TRPM7 proteins which complicates phenotypic characterization. The TRPM7-S1269A mutant cells showed expression levels comparable to those in N1E-115/TRPM7 cells. Importantly, expression of TRPM7-S1269A protein phenocopied N1E-115/TRPM7 WT cells in producing sustained Ca2+ influx, except that in these mutants raising cAMP failed to terminate Ca2+ influx. The strong and highly reproducible phenotype of S1269A mutant channels indicates that notwithstanding the modest overexpression levels, TRPM7 controls sustained Ca2+ influx and S1269 has a key role in mediating cAMP sensitivity of this influx.
We showed that the onset of inhibition of TRPM7-mediated sustained Ca2+ influx coincides with the first detectable rise (i.e., within 10–20 s) in cAMP (Fig 1E) and is complete long before cAMP peaks (usually in 2–3 minutes). This early onset of TRPM7 blockage may be because the cAMP effector kinase, PKA, has a much higher affinity for cAMP (Kd of ~230 nM, Hill coefficient >2) [48] than the FRET sensor ((Kd ~ 4 μM, Hill coefficient ~1) [32], a difference of ~ 20-fold. Thus, PKA will phosphorylate targets long before the cAMP sensor records the peak response in cAMP.
Overexpression of the PKA regulatory subunit was found to reproducibly delay the effects of raising cAMP, presumably by sequestrating free PKA catalytic subunits. This would imply that in the response termination of wtTRPM7 channels, cAMP signaling also plays a role. In this respect, it is notable that our quantitative FRET-FLIM experiments report detectable (i.e., non-zero) cAMP levels in N1E-115 cells as well as in the cells overexpressing mutant and wt TRPM7 channels (Fig 3D). Furthermore, using our newest generation of FRET sensors, we noted that BK induces a slow and minor increase in cAMP levels in a subset of cells. Therefore, involvement of cAMP/PKA signaling in the termination of wt TRPM7 channels is a formal possibility, but the current data sets do not allow drawing solid conclusions, and further experimentation is needed to resolve this issue.
We note that the inhibitory effects of cAMP on TRPM7 found in our study contrast with the stimulatory effects documented by Takezawa et al. [49]. These authors employed whole-cell patch clamping to show that cells, internally perfused with 100 μM cAMP showed enhanced outwardly rectifying TRPM7 currents in HEK293 cells. Noting that we addressed a similar discrepancy between whole-cell patch clamp results and intact-cell recording (Ca2+, perforated patch) with respect to PLC signaling (see Introduction), we propose that several factors may underlie this difference. First, in whole-cell patch clamp experiments TRPM7 currents are evoked by perfusing the cell interior with Mg2+-free internal solution. This triggers large TRPM7 currents that do not necessarily reflect the quite small Ca2+ currents we studied in intact cells [18]. Indeed, in the study by Takezawa and colleagues, the stimulatory effect was lost when 6 mM of Mg.ATP was included in the pipette solution. Furthermore, internal perfusion may also alter signaling pathways, e.g. by washout of soluble signaling components. In addition, Takezawa and colleagues used HEK293 human embryonal kidney cells rather than the neuroblastoma cells used in this study. HEK293 cells allow substantial overexpression before they eventually die from TRPM7 expression. This allows electrophysiological characterization of channel properties with exceptional signal-to-noise ratio, but it may also cause differences in response to cellular signals. It will be interesting to include TRPM7 S1269A in such studies.
Finally, please note that just before submission of the revised version of this manuscript, Tian and colleagues published significant data that strongly support the importance of S1269 and PKA in the regulation of TRPM7[50]. Remarkably, despite using whole-cell patch clamping, the data of these authors support our model of inhibitory cAMP action, although the study lacked controls to show how PGE2 affects cAMP in their cells.
In summary, our data reveal a new level of complexity in the cAMP-dependent regulation of TRPM7, the full elucidation of which awaits further experimentation.
Supporting information
Individual data and traces of Ca2+ and cAMP measurements in single cells as mentioned in Fig 1E. Despite poor signal-to-noise and cell-to-cell variability, the responses in 23 cells clearly demonstrate tight temporal correlation of TRPM7 inhibition and cAMP increase.
(ZIP)
Acknowledgments
We thank Joachim Goedhart for providing the Twitch-2B DNA and Bram van den Broek for technical assistance.
Data Availability
All relevant data are in the paper, its Supporting Information files, or on Open Science Framework at: https://osf.io/8twrn/.
Funding Statement
Funded by KWF kankerbestrijding grant NKI 2010-4626. To dr K. Jalink.
References
- 1.Yee NS, Kazi AA, Yee RK (2014) Cellular and Developmental Biology of TRPM7 Channel-Kinase: Implicated Roles in Cancer. Cells 3:751–77 10.3390/cells3030751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visser D, Middelbeek J, van Leeuwen FN, Jalink K (2014) Function and regulation of the channel-kinase TRPM7 in health and disease. Eur J Cell Biol 93:455–465 10.1016/j.ejcb.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 3.Aarts M, Iihara K, Wei W-L, Xiong Z-G, Arundine M, Cerwinski W, MacDonald JF, Tymianski M (2003) A Key Role for TRPM7 Channels in Anoxic Neuronal Death. Cell 115:863–877 [DOI] [PubMed] [Google Scholar]
- 4.Sun H-S, Jackson MF, Martin LJ, et al. (2009) Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci 12:1300–7 10.1038/nn.2395 [DOI] [PubMed] [Google Scholar]
- 5.Sah R, Mesirca P, Van den Boogert M, Rosen J, Mably J, Mangoni ME, Clapham DE (2013) Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc Natl Acad Sci U S A 110:E3037–46 10.1073/pnas.1311865110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, Chen S, Xiao C, Jia Y, Guo J, Jiang J, Liu P (2014) TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium 55:252–60 10.1016/j.ceca.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 7.Middelbeek J, Kuipers AJ, Henneman L, et al. (2012) TRPM7 is required for breast tumor cell metastasis. Cancer Res 72:4250–61 10.1158/0008-5472.CAN-11-3863 [DOI] [PubMed] [Google Scholar]
- 8.Guilbert A, Gautier M, Dhennin-Duthille I, Rybarczyk P, Sahni J, Sevestre H, Scharenberg AM, Ouadid-Ahidouch H (2013) Transient receptor potential melastatin 7 is involved in oestrogen receptor-negative metastatic breast cancer cells migration through its kinase domain. Eur J Cancer 49:3694–707 10.1016/j.ejca.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 9.Meng X, Cai C, Wu J, Cai S, Ye C, Chen H, Yang Z, Zeng H, Shen Q, Zou F (2013) TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett 333:96–102 10.1016/j.canlet.2013.01.031 [DOI] [PubMed] [Google Scholar]
- 10.Shen B, Sun L, Zheng H, Yang D, Zhang J, Zhang Q (2014) The association between single-nucleotide polymorphisms of TRPM7 gene and breast cancer in Han Population of Northeast China. Med Oncol 31:51 10.1007/s12032-014-0051-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW, Goodhill GJ, Thompson EW, Roberts-Thomson SJ, Monteith GR (2014) Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene 33:2307–16 10.1038/onc.2013.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monteilh-Zoller MK, Hermosura MC, Nadler MJS, Scharenberg AM, Penner R, Fleig A (2003) TRPM7 Provides an Ion Channel Mechanism for Cellular Entry of Trace Metal Ions. J Gen Physiol 121:49–60 10.1085/jgp.20028740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abiria SA, Krapivinsky G, Sah R, Santa-Cruz AG, Chaudhuri D, Zhang J, Adstamongkonkul P, DeCaen PG, Clapham DE (2017) TRPM7 senses oxidative stress to release Zn(2+) from unique intracellular vesicles. Proc Natl Acad Sci U S A 114:E6079–E6088 10.1073/pnas.1707380114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN (2006) TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J 25:290–301 10.1038/sj.emboj.7600931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middelbeek J, Visser D, Henneman L, Kamermans A, Kuipers AJ, Hoogerbrugge PM, Jalink K, van Leeuwen FN (2015) TRPM7 maintains progenitor-like features of neuroblastoma cells: implications for metastasis formation. Oncotarget 6:8760–76 10.18632/oncotarget.3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrighton KH (2014) Epigenetics: the TRPM7 ion channel modifies histones. Nat Rev Mol Cell Biol 15:427 10.1038/nrm3824 [DOI] [PubMed] [Google Scholar]
- 17.Krapivinsky G, Krapivinsky L, Manasian Y, Clapham DE (2014) The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell 157:1061–72 10.1016/j.cell.2014.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K (2007) Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J Biol Chem 282:232–9 10.1074/jbc.M605300200 [DOI] [PubMed] [Google Scholar]
- 19.Wei C, Wang X, Chen M, Ouyang K, Song L-S, Cheng H (2009) Calcium flickers steer cell migration. Nature 457:901–5 10.1038/nature07577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai F-C, Meyer T (2012) Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr Biol 22:837–42 10.1016/j.cub.2012.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser D, Langeslag M, Kedziora KM, Klarenbeek J, Kamermans A, Horgen FD, Fleig A, van Leeuwen FN, Jalink K (2013) TRPM7 triggers Ca2+ sparks and invadosome formation in neuroblastoma cells. Cell Calcium 54:404–15 10.1016/j.ceca.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Runnels LW, Yue L, Clapham DE (2002) The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol 4:329–36 10.1038/ncb781 [DOI] [PubMed] [Google Scholar]
- 23.Callera GE, He Y, Yogi A, Montezano AC, Paravicini T, Yao G, Touyz RM (2009) Regulation of the novel Mg2+ transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J Hypertens 27:155–166 [DOI] [PubMed] [Google Scholar]
- 24.Kim BJ, Lim H-H, Yang DK, Jun JY, Chang IY, Park C-S, So I, Stanfield PR, Kim KW (2005) Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology 129:1504–17 10.1053/j.gastro.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 25.Chen J-P, Luan Y, You C-X, Chen X-H, Luo R-C, Li R (2010) TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca2+ influx. Cell Calcium 47:425–432 10.1016/j.ceca.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Nadler MJS, Hermosura MC, Inabe K, et al. (2001) LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 411:590–595 10.1038/35079092 [DOI] [PubMed] [Google Scholar]
- 27.Langeslag M (2006) Doctoral thesis; TRPM7, Calcium and the cytoskeleton. Division of Cellbiology, Faculty of Medicine, Leiden University
- 28.Visser D (2014) Doctoral thesis; TRPM7: Ca2+ signaling, actomyosin remodeling and metastasis. University of Amsterdam [Google Scholar]
- 29.Yogi A, Callera GE, Tostes R, Touyz RM (2009) Bradykinin regulates calpain and proinflammatory signaling through TRPM7-sensitive pathways in vascular smooth muscle cells. Am J Physiol Integr Comp Physiol 296:R201–R207 [DOI] [PubMed] [Google Scholar]
- 30.Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T (2000) A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol 2:25–9 10.1038/71345 [DOI] [PubMed] [Google Scholar]
- 31.Thestrup T, Litzlbauer J, Bartholomäus I, et al. (2014) Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat Methods 11:175–82 10.1038/nmeth.2773 [DOI] [PubMed] [Google Scholar]
- 32.Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K (2015) Fourth-Generation Epac-Based FRET Sensors for cAMP Feature Exceptional Brightness, Photostability and Dynamic Range: Characterization of Dedicated Sensors for FLIM, for Ratiometry and with High Affinity. PLoS One 10:e0122513 10.1371/journal.pone.0122513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4:1633–1649 10.1002/pmic.200300771 [DOI] [PubMed] [Google Scholar]
- 34.Neuberger G, Schneider G, Eisenhaber F (2007) pkaPS: prediction of protein kinase A phosphorylation sites with the simplified kinase-substrate binding model. Biol Direct 2:1 10.1186/1745-6150-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X (2008) GPS 2.0, a Tool to Predict Kinase-specific Phosphorylation Sites in Hierarchy. Mol Cell Proteomics 7:1598–1608 10.1074/mcp.M700574-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vliem MJ, Ponsioen B, Schwede F, Pannekoek W-J, Riedl J, Kooistra MRH, Jalink K, Genieser H-G, Bos JL, Rehmann H (2008) 8-pCPT-2’-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. Chembiochem 9:2052–4 10.1002/cbic.200800216 [DOI] [PubMed] [Google Scholar]
- 37.Lochner A, Moolman JA (2006) The many faces of H89: a review. Cardiovasc Drug Rev 24:261–74 10.1111/j.1527-3466.2006.00261.x [DOI] [PubMed] [Google Scholar]
- 38.Kim TY, Shin SK, Song M-Y, Lee JE, Park K-S (2012) Identification of the phosphorylation sites on intact TRPM7 channels from mammalian cells. Biochem Biophys Res Commun 417:1030–4 10.1016/j.bbrc.2011.12.085 [DOI] [PubMed] [Google Scholar]
- 39.Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC (1994) Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol 4:973–982 [DOI] [PubMed] [Google Scholar]
- 40.Middelbeek J, Vrenken K, Visser D, Lasonder E, Koster J, Jalink K, Clark K, van Leeuwen FN (2016) The TRPM7 interactome defines a cytoskeletal complex linked to neuroblastoma progression. Eur J Cell Biol 95:465–474 10.1016/j.ejcb.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 41.Cai N, Bai Z, Nanda V, Runnels LW (2017) Mass Spectrometric Analysis of TRPM6 and TRPM7 Phosphorylation Reveals Regulatory Mechanisms of the Channel-Kinases. Sci Rep 7:42739 10.1038/srep42739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prakriya M, Lewis RS (2015) Store-Operated Calcium Channels. Physiol Rev 95:1383–1436 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zierler S, Yao G, Zhang Z, Kuo WC, Pörzgen P, Penner R, Horgen FD, Fleig A (2011) Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J Biol Chem 286:39328–35 10.1074/jbc.M111.264341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faouzi M, Kilch T, Horgen FD, Fleig A, Penner R (2017) The TRPM7 channel kinase regulates store-operated calcium entry. J Physiol 595:3165–3180 10.1113/JP274006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuruda PR, Julius D, Minor DL (2006) Coiled Coils Direct Assembly of a Cold-Activated TRP Channel. Neuron 51:201–212 10.1016/j.neuron.2006.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen C, Sahni J, Suzuki S, Horgen FD, Penner R, Fleig A (2016) The coiled-coil domain of zebrafish TRPM7 regulates Mg·nucleotide sensitivity. Sci Rep 6:33459 10.1038/srep33459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortega A, Roselló-Lletí E, Tarazón E, Gil-Cayuela C, Lago F, González-Juanatey J-R, Martinez-Dolz L, Portolés M, Rivera M (2016) TRPM7 is down-regulated in both left atria and left ventricle of ischaemic cardiomyopathy patients and highly related to changes in ventricular function. ESC Hear Fail 3:220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tank D, Sugimori M, Connor J, Llinas R, Kaang B, Kandel E, Tsien R (1988) Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science (80-) 242:773–777 [DOI] [PubMed] [Google Scholar]
- 49.Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A (2004) Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci U S A 101:6009–14 10.1073/pnas.0307565101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian Y, Yang T, Yu S, Liu C, He M, Hu C (2018) Prostaglandin E2 increases migration and proliferation of human glioblastoma cells by activating transient receptor potential melastatin 7 channels. J Cell Mol Med. 10.1111/jcmm.13931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual data and traces of Ca2+ and cAMP measurements in single cells as mentioned in Fig 1E. Despite poor signal-to-noise and cell-to-cell variability, the responses in 23 cells clearly demonstrate tight temporal correlation of TRPM7 inhibition and cAMP increase.
(ZIP)
Data Availability Statement
All relevant data are in the paper, its Supporting Information files, or on Open Science Framework at: https://osf.io/8twrn/.