Abstract
Chlamydia pecorum is responsible for causing ocular infection and disease which can lead to blindness in koalas (Phascolarctos cinereus). Antibiotics are the current treatment for chlamydial infection and disease in koalas, however, they can be detrimental for the koala’s gastrointestinal tract microbiota and in severe cases, can lead to dysbiosis and death. In this study, we evaluated the therapeutic effects provided by a recombinant chlamydial major outer membrane protein (MOMP) vaccine on ocular disease in koalas. Koalas with ocular disease (unilateral or bilateral) were vaccinated and assessed for six weeks, evaluating any changes to the conjunctival tissue and discharge. Samples were collected pre- and post-vaccination to evaluate both humoral and cell-mediated immune responses. We further assessed the infecting C. pecorum genotype, host MHC class II alleles and presence of koala retrovirus type (KoRV-B). Our results clearly showed an improvement in the clinical ocular disease state of all seven koalas, post-vaccination. We observed increases in ocular mucosal IgA antibodies to whole C. pecorum elementary bodies, post-vaccination. We found that systemic cell-mediated immune responses to interferon-γ, interleukin-6 and interleukin-17A were not significantly predictive of ocular disease in koalas. Interestingly, one koala did not have as positive a clinical response (in one eye primarily) and this koala was infected with a C. pecorum genotype (E’) that was not used as part of the vaccine formula (MOMP genotypes A, F and G). The predominant MHC class II alleles identified were DAb*19, DAb*21 and DBb*05, with no two koalas identified with the same genetic sequence. Additionally, KoRV-B, which is associated with chlamydial disease outcome, was identified in two (29%) ocular diseased koalas, which still produced vaccine-induced immune responses and clinical ocular improvements post-vaccination. Our findings show promise for the use of a recombinant chlamydial MOMP vaccine for the therapeutic treatment of ocular disease in koalas.
Introduction
Chlamydia pecorum is responsible for causing debilitating disease in the koala (Phascolarctos cinereus) infecting the urinary tract, reproductive tract, and ocular sites [1]. Infections at the urinary and reproductive sites can cause cystitis and reproductive disease, which can often lead to infertility [1–4]. Infections at the ocular site of koalas can cause kerato-conjunctivitis, which if left untreated, can lead to blindness [1, 2, 5]. Clinical signs of ocular disease in koalas can range broadly from mild to severe and can present either unilaterally or bilaterally. Typically, signs of clinical ocular disease in koalas include redness and inflammation of the conjunctiva, proliferation of the conjunctival tissue and corneal scaring [1, 2]. Inflammation and proliferation of conjunctival tissue can become quite pronounced, swelling beyond the lid margins and obscuring the eye completely [2]. Additionally, the presence of a mucoid or purulent ocular discharge can become encrusted around the eye resulting in the koala’s inability to open its eye [2]. This can all lead to rubbing and scratching of the inflamed ocular site, which can additionally put the koala at risk of sustaining further damage and injury to the eye. Other species of Chlamydia can also infect the eyes of their host, with the most important being C. trachomatis which infects the eyes in humans, causing debilitating disease and is recognised as the number one cause of preventable blindness [6, 7].
The mechanisms that drive the progression of a chlamydial infection to a diseased state in koalas remains unclear, with characteristics of the infecting Chlamydia strain, host genetic factors and host immune response being three factors implicated [8]. Koalas are known to be infected with a genetically diverse range of C. pecorum genotypes [9–11] and reports suggest that particular C. pecorum genotypes may be more prevalent at distinct anatomical sites [10, 12]. This is similar to what is reported in humans, where different C. trachomatis strains have different virulence characteristics and different tissue site trophisms [6, 13–15]. It has also been shown that koalas residing at different geographical locations are infected with different C. pecorum genotypes, with varying levels of disease observed [9, 11]. Koalas located in Victoria (southern Australia) have been identified as predominantly being infected with C. pecorum genotype B and to a lesser degree, genotypes C, F, L, M and N [10], and report a relatively low prevalence of disease [16]. In comparison, C. pecorum infected koalas located in Queensland (north eastern Australia) have been identified with genotypes A, E’, F, G and H [9] and report a higher prevalence of disease among wild koala populations [11, 12, 17]. Furthermore, it remains unclear what bearing the level of chlamydial load has on the clinical disease outcome, with reports identifying some diseased koalas with high chlamydial loads while other diseased koalas have no detectable levels of a current chlamydial infection [12].
From the host side, host genetic variability of the koala, such as differences in Major Histocompatibility Complex (MHC) gene alleles, will impact which chlamydial antigens are presented to T-cells in different koalas, influencing the immune response mounted and clinical disease outcome achieved [8, 18]. This has been demonstrated with studies identifying individual MHC class II gene alleles and linking them to antibody production, chlamydial load and disease outcome within wild koala populations [8, 18]. Humoral responses are important immune factors to consider when evaluating the reduction in inflammation and swelling of the conjunctival tissue. The impact of antibodies, in particular immunoglobulin-A (IgA) at the mucosal site, is thought to: a) inhibit bacterial adherence to epithelial cells [19], b) neutralise intracellular pathogens [20], and c) remove bacterial cells from the epithelium [21]. Importantly, secretory IgA (sIgA) plays a role in intracellular neutralisation, reduction of bacterial-induced pro-inflammatory responses, and contributes to localised homeostasis and reduced inflammation and swelling [20, 22]. Finally, the important role of IgG has been demonstrated in post-vaccinated koalas showing Chlamydia-specific neutralisation abilities [23] and possible protection against chlamydial infection [24].
Cell-mediated immune responses are pivotal in inflammatory responses, with interferon-gamma (IFN-γ) providing a protective role via eradication of chlamydial pathogens [25–28]. In addition to this, using the mouse model, it has been demonstrated that IFN-γ was required to prevent the progression of disease post chlamydial infection [29]. Interleukins (IL) also have effects, with the dual role played by IL-6 as both a pro and anti-inflammatory cytokine providing an additional mechanism in controlling inflammation and maintaining homeostasis [30, 31]. IL-6 has further been shown to be important for reducing and limiting a C. muridarum infection in the genital tract of mice [32]. The role of IL-17A is via a pro-inflammatory response with increased expression of IL-17A associated with active C. trachomatis [33], and higher levels of IL-17A have also been observed in diseased koalas compared to non-diseased koala [34]. In addition to this, IL-17 has been shown to play a protective role against bacterial infections [35] and could be essential for inducing immune responses against bacterial infections [36]. Maher et al. (2016), have further noted that IL-17A expression was significantly increased in koala retrovirus-B (KoRV-B) positive koalas [37].
KoRV is a gamma retrovirus identified in both captive and wild koala populations [38]. The prevalence of KoRV varies, depending on geographical location, with a higher prevalence identified in Queensland koalas compared to Victorian koalas [39]. Variations of KoRV genotype are also seen within koala populations with KoRV-B being previously linked to disease outcome [40]. Increased levels of KoRV have been identified in koalas suffering from leukaemia or lymphoma [41] and KoRV-B has further been associated with chlamydial disease outcomes in wild koala populations [40, 42]. Importantly, KoRV-B has been implicated as a high-risk factor for the progression to ocular disease in C. pecorum infected koalas [8].
C. pecorum infections in koalas are a major threat to long-term viability of wild koala populations, with debilitating disease outcomes and reduced fecundity, which ultimately threatens their survival. Further investigation is still required to understand the mechanisms driving the progression of chlamydial infection to disease and monitoring clinical changes as disease progresses through the various stages. Currently, treatment of chlamydiosis in koalas is by antibiotics [43–45]. Although antibiotics have been shown to be beneficial in the treatment of C. pecorum infected koalas [43], evidence has emerged that antibiotic treatment alters the microflora of the koala’s gastrointestinal tract, which is essential for the digestion of eucalyptus leaves [46, 47]. Consequently, the use of antibiotics in koalas has often been associated with gastrointestinal dysbiosis [48, 49], which can be fatal [50]. As well, antibiotic treatment has been reported to only be effective in koalas with low levels of chlamydial infections [43]. Alternatives to antibiotic treatment of chlamydial infections in koalas is currently not available despite being desperately needed; a chlamydial vaccine is a logical choice to treat disease and overcome unwanted side effects.
Our group has progressively been assessing the immunological responses to a recombinant major outer membrane protein (rMOMP) vaccine in koalas, with promising results [23, 24, 51–58]. In short, this work has demonstrated, a) Chlamydia-specific vaccine induces both systemic IgG and mucosal IgA antibodies [23, 24, 51, 53–56, 59], b) strong cell-mediated responses, including the production of IFN-γ and IL-17A [23, 54, 58], and c) immune cross recognition of other koala-C. pecorum genotypes [51–53, 59]. Additionally, a pilot study revealed a potential therapeutic effect of a rMOMP vaccine for ocular diseased koalas [57]. The current project aimed to significantly extend previous preliminary work by evaluating both humoral and cell-mediated responses and the possible influences of both bacterial and host genetics on ocular diseased koalas. This trial showed that it is possible to vaccinate diseased koalas and reverse their clinical disease at the ocular site. Immune parameter analysis suggests that one key positive vaccine response involved was a strong ocular IgA response.
Materials and methods
Animals
Seven koalas (3 males, 4 females) with either unilateral or bilateral ocular disease were selected from wild populations residing in south east Queensland, Australia. They were recruited into the trial after presentation to Australia Zoo Wildlife Hospital (AZWH) requiring treatment for chlamydial ocular disease. All koalas received a full veterinary examination upon presentation and only koalas with either grade 1 or grade 2 ocular disease (Fig 1), as assessed by the veterinarian, were recruited into the trial. All koalas had no signs of urogenital tract disease, neither overtly nor sub-clinically and were housed at AZWH for the seven-week duration of the trial. Koalas were housed individually in enclosures that comply with the Code of Practice for Wildlife Care (Queensland) and either met or exceeded zoo standards for koala enclosures. All koalas were individually checked daily by an experienced wildlife veterinary nurse. At the completion of the trial, if required, koalas received further treatment, and when possible were released back to the wild where they were originally located. All procedures relating to this study were approved by the University of the Sunshine Coast (USC) Animal Ethics Committee (Animal Ethics permit number AN/S/15/40) and by the Queensland Government (Scientific Purposes Permit number WISP16501015).
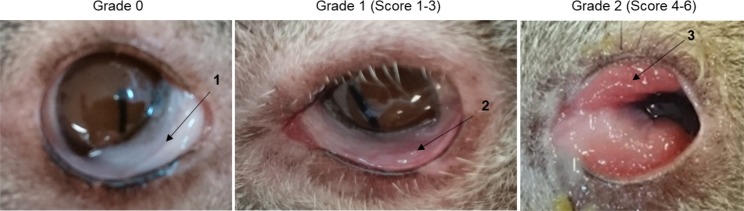
Fig 1. Range of clinical ocular disease observed in koalas infected with Chlamydia pecorum.
(1) Grade 0, clinically healthy conjunctiva from koalas with no evidence of ocular disease. (2) Grade 1 ocular disease with mild redness and swelling of the conjunctiva. (3) Grade 2 ocular disease with redness, swelling and proliferation of the conjunctiva.
Veterinary examination and sample collection
Koalas underwent a comprehensive veterinary examination under anaesthesia on initial presentation to AZWH, and then weekly for six weeks. During each of these examinations, the ocular site of each eye was examined and assessed by the veterinarian who graded the severity and any changes to the ocular disease state. Since ocular disease grading categories can be phenotypically broad, to further categorise changes in clinical state during this trial, we subdivided the grading categories. Healthy eyes were assigned to grade 0. Eyes with redness and swelling of the conjunctiva, with or without mucoidal discharge, were assigned to grade 1 with a score of 1–3, depending on severity. Eyes that presented with proliferation of the conjunctiva (lumps appearing within the conjunctival tissue), along with increased redness and swelling, and with or without purulent discharge, were assigned to grade 2 with a score of 4–6, depending on severity (Fig 1). Changes in ocular disease state were recorded independently for each eye. Biological samples were collected at each examination, pre-vaccination and weekly for six weeks. Whole blood (5 mL) was collected and placed into EDTA collection tubes (Interpath Services) and stored at 4°C until centrifugation where the plasma was then removed and stored at -20°C. The remaining blood was processed immediately for the collection of peripheral blood mononuclear cells (PBMC) (detailed below). Two swab samples were collected from each eye containing cells from the epithelial surface (Aluminium rayon swabs; Copan). One ocular swab was placed into 1 mL PBS containing 1% protease inhibitor cocktail (PIC) and stored at -20°C for later use in the IgA ELISA assay (detailed below). The other ocular swab was placed back into the sterile collection tube and stored at -20°C for later use in the quantification of chlamydial load by PCR (detailed below).
Vaccine preparation and administration
The vaccine antigen consisted of three different genotypes of Chlamydia pecorum recombinant major outer membrane proteins (rMOMP) A, F and G [9]. The method used for purifying the rMOMP proteins is as previously described by Kollipara et al. (2012) [51]. The vaccine antigen containing rMOMPs A, F and G (50 μg each rMOMP protein) was combined with Tri-Adjuvant, containing Poly I;C (250 μg), Host Defence Peptide-Innate Defence Regulator IDR-1002 (500 μg), and Polyphosphazene EP3 (250 μg) (VIDO-Intervac, University of Saskatchewan, Canada), to a total vaccine volume of 500 μL with sterile endotoxin-free PBS. Each vaccine was prepared in a sterile endotoxin-free amber glass vial, stored on ice and administered within 2 hours of preparation. Post veterinary examination and sample collection, koalas received a single subcutaneous injection. No antibiotic treatment was administered to any of the koalas during the trial period.
Koala specific Chlamydia pecorum IgG ELISA
C. pecorum-specific systemic IgG titres were determined from koala plasma by ELISA assay targeting using either recombinant MOMP G protein or heat inactivated semi-purified C. pecorum G elementary bodies (EBs) (purified as per [60]). Initially, 96 well plates (Greiner Bio-One medium binding) where coated with 50 μL of carbonate-bicarbonate coating buffer containing either 2 μg/well of recombinant MOMP G or 50,000 IFU/well of heat inactivated semi-purified C. pecorum G EBs and incubated at 4°C overnight. Wells were emptied and then coated with 100 μL per well of blocking buffer consisting of 5% skim milk in PBS containing 0.01% Tween-20 and incubated for an additional 2 hours at 37°C. Post incubation, wells were emptied and 1:3 serially diluted plasma, with dilutions starting at 1:50, was added in duplicate and incubated for 1 hour at 37ºC. Next, wells were washed 3 times with PBS containing 0.05% Tween-20 and coated with 50 μL/well of sheep anti-koala IgG diluted 1:8,000 in PBS containing 0.01% Tween-20, followed by incubation for 1 hour at 37˚C. Wells were again washed 3 times with PBS containing 0.05% Tween-20 and coated with 50 μL/well of HRP-conjugated donkey anti-sheep IgG diluted 1:20,000 (Abcam) in PBS containing 0.01% Tween-20, followed by incubation for 1 hour at 37°C. Wells were then washed 3 times with PBS alone then 50 μL/well of 3,3’,5,5’-Tetramethylbenzidine substrate (Sigma-Aldrich) was added and incubated at room temperature for 30 mins before stopping the reaction with 50 μL/well of 1 M sulphuric acid. Plates were read using an EnSpire Multimode plate reader (PerkinElmer) and the end point titre (EPT) was calculated as per [58].
Koala specific Chlamydia pecorum IgA ELISA
The mucosal IgA response was determined from ocular swab samples stored in 1% PIC by ELISA assay targeting either using recombinant MOMP G protein or heat inactivated semi-purified C. pecorum G EBs. Initially, 96 well plates were coated with antigen and blocked as above for systemic IgG. Prepared plates received 50 μL/well of swab sample solution was added in duplicate (defrosted at room temperature and vortexed for 3 minutes prior to addition) and incubated for 1 hour at 37°C. Post incubation, wells were washed 3 times with PBS containing 0.05% Tween-20 then coated with 50 μL/well of rabbit anti-koala IgA diluted 1:3,000 in PBS containing 0.01% Tween-20 and incubated for 1 hour at 37°C. Wells were then washed 3 times with PBS containing 0.05% Tween-20 and coated with 50 μL/well of HRP-conjugated goat anti-rabbit IgG (ab6721; Abcam) diluted 1:20,000 in PBS containing 0.01% Tween-20 and incubated for 1 hour at 37°C. Finally, wells were washed, colour developed and read as above for systemic IgG. The optical density (OD) was measured at 450 nm and the absorbance value was calculated as the mean of duplicate samples minus the mean of the no sample control wells.
PBMC purification and antigen stimulation
Peripheral blood mononuclear cells (PBMCs) were extracted from whole blood for further use in determining cell-mediated responses. Whole blood sample was centrifuged at 1000 rpm for 5 mins at 15°C. Plasma was removed and stored at -20°C. The remaining cellular fraction were resuspended in an equal volume of PBS. The cell suspension was then overlayed onto 10ml of Ficoll-paque (GE Healthcare, Australia) and centrifuged at 400 g for 25 min, brakes off, at 18°C. Cells were removed from the middle buffy coat fraction, placed into 10 ml of PBS, and centrifuged at 400 g for 10 min at 18°C. Pelleted cells were washed three times by resuspending in 10 mL PBS, followed by centrifugation at 400 g for 10 min at 18°C. Final cell pellets were resuspended in 1ml RPMI 1640 media (Gibco Life technologies, Australia) containing 10% heat-inactivated foetal calf serum (Life technologies, Australia), 120 μg/mL streptomycin (Sigma-Aldrich, Australia) and 50 μg/mL gentamycin (Gibco Life technologies, Australia). An aliquot of cell suspension was mixed with Trypan blue solution (Gilco Life technologies, Australia) at 1:10 dilution and placed onto a haemocytometer for counting using light microscope. The remaining PBMC cell suspension was diluted to 2 x 106 cells/mL with RPMI 1640 media containing 10% heat-inactivated foetal calf serum, 120 μg/mL streptomycin, and 50 μg/mL gentamycin and transferred to a 96 well plate (Greiner Bio-One medium binding) at 100 μL/well. Half the PBMCs were used as control and the other half were stimulated with 100 μL/well of 2 μg/100 μL of rMOMP in RPMI 1640 media containing 10% heat-inactivated foetal calf serum, 120 μg/mL streptomycin and 50 μg/mL gentamycin and incubated at 37°C in 5% CO2 for 12 hours. Following 12 hour incubation, cells were collected and centrifuged at 2,000g for 10 minutes at 18°C. PBMCs were then suspended in 1mL of TRIzol reagent (Invitrogen, Australia) and store at -80°C. PBMCs were not extracted for koala K1.
RNA extraction from PBMCs and reverse transcription
For the extraction of RNA from PBMCs, 200 μL of chloroform (Sigma-Aldrich, Australia) was first added to PBMCs suspended in TRIzol, then centrifuged at 12,000 g for 15 mins at 4°C. The top aqueous layer was removed and added to an equal volume of 100% ethanol. All further RNA extraction procedures were as per manufacturer’s instructions for RNeasy mini kit (Qiagen, Australia). For the elimination of DNA, DNase I (Sigma-Aldrich, Australia) was used, as per manufacturer’s instructions. Reverse transcription was performed using iScript reverse transcription supermix (Bio-Rad, Australia), as per manufacturer’s instructions. cDNA was then stored at -20°C until utilised in qRT-PCR for gene expression.
Quantification of cytokine gene expression
cDNA, extracted from PBMCs, was used to measure the level of koala gene expression using quantitative real-time PCR (qPCR). Gene expression of IFN-γ, IL-6, IL-17A was measured and compared to the house-keeping gene GAPDH. All forward and reverse primers are listed in Table 1. Each PCR reaction contained 2 μL of cDNA template added to a mastermix containing, 1x Quantitect SYBR Green (Qiagen), 0.3 μM of each forward and reverse primer and molecular grade water making a final volume of 20 μL per reaction. For PCR amplification, there was an initial denaturation at 95°C for 15 mins, followed by 40 cycles of 94°C for 15 secs, 59°C for 30 secs and 72°C for 30 secs, followed by melt curve analysis (72°C to 95°C in 0.5°C increments). All reactions were performed in duplicate and carried out on a Rotor-Gene Q 5-plex HRM platform (Qiagen). Koala gene expressions were normalised to GAPDH using the 2-ΔΔCT equation (where CT is threshold cycle): ΔCT = (CT of IFN-γ, IL-6 or IL-17A gene–CT of GAPDH gene) and ΔΔCT = (ΔCT of stimulated sample - ΔCT of unstimulated sample) [61, 62].
Table 1. PCR primer sequences for gene expression.
| Gene Target | Primer orientation | Sequence (5'-3') | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| IFN-γ | Forward | TGAACATGATGGATCGTTGG | 182 | [63] |
| IFN-γ | Reverse | CACTTTGCTGGCAGTGTTGT | This study | |
| IL-6 | Forward | CACCTGTTTGGCTTTTAGCA | 187 | This study |
| IL-6 | Reverse | GCAGAGCTTGTATGGCTCCT | This study | |
| IL-17A | Forward | TCCCTAATGAGGATGCCAAC | 173 | This study |
| IL-17A | Reverse | TTGGAAGGAAGTGGAGCAGT | This study | |
| GAPDH | Forward | AACTTTGGCATTGTGGAAGG | 188 | This study |
| GAPDH | Reverse | GTGAGCTTCCCATTCAGCTC | This study |
Gene targets with corresponding forward and reverse primer and amplicon size (bp).
DNA extraction and qPCR quantification of Chlamydia pecorum load
Swab samples collected from both the left and right eyes of koalas were thawed at room temperature and added to 1.5 mL sucrose phosphate glutamate at pH 7.4 (0.2 M sucrose, 3.8 mM potassium phosphate monobasic, 8.6 mM disodium phosphate, 4.9 mM glutamic acid). Swabs were vortexed for 3 mins before 1 mL of solution was removed and centrifuged at 18,000 rpm for 20 mins. The resulting cell pellet was re-suspended in 50 μL TE buffer and heated at 95°C for 10 mins. DNA extraction was then performed using QIAmp DNA mini kit (Qiagen), as per manufactures instructions, with the exception of the proteinase K digestion being extended to 12 hours. Extracted DNA was screened for the presence and load of C. pecorum using quantitative real-time PCR (qPCR) with the forward primer: 5’ AGTCGAACGGAATAATGGCT 3’, and the reverse primer: 5’ CCAACAAGCTGATATCCCAC 3’, which targeted a 204 bp fragment of the C. pecorum 16S rRNA gene. Each PCR reaction contained 5 μL of DNA template added to a mastermix containing, 1x Quantitect SYBR Green (Qiagen), 0.5 μM of each forward and reverse primer and molecular grade water making a final volume of 20 μL per reaction. For PCR amplification, there was an initial denaturation at 95°C for 15 mins, followed by 35 cycles of 94°C for 15 secs, 57°C for 15 secs and 72°C for 30 secs, followed by melt curve analysis (55°C to 95°C in 0.5°C increments). All reactions were performed in duplicate and samples of ˃ 50 copies/μL were considered positive. All reactions were carried out on a Rotor-Gene Q 5-plex HRM platform (Qiagen).
Chalmydia pecorum ompA genotyping
Outer membrane protein A (ompA) sequencing was performed using DNA extracted from both eyes to determine the genotype infecting each ocular sites and genetic diversity among our koalas. DNA extracted from the ocular swabs was used as template for conventional PCR amplification. The forward primer: CpeNTVD3 5’-GTTCTTTCTAACGTAGC-3’, and the reverse primer: CpeNTVD4 5’-TGAAGAGAAACAATTTG-3’ were used to amplify the VD3 and VD4 regions of the ompA gene targeting the 359 bp fragment located at 670–1,028 bp region of the full length ompA gene. Each PCR reaction contained 1 μL of gDNA template added to a mastermix containing, 12.5 μL of 2x HotStar Taq (Qiagen), 0.3 mM of each forward and reverse primer and molecular grade water making a final volume of 25 μL per reaction. For PCR amplification, there was an initial denaturation at 95°C for 5 mins, then 40 cycles of denaturation at 95°C for 30 secs, primer annealing at 46°C for 30 secs, primer extension at 72°C for 30 secs, followed by a final extension at 72°C for 5 mins. All ompA sequences were determined by direct sequencing of the PCR product using CpeNTVD3/CpeNTVD4 performed by Macrogen Inc. (Korea).
MHC class II gene identification
MHC class II DAb and DBb gene alleles were determined as previously described by Quigley et al. (2018) [8] with the exception that DNA was extracted from swabs collected from the eyes, as described in this paper.
KoRV-A and KoRV-B identification
The identification of KoRV-A and KoRV-B in our trial koalas was determined as previously described by Quigley et al. (2018) [40] with the exception that DNA was extracted from swabs collected from the eyes, as described in this paper.
Statistical analysis
All statistical analysis was performed using GraphPad Prism version 7 (GraphPad Software, LaJolla, CA, USA). A Wilcoxon signed rank test, and paired t test were used to determine the difference in clinical ocular score, mucosal IgA antibodies, and systemic IgG antibody titres between pre- and post-vaccination, as appropriate.
Results
Clinical improvement was observed in the eyes of all koalas at six-weeks post-vaccination
Clinical differences in the eyes of the trial koalas were examined pre-vaccination and then weekly for six-weeks to observe any pathological changes. Pathological changes were recorded as; a) changes in colour to the conjunctival tissue, b) the amount of inflammation within and around the ocular site, c) the onset or changes in the severity of proliferation seen in the conjunctival tissue, and d) the amount of discharge exuded. Where proliferation of the conjunctival tissue was present, it could be seen in either the upper or lower eyelid and predominantly towards the distal end of the conjunctiva. Mucoid and purulent discharge was seen in some koalas with purulent discharge, observed more in grade 2 ocular disease. The level of clinical disease varied between each eye and between koalas, with examples of different ocular disease stages shown in Fig 1 (Fig 1). All the koalas in this trial were from a wild population and hence there was no available history of the disease state of each koala (acute or chronic) prior to arriving at the hospital. Each eye was examined and evaluated individually, and our observations showed that post-vaccination, each eye responded independently of the other eye.
All seven koalas made some level of clinical improvement, in at least one eye, after vaccination (Fig 2; Table 2). Six out of seven koalas (K1, K2, K3, K4, K5 and K6) either maintained (i.e. no worsening) or improved their ocular disease state, in both eyes, across the trial (Fig 2A, 2B, 2C, 2D, 2E and 2F). The ocular disease score average for both eyes (n = 14) at pre-vaccination, was 2.3 (SE = 0.38), and the ocular disease score average for both eyes (n = 14) at six-weeks post-vaccination, was 0.9 (SE = 0.32). Importantly, there was a highly statistically significant decrease in the average ocular disease score from pre-vaccination, to six-weeks post-vaccination (P = 0.0034). An example of the clinical ocular disease improvement post-vaccination is shown in Fig 3 (Fig 3). Variations in clinical response were observed over time and, notably, five out of seven koalas (K1, K2, K3, K5 and K7) showed an initial worsening (for the initial 1 to 2 weeks of the trial only) of diseased state, in at least one eye (Fig 2A, 2B, 2C, 2E and 2G). Despite this, from 3–4 weeks post-vaccination, we observed more general clinical improvements, which in most koalas, continued to improve out to the six-week endpoint. Five of the koalas (K1, K2, K4, K5 and K6) showed no signs of clinical disease, in at least one eye (and often both eyes) at the end of the trial (Fig 2A, 2B, 2D, 2E and 2F), while six of the koalas (K1, K2, K3, K4, K5 and K6) had a clinical ocular disease score of 1 or below in both eyes at the six-week endpoint (Fig 2A, 2B, 2C, 2D, 2E and 2F). Only one koala (K7), had a final clinical score above 1 in both eyes (Fig 2G).
Fig 2. Changes in clinical ocular disease score in koalas post-vaccination.
Clinical ocular disease scores measured at pre-vaccination and weekly for six weeks (1–6) post-vaccination. The clinical score represents the ocular disease state in each eye at that timepoint.
Table 2. Koala ocular disease scores at pre-vaccination and post-vaccination.
| Koala | Ocular disease score pre-vaccination | Ocular disease score six-weeks post-vaccination | Change in ocular disease score (+/-) |
|---|---|---|---|
| K1—Left eye | 2 | 1 | -1 |
| K1—Right eye | 0 | 0 | 0 |
| K2—Left eye | 1.5 | 0 | -1.5 |
| K2—Right eye | 4 | 1 | -3 |
| K3—Left eye | 4 | 1 | -3 |
| K3—Right eye | 3 | 1 | -2 |
| K4—Left eye | 3 | 0 | -3 |
| K4—Right eye | 3 | 0 | -3 |
| K5—Left eye | 1.5 | 1 | -0.5 |
| K5—Right eye | 0 | 0 | 0 |
| K6—Left eye | 3 | 1 | -2 |
| K6—Right eye | 1 | 0 | -1 |
| K7—Left eye | 2 | 3 | +1 |
| K7—Right eye | 4.5 | 4 | -0.5 |
Change in clinical ocular disease score, as assessed by expert wildlife veterinarian, in both left and right eyes at pre-vaccination and again at six-weeks post-vaccination.
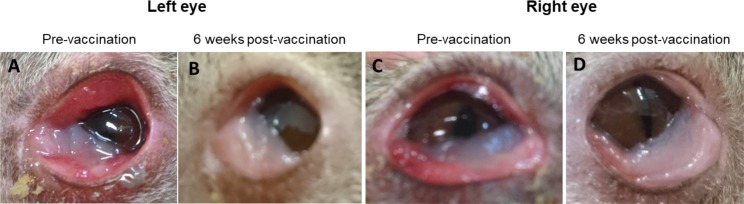
Fig 3. Clinical ocular disease improvement seen post-vaccination.
An example of clinical ocular disease changes observed from pre-vaccination to six-weeks post-vaccination in koala K3. (A) Left eye at pre-vaccination showing inflammation, swelling and proliferation of the conjunctival tissue with a clinical score of 4. (B) Left eye six-weeks post-vaccination showing reduced inflammation and swelling, with a clinical score of 1. (C) Right eye at pre-vaccination showing inflammation and swelling of the conjunctiva with a clinical score of 3. (D) Right eye six-weeks post-vaccination showing reduced inflammation and swelling of the conjunctiva with a clinical score of 1.
Vaccination resulted in increased Chlamydia pecorum-specific systemic IgG and ocular IgA antibody levels
Systemic IgG and mucosal IgA antibodies were measured against C. pecorum recombinant MOMP G protein as well as against whole C. pecorum G elementary bodies (EBs) at pre-vaccination and weekly for the duration of the trial. Although there was variation between koalas, a statistically significant increase in C. pecorum-specific systemic IgG antibodies (measured against both rMOMP G protein and whole C. pecorum EBs) was observed from pre-vaccination to six-week post-vaccination (P = 0.0156) (Fig 4; S1 Fig). Notably, koalas K1, K2 and K3 had the highest levels of systemic IgG antibodies that reacted with whole chlamydial EBs (S1A, S1B and S1C Fig), and were also among the animals with the most clinically severe natural disease.
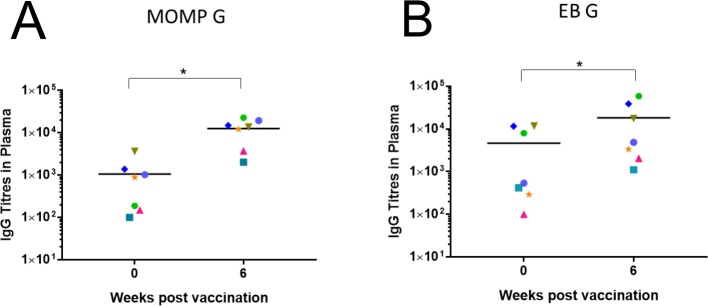
Fig 4. Systemic IgG antibody levels to rMOMP protein and whole Chlamydia pecorum elementary bodies.
Systemic IgG antibodies (from plasma) were measured at pre-vaccination and six-weeks post-vaccination by ELISA assay and reported as end point titre (EPT). (A) Systemic IgG measured at pre-vaccination and six-weeks post-vaccination, against rMOMP G protein (n = 7) with a P value 0.0156. (B) Systemic IgG measured at pre-vaccination and six-weeks post-vaccination, against whole C. pecorum G EBs with a P value 0.0156.
Mucosal IgA antibody levels were analysed in each eye, to evaluate the local vaccine-induced response. Koalas with grade 1 ocular disease at time of entry into the trial showed an increase in C. pecorum-specific mucosal IgA antibodies, in that specific eye, to both rMOMP G and C. pecorum EBs from pre-vaccination to six-weeks post-vaccination (Fig 5). This increase was statistically significant for the rMOMP G antibody response with a P value of 0.0042. (Fig 5A). The grade 2 ocular diseased sites of koalas (at pre-vaccination) also showed an increase in C. pecorum-specific mucosal IgA antibodies, in most eyes, to both rMOMP G and C. pecorum EBs, from pre-vaccination to six-weeks post-vaccination (Fig 5). Although antibody levels varied throughout the trial, all koalas (7/7) showed an increase in C. pecorum-specific mucosal IgA antibodies to both rMOMP G protein and C. pecorum EBs, in at least one eye, post-vaccination (S2 Fig). In contrast to the systemic IgG levels, most koalas (6/7; K1, K2, K4, K5, K6 and K7) had either low or no detectable levels of C. pecorum-specific IgA antibodies (measured by both C. pecorum rMOMP G protein and whole EBs) at pre-vaccination (Fig 5). The overall trend was for C. pecorum-specific IgA antibody levels to increase by week 2 and peak at around 3 to 4 weeks (S2 Fig).
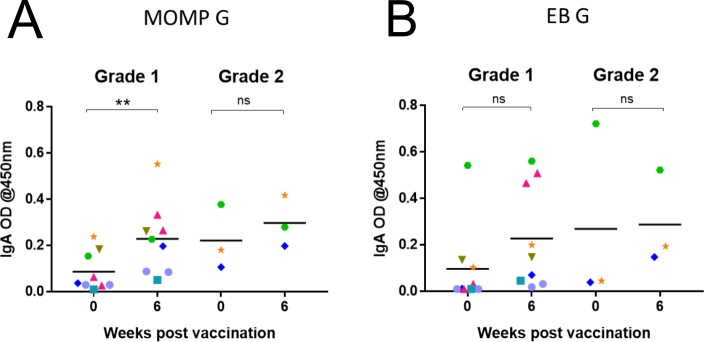
Fig 5. Mucosal IgA antibodies to rMOMP protein and whole Chlamydia pecorum elementary bodies.
Mucosal C. pecorum-specific IgA antibodies (from ocular swabs) were analysed by ELISA assay and reported as optical density (OD) measured at 450 nm. IgA responses are shown against rMOMP G protein and whole C. pecorum EBs. (A) Mucosal IgA antibody responses measured at pre-vaccination and six-weeks post-vaccination, against rMOMP G in either grade 1 (P = 0.0042) or grade 2 ocular sites. (B) Mucosal IgA antibody responses measured at pre-vaccination and six-weeks post-vaccination, against EB G in either grade 1 or grade 2 ocular sites.
Chlamydial qPCR load was low in most koalas
All koalas were assessed for ocular C. pecorum presence and load using qPCR (S3 Fig). All koalas except one (K4 right eye) (S3D Fig) had low (< 300 copies/μL) to undetectable levels of C. pecorum pre-vaccination (S3 Fig). The C. pecorum load in an eye did not always correlate with the clinical disease observed. Some koalas with detectable C. pecorum showed no signs of ocular disease and conversely, some koalas with no detectable C. pecorum were found to have grade 1 or grade 2 ocular disease. Koala K4 had the highest initial C. pecorum load of over 30,000 copies/μL in the right eye at pre-vaccination however, this subsequently decreased to undetectable C. pecorum levels in both eyes, at six-weeks post-vaccination (S3D Fig). All other koalas (K1, K2, K3, K5, K6 and K7) recorded either no detectable levels or very low (< 600 copies/μL) detectable levels of chlamydial load at six-weeks post-vaccination for both eyes (S3A, S3B, S3C, S3E, S3F and S3G Fig).
Cytokine expression increased post-vaccination
Cell mediated responses play an important role in the mediation of inflammatory responses. To evaluate the cell mediated responses in our vaccinated koalas, we compared the cytokine expression of IFN-γ, IL-6 and IL-17A at pre-vaccination and weekly for six-weeks post-vaccination (S1 Table). Cytokine expression was measured as fold change using RT-qPCR performed on RNA extracted from PBMCs stimulated with rMOMP G protein. There was considerable variation between koalas, expressing different levels of IFN-γ, IL-6 and IL-17A at different timepoints, post-vaccination (S1 Table). Collectively, the mean-expression, for all three cytokines (IFN-γ, IL-17A and IL-6), remained relatively similar at pre-vaccination and at six-weeks post-vaccination. For IFN-γ gene expression, we did not observe any elevated expression levels in koalas to their natural infected and diseased state, at pre-vaccination. Increases in IFN-γ expression were seen in some koalas post-vaccination, at varying time-points, but this was not consistent. Post-vaccination fold-change increases were observed to be highest in koala K4 at weeks 3 and 4 (43.5 and 25.7 respectively) (S2 Table). Similarly, we did not observe any elevated IL-6 expression levels in any koalas at pre-vaccination (S1 Table). The expression levels of IL-6 did not appear to vary in most koalas post-vaccination (S1 Table). Koala K4 again showed the highest fold-change increases post-vaccination seen from week 1 (161.0), which peaked at week 3 post-vaccination (403.0) (S2 Table). For IL-17A expression, only one koala (K7) showed a higher expression, compared to the other koalas, at pre-vaccination (S1 Table). The IL-17A expression levels observed in koala K7 then reduced from one-week post-vaccination (S1 Table). Fold-change increases were then observed in some koalas at varying timepoints post-vaccination, and once again koala K4 showed the highest fold-change increase (143.8) at two-weeks post-vaccination (S2 Table).
Effect of chlamydial genotype of the infecting strain on response to vaccination, MHC class II allele diversity and KoRV-A and KoRV-B status
For our current study, we used ompA sequencing to type the strains of C. pecorum present in the animals in our trial, including to determine if the same strain was infecting both eyes of each koala. We also wanted to determine how well the types of MOMP protein used in the vaccine matched the infecting strains in the vaccinated animals. In our seven vaccine trial koalas, there were three different C. pecorum strains infecting the animals’ eyes (G–three koalas, F–two koalas, E’–one koala, and mixed infection–one koala) (Table 3). However, each individual koala was found to have the same C. pecorum genotype present in both eyes. Three MOMP genotypes were used in the vaccine formulation (A, F and G) and hence, six out of the seven trial koalas (K1, K2, K3, K4, K5 and K6) were infected with a C. pecorum genotype that matched the MOMPs used in the vaccine formula (Table 3). These six koalas were the same koalas that we observed clinical ocular disease improvements in by the trial endpoint. However, the koala infected with C. pecorum genotype E’ (K7), which was not used in the vaccine formula (Table 3), showed worsening clinical disease by the trial endpoint.
Table 3. Genetic diversity of the chlamydial strains, host MHC type and KoRV status of trial koalas.
| Koala | ompA genotype | Strain included in vaccine | MHC class II DAb gene allele | MHC class II DBb gene allele | KoRV-B | Koala Age and (sex) |
|---|---|---|---|---|---|---|
| K1 | G | Y | 10, 19, 30 | 2, 3, 5 | + | 10 (M) |
| K2 | G | Y | 10, 15, 19, 21 | 1, 5 | - | 4 (F) |
| K3 | F | Y | 10, 15, 19, 21 | 2, 4, 5 | - | 3 (M) |
| K4 | F | Y | 15, 19, 21 | 2, 3, 5 | + | 1 (F) |
| K5 | G | Y | 15, 21 | 2, 5 | - | 3 (M) |
| K6 | MIXED | Y | 10, 19, 21 | 3 | - | 7 (F) |
| K7 | E' | N | 15, 19, 21 | 3 | - | 8 (F) |
Chlamydia pecorum genotype infecting each koala, at the ocular site, as determined by ompA sequencing. Association between infecting genotype and vaccine antigen, yes (Y) or no (N). MHC class II DAb and DBb gene alleles detected in each koala showing their genetic diversity. Presence (+) or absence (-) of KoRV-B and. Koala age and sex male (M) and female (F).
To evaluate the host genetic diversity levels of our ocular diseased koalas, we determined the MHC class II DAb and DBb gene alleles [8]. We detected five different DAb alleles and five different DBb alleles amongst our seven koalas (Table 3). The most common DAb alleles detected were Dab*19 and *21, and the most common DBb allele detected was DBb*05 (Table 3). Dab*30 and DBb*01 and *04 were each only detected in one koala (K1, K2 and K3, respectively) (Table 3). All seven koalas were genetically different with no two koalas identified with the same genetic sequence.
KoRV-A and KoRV-B status was determined, at the ocular site, with particular interest in KoRV-B prevalence due to the previous association with chlamydial disease outcome [40, 42]. KoRV-A was present in all seven koalas (K1, K2, K3, K4, K5, K6 and K7) however, KoRV-B was only present in two (29%) of the koalas (K1 and K4). The two KoRV-B positive koalas were both identified as having the same DBb gene alleles (2, 3 and 5) but had different DAb gene alleles.
Discussion
Vaccine development usually focuses on prophylactic vaccines, with relatively little effort directed towards the development of therapeutic vaccines for infectious diseases [64]. The prophylactic properties of an anti-chlamydial vaccine are no doubt key for providing long-term protection against C. pecorum infections in wild koalas. However, in addition, therapeutic effects of a vaccine could be beneficial in koalas that have progressed to disease. As a significant percentage of wild koalas have sub-clinical disease [12], the possibility of a vaccine being effective against mild disease is a promising prospect as they often go undetected and progress to severe disease. Furthermore, due to unwanted side effects following antibiotic treatment, a therapeutic vaccine would be beneficial in the treatment of koalas [47–50]. The potential to halt or even reverse clinical disease in the koala through vaccination has the potential to overcome the unwanted side effects of antibiotic usage.
Previous prophylactic studies in both captive and wild koala populations have shown that a rMOMP vaccine induces both humoral and cell-mediated immunity with long lasting (12 and six months respectively) responses [23, 24, 51, 52, 54, 55, 58, 60]. This current study expands on this research, by now evaluating the therapeutic effects of a rMOMP vaccine on ocular diseased koalas. Our current findings demonstrate that a therapeutic effect is possible, with all vaccinated koalas showing clinical ocular improvements without administering antibiotics. Additionally, we found that minor changes in the C. pecorum strain may have an impact on vaccine success, with the one koala infected with a strain not used in the vaccine formulation, exhibiting the poorest clinical response.
Pathogenesis at the ocular site of our diseased koalas varied and, notably, each eye responded independently of the other. A time lag was evident with some koalas showed an initial worsening of clinical signs within the first three weeks however, in most koalas, the clinical diseased state subsequently improved to either very low or no detectable disease by the trial endpoint. As these are koalas from a wild population, we have no known history of their previous chlamydial infections and whether the koala was in a natural progressive stage of the disease. If some koalas were in a progressive stage of disease, while others were experiencing a more stable stage of disease, then this could explain the initial increase in clinical signs seen in some koalas but not in others. This would then imply that if the clinical state was in a natural progressive stage, the vaccine has potentially halted and then reversed this natural disease progression. Furthermore, changes in the ocular disease state were found to be unrelated to the koalas’ C. pecorum load for that particular eye. This would imply that the changes in clinical ocular disease state are primarily driven by immune responses and not chlamydial load.
Immune responses should not only be specific to the prevention and clearance of the bacteria but should also contribute to the reduction in the clinical diseased state. As C. pecorum is an intracellular pathogen, infecting the epithelial cells of the mucosal surface, a primary area of interest is the mucosal immune response, including the ability to induce secretory IgA antibodies. The results from this trial show that naturally infected and diseased koalas do not produce a strong mucosal anti-chlamydial IgA response. However, following vaccination of these koalas, we observed an increase in their secretory IgA antibodies, which peaked in most koalas, at around 3–4 weeks post-vaccination. Interestingly, this was also around the time that we observed improvement of the clinical ocular diseased state of most koalas. We have previously demonstrated mucosal IgA increases in the koala at 12 and 26 weeks post-vaccination [58, 59] however, this is the first trial to analyse the mucosal IgA response in koalas at weekly intervals post-vaccination. The variation in mucosal IgA antibody response could potentially be explained by the low levels of C. pecorum load detected in our koalas. This has previously been demonstrated in a study conducted on guinea pigs where they demonstrated that the IgA response post-inoculation, was dose dependent [65]. Furthermore, we noticed that for most koalas, the eye that showed the higher IgA increase also corresponded with the eye that had the worst disease state. Regardless of grade 1 or grade 2 ocular disease, koalas made similar increases in mucosal IgA antibody response post-vaccination.
It has been shown in C. trachomatis infected humans, that there is an inverse relationship between IgA levels and chlamydial load [66], suggesting that IgA plays a direct role in decreasing and eliminating chlamydial infection at the mucosal site. The koalas in our study presented with either low or no detectable levels of C. pecorum, as determined by qPCR, with the exception of the individual K4. Low levels of C. pecorum load were not surprising, as Nyari et al. (2017) previously reported that the C. pecorum load was significantly lower in wild koalas with clinical disease, compared to koalas with no clinical disease [12]. Similarly, Patterson et al. (2015) also reported the absence of a chlamydial infection in diseased koalas from a Victorian population [16]. Notably, in our current study, koala K4 had a C. pecorum load of >30,000 copies/μL with minimal detectable levels of mucosal IgA pre-vaccination. Koala K4 subsequently increased mucosal IgA antibodies and decreased the C. pecorum load to no detectable levels post-vaccination. Taken together, this is supportive of an inverse relationship between IgA levels and chlamydial load. Similarly, in post-vaccinated challenged mice, increased levels of IgA antibodies have additionally been shown to enhance protection against a chlamydial infection [67].
Developing strong cell-mediated responses is another important factor in vaccine development [68] including anti-chlamydial vaccines [69]. In this study, we evaluated the ability of a rMOMP vaccine to induce the expression of IFN-γ, IL-6 and IL-17A. We observed that for this group of ocular diseased koalas the mean cytokine expression for all three cytokines (IFN-γ, IL-6 and IL-17A) was similar at pre-vaccination to six-weeks post-vaccination. Although we observed higher cytokine expressions in some koalas at varying time-points, post-vaccination, this was not consistent. For IFN-γ gene expression, we did not observe any elevated expression levels in koalas to their natural infected and diseased state, at pre-vaccination. This was surprising as the importance of IFN-γ in mediating immune responses for the control of chlamydial infections has been previously shown [26, 27, 70–73] with a strong correlation between the production of IFN-γ and protection against C. trachomatis [74]. In addition, IFN-γ responses have been demonstrated to be involved in both the clearance of a Chlamydia infection and the resistance to reinfection [75]. Importantly, the anti-inflammatory properties of IFN-γ have also been described, through the ability to modulate other pro-inflammatory cytokine production, resulting in self-regulation of inflammation [76, 77]. Given the relationship between IFN-γ expression and chlamydial infection, the low levels of IFN-γ expression seen in our vaccinated koalas could possibly be explained by the low to no detectable levels of C. pecorum. Therefore, from this data, it appears that IFN-γ is not a direct marker for infection or disease and does not seem to be responsible for the resolution of infection, in these ocular diseased koalas.
Interleukin-6 is well known as a pro-inflammatory cytokine, playing an important role in acute inflammatory responses triggered by infection and tissue damage [78–80]. The beneficial role of IL-6 has been demonstrated in a previous study where higher levels of bacterial burden along with an increased mortality rate, were observed in IL-6 depleted mice [81]. Conversely, IL-6 also has anti-inflammatory activities, which play a role in returning the host to a homeostatic state [82–84] and contributes to the processes involved in tissue repair [30]. The anti-inflammatory properties of IL-6 have been shown by Xing et al. (1997) where they demonstrated, in mice, how IL-6 controlled the levels of other pro-inflammatory cytokines but had no effect on anti-inflammatory cytokine responses [31]. These attributes are paramount for an anti-chlamydial vaccine to provide a therapeutic effect and, until now, have not been observed within diseased koalas. In this study, we did not observe any significant increased IL-6 expression in any koala to natural infection, at pre-vaccination. However, post-vaccination, one koala (K4) showed a high fold-change increase from one-week post-vaccination. Notably, the level of cytokine expression measured in koala K4 was not necessarily higher than other ocular disease koalas, but this koala had the lowest IL-6 expression pre-vaccination. Interestingly, this koala (K4) also showed a high C. pecorum load (> 30 000 copies/μL) at pre-vaccination and was the only koala to consistently show increased cytokine expression fold-increases, post-vaccination, to all three cytokines.
The cytokine IL-17 has been shown to be important in the defence against bacterial pathogens [36, 85]. In particular, this defensive role to intracellular bacteria was demonstrated previously by showing that IL-17 neutralised mice had significantly higher levels of chlamydial infection and severe disease outcomes [86]. Interestingly, our current study found that the one koala (K4) who had a high C. pecorum load, pre-vaccination, also expressed the lowest IL-17A response, at pre-vaccination. The IL-17A expression for koala K4 subsequently increased post-vaccination, as we observed a decrease in the C. pecorum load, along with improved clinical ocular disease state in both eyes. Evaluating the expression levels of IL-17A in the other ocular diseased koalas revealed that only one koala (K7) had elevated IL-17A expression, compared to the other koalas, at pre-vaccination. As IL-17A is a pro-inflammatory cytokine, it is not surprising that koala K7 also had the highest clinical disease score at pre-vaccination, but this does not explain the lower levels expressed in other koalas with clinical disease. Surprisingly, for koala K7 the expression levels of IL-17A decreased, post-vaccination, as clinical signs increased. All other koalas that showed increases in IL-17A expression post-vaccination, showed decreases in clinical inflammation. This is interesting and possibly indicates that IL-17A expression in koalas, may not be the main contributor to inflammation at the conjunctival site, despite reports of active trachoma in children being associated with increased expression of IL-17A at the conjunctival site [33]. This study observed cytokine expression in ocular diseased koalas and then again post-vaccination however, due to the small group of koalas used in this study, it is not possible to draw any conclusions. Additionally, cell-mediated responses were measured as systemic responses and a different profile of cytokine expression could therefore be shown at the mucosal site and should be assessed in the future.
Another interesting finding in this study was when we determined the strain of C. pecorum at the ocular site, as determined by ompA sequencing. We found that the one koala (K7) who showed the least amount of clinical improvement was infected with genotype E’. Genotype E’ was not used as part of the vaccine formulation. This was interesting, as we have previously shown that our rMOMP vaccine can induce antibodies in post-vaccinated koalas that cross-recognise other C. pecorum genotypes [59] however, this has not been shown for genotype E’. Additionally, we have previously reported, in an epidemiology study conducted on koalas in south east Queensland, that koalas infected with genotype E’ were more likely to have disease than koalas infected with genotype G [12]. Surprisingly, genotype E’ has 99.7% similarity to genotype F, which was used in the vaccine formulation. There are four single nucleotide differences between genotypes E’ and F, two synonymous and two non-synonymous changes. The relevance of minor ompA gene sequence changes and any subsequent impact on pathogenicity has been outlined previously by Sturdevant et al. (2010) [87] who identified single frameshift mutations within C. trachomatis serovar D. They showed that these single mutations within C. trachomatis serovar D resulted in different virulence factors which influenced the duration of the diseased state. It is therefore recommended that future C. pecorum MOMP vaccine studies consider incorporating a broader variety of MOMP genotypes focusing on specific genotypes identified within particular koala populations.
The importance of the role played by host genetics in disease progression within koalas remains unclear, with some studies suggesting that particular MHC alleles are associated with clinical disease outcomes [8, 18]. Given this, we assessed the level of variation of MHC class II alleles within our ocular diseased koalas and found that no two koalas had identical MHC class II alleles. However, we did identify the MHC class II DAb*21 allele in 6 (86%) of our koalas, which has previously been linked to chlamydial disease in wild koalas. We further identified DAb*19 allele in six (86%) of the ocular diseased koalas with five koalas having both the DAb*19 and DAb*21 allele. Surprisingly, we identified the MHC class II DBb*03 allele in four (57%) of our koalas but, interestingly, the absence of this allele has been associated with chlamydial disease in koalas [8]. In addition to the DBb alleles, DBb*05 allele was the most common allele, identified in five ocular diseased koalas. Due to the variation in MHC class II gene alleles identified within our diseased koalas, we were unable to determine if any one particular allele or set of alleles, was associated with the presence or severity of clinical ocular disease. However, given that MHC class II molecules influence antigen presentation to CD4+ T cells, which in turn influence B cell differentiation, antibody production and cytokine secretion, it is not surprising that we would then see differences in immune responses within each koala. This data provides further information on the identification of MHC class II alleles, identified within ocular diseased koalas, adding value to future research.
Additionally, as KoRV has been suggested to be linked with disease outcome for Chlamydia infected koalas [40, 42], we evaluated each koala’s KoRV-B status, comparing it to their ocular disease state. We identified KoRV-B in only 29% (2/7) of the ocular diseased koalas (K1 and K4) in this trial. Surprisingly, despite KoRV-B being associated with inducing immunosuppressive properties within the koala [88], this did not appear to have any effect on post-vaccine immune responses for koala K4, who showed some of the strongest humoral and cell mediated immune responses. However, koala K4 did exhibit low humoral and cell mediated responses pre-vaccination.
In conclusion, the vaccinated koalas from this trial showed a reduction in their clinical ocular disease state by six-weeks post-vaccination, without the intervention of antibiotic treatment. This has clearly demonstrated that a rMOMP vaccine can have a therapeutic effect on koala ocular disease. Vaccine development for preventing chlamydial infections in the koala is continuing, with this study now adding valuable insight into the therapeutic effects provided by a rMOMP vaccine on ocular disease. Given the detrimental effects resulting from the use of antibiotics in koalas, this study provides new evidence that a rMOMP vaccine could potentially be used in the treatment of ocular disease in the koala.
Supporting information
(TIF)
(TIF)
(TIF)
No sample = N/A; Below detection level = B/D.
(DOCX)
No sample = N/A; Below detection level = B/D.
(DOCX)
Acknowledgments
The authors thank all the staff at Australia Zoo Wildlife Hospital who contributed to the care and handling of the koalas involved in this trial. We thank the Vaccine and Infectious Diseases Organization (VIDO), Canada for supplying the adjuvant. We thank the many groups that have supported the overall koala Chlamydia vaccine development work, including the Australian Research Council, the Queensland Government (Department of Transport and Main Roads, particularly the Moreton Bay Rail Project Team and Department of Environment and Heritage Protection), Moreton Bay Regional Council, Friends of Koala (Lismore), Koala Action Inc, Endeavour Veterinary Ecology, Lone Pine Koala Sanctuary, City of Gold Coast, and Redland City Council. We would further like to thank Olusola Olagoke for his assistance with the koala retrovirus qPCR assay and Dr Martina Jelocnik for her assistance with bioinformatics.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by an Australian Research Council Linkage program received by PT, the Redlands City Council and Koala action Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Blanshard W, Bodley K. In 'Medicine of Australian Mammals'. Vogelnest L, Woods R, editors. Collingwood: CSIRO; 2008. [Google Scholar]
- 2.Obendorf DL. Causes of Mortality and Morbidity of Wild Koalas, Phascolarctos Cinereus (Goldfuss), in Victoria, Australia. Journal of wildlife diseases. 1983;19(2):123–31. 10.7589/0090-3558-19.2.123 [DOI] [PubMed] [Google Scholar]
- 3.Johnston SD, Deif HH, McKinnon A, Theilemann P, Griffith JE, Higgins DP. Orchitis and Epididymitis in Koalas (Phascolarctos cinereus) Infected With Chlamydia pecorum. Veterinary pathology. 2015;52(6):1254–7. 10.1177/0300985815570069 . [DOI] [PubMed] [Google Scholar]
- 4.McColl KA, Martin RW, Gleeson LJ, Handasyde KA, Lee AK. Chlamydia infection and infertility in the female koala (Phascolarctos cineteus). Veterinary record. 1984;115:25–6. [DOI] [PubMed] [Google Scholar]
- 5.Wan C, Loader J, Hanger J, Beagley K, Timms P, Polkinghorne A. Using quantitative polymerase chain reaction to correlate Chlamydia pecorum infectious load with ocular, urinary and reproductive tract disease in the koala (Phascolarctos cinereus). Australian veterinary journal. 2011;89(10):409–12. 10.1111/j.1751-0813.2011.00827.x . [DOI] [PubMed] [Google Scholar]
- 6.Hu VH, Holland MJ, Burton MJ. Trachoma: protective and pathogenic ocular immune responses to Chlamydia trachomatis. PLoS Negl Trop Dis. 2013;7(2):e2020 10.1371/journal.pntd.0002020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton MJ. Trachoma: an overview. Br Med Bull. 2007;84:99–116. 10.1093/bmb/ldm034 . [DOI] [PubMed] [Google Scholar]
- 8.Quigley BL, Carver S, Hanger J, Vidgen ME, Timms P. The relative contribution of causal factors in the transition from infection to clinical chlamydial disease. Sci Rep. 2018;8(1):8893 10.1038/s41598-018-27253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollipara A, Polkinghorne A, Wan C, Kanyoka P, Hanger J, Loader J, et al. Genetic diversity of Chlamydia pecorum strains in wild koala locations across Australia and the implications for a recombinant C. pecorum major outer membrane protein based vaccine. Veterinary microbiology. 2013;167(3–4):513–22. 10.1016/j.vetmic.2013.08.009 . [DOI] [PubMed] [Google Scholar]
- 10.Legione AR, Patterson JL, Whiteley PL, Amery-Gale J, Lynch M, Haynes L, et al. Identification of unusual Chlamydia pecorum genotypes in Victorian koalas (Phascolarctos cinereus) and clinical variables associated with infection. J Med Microbiol. 2016;65(5):420–8. 10.1099/jmm.0.000241 . [DOI] [PubMed] [Google Scholar]
- 11.Polkinghorne A, Hanger J, Timms P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Veterinary microbiology. 2013;165(3–4):214–23. 10.1016/j.vetmic.2013.02.026 . [DOI] [PubMed] [Google Scholar]
- 12.Nyari S, Waugh CA, Dong J, Quigley BL, Hanger J, Loader J, et al. Epidemiology of chlamydial infection and disease in a free-ranging koala (Phascolarctos cinereus) population. PloS one. 2017;12(12):e0190114 10.1371/journal.pone.0190114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satpathy G, Behera HS, Ahmed NH. Chlamydial eye infections: Current perspectives. Indian J Ophthalmol. 2017;65(2):97–102. 10.4103/ijo.IJO_870_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikehata M, Numazaki K, Chiba S. Analysis of Chlamydia trachomatis serovars in endocervicalspecimens derived from pregnant Japanese women. Immunology and madical microbiology. 2000;27:35–41. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell HD, Wood H, Crane D, Bailey R, Jones RB, Mabey D, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. Journal of Clinical Investigation. 2003;111(11):1757–69. 10.1172/JCI17993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson JL, Lynch M, Anderson GA, Noormohammadi AH, Legione A, Gilkerson JR, et al. The prevalence and clinical significance of Chlamydia infection in island and mainland populations of Victorian koalas (Phascolarctos cinereus). Journal of wildlife diseases. 2015;51(2):309–17. 10.7589/2014-07-176 . [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Astudillo V, Allavena R, McKinnon A, Larkin R, Henning J. Decline causes of Koalas in South East Queensland, Australia: a 17-year retrospective study of mortality and morbidity. Sci Rep. 2017;7:42587 10.1038/srep42587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau Q, Griffith JE, Higgins DP. Identification of MHCII variants associated with chlamydial disease in the koala (Phascolarctos cinereus). PeerJ. 2014;2:e443 10.7717/peerj.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turula H, Wobus CE. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses. 2018;10(5). 10.3390/v10050237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthesy B, et al. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol. 2009;183(9):5879–85. 10.4049/jimmunol.0901838 . [DOI] [PubMed] [Google Scholar]
- 21.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8(9):656–67. 10.1038/nrmicro2384 . [DOI] [PubMed] [Google Scholar]
- 22.Mestecky J, Russell MW, Elson CO. Intestinal IgA novel views on its function in the defence of the large mucosal surface. Gut. 1999;44:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan SA, Desclozeaux M, Waugh C, Hanger J, Loader J, Gerdts V, et al. Antibody and Cytokine Responses of Koalas (Phascolarctos cinereus) Vaccinated with Recombinant Chlamydial Major Outer Membrane Protein (MOMP) with Two Different Adjuvants. PloS one. 2016;11(5):e0156094 10.1371/journal.pone.0156094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SA, Polkinghorne A, Waugh C, Hanger J, Loader J, Beagley K, et al. Humoral immune responses in koalas (Phascolarctos cinereus) either naturally infected with Chlamydia pecorum or following administration of a recombinant chlamydial major outer membrane protein vaccine. Vaccine. 2016;34(6):775–82. 10.1016/j.vaccine.2015.12.050 . [DOI] [PubMed] [Google Scholar]
- 25.Beatty W, Belanger TA, Desai AA, Morrison RP, Byrne GE. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistance. Infection and immunity. 1994;62:3705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rottenberg ME, Gigliotti-Rothfuchs A, Wigzell H. The role of IFN-gamma in the outcome of chlamydial infection. Current opinion in Microbiology. 2002;14:444–51. [DOI] [PubMed] [Google Scholar]
- 27.Gondek DC, Olive AJ, Stary G, Starnbach MN. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. J Immunol. 2012;189(5):2441–9. 10.4049/jimmunol.1103032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito JI, Lyons JM. Role of gamma interferon in controlling murine chlamydial genetal tract infecton. Infection and immunity. 1999;67(10):5518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. The Journal of Immunology. 1997;158(7):3344–52. [PubMed] [Google Scholar]
- 30.Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PloS one. 2014;9(4):e94188 10.1371/journal.pone.0094188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. Journal of clinical investigation. 1998;101:311–20. 10.1172/JCI1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Tian Q, Wang L, Xue M, Zhong G. IL-6-mediated signaling pathways limit Chlamydia muridarum infection and exacerbate its pathogenicity in the mouse genital tract. Microbes Infect. 2017;19(11):536–45. 10.1016/j.micinf.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton MJ, Ramadhani A, Weiss HA, Hu V, Massae P, Burr SE, et al. Active trachoma is associated with increased conjunctival expression of IL17A and profibrotic cytokines. Infection and immunity. 2011;79(12):4977–83. 10.1128/IAI.05718-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew M, Waugh C, Beagley KW, Timms P, Polkinghorne A. Interleukin 17A is an immune marker for chlamydial disease severity and pathogenesis in the koala (Phascolarctos cinereus). Developmental and comparative immunology. 2014;46(2):423–9. 10.1016/j.dci.2014.05.015 . [DOI] [PubMed] [Google Scholar]
- 35.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-Mediated Regulation of Innate and Acquired Immune Response against Pulmonary Mycobacterium bovis Bacille Calmette-Guerin Infection. The Journal of Immunology. 2007;178(6):3786–96. 10.4049/jimmunol.178.6.3786 [DOI] [PubMed] [Google Scholar]
- 36.Lin Y, Slight SR, Khader SA. Th17 cytokines and vaccine-induced immunity. Semin Immunopathol. 2010;32(1):79–90. 10.1007/s00281-009-0191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maher IE, Higgins DP. Altered Immune Cytokine Expression Associated with KoRV B Infection and Season in Captive Koalas. PloS one. 2016;11(10):e0163780 10.1371/journal.pone.0163780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanger JJ, Bromham LD, McKee JJ, O'Brien TM, Robinson WF. The nucleotide sequence of koala (Phascolarctos cinereus) retrovirus a novel type C endogenous virus related to Gibbon ape leukemia virus. Journal of virology. 2000;74(9):4264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons GS, Young PR, Hanger JJ, Jones K, Clarke D, McKee JJ, et al. Prevalence of koala retrovirus in geographically diverse populations in Australia. Australian veterinary journal. 2012;90(10):404–9. 10.1111/j.1751-0813.2012.00964.x . [DOI] [PubMed] [Google Scholar]
- 40.Chaban B, Ong VA, Hanger J, Timms P. Molecular dynamics and mode of transmission of Koala Retrovirus (KoRV) as it invades and spreads through a wild Queensland koala population. Journal of virology. 2017. 10.1128/JVI.01871-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarlinton R, Meers J, Hanger J, Young P. Real-time reverse transcriptase PCR for the endogenous koala retrovirus reveals an association between plasma viral load and neoplastic disease in koalas. The Journal of general virology. 2005;86(Pt 3):783–7. 10.1099/vir.0.80547-0 . [DOI] [PubMed] [Google Scholar]
- 42.Waugh CA, Hanger J, Loader J, King A, Hobbs M, Johnson R, et al. Infection with koala retrovirus subgroup B (KoRV-B), but not KoRV-A, is associated with chlamydial disease in free-ranging koalas (Phascolarctos cinereus). Sci Rep. 2017;7(1):134 10.1038/s41598-017-00137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Govendir M, Hanger J, Loader JJ, Kimble B, Griffith JE, Black LA, et al. Plasma concentrations of chloramphenicol after subcutaneous administration to koalas (Phascolarctos cinereus) with chlamydiosis. J Vet Pharmacol Ther. 2012;35(2):147–54. 10.1111/j.1365-2885.2011.01307.x . [DOI] [PubMed] [Google Scholar]
- 44.Black LA, McLachlan AJ, Griffith JE, Higgins DP, Gillett A, Krockenberger MB, et al. Pharmacokinetics of chloramphenicol following administration of intravenous and subcutaneous chloramphenicol sodium succinate, and subcutaneous chloramphenicol, to koalas (Phascolarctos cinereus). J Vet Pharmacol Ther. 2013;36(5):478–85. 10.1111/jvp.12024 . [DOI] [PubMed] [Google Scholar]
- 45.Griffith JE, Higgins DP, Li KM, Krockenberger MB, Govendir M. Absorption of enrofloxacin and marbofloxacin after oral and subcutaneous administration in diseased koalas (Phascolarctos cinereus). J Vet Pharmacol Ther. 2010;33(6):595–604. 10.1111/j.1365-2885.2010.01169.x . [DOI] [PubMed] [Google Scholar]
- 46.Dahlhausen KE, Doroud L, Firl AJ, Polkinghorne A, Eisen JA. Characterization of shifts of koala (Phascolarctos cinereus) intestinal microbial communities associated with antibiotic treatment. PeerJ. 2018;6:e4452 10.7717/peerj.4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osawa R, Bird PS, Harbrow DJ, Ogimoto K, Seymour GJ. Microbiological Studies of the Intestinal Microflora of the Koala, Phascolarctos cinereus. I. Colonisation of the Caecal Wall by Tannin-protein-complexdegrading Enterobacteria. Australian Journal of Zoology. 1993;41:599–609. [Google Scholar]
- 48.Lange K, Buerger M, Stallmach A, Bruns T. Effects of Antibiotics on Gut Microbiota. Dig Dis. 2016;34(3):260–8. 10.1159/000443360 . [DOI] [PubMed] [Google Scholar]
- 49.Neuman H, Forsythe P, Uzan A, Avni O, Koren O. Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol Rev. 2018;42(4):489–99. 10.1093/femsre/fuy018 . [DOI] [PubMed] [Google Scholar]
- 50.Budd C, Flanagan C, Gillett A, Hanger J, Loader JJ, Govendir M. Assessment of florfenicol as a possible treatment for chlamydiosis in koalas (Phascolarctos cinereus). Australian veterinary journal. 2017;95(9):343–9. 10.1111/avj.12617 . [DOI] [PubMed] [Google Scholar]
- 51.Kollipara A, George C, Hanger J, Loader J, Polkinghorne A, Beagley K, et al. Vaccination of healthy and diseased koalas (Phascolarctos cinereus) with a Chlamydia pecorum multi-subunit vaccine: evaluation of immunity and pathology. Vaccine. 2012;30(10):1875–85. 10.1016/j.vaccine.2011.12.125 . [DOI] [PubMed] [Google Scholar]
- 52.Kollipara A, Wan C, Rawlinson G, Brumm J, Nilsson K, Polkinghorne A, et al. Antigenic specificity of a monovalent versus polyvalent MOMP based Chlamydia pecorum vaccine in koalas (Phascolarctos cinereus). Vaccine. 2013;31(8):1217–23. 10.1016/j.vaccine.2012.12.057 . [DOI] [PubMed] [Google Scholar]
- 53.Kollipara A, Polkinghorne A, Beagley K, Timms P. Vaccination of koalas with a recombinant Chlamydia pecorum Major Outer Membrane Protein induces antibodies of different specificity compared to those following a natural live infection. PlosOne. 2013;8(9):e74808 10.1371/10.1371/journal.pone.0074808.t001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan SA, Waugh C, Rawlinson G, Brumm J, Nilsson K, Gerdts V, et al. Vaccination of koalas (Phascolarctos cinereus) with a recombinant chlamydial major outer membrane protein adjuvanted with poly I:C, a host defense peptide and polyphosphazine, elicits strong and long lasting cellular and humoral immune responses. Vaccine. 2014;32(44):5781–6. 10.1016/j.vaccine.2014.08.037 . [DOI] [PubMed] [Google Scholar]
- 55.Waugh CA, Timms P, Andrew D, Rawlinson G, Brumm J, Nilsson K, et al. Comparison of subcutaneous versus intranasal immunization of male koalas (Phascolarctos cinereus) for induction of mucosal and systemic immunity against Chlamydia pecorum. Vaccine. 2015;33(7):855–60. 10.1016/j.vaccine.2014.12.052 . [DOI] [PubMed] [Google Scholar]
- 56.Waugh C, Khan SA, Carver S, Hanger J, Loader J, Polkinghorne A, et al. A Prototype Recombinant-Protein Based Chlamydia pecorum Vaccine Results in Reduced Chlamydial Burden and Less Clinical Disease in Free-Ranging Koalas (Phascolarctos cinereus). PloS one. 2016;11(1):e0146934 10.1371/journal.pone.0146934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waugh C, Austin R, Polkinghorne A, Timms P. Treatment of Chlamydia-associated ocular disease via a recombinant protein based vaccine in the koala (Phascolarctos cinereus). Biologicals. 2016;44(6):588–90. 10.1016/j.biologicals.2016.09.006 . [DOI] [PubMed] [Google Scholar]
- 58.Desclozeaux M, Robbins A, Jelocnik M, Khan SA, Hanger J, Gerdts V, et al. Immunization of a wild koala population with a recombinant Chlamydia pecorum Major Outer Membrane Protein (MOMP) or Polymorphic Membrane Protein (PMP) based vaccine: New insights into immune response, protection and clearance. PloS one. 2017;12(6):e0178786 10.1371/journal.pone.0178786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nyari S, Khan SA, Rawlinson G, Waugh CA, Potter A, Gerdts V, et al. Vaccination of koalas (Phascolarctos cinereus) against Chlamydia pecorum using synthetic peptides derived from the major outer membrane protein. PloS one. 2018;13(6):e0200112 10.1371/journal.pone.0200112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carey AJ, Timms P, Rawlinson G, Brumm J, Nilsson K, Harris JM, et al. A multi-subunit chlamydial vaccine induces antibody and cell-mediated immunity in immunized koalas (Phascolarctos cinereus): comparison of three different adjuvants. Am J Reprod Immunol. 2010;63(2):161–72. 10.1111/j.1600-0897.2009.00776.x . [DOI] [PubMed] [Google Scholar]
- 61.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3(6):1101–8. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 62.Islam MM, Jelocnik M, Huston WM, Timms P, Polkinghorne A. Characterization of the In Vitro Chlamydia pecorum Response to Gamma Interferon. Infection and immunity. 2018;86(4):714–7. doi: 10.1128/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maher IE, Griffith JE, Lau Q, Reeves T, Higgins DP. Expression profiles of the immune genes CD4, CD8beta, IFNgamma, IL-4, IL-6 and IL-10 in mitogen-stimulated koala lymphocytes (Phascolarctos cinereus) by qRT-PCR. PeerJ. 2014;2:e280 10.7717/peerj.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gulley JL. Therapeutic vaccines: the ultimate personalized therapy? Hum Vaccin Immunother. 2013;9(1):219–21. 10.4161/hv.22106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filipovic A, Ghasemian E, Inic-Kanada A, Lukic I, Stein E, Marinkovic E, et al. The effect of infectious dose on humoral and cellular immune responses in Chlamydophila caviae primary ocular infection. PloS one. 2017;12(7):e0180551 10.1371/journal.pone.0180551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunham R, Kuo CC, Cles L, Holmes KK. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infection and immunity. 1982;39(3):1491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dong-Ji Z, Yang X, Shen C, Lu H, Murdin A, Brunham R. Priming with Chlamydia trachomatis Major Outer Membrane Protein (MOMP) DNA followed by MOMP ISCOM Boosting Enhances Protection and Is Associated with Increased Immunoglobulin A and Th1 Cellular Immune Responses. Infection and immunity. 2000;68(6):3074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumari J, Selvan SR, Becart S, Chattopadhyay S, Dalmo RA. Cell-mediated immunity and vaccines. J Immunol Res. 2014;2014:632632 10.1155/2014/632632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasilevsky S, Greub G, Nardelli-Haefliger D, Baud D. Genital Chlamydia trachomatis: understanding the roles of innate and adaptive immunity in vaccine research. Clin Microbiol Rev. 2014;27(2):346–70. 10.1128/CMR.00105-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gondek DC, Roan NR, Starnbach MN. T cell responses in the absence of IFN-gamma exacerbate uterine infection with Chlamydia trachomatis. J Immunol. 2009;183(2):1313–9. 10.4049/jimmunol.0900295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loomis WP, Starnbach MN. T cell responses to Chlamydia trachomatis. Current opinion in Microbiology. 2002;5:87–91. [DOI] [PubMed] [Google Scholar]
- 72.Longbottom D. Chlamydial vaccine development. J Med Microbiol. 2003;52(Pt 7):537–40. 10.1099/jmm.0.05093-0 . [DOI] [PubMed] [Google Scholar]
- 73.Redgrove KA, McLaughlin EA. The Role of the Immune Response in Chlamydia trachomatis Infection of the Male Genital Tract: A Double-Edged Sword. Front Immunol. 2014;5:534 10.3389/fimmu.2014.00534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen CR, Koochesfahani KM, Meier AS, Shen C, Karunakaran K, Ondondo B, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon- gamma. Journal of infectious diseases. 2005;192:591–9. 10.1086/432070 [DOI] [PubMed] [Google Scholar]
- 75.Karunakaran KP, Yu H, Foster LJ, Brunham RC. Development of a Chlamydia trachomatis T cell Vaccine. Human Vaccines. 2014;6(8):676–80. 10.4161/hv.6.8.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mühl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-γ. International Immunopharmacology. 2003;3(9):1247–55. 10.1016/S1567-5769(03)00131-0 [DOI] [PubMed] [Google Scholar]
- 77.Zhang J. Yin and yang interplay of IFN-gamma in inflammation and autoimmune disease. J Clin Invest. 2007;117(4):871–3. 10.1172/JCI31860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka T, Narazaki M, Kishimoto T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harbor Perspectives in Biology. 2014;6(10):a016295–a. 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schett G. P hysiological effects of modulating the interleukin-6 axis. Rheumatology (Oxford). 2018;57(suppl_2):ii43–ii50. 10.1093/rheumatology/kex513 . [DOI] [PubMed] [Google Scholar]
- 80.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005(5):273–9. 10.1155/MI.2005.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Albrecht LJ, Tauber SC, Merres J, Kress E, Stope MB, Jansen S, et al. Lack of Proinflammatory Cytokine Interleukin-6 or Tumor Necrosis Factor Receptor-1 Results in a Failure of the Innate Immune Response after Bacterial Meningitis. Mediators Inflamm. 2016;2016:7678542 10.1155/2016/7678542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. 10.1016/j.bbamcr.2011.01.034 . [DOI] [PubMed] [Google Scholar]
- 83.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Research & Therapy. 2006;8 10.1186/ar1917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6 a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends in Immunology. 2003;24. [DOI] [PubMed] [Google Scholar]
- 85.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2(5):403–11. 10.1038/mi.2009.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183(9):5886–95. 10.4049/jimmunol.0901584 . [DOI] [PubMed] [Google Scholar]
- 87.Sturdevant GL, Kari L, Gardner DJ, Olivares-Zavaleta N, Randall LB, Whitmire WM, et al. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infection and immunity. 2010;78(9):3660–8. 10.1128/IAI.00386-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fiebig U, Hartmann MG, Bannert N, Kurth R, Denner J. Transspecies transmission of the endogenous koala retrovirus. Journal of virology. 2006;80(11):5651–4. 10.1128/JVI.02597-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
No sample = N/A; Below detection level = B/D.
(DOCX)
No sample = N/A; Below detection level = B/D.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.