Abstract
Objective
Special attention has been given to verbal memory deficits in schizophrenia because they are apparent in healthy biological relatives of affected individuals, indicating a link to genetic risk for the disorder. Despite a growing consensus that encoding abnormalities contribute to poor verbal memory in the disorder, few studies have directly examined how neural responses during encoding contribute to later memory performance.
Method
We evaluated event-related potentials (ERPs) during encoding of verbal material by patients with schizophrenia, healthy first-degree biological relatives of patients, and healthy controls. The extent to which N1, N400, and anterior and parietal Late Positive Components (LPCs) explained encoding accuracy and later memory of material was investigated.
Results
Encoding accuracy was associated with asymmetry in anterior LPCs toward right frontal brain regions and was most evident in relatives. N1 was abnormal at encoding in schizophrenia and differentially accounted for later memory performance. In controls better recall of verbal material was predicted by a larger early occipital (N1) encoding response; however, in patients with schizophrenia smaller N1 encoding responses were related to better recall. Interestingly, better recognition of verbal material across groups was also predicted by smaller N1 amplitudes during encoding of word stimuli.
Conclusion
Separable patterns of electrophysiological response during encoding appear to differentially support recall and recognition of material from memory. Similar patterns of electrophysiological response across patient and relative groups suggest that those who carry genetic liability for schizophrenia share deviations in the neural activity related to encoding of material into episodic memory.
Keywords: Psychopathology, Electroencephalography, Cognition, Memory, Episodic, Attention, Neurobehavioral manifestations, Schizophrenia
Introduction
Neural responses at initial exposure to a word contribute to whether that word will be remembered. People with schizophrenia exhibit notable difficulties remembering verbal information, which has the apparent consequence of creating obstacles for functioning in community and work settings (Green, 1996; Laes & Sponheim, 2006; Libby, Yonelinas, Ranganath, & Ragland, 2013). Most functional magnetic resonance imaging (fMRI) and electrophysiological studies of verbal memory in schizophrenia have focused on neural response during retrieval of previously presented information. Less is known about how neural processes during the initial exposure to verbal material lead to successful memory (Yonelinas, 2002). Studies in healthy individuals suggest event-related potentials (ERPs) during encoding predicts later memory performance, but similar studies have yet to be completed in persons with schizophrenia (Paller, McCarthy, & Wood, 1988). Abnormal processing is evident in the few fMRI studies that have investigated word encoding in schizophrenia (Aleman, Hijman, de Haan, & Kahn, 1999; Bonner-Jackson, Yodkovik, Csernansky, & Barch, 2008). The present study explores the origins of episodic memory deficits in schizophrenia by investigating ERPs during incidental encoding of verbal material that may contribute to subsequent detriment in retrieval performance in people affected by schizophrenia. The findings are expected to fit in overarching neural models of episodic memory, such as the hemispheric encoding/retrieval asymmetry (HERA) model (Tulving, Kapur, Craik, Moscovitch, & Houle, 1994), which have identified abnormalities in both encoding and retrieval cognitive processes that contribute to retrieval performance deficits. Encoding processes are hypothesized to be the primary source of deficits in retrieval performance in schizophrenia, though there is a dearth of relevant studies in psychiatric populations (Cirillo & Seidman, 2003). Direct evidence of errant brain processes during encoding would highlight how the initial stages of bringing material into memory may be one important target for interventions intended to improve cognition in schizophrenia.
Verbal memory deficits also appear to reflect genetic liability for the disorder. Biological relatives of individuals with schizophrenia exhibit deficits in free-recall performance, although recognition of verbal material is comparable for biological relatives and healthy controls (Egan, Goldberg, & Gscheidle, 2001; Sponheim, Steele, & McGuire, 2004). The selective recall deficit in biological relatives suggests that impairment in a specific aspect of episodic memory is related to genetic factors that contribute to risk for psychosis. Establishing the relationship between event-related potentials (ERPs) during encoding and later memory recall can elucidate how genetic liability might be expressed in the brain function of biological relatives of people with schizophrenia.
Overarching theories of memory dysfunction in schizophrenia posit that deficits occur via disruptions in both encoding and retrieval processes (Cirillo & Seidman, 2003). Patients retain most items between immediate and delayed recall in story and list learning tests, demonstrating that schizophrenia does not consistently interfere with prolonged storage (Ranganath, Minzenberg, & Ragland, 2008). Instead, various findings demonstrate how abnormalities at encoding may contribute to later memory. People with schizophrenia show steeper learning curves, taking longer to retain new material, which suggests that poor subsequent retrieval occurs when verbal material is insufficiently encoded to memory during initial exposure (Aleman et al., 1999). An inability to indicate whether presented words are old or new in recognition paradigms has also, in part, been attributed to disrupted encoding (Ragland, 2004). These behavioral patterns are often understood as reflecting interplay of encoding and retrieval processes. Functional imaging and psychophysiological recordings point to disruptions in the network of brain regions that support retrieval, including the prefrontal cortex (PFC), medial temporal lobe (MTL), and parietal cortex (Bridger, Bader, Kriukova, Unger, & Mecklinger, 2012; Shimamura, 2014; Yonelinas, 2002). Yet, few studies have considered abnormalities at encoding by examining the brain responses of participants when first exposed to verbal stimuli.
Paller and colleagues introduced the idea that certain neurophysiological responses during encoding will predict later behavioral responses (Paller et al., 1988). Using incidental word learning experiments they identified the “Differences based on subsequent memory performance” (Dm) event-related potential (ERP). The Dm component is a subtraction of ERPs for recognized and unrecognized words, most visible at midline sites (Fz, Cz, Pz) from 400 to 700 ms after word presentation. Amplitude of the brain response at encoding for words later recognized is more positive than that of unrecognized words. The waveforms at frontal sites (e.g., Fz) can be highly affected by encoding strategy (e.g. engaging mnemonics; Fabiani, Karis, & Donchin, 1990), consistent with neuroimaging findings that prefrontal activation is reduced when persons with schizophrenia are not instructed to use a particular encoding strategy (Guimond, Hawco, & Lepage, 2017). Contemporary studies more commonly quantify late positive components (LPCs). The LPC component occurs in the same time window as Dm; unlike Dm, it is not calculated as a difference waveform. The parietal LPC can occur in a wide swath of time after the stimulus, from 400 ms on, and is associated with memory processing and consolidation, and correlates with recollection in incidental word learning tasks (Voss & Paller, 2009a; Wilding & Ranganath, 2011). More positive amplitude indicates more features of an item were remembered (Bridger et al., 2012). LPCs have been widely observed during episodic memory tasks and are thought to reflect the extent to which a stimulus is recognized (Allen, 2002; Niznikiewicz & O’Donnell, 1997). As with the Dm component, the task approach of the individual affects LPCs. LPCs are related to planning goal-directed behaviors, which may be more useful than a semantic strategy in some tasks (Sitnikova, Perrone, Goff, & Kuperberg, 2010).
ERP studies show that word encoding also elicits negative components at centro-parietal sites around 400 ms (N400; Paller & Wagner, 2002). The N400 component consists of a negative going amplitude that occurs during processing of semantic information and, in part, reflects the integration of word meaning into the current semantic context. Disrupted N400s are consistently observed in patients with schizophrenia (Voss & Paller, 2009b). Given the timing and location, N400s are thought to reflect engagement of the medial temporal lobes (MTL; Glisky, Polster, & Routhieaux, 1995) and facilitation of memory through encoding semantic information and contextual cues. Our exploration of N400, parietal LPC, and frontal LPC components in the current study allow us to model the temporal progression of memory encoding processes that have been localized to activation in temporal, parietal, and frontal regions, respectively.
Perceptual functions are also known to contribute to encoding but many models of verbal memory do not incorporate these early attentional processes. Early differences in visual processing can be captured by the N1 ERP component which has long been associated with visual selective attention (Mangun, 1995). Specifically, greater negativity at occipital and parietal sites approximately 150 ms after stimulus onset reflects a person’s ability to discriminate between attended and unimportant visual stimuli (Vogel & Luck, 2000). Persons with schizophrenia often have attenuated N1 amplitude, reflecting disturbances in visual perceptual pathways that affect pre-attentive sensory processing (O’Donnell, Salisbury, Niznikiewicz, Brenner, & Vohs, 2011). In the current study we additionally explored how early attentional processes at encoding contribute to later verbal memory performance.
The present study investigates the neurophysiological correlates of verbal memory during encoding through addressing two central research questions: (1) Are ERP components during encoding predictive of later verbal memory performance? (2) Do patients with schizophrenia and their first-degree relatives show similar disruptions in ERP activity during verbal encoding reflecting an underlying genetic liability? In addressing the first question, we hypothesized that ERP activations during encoding, particularly N1 and anterior LPC, would be predictive of later recognition. We expected N400 and parietal LPC to predict explicit recall performance. With respect to the second inquiry, we hypothesized that ERPs indicative of attentional discrimination (N1), semantic elaboration (N400), and episodic memory (parietal LPC) would be disrupted in patients. Both early and late processes are likely to be relevant to encoding and clarification of which are disrupted in schizophrenia may shed light on neuropathology related to the disorder. Relatives of patients with schizophrenia were predicted to exhibit similar, but less prominent abnormalities in neural responses during encoding. In particular, we expected N400s to be attenuated given structural abnormalities in relatives’ MTL volume (Boos, Aleman, Cahn, Hulshoff Pol, & Kahn, 2007).
Methods
Participants and Diagnostic Assessment
Twenty-three patients with schizophrenia diagnoses (SZ), 17 first-degree biological relatives of patients with schizophrenia (SZ-REL), and 32 non-psychiatric control participants were included in data analysis (see Table 1). Participants were a subset of a larger family study of psychosis (Sponheim et al., 2004) for whom EEG data was collected during the verbal memory task. All participants completed an informed consent process consistent with the Declaration of Helsinki, and Minneapolis VA Medical Center and University of Minnesota Institutional Review Boards approval.
Table 1.
Sample characteristics and Memory Task Performance
| Controls | SZ-REL | SZ | Test value | |
|---|---|---|---|---|
| n = 32 | n = 17 | n = 23 | ||
| Age | 42.69 (15.20) | 53.88 (7.70) | 47.87 (7.58) | F(2, 69) = 5.22**b |
| Percent male | 56% | 41% | 83% | χ2 = 7.63*c |
| Percent right-handed | 94% | 100% | 83% | n.s. |
| Years of education | 14.84 (1.80) | 15.35 (2.15) | 14.13 (1.98) | n.s. |
| Estimated IQ | 111.56 (14.06) | 111.35 (12.85) | 97.17 (10.73) | F(2, 69) = 9.79**ac |
| Chlorpromazine equivalents (mg) | — | — | 672.10 (567.24) | — |
| BPRS total score | — | — | 42.91 (11.71) | — |
| SPQ total score | 11.12 (5.90) | 15.47 (6.50) | — | F(1, 47) = 5.62*b |
| Verbal Memory Task | ||||
| Encoding | 78.09 (7.94) | 78.12 (5.01) | 69.00 (11.42) | F(2, 69) = 8.56**ac |
| Recall | 21.03 (8.18) | 15.82 (6.93) | 9.00 (4.89) | F(2, 69) = 19.85**abc |
| Recognition | 74.28 (17.65) | 73.59 (18.98) | 62.74 (15.82) | F(2, 69) = 3.31*a |
| Recognition without recall | 55.28 (13.60) | 58.71 (15.86) | 55.48 (13.63) | n.s. |
Note: Age and gender percentages differed across the groups and were included as covariates in all analyses. Intelligence quotient (IQ) was estimated from WASI-III Block Design and Vocabulary Subtests. The Brief Psychiatric Rating Scale (BPRS) was collected only for patients as a measure of current symptoms; the Schizotypal Personality Questionnaire (SPQ) was collected only for relatives and controls. Verbal memory performance metrics are reported as correct responses across the encoding, recall and recognition portions of the verbal memory task (maximum of 90 for each index).
n.s. = not significant (p > .05); *p < .05; **p < .01. Post-hoc group comparisons: a = patients with schizophrenia differed from healthy controls; b = relatives of patients differed from controls; c = relatives differed from patients.
Stable psychiatric outpatients ages 18–59 were recruited from the Minneapolis VA Medical Center and community mental health agencies. Patients completed the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994) administered by a trained doctoral-level clinical psychologist. The psychologist rated current symptomatology using the 24-item version of the Brief Psychiatric Rating Scale (BPRS; Lukoff, Liberman, & Nuechterlein, 1986). Final diagnosis was determined by doctoral-level psychologists and trained advanced graduates students through a consensus process consistent with published guidelines (Leckman, 1982). Chlorpromazine equivalent values were determined for all antipsychotic medications (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010). All patients were taking antipsychotic medication (74% novel). Concurrent prescriptions were anti-parkinsonian (22%), anti-depressant (52%), and anti-anxiety (9%).
Control participants were screened by study staff via a telephone interview using the same age range as relatives and the same exclusion criteria as volunteers with schizophrenia. In addition to exclusion criteria above, staff excluded control participants if they had a personal or family history of psychotic symptoms or an affective disorder as defined by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition text revision (DSM-IV-TR; American Psychiatric Association, 2000), the current DSM version at the time of data collection. We excluded potential schizophrenia participants and controls if they were adopted or had English as a second language, estimated IQ less than 70 or a diagnosis of an intellectual disability, current alcohol or drug abuse, past drug dependence, a medical condition or disease with likely significant central nervous system effects, history of head injury with skull fracture or loss of consciousness of greater than 20 min, a physical problem that would render study measures difficult or impossible to administer or interpret (e.g., blindness), significant tardive dyskinesia as indicated by a Dyskinesia Identification System: Condensed User Scale (Sprague & Kalachnik, 1991), or history of electroconvulsive therapy.
Relatives of patients were identified for participation through a pedigree from the patient’s report. Interested relatives completed a telephone interview to determine their eligibility. To maximize the number of participating relatives, first-degree biological relatives were excluded from participating only if they had a personal history of schizophrenia spectrum diagnosis, a physical problem that would render study measures impossible to measure, or were not aged 18–68 years. Additionally, none of the relatives meets diagnostic criteria for a bipolar affective or schizophrenia spectrum disorder. All relatives and patients in this sample are from different families. See a previously published report for full information regarding clinical assessment of relatives and control participants (Sponheim, McGuire, & Stanwyck, 2006).
Verbal Memory Task
The verbal memory assessment contained three encoding-recall-lexical decision blocks and one recognition block (Sponheim et al., 2004). This study focuses on conceptual (“deep”) encoding in verbal memory rather than priming effects, thus we include only encoding, recall and recognition in the analysis. Each encoding-recall-priming block consisted of 30 words presented in a size judgment task, free-recall of as many of the 30 words as possible, and a lexical decision task including the 30 encoded words, 15 new words, and 15 pronounceable non-words derived from Paller et al. (1995). During encoding, words were presented via computer in upper case letters (e.g., DAISY) for 244 milliseconds (ms). Participants had 2500 ms to decide if the named object was larger or smaller than the computer monitor. Encoding accuracy was the number of words for which size judgments were correct. Words were separated by 4410 ± 10 ms intertrial intervals. Next, participants verbally recalled the words presented in the size judgment task while the experimenter recorded responses. No cues were provided. Recall accuracy was the total number of words freely recalled across all three blocks. The participants then completed a lexical decision task that was not included in the current analysis. All words are seen in lexical decision so any effect on verbal memory is equivalent across all stimuli. After the three encoding-recall-priming blocks, the final step was to test for recognition of words presented in the size judgment task. Participants were presented all 90 words from the size judgment task, the 45 words presented as new in the lexical decision task, and 45 new foil words and indicated whether or not each word had been presented at encoding. The total correct “yes” responses formed recognition accuracy. Words appeared for 244 ms, with 1500 ms allowed for a response and 342 ± 1 ms intertrial intervals. All stimuli were presented on a CRT monitor using DMASTR software developed at the University of Arizona by K.I. Forster and J.C. Forster.
Intelligence was measured using the Wechsler Adult Intelligence Scale, Third Edition (Wechsler, 1997), which was the most recent version at the time of data collect; IQ was derived from the Vocabulary and Block Design subtests according to a short-form formula that has been deemed appropriate for WAIS-III (Jeyakumar, Warriner, Raval, & Ahmad, 2004; Tellegen & Briggs, 1967).
Electroencephalography Collection and Processing
Electroencephalograms (EEG) were collected utilizing an elastic electrode cap with 29 tin electrodes placed on the scalp conforming to a subset of locations in the 10–10 International System (Chatrian et al., 1988) using Neuroscan amplifiers and software. Vertical and horizontal electro-oculograms (VEOG and HEOG) were monitored by electrodes placed above and below the right eye and on the left and right temples, respectively. Electrodes were filled with conductive gel and the sites were abraded to reduce impedances to less than 5 kΩ. EEG signals were digitized on-line at 250 Hz during data collection with an analog band pass filter of 0.01–50 Hz. Offline, continuous EEG recordings were re-referenced to linked-ears using Neuroscan EDIT software (version 4.0). While the average reference offers theoretical as well as practical advantages, it is effective only if there are a sufficient number of electrodes distributed over the entire scalp (Nunez & Srinivasan, 2006). Recordings were band pass filtered with 0.1 Hz low-frequency (48 dB/octave roll-off) and 30 Hz high-frequency (48 dB/octave roll-off) filters. Eye blink artifacts were corrected using the algorithm described by Semlitsch, Anderer, Schuster, and Presslich (1986), as implemented within the EDIT software. Data were next epoched from 100 ms pre-stimulus to 1500 ms post-stimulus. Segments with voltages exceeding ±100 µV at any EEG site of the HEOG recording were excluded from further analysis, and all remaining data were visually inspected for bioelectrical artifact including verifying removal of EEG containing excessive eye movements. The epoched and eye-blink corrected EEG data were then imported to Matlab (Mathworks, Inc.) for subsequent data processing, including downsampling to 128 Hz using the Matlab resample command, which first applies a low pass anti-aliasing filter. Baseline correction was made based on the median amplitude 100 ms pre-stimulus. For each participant, trials were averaged for the encoding condition and grand averages were computed by averaging waveforms within conditions across participants.
ERP component windows were defined through inspection of grand average waveforms, histograms depicting the frequency of peak amplitude timing, and review of the literature. The N1 component was defined as the mean negative voltage occurring between 150 and 210 ms post-stimulus at the occipital electrode site O1. The O1 was chosen as approximating midline occipital due to no central electrode in the occipital array; missing data for two participants at O2 prevented an average of the two electrodes. The N400 component was defined as the mean voltage occurring between 300 and 500 ms post-stimulus at electrode Cz. LPC was investigated at 500–700 ms across parietal (PZ, P7, and P8) and 450–700 ms across frontal (FZ, F7, and F8) sites. Mean amplitude differed at left and right anterior scalp (β = 3.10, t139 = 5.22, p < .05). To quantify observed laterality effects, the final anterior LPC component was computed as difference in peak amplitude between homologous electrode sites for each anterior hemisphere (F8 [right] minus F7 [left]), referred to as the LPC anterior lateralization component. The parietal LPC component showed no lateralization effect (β = 0.66, t139 = 1.55, p = .12) and was quantified as mean amplitude at the central Pz electrode.
Statistical Analysis
Sample characteristics were compared using one-way ANOVA and Tukey HSD post-hoc tests for continuous variables. Pearson’s chi-squared tests were applied to categorical variables. Group differences in ERP components were modeled with linear regressions, followed by group contrasts when predictors were significant. For the main analyses, variable selection was performed using the genetic algorithm method of the glmulti function (Calcagno & Mazancourt, 2010) in R statistical software so that all combinations of variables could be considered, as opposed to stepwise regression where the order of adding variables must be predetermined. The predictor variables were participant group, gender, and the ERP components. Interactions between ERP components were not permitted. All continuous predictors were centered. Males and controls served as the reference groups in categorical variables gender and group, respectively. The best model was that with the lowest Akaike information criterion (AIC) value. Using this approach prevented inflating the predictive value of the models if we had included all variables as predictors. Models were determined separately for encoding, recognition without recall, and recall. For each, four iterations of the genetic algorithm method were applied to the regression function in R and submitted to the consensus function to ensure that the models matched. The three models—encoding, recognition without recall, and recall—then underwent standard multiple regression techniques. Holm–Bonferroni corrections for multiple comparisons were applied to the regression results (Holm, 1979). Variation inflation factor (VIF) for all regression coefficients was well below the suggested threshold of 10, indicating collinearity was minimal in the models.
Results
Participant Characteristics
Demographic and clinical factors were compared across the three groups, as shown in Table 1. Handedness and years of education were similar in all groups. The groups were not matched on age or gender proportions (see Table 1), owing to practical limitations in the sample. Relatives were older than controls, due to the enrollment of parents of persons with schizophrenia. There were fewer females in the schizophrenia group compared to relatives, consistent with sex differences in the incidence of schizophrenia (Aleman, Kahn, & Selten, 2003). Therefore, we took extra steps to explore the affect of these two variables on other variables of interest. We also computed Pearson correlations and asymptotic p-values to explore the relationship between age and verbal memory performance. There were no significant associations between age and encoding, recognition, or recall performance. As well, the memory task emphasizes incidental encoding, which is less vulnerable to age-related effects than associative memory (Chalfonte & Johnson, 1996). Age also failed to predict significant variance in any of the ERP components with the exception of the LPC at the parietal site (r = −0.26, p < .05). Nonetheless, age and gender were further investigated by including them as predictor variables in regression analyses of ERP variables.
Participants with schizophrenia had lower estimated IQs than relatives and controls. Because lower IQ is a recognized developmental risk factor for schizophrenia, it was not entered as a covariate in analyses (Miller & Chapman, 2001). Patients were stable, with mild to moderate levels of symptomatology as measured by the BPRS. Relatives showed a significant elevation in self-reported schizotypal characteristics assessed through the SPQ, consistent with greater expression of subthreshold symptoms of psychosis in those genetically related to a person with psychosis (Calkins, Curtis, Grove, & Iacono, 2004).
Encoding and Memory Performance
Accuracy on the three relevant components of the memory task was examined in the groups. Means, standard deviations, and statistics from contrasts are reported in Table 1. During the size judgment encoding task, the schizophrenia group incorrectly judged the size of the named object more often than the relatives or controls. The size of named objects was subjective to a degree, given that items like “pizza” or “pajamas” range in size. Overall response rate (correct plus incorrect judgments) was comparable across all participants. Persons with schizophrenia and relatives had poorer free recall than controls, suggesting that either the presence of the disorder or greater genetic liability further impaired episodic memory. Recall performance of the schizophrenia group was worse than the relative group, suggesting that either the presence of the disorder or greater genetic liability further impaired episodic memory.
The memory processes supporting recall may also aid recognition. To better isolate the processes unique to recognition within the limits of a traditional recall/recognize task, we employed a count of words that were recognized but not freely recalled (i.e., “recognition without recall” in Table 1). Recognition without recall, unlike total recognition, does not share a strong positive correlation with recall performance (r = −0.06, as compared to 0.44 with recognition). Recognition without recall did not differ across participant groups indicating that deficits associated with schizophrenia were mostly evident during the free recall of material from memory. For completeness, we also examined the traditional recognition metric. There was a main effect of group for total number of words recognized; participants with schizophrenia correctly responded to fewer words than controls. Relatives did not differ from either patients or controls, though their performance was intermediate to the other two groups. False alarm rates did not differ across the groups (F(2, 60) = 0.62, p = .54; 10% of participants did not have complete data), nor was there a main effect of group for false alarms when the two foil categories were examined separately (lexical decision foils: F(2, 60) = 0.08, p = .92; new foils: F(2, 60) = 2.02, p = .12).
Electrophysiological Responses at Encoding: Abnormalities in Schizophrenia
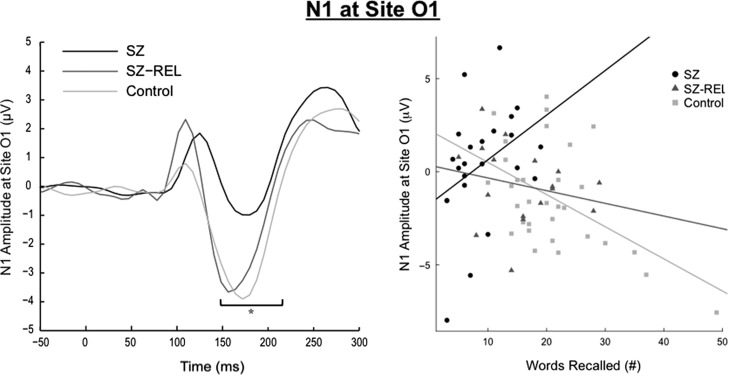
Group differences were examined by linear regressions for each ERP component: N1, N400, LPC anterior lateralization, and LPC parietal. The early posterior brain response N1 was diminished during encoding in schizophrenia compared to controls during encoding (see Fig. 1; β = 1.70, t71 = 2.03, p ≤ .05). The difference between relatives and controls (β = 0.35, t71 = 0.39, p = .70), and relatives and patients (β = 1.24, t71 = 1.28, p = .21) did not reach significance. Diminished N1 in schizophrenia supports the hypothesis that attentional discrimination during encoding is disrupted in the disorder. Neither relatives (β = −1.20, t71 = −1.11, p = .27) nor patients (β = 1.51, t71 = 1.62, p = .11) differed from controls in the N400 component. Relatives showed larger N400 response than patients (β = 2.70, t71 = 2.37, p < .05), which suggests that MTL directed semantic processing of word stimuli during encoding was heightened in relatives as compared to patients. Females had significantly smaller N400 amplitudes than males (β = 1.72, t71 = 2.04, p < .05), which may partially account for the observed group difference.
Fig. 1.
Left: Patients with schizophrenia had significantly smaller N1 than controls in response to words at encoding, as seen in the waveform plot at left. This was in part explained by the interaction between participant group and N1 in predicting later free recall performance. Right: As shown in the scatterplot at right, “smaller” (i.e., less negative) encoding N1 amplitudes predicted better free recall performance in the patient group while a “larger” encoding N1 predicted better recall in controls.
We investigated group differences in late encoding processes by examining midline parietal and lateralized anterior scalp response. Electrophysiological response did not differ between any of the groups for the LPC parietal component, in contrast to our hypothesis (SZ vs. SZ-REL: β = 0.27, t71 = 0.29, p = .77; SZ vs. Control: β = −0.61, t71 = −0.80, p = .43; SZ-REL vs. Control: β = −0.88, t71 = −1.00, p = .32). However, females had a greater amplitude response than males (β = 2.21, t71 = 3.13, p < .05). In earlier examinations, age correlated with the LPC parietal component but did not predict significant variation in the memory task; here we find no effect of age on parietal response. Therefore, age was not included in analyses of ERPs and memory performance.
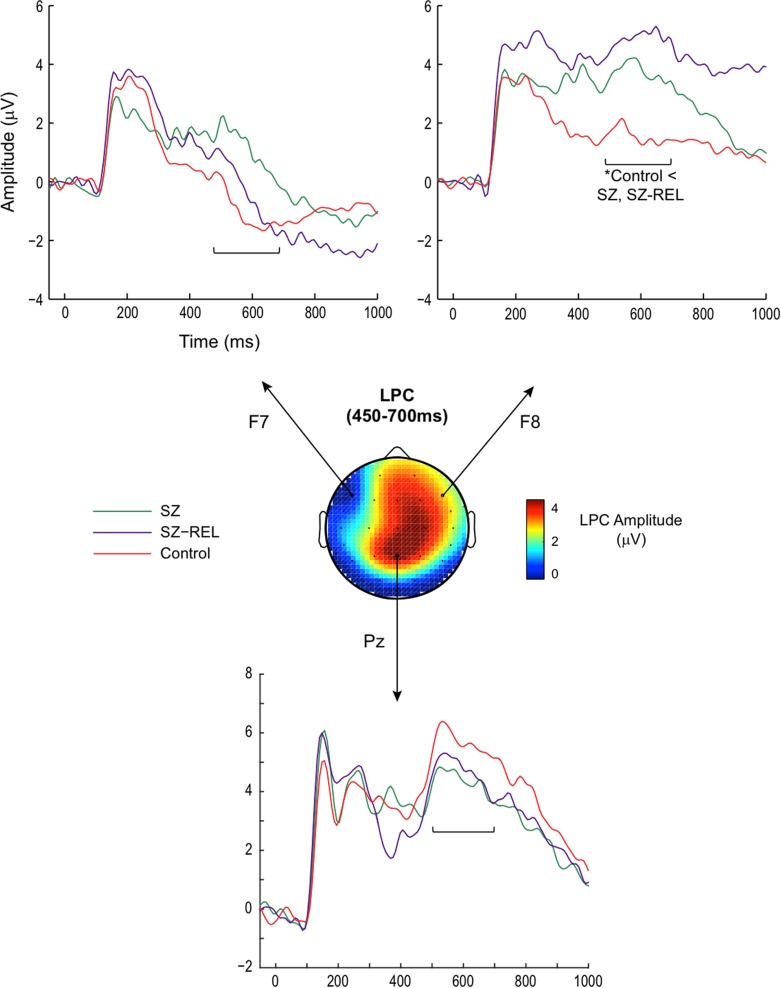
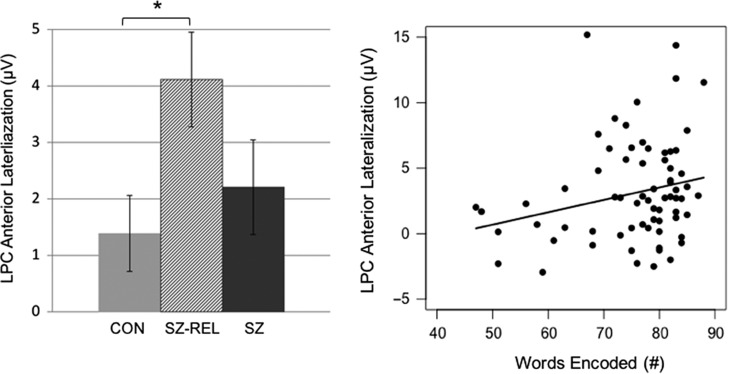
Inspection of ERPs revealed markedly lateralized late activity over frontal brain regions. Hence, we examined group differences between the left (F7), right (F8) anterior sites, as well as calculated the amplitude difference between the two sites (LPC anterior lateralization; depicted in Fig. 2). At the left hemisphere site (F7), there were no effects of age, (β = 0.03, t71 = 0.71, p = .48) gender, (β = −0.10, t71 = −0.12, p = .91) or group (SZ vs. SZ-REL: β = 1.51, t71 = 1.26, p = .21; SZ-REL vs. Control: β = 0.23, t71 = 1.15, p = .84). At the right hemisphere site (F8), the relatives and schizophrenia groups had larger LPC on encoding compared to controls (SZ-REL: β = 3.05, t71 = 3.03, p < .05; SZ: β = 2.69, t71 = 2.84, p < .05). Females also showed larger LPC responses at F8 (β = 1.81, t71 = 2.11, p < .05). When considering the amplitude difference between left and right hemispheres in the anterior LPC, relatives showed greater lateralization than controls (β = 2.51, t71 = 2.15, p < .05; see Fig. 3a). Females also showed greater mean lateralization than males (β = 2.08, t71 = 2.10, p < .05). The area of lateralization is generally consistent with cortical regions implicated in imaging studies of verbal memory (Ragland, 2004).
Fig. 2.
Late brain responses over anterior scalp sites during the encoding of words were lateralized toward the right hemisphere. Group contrasts showed that patients and relatives had larger right anterior LPCs than controls (site F8). The groups did not differ at midline parietal LPC. The scalp topography illustrates the grand average across all participants across the full LPC time course (450–700 ms).
Fig. 3.
Left: The LPC anterior lateralization, calculated as the difference between amplitude at sites F8 and F7 (450–700 ms), was significantly greater for relatives than controls. This represents substantially greater voltage response at right versus left hemisphere anterior scalp sites during the encoding of words. Right: Greater right lateralization was generally associated with better performance on the size judgment task during encoding. There were no group differences in the association between LPC anterior lateralization and encoding task performance.
Electrophysiological Responses at Encoding: Predictions of Later Memory Performance
We used regression analyses to examine whether ERP components evident during encoding predicted later recall and recognition of the verbal material. To consider how brain responses during encoding were associated with memory performance, empirical variable selection was used to design the models from the four ERP component amplitudes (N1, N400, LPC parietal, and LPC anterior lateralization) and sample characteristics (participant group and gender). Gender was included because males and females showed different patterns of response for three of the four ERP components. Due to the sample size, we determined there was not sufficient power for separate group analyses of males and females. Instead, gender was maintained as a variable in the final regression analyses. Variable selection was based on AIC values (Calcagno & Mazancourt, 2010).
The selected models and regression results are shown in Table 2. Accuracy of size judgments at encoding was optimally predicted by gender, group, and LPC anterior lateralization (F(4, 65) = 5.95, p < .001, R2adj = .22). Greater positive voltage deflection in the right hemisphere during encoding predicted more accurate size judgments (β = 0.57, t71 = 2.08, p < .05, ηp2 = 0.05). The main effect of group, with a deficit in the schizophrenia group, was evident in the regression results (ηp2 = 0.22), with schizophrenia predicting lower accuracy (β = −10.44, t71 = −4.27, p < .025). Gender and relative group membership did not significantly affect encoding.
Table 2.
Prediction of encoding recall and recognition of verbal material by encoding ERP responses
| β | Std. error | t | 95% CI | p-Value | η2 | |
|---|---|---|---|---|---|---|
| Model 1: Encoding | ||||||
| Gender | −3.50 | 2.39 | −1.46 | [−8.29, 1.29] | .15 | 0.04 |
| Group | ||||||
| SZ-REL | −0.64 | 2.75 | −0.23 | [−6.15, 4.87] | .82 | 0.25 |
| SZ | −10.87 | 2.56 | −4.24 | [−16.00, −5.74] | <.025* | |
| LPC anterior lateralization | 0.56 | 0.28 | 2.03 | [0.01, 1.12] | <.05* | 0.07 |
| Model 2: Recall | ||||||
| Group | ||||||
| SZ-REL | −3.28 | 1.96 | −1.67 | [−7.19, 0.64] | .09 | 0.38 |
| SZ | −10.54 | 1.74 | −6.06 | [−14.02, −7.06] | <.017* | |
| N1 | −1.30 | 0.41 | −3.20 | [−2.11, −0.48] | <.025* | 0.15 |
| LPC parietal | 0.34 | 0.36 | 0.94 | [−0.38, 1.06] | .34 | 0.01 |
| LPC anterior lateralization | 0.39 | 0.25 | 1.60 | [−0.10, 0.89] | .12 | 0.04 |
| N1 × Group | ||||||
| SZ-REL | 0.42 | 0.81 | 0.52 | [−1.19, 2.03] | .61 | 0.14 |
| SZ | 1.82 | 0.59 | 3.06 | [0.63, 3.00] | <.05* | |
| LPC parietal × gender | 1.05 | 0.54 | 1.93 | [−0.04, 2.13] | .06 | 0.06 |
| LPC anterior × gender | −0.46 | 0.30 | −1.51 | [−1.07, 0.14] | .13 | 0.04 |
| Model 3: Recognition without recall | ||||||
| N1 | 1.60 | 0.56 | 2.86 | [0.48, 2.71] | <.05* | 0.11 |
| LPC anterior lateralization | −0.75 | 0.40 | −1.87 | [−1.54, 0.05] | .06 | 0.05 |
Note: Three multiple linear regressions modeled the relationship between ERP response and performance in the verbal memory task. Coefficients, standard error, t-values 95% confidence intervals, significance levels, and partial eta-squared are reported. For categorical predictors, partial eta-squared was calculated for the overall variable rather though beta coefficients are reported for the individual dummy codes. Variables are centered so that beta coefficients reflect units from the predictor mean.
*Significant after Holm–Bonferroni correction for multiple comparisons.
The optimal model of free recall performance included group, N1, LPC parietal, and LPC anterior lateralization, an interaction between LPC parietal and gender, an interaction between LPC anterior lateralization and group, and an interaction between N1 and group (F(9, 60) = 10.47, p < .001, R2adj = .55). Participant group predicted recall most strongly (ηp2 = 0.24), reflecting group differences reported in Table 1. In terms of ERPs, a larger early negative posterior brain response (N1) was most associated with better free recall performance across all participants (ηp2 = 0.07). The N1 component interacted with group membership; that is, patients showed the opposite association between N1 and recall, with an attenuated N1 predicting better recall performance (ηp2 = 0.07; see Fig. 1). Thus, we found that attentional discrimination at encoding (i.e., N1) was generally predictive of later recall, but the association was reversed in individuals with schizophrenia.
A subset of variables, N1 and LPC anterior lateralization, predicted the number of words that were recognized and not recalled (F(2, 67) = 5.62, p < .01, R2adj = 0.12; see Table 2). As with free recall, N1 was the ERP associated most strongly with performance (ηp2 = 0.10). Counter to findings in the free recall model, attenuated early voltage response at occipital sites predicted recognition. In the encoding model, LPC anterior lateralization beta coefficients were positive, suggesting right frontal scalp sites were more engaged than left (ηp2 = 0.07; p < .05). LPC anterior lateralization during encoding did not significantly predict the number of words recognized without being recalled (ηp2 = 0.05; p = .06). Group failed to be selected as a significant predictor of recognition of words that were not previously recalled, consistent with similar performance across the three groups as reported in Table 2. This suggests the electrophysiological responses at encoding contributed to the words that were solely recognized similarly across all groups.
Discussion
The current study identified abnormal brain responses (ERPs) in schizophrenia that were evident during encoding of words. We used regression analyses to identify how these brain responses during encoding predicted deficits in later retrieval of verbal material. Encoding processes that are typically invisible to behavioral measures were made accessible with the electrophysiological recordings. Patients with schizophrenia showed attenuated negative amplitudes (N1) within 200 ms of being shown a word at encoding, which is consistent with reduced selective attention toward the words when initially presented. A negative going brain potential at mid-latency (N400) associated with semantic aspects of stimuli was largely intact in patients and relatives. Amplitude of the late parietal component that has been associated with later recollection of verbal material (LPC parietal) was comparable across groups. However, hemispheric differences over frontal brain regions (LPC anterior lateralization) were increased in relatives, perhaps reflecting a compensatory engagement of processes that will support later response retrieval and executive control (Landro, Pape-Ellefsen, Hagland, & Odland, 2001; Shimamura, 2014).
Three of the four ERP variables of interest contributed to different aspects of behavioral indices of memory retrieval on the task. Asymmetric, positive electrophysiological response across frontal scalp areas (i.e., LPC anterior lateralization) increased with greater encoding accuracy; groups showed a similar relationship between anterior response and encoding regardless of diagnosis or genetic liability for schizophrenia. The prefrontal cortex may be particularly important to selection of task relevant perceptual information during encoding (Shimamura, 2014). Lateralization of the prefrontal cortex is typical during encoding of verbal stimuli, but is less specifically studied with respect to memory. Right lateralization of brain activity, as observed here, has predicted recall of more complex visual stimuli while left lateralization is tied to memory for word stimuli (Golby et al., 2001; Habib, Nyberg, & Tulving, 2003; McDermott, Buckner, Petersen, Kelley, & Sanders, 1999; but see Guimond et al., 2017). In contrast, deeper processing in verbal episodic memory has been associated with both better recognition performance and left prefrontal lateralization during encoding (Ragland et al., 2005). The size estimation task is an experimental design that has been shown to instill deeper processing, whereby the participant considers semantic qualities of the words rather than favoring superficial (e.g., mnemonic) qualities of the stimuli (Bonner-Jackson et al., 2008). Given the association between lateralization and stimulus content, asymmetry during encoding and retrieval may be related to the way in which stimulus features were processed. It appears anterior lateralization may be deployed in distinct ways during encoding.
Occipital brain potentials during encoding were associated with free recall and the number of words recognized but not recalled (i.e., recognition hits minus recall). As stated in the methods, we utilized the metric “recognition without recall” in an effort to separate the processes that are unique to recognition and recall within the confines of the task design. That is, what brain processes are associated with the ability to accurately recognize a word after not being able to freely recall the same word? Thus, recognition findings in the current study refer to “recognition without recall.” With respect to N1, larger negative amplitude of the posterior brain response at encoding predicted better recall while a smaller encoding N1 amplitude predicted better recognition of words that had not previously been recalled. The prediction of recall generally conforms to the understanding of N1 marking discriminant attention to a sensory cue, which presumably facilitates explicit encoding. The association between better recognition without recall performance and smaller N1 amplitudes (i.e., less negative) suggests a disengagement of deliberate attentional discrimination processes during encoding of these words and reliance on an alternate process perhaps tied to implicit memory and familiarity. Familiarity is one of two episodic memory processes that has been isolated using an alternate experimental paradigm. Though the current paradigm prevents clean distinctions, recollection is involved in free recall and recognition while familiarity is exclusively involved in recognition (Libby et al., 2013). Likewise, the models derived for recognition and recall data in this study support a dual-process conceptualization of verbal memory, whereby recognition and recall occur via unique processes (Wilding & Ranganath, 2011). The alternative single process model proposes that recall occurs when a stronger memory trace is created than in recognition through superior engagement of a common cognitive process. We observed opposing relationships between neural responses during encoding and the two types of retrieval, recall and recognition without recall. In the case of a single process, we would have expected the same main effects in both regression analyses, with smaller beta coefficients in the recognition without recall model.
A notable finding is that the relationship between brain potentials and free recall performance was moderated by group membership. Unlike controls, larger early occipital scalp perturbation during encoding predicted worse recall for patients with schizophrenia. This suggests that deficits in verbal recall may be caused by disrupted attentional control processes during encoding of word stimuli that occur in the disorder (Vogel & Luck, 2000). In contrast, when retrieval was intact, such as in recognition without recall, larger N1 during encoding was consistently associated with performance scores across all participant groups. One possibility is that early posterior brain response in persons with schizophrenia may reflect an ineffective deployment of memory strategy, as has been shown by other investigations in schizophrenia (Guimond et al., 2017; Iddon, McKenna, Sahakian, & Robbins, 1998). Whereby controls engage a more diffuse neural network during encoding for words that are later freely recalled, patients (and, to a lesser degree, relatives) continue to rely primarily on attentional processes across all encoding trials; this may result in less robust consolidation that ineffectively supports free recall. Abnormal early sensory attention processes may represent genetic liability for schizophrenia, as the relationship with recall is moderated by group membership, with a graded difference between the three groups (Fig. 1). However, group differences are only significant between patients and controls, suggesting early sensory attentive processes are most disrupted with presence of illness rather than shared genetic liability.
The findings strengthen the argument that the observed neurophysiology abnormalities underlie episodic memory impairments central to schizophrenia. Furthermore, we propose that there could be tradeoff between discriminative attention and anterior LPC processes, wherein the elevated LPC observed over the right hemisphere in relatives serves as a compensatory mechanism to increase effective encoding and support later memory (Libby et al., 2013). This may signify a shift in encoding strategy that results in commensurate recognition but poorer recall. Patients, on the other hand are not employing occipital N1 or LPC anterior lateralization sufficiently to compensate to the degree of relatives. Lastly, our findings indicate that N400 and LPC parietal electrophysiological response during encoding are not strongly related to encoding or later retrieval. We had hypothesized that both would predict free recall.
The generalizability of the findings may be limited by the sample. The analyses directly tested effects of age and gender, which differed between the groups. Replication in matched groups, or a sample large enough to conduct targeted subgroup analyses would bolster the results. We did not statistically account for group differences in IQ, which we consider a strength of the study with respect to recruitment and statistical issues. There is a link between onset of schizophrenia and general intellectual ability whereby lower IQ is typical of patient samples, and has been identified as a premorbid risk factor (David, Malmberg, Brandt, Allebeck, & Lewis, 1997). Thus, matching samples on IQ may inadvertently recruit people at higher risk for mental illness. With respect to quantitative concerns, adding IQ as a covariate could bias the analysis by removing variance shared between diagnostic status and IQ (Miller & Chapman, 2001). Additionally, IQ is estimated from the short form WAIS, so it may be less reliable than scores derived from all core subtests. With respect to relatives, it should be noted that the small sample and broader inclusion criteria may limit the generalizability of the findings; relatives could potentially be more heterogeneous on characteristics such as psychiatric history than the rigorously screened patient and control groups.
Together, the findings suggest that earlier stages of encoding are disrupted, preventing later binding into episodic memory. This is represented in memory models such as Cortical Binding of Relational Activity (CoBRA), in which relevant perceptual input is selected by the PFC during encoding, features are bound into a memory representation by the MTL, and maintained by the ventral posterior parietal cortex (vPPC; Shimamura, 2014). The vPPC activates in later stages of encoding, when it is thought to be connecting memory features, much like a cortical level repetition of the MTL role. Activation of vPPC coincides with the timing and role of the parietal LPC (Shimamura, 2014). Functional imaging studies have shown a strong fronto-cortical contribution, interpreted as executive processes related to response selection, but have not had sufficient temporal resolution to differentiate the time course and overlap with MTL activation (Ragland et al., 2012; Ragland, 2004). Given that schizophrenia is characterized by abnormalities in temporal and frontal lobe networks, it has been difficult to separate the contributions of each (Landro et al., 2001). Our results downplay the importance of temporal regions during encoding to later memory of verbal stimuli. Instead, early posterior regions involved in discriminant attentional processes and frontal region disruptions in later periods of encoding may affect episodic memory.
The overall results can also be considered in terms of recollection and familiarity processes, which are relevant to verbal and non-verbal stimuli (Libby et al., 2013; Ragland et al., 2012). By this model, disruptions of the prefrontal cortex are thought to be involved primarily in familiarity judgments. A late, extended right frontal positivity has been seen during retrieval and relates to recognition performance (Yonelinas, 2002). The HERA model suggests that hemispheric dominance switches in encoding and retrieval, such that left prefrontal activation orchestrates episodic encoding while right prefrontal activation is stronger during episodic retrieval processes (Habib et al., 2003; Nyberg, Cabeza, & Tulving, 1996; Tulving et al., 1994), though a meta-analysis highlights the disproportionately greater role of the left versus right hemisphere in both encoding and retrieval processes (Spaniol, 2009). We did observe opposing ERP patterns associated with encoding and recognition without recall performance. However, in contrast to lateralization proposed by the HERA model, we found greater right anterior response during encoding. Lower right anterior response (i.e., less LPC anterior lateralization) was associated with higher recognition without recall scores, but did not reach statistical significance. Furthermore, relatives had large LPC anterior lateralization during encoding, combined with intact recognition without recall scores, and perturbed recall abilities. The present findings suggest that lateralization of activity of frontal brain regions at encoding influences later recollection of verbal material and that genetic liability for schizophrenia may affect the degree of lateralization. The generalizability of the findings can be extended by comparing results across a range of verbal and visual episodic memory tasks to isolate stimulus- and process specific neural perturbations (Collier et al., 2014; McDermott et al., 1999).
The current project identified brain responses at encoding that significantly predicted later memory impairment in individuals with schizophrenia. The findings bolster theories that neural processes during encoding contribute to verbal memory deficits in schizophrenia. Future research may clarify whether late positive components arise in people with schizophrenia and their first-degree biological relatives from an alternative engagement strategy (e.g. visualization vs. phonological) or heightened consolidation processes. Enhancing strategy engagement has been identified as a promising route for cognitive remediation targeting episodic memory in schizophrenia (Kurtz et al., 2017). The inclusion of unaffected relatives in the present study provided evidence that genetic liability for schizophrenia may modify the association between early posterior brain potentials and verbal memory. Continuing to identify features shared by patients with schizophrenia and their biological relatives will help clarify how risk for psychopathology is expressed in brain function and how cognitive deficits central to the illness might be remediated.
Funding
This work was supported by a Merit Review grant received by Dr. Scott Sponheim from the Veterans Health Administration Clinical Science Research and Development Program [ICX000227A]. No author involved in this manuscript has a conflict of interest.
Conflict of Interest
None declared.
Acknowledgements
We thank Dr. Kenneth Paller for consultation and provision of word material for task design. We also gratefully acknowledge John J Stanwyck, Sarah M. Sass, and Robb Hunter for collection of electroencephalographic data, as well as the support of the Minneapolis VA Medical Center Mental and Behavioral Health Service Line.
References
- Aleman A., Hijman R., de Haan E. H. F., & Kahn R. S. (1999). Memory impairment in schizophrenia: A meta-analysis. American Journal of Psychiatry, 156, 1358–1366. 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Aleman A., Kahn R. S., & Selten J.-P. (2003). Sex differences in the risk of schizophrenia. Archives of General Psychiatry, 60, 565–571. 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Allen J. (2002). The role of psychophysiology in clinical assessment: ERPs in the evaluation of memory. Psychophysiology, 39, 261–280. https://doi.org/10.1017.S0048577201393034. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders, Text Revision (DSM-IV-TR) (4th ed.).
- Andreasen N. C., Pressler M., Nopoulos P., Miller D., & Ho B.-C. (2010). Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biological Psychiatry, 67, 255–262. 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Jackson A., Yodkovik N., Csernansky J. G., & Barch D. M. (2008). Episodic memory in schizophrenia: The influence of strategy use on behavior and brain activation. Psychiatry Research: Neuroimaging, 164 (1), 1–15. 10.1016/j.pscychresns.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos H. B. M., Aleman A., Cahn W., Hulshoff Pol H., & Kahn R. S. (2007). Brain volumes in relatives of patients with schizophrenia: A meta-analysis. Archives of General Psychiatry, 64, 297–304. 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- Bridger E. K., Bader R., Kriukova O., Unger K., & Mecklinger A. (2012). The FN400 is functionally distinct from the N400. NeuroImage, 63, 1334–1342. 10.1016/j.neuroimage.2012.07.047. [DOI] [PubMed] [Google Scholar]
- Calcagno V., & Mazancourt C. de (2010). glmulti: an R package for easy automated model selection with (generalized) linear models. Journal of Statistical Software, 34 (12), 1–29. 10.18637/jss.v034.i12. [DOI] [Google Scholar]
- Calkins M. E., Curtis C. E., Grove W. M., & Iacono W. G. (2004). Multiple dimensions of schizotypy in first degree biological relatives of schizophrenia patients. Schizophrenia Bulletin, 30, 317–325. 10.1093/oxfordjournals.schbul.a007081. [DOI] [PubMed] [Google Scholar]
- Chalfonte B. L., & Johnson M. K. (1996). Feature memory and binding in young and older adults. Memory & Cognition, 24, 403–416. 10.3758/BF03200930. [DOI] [PubMed] [Google Scholar]
- Chatrian G. E., Tsai M. L., Temkin N. R., Holmes M. D., Pauri F., & Ojemann G. A. (1998). Role of the ECoG in tailored temporal lobe resection: the University of Washington experience. Electroencephalography and Clinical Neurophysiology. Supplement, 48, 24–43. http://www.ncbi.nlm.nih.gov/pubmed/9949773. [PubMed] [Google Scholar]
- Cirillo M. A., & Seidman L. J. (2003). Verbal declarative memory dysfunction in schizophrenia: From clinical assessment to genetics and brain mechanisms. Neuropsychology Review, 13, 43–77. 10.1023/A:1023870821631. [DOI] [PubMed] [Google Scholar]
- Collier A. K., Wolf D. H., Valdez J. N., Turetsky B. I., Elliott M. A., Gur R. E., et al. (2014). Comparison of auditory and visual oddball fMRI in schizophrenia. Schizophrenia Research, 158, 183–188. 10.1016/j.schres.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. S., Malmberg A., Brandt L., Allebeck P., & Lewis G. (1997). IQ and risk for schizophrenia: A population-based cohort study. Psychological Medicine, 27, 1311–1323. 10.1017/S0033291797005680. [DOI] [PubMed] [Google Scholar]
- Egan M., Goldberg T., & Gscheidle T. (2001). Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biological Psychiatry, 50, 98–107. 10.1016/S0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Fabiani M., Karis D., & Donchin E. (1990). Effects of mnemonic strategy manipulation in a Von Restorff paradigm. Electroencephalography and Clinical Neurophysiology, 75, 22–35. 10.1016/0013-4694(90)90149-E. [DOI] [PubMed] [Google Scholar]
- Glisky E., Polster M., & Routhieaux B. (1995). Double dissociation between item and source memory. Neuropsychology, 9, 229–235. 10.1037/0894-4105.9.2.229. [DOI] [Google Scholar]
- Golby A. J., Poldrack R. A., Brewer J. B., Spencer D., Desmond J. E., Aron A. P., et al. (2001). Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain, 124, 1841–1854. 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- Green M. (1996). What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry, 153, 321–330. 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Guimond S., Hawco C., & Lepage M. (2017). Prefrontal activity and impaired memory encoding strategies in schizophrenia. Journal of Psychiatric Research, 91, 64–73. 10.1016/j.jpsychires.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Habib R., Nyberg L., & Tulving E. (2003). Hemispheric asymmetries of memory: The HERA model revisited. Trends in Cognitive Sciences, 7, 241–245. 10.1016/S1364-6613(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Holm S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6, 65–70. [Google Scholar]
- Iddon J., McKenna P., Sahakian B., & Robbins T. (1998). Impaired generation and use of strategy in schizophrenia: Evidence from visuospatial and verbal tasks. Psychological Medicine, 28, 1049–1062. 10.1017/S0033291798006758. [DOI] [PubMed] [Google Scholar]
- Jeyakumar S. L. E., Warriner E. M., Raval V. V., & Ahmad S. A. (2004). Balancing the need for reliability and time efficiency: Short forms of the Wechsler Adult Intelligence Scale-III. Educational and Psychological Measurement, 64, 71–87. 10.1177/0013164403258407. [DOI] [Google Scholar]
- Kurtz M. M., Trask C. L., Rosengard R., Hyman S., Kremen L., Mehta S., et al. (2017). Verbal learning and memory enhancement strategies in schizophrenia: A randomized, controlled investigation. Journal of the International Neuropsychological Society, 23, 352–357. 10.1017/S1355617717000042. [DOI] [PubMed] [Google Scholar]
- Laes J. R., & Sponheim S. R. (2006). Does cognition predict community function only in schizophrenia? A study of schizophrenia patients, bipolar affective disorder patients, and community control subjects. Schizophrenia Research, 84, 121–131. 10.1016/j.schres.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Landro N. I., Pape-Ellefsen E., Hagland K. O., & Odland T. (2001). Memory deficits in young schizophrenics with normal general intellectual function. Scandinavian Journal of Psychology, 42, 459–465. 10.1111/1467-9450.00259. [DOI] [PubMed] [Google Scholar]
- Leckman J. F. (1982). Best estimate of lifetime psychiatric diagnosis. Archives of General Psychiatry, 39, 879–883. 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Libby L. A., Yonelinas A. P., Ranganath C., & Ragland J. D. (2013). Recollection and familiarity in schizophrenia: A quantitative review. Biological Psychiatry, 73, 944–950. 10.1016/j.biopsych.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoff D., Liberman R., & Nuechterlein K. (1986). Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophrenia Bulletin, 12, 578–603. 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- Mangun G. R. (1995). Neural mechanisms of visual selective attention. Psychophysiology, 32, 4–18. 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- McDermott K. B., Buckner R. L., Petersen S. E., Kelley W. M., & Sanders A. L. (1999). Set-and code-specific activation in the frontal cortex: An fMRI study of encoding and retrieval of faces and words. Journal of Cognitive Neuroscience, 11, 631–640. 10.1162/089892999563698. [DOI] [PubMed] [Google Scholar]
- Miller G. A., & Chapman J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48. 10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz M., & O’Donnell B. (1997). ERP assessment of visual and auditory language processing in schizophrenia. Journal of Abnormal Psychology, 106, 85–94. 10.1037/0021-843X.106.1.85. [DOI] [PubMed] [Google Scholar]
- Nunez P. L., & Srinivasan R. (2006). Electric fields of the brain: The neurophysics of EEG, (Vol. 4). Oxford University Press; 10.1093/acprof:oso/9780195050387.001.0001. [DOI] [Google Scholar]
- Nurnberger J. I., Blehar M. C., Kaufmann C. A., York-Cooler C., Simpson S. G., Harkavy-Friedman J., et al. (1994). Diagnostic interview for genetic studies: Rationale, unique features, and training. Archives of General Psychiatry, 51, 849–859. 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Nyberg L., Cabeza R., & Tulving E. (1996). PET studies of encoding and retrieval: The HERA model. Psychonomic Bulletin & Review, 3, 135–148. 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- O’Donnell B. F., Salisbury D. F., Niznikiewicz M. A., Brenner C. A., & Vohs J. L. (2011). Abnormalities of event-related potential components in schizophrenia. The Oxford Handbook of Event-Related Potential Components. 10.1093/oxfordhb/9780195374148.013.0187. [DOI]
- Paller K. A., Kutas M., & McIsaac H. K. (1995). Monitoring conscious recollection via the electrical activity of the brain. Psychological Science, 6(2), 107–111. 10.1111/j.1467-9280.1995.tb00315.x. [DOI] [Google Scholar]
- Paller K. A., McCarthy G., & Wood C. C. (1988). ERPs predictive of subsequent recall and recognition performance. Biological Psychology, 26, 269–276. 10.1016/0301-0511(88)90023-3. [DOI] [PubMed] [Google Scholar]
- Paller K. A., & Wagner A. D. (2002). Observing the transformation of experience into memory. Trends in Cognitive Sciences, 6, 93–102. 10.1016/S1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Ragland J. D. (2004). Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. American Journal of Psychiatry, 161, 1004–1015. 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J. D., Gur R. C., Valdez J. N., Loughead J., Elliott M., Kohler C., et al. (2005). Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. American Journal of Psychiatry, 162, 1840–1848. 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J. D., Ranganath C., Barch D. M., Gold J. M., Haley B., MacDonald A. W., et al. (2012). Relational and Item-Specific Encoding (RISE): Task development and psychometric characteristics. Schizophrenia Bulletin, 38, 114–124. 10.1093/schbul/sbr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Minzenberg M. J., & Ragland J. D. (2008). The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biological Psychiatry, 64, 18–25. 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch H. V., Anderer P., Schuster P., & Presslich O. (1986). A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology, 23, 695–703. 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shimamura A. (2014). Remembering the past neural substrates underlying episodic encoding and retrieval. Current Directions in Psychological Science, 23, 257–263. 10.1177/0963721414536181. [DOI] [Google Scholar]
- Sitnikova T., Perrone C., Goff D., & Kuperberg G. (2010). Neurocognitive mechanisms of conceptual processing in healthy adults and patients with schizophrenia. International Journal of Psychophysiology, 75, 86–99. 10.1016/j.ijpsycho.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J., Davidson P. S. R., Kim A. S. N., Han H., Moscovitch M., & Grady C. L. (2009). Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia, 47(8–9), 1765–1779. 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Sponheim S. R., McGuire K. A., & Stanwyck J. J. (2006). Neural anomalies during sustained attention in first-degree biological relatives of schizophrenia patients. Biological Psychiatry, 60, 242–252. 10.1016/j.biopsych.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Sponheim S. R., Steele V. R., & McGuire K. A. (2004). Verbal memory processes in schizophrenia patients and biological relatives of schizophrenia patients: intact implicit memory, impaired explicit recollection. Schizophrenia Research, 71, 339–348. 10.1016/j.schres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Sprague R. L., & Kalachnik J. E. (1991). Reliability, validity, and a total score cutoff for the Dyskinesia Identification System: Condensed User Scale (DISCUS) with mentally ill and mentally retarded populations. Psychopharmacology Bulletin, 27, 51–58. [PubMed] [Google Scholar]
- Tellegen A., & Briggs P. F. (1967). Old wine in new skins: Grouping Wechsler subtests into new scales. Journal of Consulting Psychology, 31, 499–506. 10.1037/h0024963. [DOI] [PubMed] [Google Scholar]
- Tulving E., Kapur S., Craik F., Moscovitch M., & Houle S. (1994). Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proceedings of the National Academy of Sciences, 91, 2016–2020. 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel E. K., & Luck S. J. (2000). The visual N1 component as an index of a discrimination process. Psychophysiology, 37, 190–203. 10.1111/1469-8986.3720190. [DOI] [PubMed] [Google Scholar]
- Voss J. L., & Paller K. A. (2009. a). An electrophysiological signature of unconscious recognition memory. Nature Neuroscience, 12, 349–355. 10.1038/nn.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J. L., & Paller K. A. (2009. b). Remembering and knowing: Electrophysiological distinctions at encoding but not retrieval. NeuroImage, 46, 280–289. 10.1016/j.neuroimage.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1997). Wechsler Adult Intelligence Scale – Third Edition (WAIS–III). Psychological Corporation.
- Wilding E., & Ranganath C. (2011). Electrophysiological correlates of episodic memory processes. The Oxford Handbook of Event-Related Potential Components. 10.1093/oxfordhb/9780195374148.013.0187. [DOI]
- Yonelinas A. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46, 441–517. 10.1006/jmla.2002.2864. [DOI] [Google Scholar]