Abstract
Physiological and molecular processes initiated during implantation for pregnancy success are complex but highly organized. This review primarily highlights adverse ripple effects arising from defects during the peri-implantation period that perpetuate throughout pregnancy. These defects are reflected in aberrations in embryo spacing, decidualization, placentation and intrauterine embryonic growth, manifesting in preeclampsia, miscarriages and/or preterm birth. Understanding molecular signaling networks that coordinate strategies for successful implantation and decidualization may lead to approaches to improve the outcome of natural pregnancy and pregnancy conceived from in vitro fertilization.
Pregnancy is a complex, irreversible process that comprises discrete events including implantation, decidualization, placentation and finally birth of offspring through the process of parturition1–3. The success of each event is essential to advance toward the next stage. However, hierarchical directives that orchestrate the embryo-uterine cross-talk are not well understood. These events are primarily coordinated by ovarian estrogen and progesterone (P4)1 (Box 1), but the molecular dialogue that originates locally from the mother, embryo or both governing the orderly chronological transitions between these events is not fully appreciated.
Box 1. Uterine responses to ovarian hormones in implantation.
Ovarian estrogen and P4 regulate different phases of pregnancy by coordinating uterine cell–specific effects. These hormones bind their respective nuclear receptors and interact with specific co-chaperones and co-regulators for optimal function. P4 receptors (PR-A and PR-B) and estrogen receptors (ERα and ERβ) are expressed in the uterus. For uterine receptivity and implantation, ERα (Esr1) is the primary driver of estrogen action, as Esr1−/− mouse uteri are hypoplastic and infertile147. Mice missing both PR-A and PR-B isoforms (Pgr−/−) are also infertile with ovarian and uterine defects148, but PR-A is the key isoform for implantation, as mice lacking only PR-B have apparently normal fertility149.
In pregnant mice, uterine cell types differentially respond to changing estrogen and P4 levels. On day 1 (vaginal plug), uterine epithelial cells undergo proliferation under the influence of preovulatory estrogen and then submit to apoptosis on day 2 with declining ovarian hormone levels. When rising P4 secretion from the newly formed corpora lutea (CL) is superimposed with preimplantation ovarian estrogen secretion on day 4 (day of receptivity), stromal cells show extensive proliferation with epithelial cell differentiation and glandular secretion. P4 is an absolute requirement for implantation in all species studied. Interestingly, ovarian estrogen is not crucial for implantation in many species, including hamsters, guinea pigs, rabbits and pigs, in which embryonic estrogen is considered to have a role1. Although a rise in estrogen levels is seen prior to the receptive phase2,150, whether this mid-luteal estrogen is necessary for implantation in humans or subhuman primates remains unknown151–153; however, the evidence favors estrogen requirement in primate receptivity.
In mice, ovariectomy on day 4 morning before preimplantation estrogen secretion induces delayed implantation and embryonic diapause, a state of suspended animation. This condition can be maintained for many days with continued P4 treatment (neutral phase), but an estrogen injection can induce implantation 24–48 h after P4 priming. Molecular mechanisms that permit the uterus and blastocyst to undergo temporary quiescence while maintaining implantation competency, and resuming implantation by estrogen, remain understudied154,155. Delayed implantation models have provided a wealth of information regarding hormonal regulation of uterine gene expression during implantation. Evidence shows that autophagy, a catabolic process by which a cell’s own components are recycled under restricted nutritional supply, is activated to maintain viability of dormant blastocysts during delay in mice but is downregulated with resumption of implantation156. Whether embryonic autophagy is conserved in other diapausing mammals is not known157,158. Findings of persistent uterine Msx1 expression during delay and loss of uterine competence to implantation in delayed Msx1d/d uteri9 suggest a role for Msx in uterine quiescence.
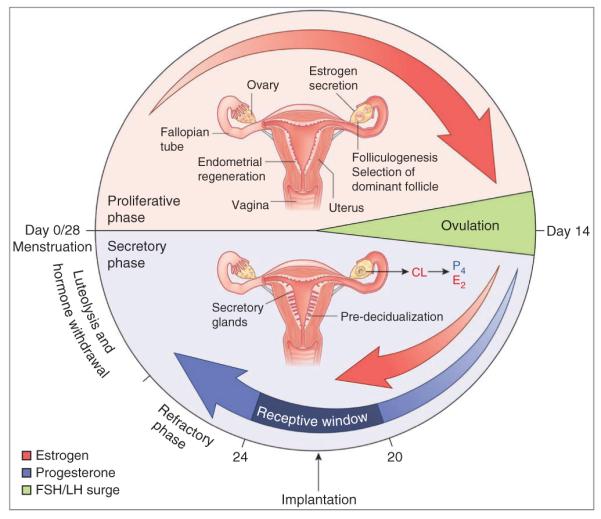
In humans, the 28- to 30-d menstrual cycle begins with menses (see illustration). The proliferative (follicular) phase is under the influence of rising estrogen levels (red arrows) from growing ovarian follicles, leading to proliferation of the epithelium, stroma and vascular endothelium to regenerate the endometrium. Numerous developing glands assume tortuous morphology during the late proliferative phase. Rising pituitary gonadotropin levels during this phase lead to folliculogenesis and selection of a dominant follicle. At midcycle, there is a surge of gonadotropins, namely follicle-stimulating hormone (FSH) and luteinizing hormone (LH), leading to ovulation on day 14 (green). The early secretory (luteal) phase is marked by thickening of the endometrium and the formation of the corpus luteum (CL) from the ruptured follicle and subsequent P4 secretion (blue arrow) in preparation for implantation. Glands become secretory with stromal cell differentiation (predecidualization) accompanied with endometrial edema in preparation for implantation159. Rising estrogen (E2) levels superimposed on P4 define the window of receptivity (dark blue) during the midluteal phase (cycle days 20–24), conducive to implantation and subsequent pregnancy. In the absence of a viable embryo, the receptive window spontaneously transits to a refractory phase, leading to luteolysis, hormone withdrawal and menstruation, thus resetting the cycle (cycle day 0/28). Conversely, an implanting blastocyst secretes chorionic gonadotropin to maintain the CL, thereby supporting pregnancy.
The window of implantation is a limited time span when blastocyst competency is superimposed on the receptive state of the uterus. If this coordination is out of phase, implantation fails or becomes defective. In humans, natural conception per cycle is poor (~30%), and 75% of failed pregnancies are considered to be due to implantation failure4. Evidence from mouse models suggests that defective implantation can generate adverse ripple effects during the course of pregnancy, leading to poor outcome. The window of receptivity is also transient in humans, and implantation beyond this window results in spontaneous miscarriages5, although a considerable number of miscarriages result from embryonic abnormalities, including aneuploidy6. Successful implantation involves close interactions between the uterus and blastocyst, but ethical restrictions and the lack of mechanistic studies have delayed and even precluded studies on embryo-uterine interactions in humans. Thus, knowledge obtained from transgenic mouse models has helped to better understand the molecular basis of uterine receptivity, implantation and decidualization in humans (Figs. 1 and 2).
Figure 1.
Signaling network for uterine receptivity and implantation. This is a hybrid cartoon, based on mouse and human studies, portraying compartment- and cell-type– specific expression of molecules and their potential functions necessary for uterine receptivity, implantation and decidualization. Interplay of ovarian P4- and/or E2-dependent and P4- and/or E2-independent factors in the pregnant uterus in specific compartments contributes to the success of implantation in a juxtacrine/paracrine/autocrine manner. During attachment, interactions between the blastocyst and luminal epithelium (LE) involve ErbB1/4 (blue) and both transmembrane (TM) and soluble (Sol) forms of HB-EGF, as well as L-selectin ligands (sLE) expressed by the luminal epithelium to L-selectin receptors on the blastocyst. The other key signaling pathways for uterine receptivity and implantation are also shown. AA, arachidonic acid; BMP2, bone morphogenetic protein 2; cPLA2α, cytosolic phospholipase A2α; COUP-TFII, chicken ovalbumin upstream promoter transcription factor-2; Cox2, cyclooxygenase-2; E, estrogens; EC, epithelial cell (luminal and glandular epithelia); ENaC, epithelium sodium channel; ER, estrogen receptor; ErbB1/4; epidermal growth factor receptor 1/4; ERK, extracellular signal–regulated kinase; FGF, fibroblast growth factor; GE, glandular epithelium; gp130, glycoprotein 130; Hand2, heart- and neural crest derivatives-expressed protein 2; HB-EGF, heparin-binding epidermal growth factor-like growth factor; Hoxa10/11, homeobox A10/11; ICM, inner cell mass; IHH, Indian hedgehog; KLF5, Kruppel-like factor 5; LIF, leukemia inhibitory factor; LIFR, LIF receptor; LPA3, lysophosphatidic acid receptor 3; MSX1, Muscle segment homeobox 1; P4, progesterone; PG, prostaglandin; PPAR-δ; peroxisome proliferators–activating receptor δ; PR, progesterone receptor; Ptc, Patched; RXR, retinoid X receptor; SC, stromal cell; SGK1, serum- and glucocorticoid-inducible kinase 1; Smo, Smoothened; STAT3, signal transducer and activator of transcription 3; Tr, trophectoderm; Wnt4/5a, Wingless-Type MMTV integration site family members 4/5a. Compartment colors: blue, stroma; pink, luminal epithelium; orange, glandular epithelium; purple, epithelium at the attachment site.
Figure 2.
Signaling networks in decidualization. Key molecules for decidualization are depicted in this hybrid cartoon as gathered from mouse and human studies. The decidua is composed of differentiated stromal (decidual) cells, many of which are terminally differentiated (polyploid). Decidualization involves coordination of several processes, including polyploidy, and several types of molecules, such as cytokine receptors, enzymes, morphogens, hormones and transcription factors. ADM, adrenomedullin; BV, blood vessel; DEDD, death effector domain– containing protein; IL-11Rα, interleukin 11 receptor α; mTORC1, mammalian target of rapamycin complex 1; SGK1, serum- and glucocorticoid-inducible kinase 1; Sphk1/2, sphingosine kinase 1/2.
The concept of uterine receptivity was first conceived by Alexandare Psychoyos. Blastocysts implant only when transferred into receptive uteri7 (Box 2). Reciprocal embryo transfer experiments in delayed implanting mouse models later showed that blastocyst activation is also a critical determinant for implantation in the receptive uterus8. Acquisition of receptivity approaching blastocyst attachment is reflected in cellular and ultrastructural changes: gradual loss of uterine epithelial cell polarity and formation of microprotrusions on the apical surface called pinopodes or uterodomes9,10. Spatiotemporal uterine RNA localization identified various genes, but their definitive roles in implantation remained speculative1,2. Transgenic mouse models moved the field toward mechanistic understanding of roles of many genes in uterine receptivity and implantation. However, the functions of many candidate genes remained uncertain because their constitutive deletion led to embryonic lethality or other systemic deficiencies. Generation of conditionally deleted mouse models advanced the field further by identifying genes crucial for implantation and decidualization. Even so, dynamic and overlapping expression patterns make it difficult to define their stage-specific roles. New mouse models that have allowed for the discovery of new regulators, that mimic aspects of human pregnancy events and that provide conceptual advances in understanding of pregnancy have come to light and are presented here.
Box 2. Dynamics of implantation.
The first physical and physiological contact between an implantation-competent blastocyst and the receptive uterus through the processes of apposition, adhesion, attachment and penetration steers successful implantation1. Implantation in mice involves homing of the blastocyst within a crypt (nidus) formed by the invagination of the luminal epithelium. Progression of implantation occurs by displacing the luminal epithelium from the basal lamina, enhancing trophoblast passage into the stroma. In humans, blastocysts are embedded within the subepithelial stroma and the process is intrusive; trophoblast cells penetrate through the luminal epithelium and basal lamina into the stroma160. One common feature between humans and mice is that both have hemochorial placentation in which trophoblasts are in direct contact with maternal blood.
In many mammals, blastocyst attachment is coincident with increased endometrial vascular permeability at the site of its apposition1. In mice, it occurs on day 4 night and can be visualized by an intravenous injection of a blue dye solution; implantation sites are demarcated by distinct blue bands7,8. In this species, blastocysts implant with their inner cell mass (ICM) oriented toward the lumen, whereas in humans, blastocysts are oriented with ICMs toward the epithelium3. Although the role of different implantation strategies adapted by different species is not clearly understood, one common feature in many animals is an increased endometrial vascular permeability at the site of blastocyst attachment. It is presumed that this also occurs in the endometrial bed during human implantation, but this has not yet been reported.
Blastocyst attachment with the luminal epithelium is followed by decidualization in the stromal bed. A requirement for a functional luminal epithelium for appropriate decidualization49 suggests that signals transmitted by the epithelium to the stroma are involved6,57. This is supported by the observation of defective decidualization in conditionally deleted mice with impaired epithelial function9,46. Ptgs2 is normally expressed in both the epithelium and stroma surrounding the implanting blastocyst63. Whereas Ptgs2 expression is restricted to the luminal epithelium in Lif−/− or Msx1d/d; Msx2d/d females9,13, its localization is limited to the stroma in Klf5d/d females46, suggesting aberrant communication between the luminal epithelium and stroma. In conclusion, a functional circuitry involving the blastocyst, luminal epithelium and stroma seems necessary for appropriate decidualization, but the identity of the blastocyst- and/or epithelial-derived signals remains to be determined.
The orderly discourse between the uterus and blastocyst, which is key to implantation, portrays many characteristics of epithelial-mesenchymal communications during embryogenesis, engaging evolutionarily conserved pathways. However, almost nothing is known regarding the interactions between the myometrium and stroma in preparation for implantation. As myometrial activity contributes to embryo spacing, it would be interesting to see whether the myometrium or myometrial-stromal interactions have a role in implantation and whether attachment signaling is coordinated with myometrial activity for appropriate embryo spacing. Myometrial- or stromal-specific gene deletion studies may prove rewarding.
Molecular marks for uterine receptivity
In mice, uterine sensitivity to implantation is divided into three principal phases: prereceptive (days 1–3), receptive (day 4) and nonreceptive (refractory; day 5 onward)1,2. On-time implantation occurs only during the receptive period. Embryos cannot implant during the prereceptive (preparatory) phase, and uterine transition into the receptive phase requires priming with P4 superimposed with estrogen7,11. Upon closure of the receptive window, the uterus spontaneously transits to the refractory phase, when the uterine milieu is hostile to blastocyst survival. Progression through these phases is unidirectional, and resetting the cycle from the refractory phase requires P4 withdrawal1. A similar sequence of events occurs in humans, although the duration of the menstrual cycle is longer (~28–30 d) than the estrous cycle in mice (~4 d)2. In humans, the prereceptive phase spans the first 7 d after ovulation (early luteal phase), and endometrial receptivity is achieved in the mid-luteal phase (~7–10 d after ovulation). The uterus then proceeds to the nonreceptive phase for the remainder of the cycle (late luteal phase), until menstruation ensues (Box 1)2,12.
The uterus is composed of three major tissue compartments: epithelium, stroma and myometrium. The individual or collective contribution of these cell types to uterine receptivity is poorly understood. As the attachment reaction rivets the blastocyst trophectoderm to the luminal epithelium, luminal epithelium is perceived as a major mediator of uterine receptivity, transmitting signals to other compartments. Conversely, it has long been held that the stroma directs luminal epithelium functions, suggesting its role in uterine receptivity, although stromal gene expression is altered in the absence of specific epithelial genes9,13. In this regard, mouse models with compartment-specific gene deletion became informative.
Uterine responsiveness to estrogen and P4 signaling
P4 and estrogen are master regulators of pregnancy success in both mice and humans (Box 1). The mechanism by which these hormones coordinate uterine functions has mostly been derived from mouse studies2. As estrogen receptor α (ERα) and PR-A are expressed in all major uterine tissue compartments, their compartment-specific contributions in uterine receptivity are difficult to ascertain. However, this has been addressed by generating mice with epithelial-specific deletion of Esr1 (ERα)14. Upon estrogenic stimulation, epithelial proliferation still occurred in Esr1−/− epithelium, and PR distribution between the compartments remained unaltered, insinuating a role for stromal ERα. These mice showed aberrant expression of estrogen-responsive genes and implantation failure, perhaps owing to uterine inability to achieve receptivity. In contrast, P4 failed to inhibit estrogen-induced proliferation of epithelial cells deleted of Pgr (PR), suggesting its direct role in suppressing epithelial estrogen action15. The infertility in these females was attributed to poor uterine receptivity with reduced expression of Indian hedgehog (Ihh) and leukemia inhibitory factor (Lif), crucial elements for implantation. This study showed direct binding of epithelial PR to the Ihh promoter; the results do not corroborate with those of recombination studies showing stromal PR regulating epithelial Ihh expression16. Sustained epithelial proliferation by estrogen suggests that loss of epithelial Pgr led to unopposed epithelial estrogen action, although the cause of downregulation of estrogen-responsive gene Lif in Pgr−/− glands remains unknown. Cell-specific localization of estrogen- and P4-responsive genes in these compartment-specific deletion mice would be more informative. Nevertheless, the results show that a bidirectional communication between the epithelium and stroma is required for normal uterine receptivity and implantation.
Estrogen and P4 coordinate uterine functions via multiple paracrine, juxtacrine and autocrine factors in a spatiotemporal manner. One major mediator of estrogen action is LIF, a member of the interleukin-6 family of cytokines. LIF is essential for uterine receptivity and implantation, as its deletion imposes implantation failure in mice13,17. LIF binds its receptor LIFR, which partners with co-receptor gp130 to activate the downstream signaling via signal transducer and activator of transcription 3 (ref. 18). This signaling is crucial because uterine deletion of the gene encoding gp130 (Il6st)9 or Stat3 (A. Bartos, X.S., T. Daikoku, J.C. and S.K.D., unpublished data) also causes implantation failure. Endometrial Lif expression is higher around the time of implantation in fertile women as opposed to lower levels in infertile women19–21. Whether LIF is essential for uterine receptivity and implantation in humans remains inconclusive. A clinical trial to improve pregnancy success by LIF administration in a relatively small cohort of hyperstimulated women with multiple etiologies of infertility did not improve pregnancy outcome22. Long-term systemic LIF delivery might not have been appropriate considering transient uterine Lif expression during receptivity in mice, and this study did not assess local LIF levels or signaling in these subjects after LIF administration.
Mice with constitutive deletion of tumor suppressor protein p53 (Trp53−/−) have been shown to have small litters with poor implantation and reduced Lif expression23. Trp53−/− mice develop wide-spread cancer at early reproductive age, and males have compromised spermatogenesis24; thus, they may not be ideal for studying reproduction. In contrast, mice with uterine deletion of Trp53 (Trp53d/d) show normal implantation but have preterm birth24 (see below). These two studies cannot be directly compared because of the different approaches (systemic versus conditional deletion) used. Contradictory reports associating Trp53 polymorphisms with human fertility suggest the need for further investigation25,26.
P4 is considered the ‘hormone of pregnancy’, and many P4-induced genes in the uterus participate in peri-implantation events. FKBP52, a P4-inducible co-chaperone, is required for optimizing PR activity. Fkbp52−/− females are infertile with impaired uterine P4 responsiveness and enhanced estrogen-like signaling27,28. Depending on the genetic background, P4 resistance is overcome with implantation and pregnancy success by excess P4 (ref. 29). In addition, Fkbp52−/− mice are more susceptible to oxidative stress due to reduced expression of a unique antioxidant peroxiredoxin 6 (PRDX6)30. If exposed to oxidative stress, Fkbp52−/− mice show implantation failure despite P4 supplementation, but this failure is reversed if the mice are supplemented with antioxidants. These results imply that FKBP52 has additional roles in uterine biology. Recent evidence also suggests FKBP52’s role in decidualization and endometriosis in mice and humans31,32. In the same vein, steroid receptor co-activator 2 (SRC-2, Ncoa2) is specifically recruited by PR for its optimal function33. Indeed, uterine deletion of Ncoa2 leads to peri-implantation failure due to defective P4 function34. SRC-2 expression in human endometria also suggests its role in optimizing P4 function35.
P4 also induces Ihh in the uterus15,36,37, and its uterine deletion leads to implantation failure due to poor uterine receptivity37. Ihh is primarily expressed in the epithelium and interacts with its receptors Patched and Smoothened in the stroma, mediating stromal cell proliferation36. These results suggest that IHH acts as a paracrine signal for epithelial-stromal interaction for achieving uterine receptivity and implantation. Notably, upregulated expression of Ihh and its receptors in human endometria by progestins implicates its role in human implantation38. Chicken ovalbumin upstream promoter-transcription factor (COUP-TFII, Nr2f2) is a proposed downstream target of IHH signaling and is expressed in the subepithelial stroma39. Its deletion in PR-expressing tissues causes infertility due to implantation failure with exaggerated epithelial estrogenic manifestation, suggesting that COUP-TFII balances ER versus PR activities required for uterine receptivity. Paradoxically, Nr2f2 deletion in the stroma and myometrium showed normal implantation, but placentation was defective. Inefficient Nr2f2 deletion in the stroma was suggested as a cause for this discrepancy40.
Hand2, a P4-induced transcription factor in the stroma, has been reported to be crucial in uterine receptivity and implantation in mice41 and is also implicated in decidualization42. Mice deficient in uterine Hand2 show high estrogenic activity and epithelial cell proliferation via upregulation of fibroblast grown factor–extracellular signal–regulated kinase (FGF-ERK) signaling41, suggesting that stromal Hand2 participates in uterine receptivity by downregulating epithelial differentiation. It would be interesting to determine whether the infertility phenotype in mice with uterine deletion of Hand2 is rescued by excess P4 or inhibitors of FGF-ERK signaling.
Reduced LIF expression is implicated as a contributor to implantation failure in several gene-deleted mouse models. However, this interpretation should be taken with caution because downregulation of Lif could be a consequence of defective uterine receptivity or implantation failure resulting from such deletion.
Uterine responsiveness to factors not induced by ovarian hormones
Although many genes necessary for uterine receptivity and implantation are induced and regulated by estrogen, P4 or both, transcription factors have recently been identified whose expressions are not greatly altered by these hormones but that profoundly influence receptivity and implantation in mice. One such gene is Msx1, encoding an ancient evolutionarily conserved homeobox transcription factor9. Msx1 was shown to be absent in the pregnant mouse uterus43, but later studies found its distinct, transient expression in the epithelium around the time of receptivity, with peak expression on day 4 morning44. The expression declined approaching the time of blastocyst attachment and remained undetected thereafter. These observations suggested Msx1’s role in uterine receptivity9. Expression of Msx2, another member in the family, is low to undetectable in the uterus during this time. However, Msx2 is upregulated in a similar fashion as Msx1 in Msx1-depleted uteri, suggesting its compensatory role. Uterine deletion of Msx genes showed graded levels of compromised fertility depending on single or double deletion9; mice deleted of uterine Msx1 (Msx1d/d) produced small litters or no litters, whereas deletion of both Msx1 and Msx2 (Msx1d/d;Msx2d/d) resulted in complete infertility due to failed or defective implantation with loss of stromal bone morphogenetic protein 2 (Bmp2) expression and cyclooxygenase-2 (Ptgs2) expression restricted to the epithelium. Interestingly, mice with uterine deletion of Msx2 have normal fertility9. The infertility phenotype in Msx1d/d; Msx2d/d mice as reported earlier was later confirmed by another group45. The role of Msx1 and Msx2 is implicated in human implantation since they are downregulated in the endometrium during the window of receptivity, similar to the situation in mice2,9.
With approaching blastocyst attachment, the luminal epithelium transits from a higher to lower state of polarity9. This transition was not evident in Msx1d/d;Msx2d/d uteri at the anticipated time of implantation. Wnt5a, a traditionally noncanonical Wnt and mediator of cell polarity, was upregulated in the epithelium and stroma in Msx1d/d and Msx1d/d;Msx2d/d mice. Wnt5a–β-catenin/E-cadherin signaling was identified as a potential downstream target of Msx to influence implantation by altering cell polarity9.
Msx1 expression persists in P4-primed delayed implanting uteri with blastocyst dormancy but is rapidly downregulated with initiation of implantation by estrogen or LIF (Box 2), which suggests an indirect effect of estrogen via LIF induction. Implantation largely fails in P4-primed delayed implanting Msx-deleted uteri after estrogen treatment, which suggests that Msx is required to maintain uterine readiness to implantation. Moreover, uterine Lif expression is downregulated in these mice, but LIF administration fails to rescue implantation, which suggests complex and nonlinear relationship between LIF and Msx. How Msx expression is so tightly regulated in the receptive uterus is yet to be explored.
Another gene necessary for implantation is that encoding Kruppel-like factor 5 (Klf5), a zinc finger–containing transcription factor. Uterine Klf5 is also unresponsive to alteration by ovarian hormones46, although it is influenced by estrogen and P4 in human breast cancer cells47. In mouse uteri, KLF5 is present in luminal and glandular epithelia until decidualization is initiated on day 5. At this time, KLF5 is expressed in proliferating stromal cells around the implantation chamber with downregulation in the epithelium. With differentiation, decidual cell expression decreases, suggesting KLF5’s participation in cell-specific proliferation and differentiation during pregnancy. Systemic deletion of Klf5 confers embryonic arrest at the blastocyst stage48. In contrast, mice with uterine deletion of Klf5 (Klf5d/d) are essentially infertile as a result of defective implantation46. Blastocysts remain entrapped within the uterine lumen beyond the anticipated time of implantation, as is evident from intact luminal epithelium. Decidualization was still initiated, albeit at a reduced extent, with Hoxa10 and Bmp2 expression being crucial for decidualization. The partial decidual response was not sustained and embryos disintegrated46. These findings corroborate with an earlier study that a functional luminal epithelium is necessary for decidual response49. Collectively, the results suggest that signals originating from a blastocyst and transmitted through the epithelium can initiate decidualization without direct contact of the blastocyst with the stroma. Nonetheless, a functional circuitry involving the blastocyst, luminal epithelium and stroma is essential for normal implantation and decidualization. Identification of such genes that regulate receptivity but are relatively unaffected by ovarian hormones may help improve in vitro fertilization (IVF)-conceived pregnancy rates or alternatively help develop nonsteroidal contraceptives.
Molecular discourse guiding attachment reaction
A two-way communication between the blastocyst and receptive uterus leads to attachment reaction and implantation. Preparation of the uterus to realize receptivity and blastocyst attachment to the luminal epithelium are endowed with distinct and overlapping gene expression. Therefore, it is difficult to appreciate the molecular dialog between these two entities during this progression.
Heparin-binding epidermal growth factor–like growth factor (HB-EGF) stands out to be an important molecular link in mediating embryo-uterine interactions with impending attachment reaction in mice. It is expressed in the luminal epithelium surrounding each blastocyst several hours before the attachment reaction50. HB-EGF is produced in soluble and transmembrane forms, and both forms influence blastocyst function in a paracrine and juxtacrine manner via the EGF family of receptors expressed on the blastocyst cell surface51,52, placing HB-EGF as a unique molecule for blastocystluminal epithelium adhesion (Fig. 1). Implantation-competent blastocysts also express HB-EGF, which can induce its own gene (Hegf1) in the uterus in a paracrine manner53,54. This auto-induction loop is the first known molecular link between the blastocyst and uterus that leads to attachment reaction. Although systemic deletion of Hegf1 causes perinatal lethality55, its uterine deletion defers implantation beyond the normal window, producing reduced litter size; partial fertility restoration is apparently due to compensation by amphiregulin, another HB-EGF–like growth factor56. Studies in humans later found that HB-EGF expression is maximal in the receptive epithelium and is concurrent with pinopodes57, and cells expressing the transmembrane form of HB-EGF can adhere to blastocysts expressing cell surface ErbB4 (refs. 58,59). Adhesion molecules such as integrins, L-selectin ligands and selectin oligosaccharides are also implicated in blastocyst attachment and placentation in humans60–62. One approach to study certain aspects of blastocyst attachment is to transfer inert blastocyst-size beads preloaded with factors as shown previously for HB-EGF and L-selectins54,60.
As Ptgs2 and Bmp2 are expressed in both the epithelium and/or underlying stroma at the site of attachment, they are also considered essential for implantation and decidualization; deletion of either gene confers infertility63,64. Owing to their overlapping expression beyond implantation, their roles in decidualization are detailed below.
Decidualization as a sequel to blastocyst-uterine attachment
Normally, the blastocyst is the stimulus for decidualization in mice. Stromal cells surrounding the implanting blastocyst undergo extensive proliferation and differentiation into specialized cell types called decidual cells (decidualization). In humans, the initiation of this process (predecidualization) does not require the presence of a blastocyst but becomes more robust with implantation. The importance of pre-decidualization is perhaps to prepare the endometrium for implantation and seems analogous to extensive stromal cell proliferation with expression of decidual marker genes before implantation in mice.
Decidualization is a complex interplay of transcription factors, morphogens, cytokines, cell cycle regulators and signaling pathways. In mice, the abdominalB-like Hox genes Hoxa10 and Hoxa11 are expressed in the stroma at receptivity and become more intense upon decidualization65–67. They are essential for decidualization, and its failure in Hoxa10−/− mice is due to reduced stromal cell proliferation to P4, although attachment reaction often occurs65,66. Hoxa11−/− mice have a similar but more robust infertility phenotype67. Defective decidualization in Hoxa10−/− mice is coincident with downregulation of a cell cycle regulatory axis involving cyclinD3, cdk4/6 and p21 (refs. 66,68). In women, both Hoxa10 and Hoxa11 are upregulated in the receptive endometrium, suggesting their roles in decidualization69,70. In addition, decidualization induced by P4, estrogen and dibutyryl cAMP in human cell culture systems is associated with expression of prolactin and insulin-like growth factor-binding protein 1 as decidual markers71–73. It would be interesting to see whether signaling molecules necessary for decidualization in mice are also necessary for human decidualization in culture.
Many decidual cells undergo endoreduplication (polyploidy), a process by which cells undergo rounds of DNA replication without cytokinesis. Endoreduplication may serve to support embryonic growth by increasing protein synthesis through enhanced gene transcription. Although decidual polyploidy is well established in rodents, early literature provides evidence for this phenotype in humans but requires validation with reliable markers74. Whether decidual polyploidy is necessary for pregnancy success was examined in mice carrying deletion of the death effector domain–containing protein (DEDD)75. Dedd deficiency resulted in defective decidualization with reduced polyploidy, which led to infertility; the attachment reaction was normal. DEDD executed its function by forming a complex with cyclin D3, cdk4/6 and AKT. Deletion of the cytokine receptor Il11ra1 gene or sphingosine kinases Sphk1 and Sphk2 in a gene dosage– dependent manner also cause defective decidualization76–78.
Serum- and glucocorticoid-inducible kinase (SGK1), involved in epithelial ion transport and cell survival, was reported to be essential for implantation and preserving decidual-placental integrity79. In mice, intraluminal delivery of an Sgk1-overexpressing vector interfered with implantation. In contrast, Sgk1 deletion had no apparent effect on implantation but led to abnormal development of the decidual-placental interface, restricted fetal growth and death. This study is clinically relevant because SGK1 was differentially expressed in different pregnancy pathologies in women. In a human in vitro system, SGK1 silencing impaired decidualization by invoking oxidative stress. These findings are intriguing, but it would be interesting to know whether the effects are specific to decidualization or are consequences of defective uterine receptivity or implantation in a mouse model. Nevertheless, the results show that some genes are differentially regulated during early pregnancy.
Factors spanning different phases of early pregnancy
Many crucial genes show overlapping uterine expression at more than one stage, and their stage-specific roles are difficult to ascertain. Hand2, Klf5, Bmp2 and Ptgs2 are such genes and are expressed at blastocyst attachment with continued expression during decidualization41,46,63,64. Implantation is considered a proinflammatory reaction, and one early discernible mark is an increased endometrial vascular permeability at the attachment site. Cyclooxygenase (Cox)-derived prostaglandins were shown to mediate these effects63 and exist in two isoforms, Cox1 (Ptgs1) and Cox2. They have differential spatiotemporal expression during pregnancy. In mice, Ptgs1 is expressed in the epithelium early on day 4 (ref. 63), suggesting its role in generalized uterine edema that may participate in luminal closure for blastocyst apposition. Ptgs1 deletion shows no apparent implantation defects due to compensation by Ptgs2 (ref. 1). Ptgs2 is induced in the luminal epithelium and underlying stroma at the attachment site63, signifying its roles in attachment and localized endometrial vascular permeability. Cox2 is also expressed in the uterus and/or blastocyst during implantation in several species including primates and humans, which indicates a conserved role of Cox2 in implantation80–82. Ptgs2−/− mice show implantation failure63, although genetic background–dependent compensation by Ptgs1 can partially rescue implantation2. Cox2-derived prostacyclin was shown to participate in implantation by activating uterine peroxisome proliferator–activated receptor-δ (PPAR-δ) and retinoid X receptor83. Whereas maternal PPAR-δ is crucial for implantation and decidualization, embryonic PPAR-δ is required for placentation84. Upon attachment, Ptgs2 is localized at the antimesometrial site but moves to the opposite side (mesometrial), the presumptive site of placentation, by day 6 of pregnancy63,83. Ptgs2−/− females also show defective ovulation, fertilization, decidualization and placentation63, asserting roles of Cox2-derived prostaglandin signaling at several stages of pregnancy. A recent report shows that activation of epithelial sodium channels induces Cox2 and is necessary for implantation85.
Bmp2 also shows overlapping expression during pregnancy in mice. It is expressed in the subepithelial stroma coincident with the attachment reaction followed by heightened expression in decidua54. Its antagonist Noggin is expressed during receptivity but disappears with stromal Bmp2 expression upon attachment and thereafter54. Uterine deletion of Bmp2 showed decidualization failure with apparently normal attachment reaction, leading to infertility64. Interestingly, beads carrying BMP2 did not elicit implantation-like responses but affected spacing of embryos when co-transferred with the beads54. Although Bmp7 is expressed during early decidualization, its function is yet to be determined54.
Cell-specific expression of Wnt ligands, receptors and inhibitors in mouse and human endometria during the reproductive cycle and pregnancy suggests roles for Wnt signaling in implantation and decidualization44,86,87. Wnt signaling is executed by canonical or noncanonical pathways depending on the subcellular localization of β-catenin, transcriptional modulation of targets and co-receptor identity. Studies in TOPGAL reporter mice found canonical Wnt signaling in the luminal epithelium and myometrium at the implantation site, suggesting a role in implantation88. Wnt4 is expressed in the subepithelial stroma at the time of implantation and becomes more robust during decidualization89. Wnt4 expression is aberrant in Lif−/− and Hoxa-10−/− mice44; its uterine deletion confers infertility with defective adenogenesis89,90. In contrast, Wnt7a is expressed in the epithelium, and its deletion incurs subfertility with defective implantation and aberrant development of oviduct, uterus, cervix and vagina along the anteroposterior axis91. Mice with Wnt7a deletion also show defective gland formation90. Similarly, uterine deletion of Foxa2, encoding a forkhead homeobox-containing transcription factor necessary for uterine gland formation, leads to infertility92. Conversely, β-catenin overexpression shows glandular hyperplasia, suggesting a role of canonical signaling in gland formation93. Collectively, subfertility and implantation failure seen with uterine deletion of Wnt4 and Wnt7a, respectively, are perhaps the consequence of structural aberrations and sparse gland formation.
Lif is expressed in a biphasic manner, first in glandular epithelia on day 4 morning and later in the subepithelial stroma at the blastocyst attachment site on day 4 night94. Pla2g4a−/− mice with defective implantation show glandular Lif expression but loss of stromal expression95. The relative contributions of glandular and stromal LIF to implantation would require cell-type–specific deletion.
Marijuana and its major psychoactive component Δ9-tetrahydrocannabinoid have been known for decades to affect reproduction in animals and humans. Identification of the endocannabinoids anandamide (AEA) and 2-arachidonylglycerol (2-AG) and their G protein–coupled receptors CB1 (Cnr1) and CB2 (Cnr2) in the uterus and embryo helped in the exploration of implications of endocannabinoid signaling in female fertility96. Indeed, this signaling has profound effects on embryo development, oviductal embryo transport, uterine receptivity, placentation and parturition in mice, suggesting that the endocannabinoid system is operative at key stages of pregnancy. There is evidence that high peripheral anandamide levels correlate with recurrent pregnancy losses in women96. AEA and 2-AG are present in the mouse uterus, the former being tightly regulated97; AEA levels are lower in receptive uteri but higher in nonreceptive uteri. Furthermore, CB1 in the blastocyst is downregulated with activation before implantation in delayed implanting mice (Box 1)96. Cnr1−/− females are subfertile with oviductal retention of blastocysts98. Whereas embryonic CB1 contributes to synchronous preimplantation embryo development, oviductal CB1 in collaboration with adrenergic receptors directs timely passage of embryos through the oviduct98. Fallopian tubes in women with ectopic pregnancy also show downregulation of CNR1 (ref. 99).
Interestingly, persistently high AEA levels resulting from deletion of the gene encoding fatty acid amide hydrolase (Faah), which degrades AEA, also lead to defective implantation100. Appropriate differentiation of trophoblast stem cells requires regulated endocannabinoid signaling; its aberrant signaling results in compromised placentation and trophoblast invasion97. Whether this model is relevant to the human preeclampsia phenotype would require further investigation. The endocannabinoid system is present in human placentas, suggesting its importance in placental function101. Therefore, tightly regulated endocannabinoid signaling is crucial for pregnancy success.
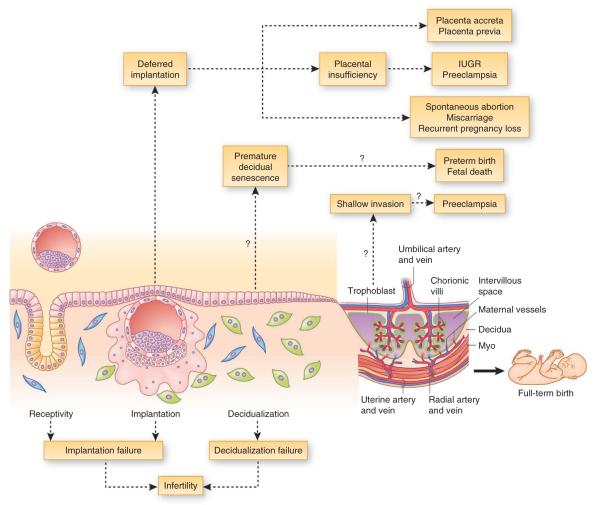
Adverse ripple effects originating from implantation defects
Pregnancy is programmed as a dynamic process and any major aberration in its normal course will either terminate pregnancy at the time of insult or perpetuate defects throughout pregnancy. Thus, ascertaining causes of late-stage disturbances requires assessment of early pregnancy defects. For instance, dysregulation of HB-EGF, a molecular link for blastocyst with the luminal epithelium, is associated with preeclampsia in humans102, a clinical correlate that suggests that early pregnancy defects can lead to pathological states later.
Several knockout mouse models helped to define propagation of early defects during the course of pregnancy (Fig. 3). The first evidence of adverse ripple effects was noted in mice missing Pla2g4a (cPLA2α). cPLA2α generates arachidonic acid from membrane phospholipids for prostaglandin synthesis via Cox enzymes. Pla2g4a is expressed in the uterus in a similar pattern as Ptgs2. Implantation in Pla2g4a−/− mice occurs beyond the normal window of implantation (deferred implantation)95, setting up adverse ripple effects reflected in embryo crowding, conjoined placenta, retarded fetoplacental development, increased resorptions and reduced litter size95. The results corroborate physiological experiments in which blastocysts were transferred in wild-type recipient uteri beyond the anticipated time of implantation95. cPLA2α’s expression in human endometrium also suggests it has a conserved role103.
Figure 3.
Potential adverse ripple effects during pregnancy arising from stage-specific defects in mice. (a) Normal pregnancy events encompass receptivity, attachment and implantation, decidualization, and placentation, leading to on-time parturition with full complement of offspring. Defective early pregnancy events veer the remainder of pregnancy off course, leading to adverse outcome. Ptgs2* mutant mice on C57BL/6J/129 genetic background have complete infertility, whereas on a CD1 background they show subfertility due to compensatory upregulation of Ptgs1. (b) Adverse pregnancy events stemming from aberrations in preceding stages in gene-deleted mice. Defective receptivity or deferred implantation (left two stages) results in embryo crowding, conjoined placenta, placental insufficiency, fetal growth restriction, fetal resorption and reduced litter size, whereas suboptimal decidualization (third stage) can lead to premature decidual senescence, resulting in preterm birth with neonatal death, or in abnormal guidance of placentation, which can lead to shallow invasion and preeclampsia. ADM, adrenomedullin; bl, blastocyst; CB1, cannabinoid receptor 1; Cox2, cyclooxygenase-2; cPLA2α, cytosolic phospholipase A2α; DB, decidua basalis; DC, decidua capsularis; dec, decidua; emb, embryo; EPC, ectoplacental cone; Klf5, Kruppel-like factor 5; LPA3, lysophosphatidic acid 3; Msx1, muscle segment homeobox 1; mTORC1, mammalian target of rapamycin complex 1; myo, myometrium; p53, transformation-related protein p53; Sgk1, serum- and glucocorticoid-inducible kinase 1; Sp, spongiotrophoblast.
A similar observation was reported in mice missing Lpar3 (LPA3), a receptor for lysophosphatidic acid104. The resemblance in the reproductive phenotypes between Pla2g4a−/− and Lpar3−/− females is attributed to reduced production of Cox2-derived prostaglandins. Depending on the genetic background, a comparable observation was also noted in Ptgs2−/− mice105. Collectively, these findings allude to LPA3-cPLA2α-Cox2 signaling as a determinant for on-time implantation; an aberration of this signaling defers initial attachment, passing along adverse effects through the rest of pregnancy. Although deferred implantation in Pla2g4a−/− or Lpar3−/− females was markedly corrected by administration of prostaglandins, embryo crowding still persisted95,104.
Aberrant embryonic spacing seen in mouse models may provide clues into the etiology of placenta previa, a complication that occurs in 1 in 200 human pregnancies. In this condition, the placenta is situated at the bottom of the womb and covers all or part of the cervix, causing mild to severe bleeding. The molecular mechanism underpinning the process remains unknown. Whether the embryo spacing program active in mice is germane to the appropriate placement of an embryo in the human uterus, especially with the rising incidence of multiples pregnancy in assisted reproductive technology programs, will require further investigation. In this context, studies on prostaglandin and/or bone morphogenetic protein (BMP) signaling in mice may be relevant54,95.
Haploinsufficiency of certain genes disrupts normal physiological function. One example is adrenomedullin (ADM), a growth-promoting, angiogenic and natriuretic factor secreted by decidua and trophoblast cells106,107. In humans, adrenomedullin levels are elevated during placentation but return to prepregnancy levels after parturition; altered adrenomedullin levels in the fetoplacental tissues are reflected in spontaneous abortion, gestational diabetes and preeclampsia108. In mice, Adm−/− fetuses prematurely die in utero with failure of neural tube closure, hydrops fetalis and cardiovascular malformation due to placental insufficiency107. Initial studies showed that Adm+/− mice have normal implantation but show embryo overcrowding and placental abnormalities, ectopic placentation beyond the decidual boundary (placenta accreta), fetal growth restriction and shallow invasion of spiral arteries indicative of preeclampsia107; but later studies found that Adm is expressed in the luminal epithelium and stroma during the peri-implantation period, and blastocyst transfer experiments in Adm+/− females showed poor uterine receptivity with reduced pinopodes formation and implantation rate109. Collectively, the results suggest that defects early in pregnancy in Adm+/− females contribute to late adverse effects.
Some transcription factors also tightly regulate the window of uterine receptivity and implantation. For example, Msx1d/d uteri show embryo crowding and increased resorption similar to those in Pla2g4a−/− or Lpar3−/− females9. Notably, whereas prostaglandin signaling permeates throughout pregnancy, Msx1 is transiently expressed in the receptive uterus. This suggests that deficits even at receptivity can derail developmental programming of pregnancy. Uterine deletion of Klf5 also results in deferred implantation with poor pregnancy outcome. However, its expression pattern is different than that of Msx1; it is first expressed in the epithelium before and during blastocyst attachment, with expression then switching to decidua with loss of epithelial expression. These results suggest that the period spanning uterine receptivity, attachment and decidualization is vulnerable to insults for initiating subsequent adverse events.
Adverse consequences of defective decidualization
Whereas the previous section recounts examples of deferred implantation leading to poor pregnancy outcome, aberrant decidualization can also give rise to adverse pregnancy phenotypes including defects in placentation, intrauterine fetal growth restriction and parturition (Fig. 3). For example, models that show shallow spiral artery invasion precludes normal placentation, a signature of preeclampsia110–112, although poor trophoblast invasion into decidua is one of several potential causes for preeclampsia97,113,114. Trophoblast migration and remodeling of decidual arterioles are also associated with human preeclampsia115. Preeclampsia is considered to be more prevalent in first pregnancies than in subsequent pregnancies. Whether reproductive hormones and other pregnancy factors during the first pregnancy prime the system for subsequent pregnancies requires further investigation. This is reminiscent of a study showing poor mammary gland development during the first, but not subsequent, pregnancies in prolactin receptor–deficient mice116.
One example of an adverse consequence of defective decidualization is preterm birth24. Mice with uterine deletion of Trp53 (Trp53d/d) show normal implantation, but 50–60% of Trp53d/d mice have preterm birth with dystocia and fetal death24. These mice have compromised decidualization with more terminally differentiated decidual cells with polyploidy, increased expression of pAkt, p21 and Cox2 and senescence-associated growth restriction. Interestingly, preterm birth in Trp53d/d mice is rescued by the selective Cox2 inhibitor celecoxib24. Many risk factors, such as gene mutation, infection and inflammation and stress, that lead to preterm birth also trigger cellular senescence via mammalian target of rapamycin complex 1 (mTORC1) signaling. Rapamycin (mTORC1 inhibitor) attenuates senescence and increases life span in mice117. Indeed, Trp53d/d decidua have increased mTORC1 activity, which is inhibited by rapamycin with attenuation of premature decidual senescence and rescue of preterm birth118. This is noteworthy, considering women with advanced age have a higher incidence of preterm birth119–121. Although p53 activation induces senescence to attenuate cancer, its loss can also activate senescence in certain cells and can be reversed by rapamycin122. It seems that p53’s role in the uterus during pregnancy is different from its role in tumorigenesis. Although there have been attempts to better understand the underlying mechanism to develop measures to prevent preterm birth, progress has been limited because of the complex nature of this pathophysiologic state that integrates both genetic predisposition and environmental factors (Box 3).
Box 3. Gene-environment interactions in pregnancy.
Environmental insults and genetic alterations are known to affect pregnancy outcome, but their cooperative effects have not been experimentally tested. Decidual senescence contributes to the timing of birth, and when it prematurely occurs as in Trp53d/d mice, it predisposes to preterm birth24,118. This model provides an opportunity to assess the effects of environmental insults such as infection or inflammation, oxidative stress or endocrine disrupters to exacerbate preterm birth. Whether these insults converge upon a senescence pathway or occur in parallel requires further investigation. Preterm birth in humans has a strong genetic component and high correlation with infection and inflammation161. Thus, Trp53d/d mice or other genetic models of spontaneous preterm birth could provide a basis for studies in gene-environment interactions during pregnancy24,162,163. There are reports of differential changes in decidual leukocyte populations in women during preterm and term labor and antiprogesterone induced decidual macrophage accumulation in rats undergoing preterm birth164. Whether P4 supplementation can neutralize this inflammatory response is not known. Inflammation and infection induce both local and systemic responses, making it difficult for appropriate interpretation. Mice constitutively deleted of Toll-like receptor 4 (Tlr4) are resistant to lipopolysaccharide-induced preterm birth165. Whether local or systemic TLR4 activation is a primary determinant of preterm birth requires evaluation.
It would be interesting to see whether other potential mediators of labor, such as gap junction protein connexin 43 or ZEB1/2, are aberrantly expressed in early pregnancy to induce preterm birth144,166. Recent clinical trials show that P4 supplementation can assuage the risk of preterm birth in women with short cervices or recurrent preterm birth167,168, suggesting P4’s beneficial effects in a subset of women with risk of preterm birth. Trp53d/d mice exposed to oxidative stress or infection or inflammation could be used to test the efficacy of P4 in combination with rapamycin and/or celecoxib in preventing preterm birth24,118. In this respect, rofecoxib (Vioxx), a Cox-2 inhibitor, used for 10 weeks during the first two trimesters failed to prevent preterm birth in high-risk women169. Notably, Vioxx was withdrawn from the market because of falsified data regarding its efficacy and adverse side effects. In contrast, celecoxib (Celebrex) has fewer adverse effects than Vioxx and is still widely used therapeutically; the dose used in Trp53d/d females to reverse preterm labor was low relative to doses used in other applications. The short-term, intermittent dosing used in this study did not show noticeable adverse effects to the dam, offspring and their growth24. These results suggest that prevention of preterm delivery in women may involve need-based low doses instead of long-term use of inhibitors, depending on the risk factors encompassing her genetic, environmental and socioeconomic history. Identification of targets downstream of Cox2 is also currently being undertaken to minimize the adverse side effects of Cox2 inhibitors.
Looking forward
Despite advances in the understanding of fertility and the overcoming of many deficiencies in human fertility by assisted reproductive technology, the implantation rate and number of ‘take-home’ babies still remain low. Limiting factors for low pregnancy success in IVF programs (~30%) include poor embryo quality and transfer of embryos into uteri of unknown state of receptivity. Another global problem is the high incidence of preterm birth and its adverse consequences on life-long health of the offspring. The fact that disturbances in early pregnancy can give rise to these enduring problems lends credence to the importance of identifying pregnancy-stage specific mediators, the aberration of which compromises pregnancy outcome (Fig. 4). As progress continues toward constructing a blueprint for normal implantation and pregnancy events, the challenges to be addressed are described below.
Figure 4.
Plausible charting of adverse ripple effects in human pregnancy. Defective receptivity, implantation, and/or decidualization can lead to infertility. Deferred implantation past the window of receptivity can lead to misguided embryo placement and implantation, resulting in placenta previa, ectopic placentation (placenta accreta) or placental insufficiency resulting in intrauterine growth restriction (IUGR) and/or preeclampsia. Implantation beyond the normal window can also give rise to spontaneous abortion, miscarriage and recurrent pregnancy loss, leading to infertility. Premature decidual senescence can lead to preterm birth and fetal death, whereas shallow trophoblast invasion into maternal decidua and/or blood vessels can lead to preeclampsia.
In IVF programs, the major barriers are poor rate of embryo development to blastocysts in culture, high aneuploidy rates and the lack of reliable markers of uterine receptivity for embryo transfer2,6. Mouse embryos cultured in very small volumes of medium show superior development to blastocysts123,124, and pregnancy outcome in IVF programs is superior when blastocysts are transferred rather than preblastocyst embryos. Therefore, IVF clinics may benefit from adopting and refining these approaches to reduce aneuploidy and enhance embryo development to improve implantation rates. Increasing pregnancy success by blastocyst transfer would also limit the number of embryos transferred, precluding the risk of multiple pregnancies. A clinical trial suggests that addition of granulocyte-macrophage colony–stimulating factor to culture medium improves implantation rate125 (Origio A/S). Likewise, addition of HB-EGF to culture medium is known to show beneficial effects on embryo development and attachment in vitro126,127. A clinical trial assessing HB-EGF’s effects on pregnancy improvement could be worthwhile.
Identification of embryonic signals that promote implantation also remains elusive. With advances in high-efficiency mass spectrometry (MALDI-MS) technology, it may be possible to identify new low-abundance proteins and/or lipid mediators secreted by embryos in culture that promote their own growth or signal the endometrium for implantation. This can be attempted by analyzing media in which IVF-derived human embryos were cultured. Another pressing need is to identify reliable biomarkers to ascertain the uterine receptive state. Although DNA microarrays have identified potential biomarkers for human endometrial receptivity128, the use of MALDI-MS could also be a powerful approach to map uterine proteomics and lipidomics profiles at defined physiological states to identify biomarkers of receptivity and better understand human implantation to improve fertility. Recent application of in situ mass spectrometry in periimplantation mouse uteri also provides opportunities to generate spatiotemporal maps of proteins and lipids and their modifications on human endometrium129,130.
Numerous mediators are crucial for conferring uterine receptivity and implantation (Fig. 2). However, the exact molecular cross-talk between these pathways is far from clear. In particular, the molecular mechanism behind spontaneous transition of the receptive uterus to the refractory phase remains unknown, and much work is needed to understand and manipulate this transition. Coordinated interactions between estrogen and P4 are crucial for conferring uterine receptivity and for moving the uterus to the nonreceptive phase in mice11,131. As high estrogen levels provoke uterine nonreceptivity, one cause of the high implantation failure rate of IVF may be high estrogen levels arising from ovarian hyperstimulation by gonadotropins to retrieve multiple oocytes131. By manipulating estrogen levels, it may be possible to prolong the receptive window to improve IVF-conceived pregnancy rates11.
More knowledge regarding the signaling network will help in manipulating the receptive window. In this respect, Msx1 is an attractive candidate for extending the receptive phase. Given that Msx genes are also expressed in human endometria, similarly to the situation in mice, identifying small-molecule targets that influence Msx signaling is an approach worth exploring. Cell-specific expression of Msx genes should also be evaluated throughout the menstrual cycle to better understand their roles and regulation.
One aspect of pregnancy success is maternal immunological adaptation to the fetus, which is considered an allograft132. Multiple mechanisms have been proposed to explain the fetus’s competency to escape rejection by the maternal immune system133. In mice, these include entrapment of dendritic cells within decidua, preventing immunogenic exposure of maternal T cells to fetal or placental antigens in uterine draining lymph nodes and systemic immunosuppression by regulatory T cells (Treg cells)134. A recent work on Treg cells during pregnancy describes enhancer elements that drive the Treg-specific transcription factor FOXP3 (ref. 135). The enhancer element CNS1 differentially induces Foxp3 expression in peripheral Treg cells (pTreg cells) upon exposure to antigens and thymic Treg cells. When pTreg-specific CNS1 was deleted, females lost their pTreg cell population and showed somewhat higher embryo resorption rates when mated with allogeneic males. However, the rate remained unaltered when CNS1-deficient females were mated to syngenic males, implicating pTreg cells in maintaining maternal immunological restraint to fetal and placental alloantigens. Intriguingly, CNS1 exists only in eutherian mammalian genomes, linking the evolution of pTreg cells with placentation. Another recent study in mice shows that decidua has an important role in preventing T cell–mediated immunological assault on the fetus and placenta136. An epigenetic program in decidua silences the expression of many inflammatory chemokine genes that recruit activated T helper type 1 CD4+ T cells and CD8+ cytotoxic T lymphocytes to inflammation sites, thus restricting chemokine expression in decidual cells, even under inflammation, and preventing activated T cell recruitment with fetal and placental specificity. In sum, the decidua provides immunological protection to the fetus and placenta. This evolving area of research warrants further investigation.
The genome-epigenome relationship and transmission of epigenomic information across generations are areas of intense research. Exposure to environmental endocrine disrupters in utero or varying the composition of maternal or paternal diets during pregnancy have profound effects on adult-onset diseases (Barker’s hypothesis)137,138 and impart transgenerational consequences on the offspring’s health, ranging from metabolism to sex determination139–141. It would be interesting to know whether epigenetic changes affecting the mother’s fertility can alter the offspring’s fertility.
Advances in research on noncoding RNAs including long noncoding RNAs, PIWI-interacting RNAs, miRNAs and siRNAs with parallel leaps in sequencing technology could be exploited to study gene regulation during pregnancy142–144. Until more information is gathered to identify targets and the underlying regulatory mechanisms, the functions of noncoding RNAs in pregnancy will remain limited. Furthermore, predictive gene regulation by cis-regulatory elements and transposons and their evolutionary adaptations in eutherian pregnancies have come to light145,146. It remains to be seen whether these elements can be validated in a physiological setting. Nonetheless, this area of research may have bearing on the different implantation strategies adapted by various species.
In this Review, we discuss adverse pregnancy outcomes stemming from aberrant implantation and defective decidualization. Sensitive noninvasive methods to identify the more precise timing of implantation in humans will help to identify whether adverse pregnancy effects arise from defective implantation and decidualization.
Acknowledgments
We regret that space limitations precluded us from citing many relevant references. We thank K. Yoshinaga and A. Erlebacher for helpful discussions. Work embodied in this article from S.K.D.’s group was supported in part by US National Institutes of Health (NIH) grants (HD12304, HD068524 and DA06668), the March of Dimes, and the Grand Challenges Explorations Initiative through the Bill & Melinda Gates Foundation. J.C. is supported by an NIH National Research Service Award Fellowship (F30AG040858) and the University of Cincinnati Medical Scientist Training Program (T32 GM063483), and X.S. is supported by a Lalor Foundation Postdoctoral Fellowship.
Footnotes
Note added in proof. At the time of uterine receptivity, nuclear receptor coactivator-6 (Ncoa6) was shown to attenuate estrogen sensitivity via ERα ubiquitination and subsequent degradation. Uterine deletion of Ncoa6 shows heightened estrogen sensitivity with ERα accumulation and upregulation of the ERα coactivator, SRC-3, resulting in implantation failure. Administration of the ER antagonist ICI 182,780 rescued implantation failure170. It would be interesting to see the extent of decidualization and long-term viability of implanting embryos under antiestrogen influence.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/ reprints/index.html.
References
- 1.Dey SK, et al. Molecular cues to implantation. Endocr. Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 3.Carson DD, et al. Embryo implantation. Dev. Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 4.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N. Engl. J. Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 6.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Psychoyos A. Endocrine Control of Egg Implantation. American Physiology Society; Washington, D.C.: 1973. [Google Scholar]
- 8.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc. Natl. Acad. Sci. USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daikoku T, et al. Conditional deletion of MSX homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev. Cell. 2011;21:1014–1024. doi: 10.1016/j.devcel.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikas G, Psychoyos A. Uterine pinopodes in peri-implantation human endometrium. Clinical relevance. Ann. NY Acad. Sci. 1997;816:129–142. doi: 10.1111/j.1749-6632.1997.tb52136.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. USA. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giudice LC. Potential biochemical markers of uterine receptivity. Hum. Reprod. 1999;14(suppl. 2):3–16. doi: 10.1093/humrep/14.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 13.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol. Endocrinol. 2000;14:1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 14.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor α is dispensable for proliferation but essential for complete biological and biochemical responses. Proc. Natl. Acad. Sci. USA. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco HL, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26:1218–1227. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon L, et al. Stromal progesterone receptors mediate induction of Indian Hedgehog (IHH) in uterine epithelium and its downstream targets in uterine stroma. Endocrinology. 2009;150:3871–3876. doi: 10.1210/en.2008-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart CL, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 18.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird SM, et al. The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum. Reprod. 1997;12:569–574. doi: 10.1093/humrep/12.3.569. [DOI] [PubMed] [Google Scholar]
- 20.Piccinni MP, et al. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat. Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 21.Hambartsoumian E. Endometrial leukemia inhibitory factor (LIF) as a possible cause of unexplained infertility and multiple failures of implantation. Am. J. Reprod. Immunol. 1998;39:137–143. doi: 10.1111/j.1600-0897.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 22.Brinsden PR, Alam V, de Moustier B, Engrand P. Recombinant human leukemia inhibitory factor does not improve implantation and pregnancy outcomes after assisted reproductive techniques in women with recurrent unexplained implantation failure. Fertil. Steril. 2009;91:1445–1447. doi: 10.1016/j.fertnstert.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 23.Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450:721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 24.Hirota Y, et al. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J. Clin. Invest. 2010;120:803–815. doi: 10.1172/JCI40051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang HJ, et al. Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc. Natl. Acad. Sci. USA. 2009;106:9761–9766. doi: 10.1073/pnas.0904280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patounakis G, et al. The p53 codon 72 single nucleotide polymorphism lacks a significant effect on implantation rate in fresh in vitro fertilization cycles: an analysis of 1,056 patients. Fertil. Steril. 2009;92:1290–1296. doi: 10.1016/j.fertnstert.2008.07.1783. [DOI] [PubMed] [Google Scholar]
- 27.Tranguch S, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, et al. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol. Endocrinol. 2006;20:2682–2694. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tranguch S, et al. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J. Clin. Invest. 2007;117:1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota Y, et al. Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc. Natl. Acad. Sci. USA. 2010;107:15577–15582. doi: 10.1073/pnas.1009324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirota Y, et al. Deficiency of immunophilin FKBP52 promotes endometriosis. Am. J. Pathol. 2008;173:1747–1757. doi: 10.2353/ajpath.2008.080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, et al. FKBP52 is regulated by HOXA10 during decidualizaton and in endometriosis. Reproduction. 2012;143:531–538. doi: 10.1530/REP-11-0438. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat. Rev. Cancer. 2009;9:615–630. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee A, et al. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol. Cell. Biol. 2006;26:6571–6583. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee A, Amato P, Allred DC, DeMayo FJ, Lydon JP. Steroid receptor coactivator 2 is required for female fertility and mammary morphogenesis: insights from the mouse, relevance to the human. Nucl. Recept. Signal. 2007;5:e011. doi: 10.1621/nrs.05011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev. Biol. 2002;245:280–290. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- 37.Lee K, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat. Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 38.Wei Q, Levens ED, Stefansson L, Nieman LK. Indian Hedgehog and its targets in human endometrium: menstrual cycle expression and response to CDB-2914. J. Clin. Endocrinol. Metab. 2010;95:5330–5337. doi: 10.1210/jc.2010-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurihara I, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petit FG, et al. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc. Natl. Acad. Sci. USA. 2007;104:6293–6298. doi: 10.1073/pnas.0702039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huyen DV, Bany BM. Evidence for a conserved function of heart and neural crest derivatives expressed transcript 2 in mouse and human decidualization. Reproduction. 2011;142:353–368. doi: 10.1530/REP-11-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavlova A, Boutin E, Cunha G, Sassoon D. Msx1 (Hox-7.1) in the adult mouse uterus: cellular interactions underlying regulation of expression. Development. 1994;120:335–345. doi: 10.1242/dev.120.2.335. [DOI] [PubMed] [Google Scholar]
- 44.Daikoku T, et al. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol. Endocrinol. 2004;18:1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- 45.Nallasamy S, Li Q, Bagchi MK, Bagchi IC. Msx homeobox genes critically regulate embryo implantation by controlling paracrine signaling between uterine stroma and epithelium. PLoS Genet. 2012;8:e1002500. doi: 10.1371/journal.pgen.1002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, et al. Kruppel-like factor 5 (KLF5) is critical for conferring uterine receptivity to implantation. Proc. Natl. Acad. Sci. USA. 2012;109:1145–1150. doi: 10.1073/pnas.1118411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu R, Zhou Z, Zhao D, Chen C. The induction of KLF5 transcription factor by progesterone contributes to progesterone-induced breast cancer cell proliferation and dedifferentiation. Mol. Endocrinol. 2011;25:1137–1144. doi: 10.1210/me.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ema M, et al. Kruppel-like factor 5 is essential for blastocyst development and the normal self-renewal of mouse ESCs. Cell Stem Cell. 2008;3:555–567. doi: 10.1016/j.stem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Lejeune B, Van Hoeck J, Leroy F. Transmitter role of the luminal uterine epithelium in the induction of decidualization in rats. J. Reprod. Fertil. 1981;61:235–240. doi: 10.1530/jrf.0.0610235. [DOI] [PubMed] [Google Scholar]
- 50.Das SK, et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 51.Paria BC, Elenius K, Klagsbrun M, Dey SK. Heparin-binding EGF-like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- 52.Raab G, et al. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development. 1996;122:637–645. doi: 10.1242/dev.122.2.637. [DOI] [PubMed] [Google Scholar]
- 53.Hamatani T, et al. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc. Natl. Acad. Sci. USA. 2004;101:10326–10331. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paria BC, et al. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc. Natl. Acad. Sci. USA. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwamoto R, et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc. Natl. Acad. Sci. USA. 2003;100:3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie H, et al. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc. Natl. Acad. Sci. USA. 2007;104:18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stavreus-Evers A, et al. Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol. Hum. Reprod. 2002;8:765–769. doi: 10.1093/molehr/8.8.765. [DOI] [PubMed] [Google Scholar]
- 58.Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev. Genet. 1997;21:102–108. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 59.Chobotova K, et al. Heparin-binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mech. Dev. 2002;119:137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
- 60.Genbacev OD, et al. Trophoblast L-selectin–mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 61.Lessey BA. Assessment of endometrial receptivity. Fertil. Steril. 2011;96:522–529. doi: 10.1016/j.fertnstert.2011.07.1095. [DOI] [PubMed] [Google Scholar]
- 62.Prakobphol A, Genbacev O, Gormley M, Kapidzic M, Fisher SJ. A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev. Biol. 2006;298:107–117. doi: 10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 63.Lim H, et al. Multiple female reproductive failures in cyclooxygenase 2–deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 64.Lee KY, et al. Bmp2 is critical for the murine uterine decidual response. Mol. Cell. Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benson GV, et al. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 66.Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol. Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- 67.Gendron RL, et al. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol. Reprod. 1997;56:1097–1105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- 68.Tan J, et al. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech. Dev. 2002;111:99–113. doi: 10.1016/s0925-4773(01)00614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J. Clin. Invest. 1998;101:1379–1384. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J. Clin. Endocrinol. Metab. 1999;84:1129–1135. doi: 10.1210/jcem.84.3.5573. [DOI] [PubMed] [Google Scholar]
- 71.Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141:3510–3513. doi: 10.1210/endo.141.9.7789. [DOI] [PubMed] [Google Scholar]
- 72.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 73.Brar AK, et al. Laminin decreases PRL and IGFBP-1 expression during in vitro decidualization of human endometrial stromal cells. J. Cell. Physiol. 1995;163:30–37. doi: 10.1002/jcp.1041630105. [DOI] [PubMed] [Google Scholar]
- 74.Arias-Stella J. The Arias-Stella reaction: facts and fancies four decades after. Adv. Anat. Pathol. 2002;9:12–23. doi: 10.1097/00125480-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Mori M, et al. Death effector domain-containing protein (DEDD) is required for uterine decidualization during early pregnancy in mice. J. Clin. Invest. 2011;121:318–327. doi: 10.1172/JCI44723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bilinski P, Roopenian D, Gossler A. Maternal IL-11Rα function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12:2234–2243. doi: 10.1101/gad.12.14.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robb L, et al. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat. Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 78.Mizugishi K, et al. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Invest. 2007;117:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salker MS, et al. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat. Med. 2011;17:1509–1513. doi: 10.1038/nm.2498. [DOI] [PubMed] [Google Scholar]
- 80.Kim JJ, et al. Expression of cyclooxygenase-1 and -2 in the baboon endometrium during the menstrual cycle and pregnancy. Endocrinology. 1999;140:2672–2678. doi: 10.1210/endo.140.6.6716. [DOI] [PubMed] [Google Scholar]
- 81.Critchley HO, et al. Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. J. Clin. Endocrinol. Metab. 1999;84:240–248. doi: 10.1210/jcem.84.1.5380. [DOI] [PubMed] [Google Scholar]
- 82.Marions L, Danielsson KG. Expression of cyclo-oxygenase in human endometrium during the implantation period. Mol. Hum. Reprod. 1999;5:961–965. doi: 10.1093/molehr/5.10.961. [DOI] [PubMed] [Google Scholar]
- 83.Lim H, et al. Cyclo-oxygenase-2–derived prostacyclin mediates embryo implantation in the mouse via PPARδ. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang H, et al. Stage-specific integration of maternal and embryonic peroxisome proliferator-activated receptor δ signaling is critical to pregnancy success. J. Biol. Chem. 2007;282:37770–37782. doi: 10.1074/jbc.M706577200. [DOI] [PubMed] [Google Scholar]
- 85.Ruan YC, et al. Activation of the epithelial Na+ channel triggers prostaglandin E2 release and production required for embryo implantation. Nat. Med. 2012;18:1112–1117. doi: 10.1038/nm.2771. [DOI] [PubMed] [Google Scholar]
- 86.Hayashi K, et al. Wnt genes in the mouse uterus: potential regulation of implantation. Biol. Reprod. 2009;80:989–1000. doi: 10.1095/biolreprod.108.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tulac S, et al. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J. Clin. Endocrinol. Metab. 2003;88:3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- 88.Mohamed OA, et al. Uterine Wnt/β-catenin signaling is required for implantation. Proc. Natl. Acad. Sci. USA. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franco HL, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dunlap KA, et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol. Reprod. 2011;85:386–396. doi: 10.1095/biolreprod.111.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- 92.Jeong JW, et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biol. Reprod. 2010;83:396–403. doi: 10.1095/biolreprod.109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeong JW, et al. β-catenin mediates glandular formation and dysregulation of β-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song H, Lim H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction. 2006;131:341–349. doi: 10.1530/rep.1.00956. [DOI] [PubMed] [Google Scholar]
- 95.Song H, et al. Cytosolic phospholipase A2α is crucial [correction of A2α deficiency is crucial] for ‘on-time’ embryo implantation that directs subsequent development. Development. 2002;129:2879–2889. doi: 10.1242/dev.129.12.2879. [DOI] [PubMed] [Google Scholar]
- 96.Wang H, Dey SK, Maccarrone M. Jekyll and Hyde: two faces of cannabinoid signaling in male and female fertility. Endocr. Rev. 2006;27:427–448. doi: 10.1210/er.2006-0006. [DOI] [PubMed] [Google Scholar]
- 97.Sun X, et al. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc. Natl. Acad. Sci. USA. 2010;107:16887–16892. doi: 10.1073/pnas.1010892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H, et al. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat. Med. 2004;10:1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- 99.Horne AW, et al. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS ONE. 2008;3:e3969. doi: 10.1371/journal.pone.0003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang H, et al. Fatty acid amide hydrolase deficiency limits early pregnancy events. J. Clin. Invest. 2006;116:2122–2131. doi: 10.1172/JCI28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trabucco E, et al. Endocannabinoid system in first trimester placenta: low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta. 2009;30:516–522. doi: 10.1016/j.placenta.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 102.Leach RE, et al. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet. 2002;360:1215–1219. doi: 10.1016/S0140-6736(02)11283-9. [DOI] [PubMed] [Google Scholar]
- 103.Stavreus-Evers A, Koraen L, Scott JE, Zhang P, Westlund P. Distribution of cyclooxygenase-1, cyclooxygenase-2, and cytosolic phospholipase A2 in the luteal phase human endometrium and ovary. Fertil. Steril. 2005;83:156–162. doi: 10.1016/j.fertnstert.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 104.Ye X, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang H, et al. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J. Biol. Chem. 2004;279:10649–10658. doi: 10.1074/jbc.M312203200. [DOI] [PubMed] [Google Scholar]
- 106.Yotsumoto S, et al. Expression of adrenomedullin, a hypotensive peptide, in the trophoblast giant cells at the embryo implantation site in mouse. Dev. Biol. 1998;203:264–275. doi: 10.1006/dbio.1998.9073. [DOI] [PubMed] [Google Scholar]
- 107.Li M, Yee D, Magnuson TR, Smithies O, Caron KM. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J. Clin. Invest. 2006;116:2653–2662. doi: 10.1172/JCI28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Di Iorio R, Marinoni E, Scavo D, Letizia C, Cosmi EV. Adrenomedullin in pregnancy. Lancet. 1997;349:328. doi: 10.1016/s0140-6736(05)62827-9. [DOI] [PubMed] [Google Scholar]
- 109.Li M, Wu Y, Caron KM. Haploinsufficiency for adrenomedullin reduces pinopodes and diminishes uterine receptivity in mice. Biol. Reprod. 2008;79:1169–1175. doi: 10.1095/biolreprod.108.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J. Clin. Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hunkapiller NM, et al. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138:2987–2998. doi: 10.1242/dev.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cross JC. The genetics of pre-eclampsia: a feto-placental or maternal problem. Clin. Genet. 2003;64:96–103. doi: 10.1034/j.1399-0004.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 113.Cui Y, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dokras A, et al. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol. Reprod. 2006;75:899–907. doi: 10.1095/biolreprod.106.053603. [DOI] [PubMed] [Google Scholar]
- 115.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 116.Ormandy CJ, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 117.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirota Y, Cha J, Yoshie M, Daikoku T, Dey SK. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc. Natl. Acad. Sci. USA. 2011;108:18073–18078. doi: 10.1073/pnas.1108180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cnattingius S, Forman MR, Berendes HW, Isotalo L. Delayed childbearing and risk of adverse perinatal outcome. A population-based study. J. Am. Med. Assoc. 1992;268:886–890. [PubMed] [Google Scholar]
- 120.Krieg SA, Henne MB, Westphal LM. Obstetric outcomes in donor oocyte pregnancies compared with advanced maternal age in in vitro fertilization pregnancies. Fertil. Steril. 2008;90:65–70. doi: 10.1016/j.fertnstert.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 121.Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PLoS Med. 2011;8:e1000386. doi: 10.1371/journal.pmed.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc. Natl. Acad. Sci. USA. 2010;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc. Natl. Acad. Sci. USA. 1990;87:4756–4760. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Melin J, et al. In vitro embryo culture in defined, sub-microliter volumes. Dev. Dyn. 2009;238:950–955. doi: 10.1002/dvdy.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sjöblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony– stimulating factor promotes human blastocyst development in vitro. Hum. Reprod. 1999;14:3069–3076. doi: 10.1093/humrep/14.12.3069. [DOI] [PubMed] [Google Scholar]
- 126.Martin KL, Barlow DH, Sargent IL. Heparin-binding epidermal growth factor significantly improves human blastocyst development and hatching in serum-free medium. Hum. Reprod. 1998;13:1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- 127.Lim HJ, Dey SK. HB-EGF: a unique mediator of embryo-uterine interactions during implantation. Exp. Cell Res. 2009;315:619–626. doi: 10.1016/j.yexcr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diaz-Gimeno P, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertility and sterility. 2011;95:50–60. 60, e51–15. doi: 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 129.Burnum KE, et al. Imaging mass spectrometry reveals unique protein profiles during embryo implantation. Endocrinology. 2008;149:3274–3278. doi: 10.1210/en.2008-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burnum KE, et al. Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J. Lipid Res. 2009;50:2290–2298. doi: 10.1194/jlr.M900100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simón C, et al. Increasing uterine receptivity by decreasing estradiol levels during the preimplantation period in high responders with the use of a follicle-stimulating hormone step-down regimen. Fertil. Steril. 1998;70:234–239. doi: 10.1016/s0015-0282(98)00140-x. [DOI] [PubMed] [Google Scholar]
- 132.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 133.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol. Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 134.Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nancy P, et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 139.Carone BR, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosenfeld CS, et al. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc. Natl. Acad. Sci. USA. 2003;100:4628–4632. doi: 10.1073/pnas.0330808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chakrabarty A, et al. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc. Natl. Acad. Sci. USA. 2007;104:15144–15149. doi: 10.1073/pnas.0705917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu SJ, et al. MicroRNA expression and regulation in mouse uterus during embryo implantation. J. Biol. Chem. 2008;283:23473–23484. doi: 10.1074/jbc.M800406200. [DOI] [PubMed] [Google Scholar]
- 144.Renthal NE, et al. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc. Natl. Acad. Sci. USA. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lynch VJ, May G, Wagner GP. Regulatory evolution through divergence of a phosphoswitch in the transcription factor CEBPB. Nature. 2011;480:383–386. doi: 10.1038/nature10595. [DOI] [PubMed] [Google Scholar]
- 146.Lynch VJ, Leclerc RD, May G, Wagner GP. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat. Genet. 2011;43:1154–1159. doi: 10.1038/ng.917. [DOI] [PubMed] [Google Scholar]
- 147.Lubahn DB, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lydon JP, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 149.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 150.Thomas K, De Hertogh R, Pizarro M, Van Exter C, Ferin J. Plasma LH-HCG, 17 -estradiol, estrone and progesterone monitoring around ovulation and subsequent nidation. Int. J. Fertil. 1973;18:65–73. [PubMed] [Google Scholar]
- 151.Ghosh D, De P, Sengupta J. Luteal phase ovarian oestrogen is not essential for implantation and maintenance of pregnancy from surrogate embryo transfer in the rhesus monkey. Hum. Reprod. 1994;9:629–637. doi: 10.1093/oxfordjournals.humrep.a138561. [DOI] [PubMed] [Google Scholar]
- 152.Smitz J, et al. A prospective randomized study on oestradiol valerate supplementation in addition to intravaginal micronized progesterone in buserelin and HMG induced superovulation. Hum. Reprod. 1993;8:40–45. doi: 10.1093/oxfordjournals.humrep.a137871. [DOI] [PubMed] [Google Scholar]
- 153.Rao AJ, et al. Establishment of the need for oestrogen during implantation in non-human primates. Reprod. Biomed. Online. 2007;14:563–571. doi: 10.1016/s1472-6483(10)61047-4. [DOI] [PubMed] [Google Scholar]
- 154.McLaren A. A study of balstocysts during delay and subsequent implantation in lactating mice. J. Endocrinol. 1968;42:453–463. doi: 10.1677/joe.0.0420453. [DOI] [PubMed] [Google Scholar]
- 155.Yoshinaga K, Adams CE. Delayed implantation in the spayed, progesterone treated adult mouse. J. Reprod. Fertil. 1966;12:593–595. doi: 10.1530/jrf.0.0120593. [DOI] [PubMed] [Google Scholar]
- 156.Lee JE, et al. Autophagy regulates embryonic survival during delayed implantation. Endocrinology. 2011;152:2067–2075. doi: 10.1210/en.2010-1456. [DOI] [PubMed] [Google Scholar]
- 157.Lopes FL, Desmarais JA, Murphy BD. Embryonic diapause and its regulation. Reproduction. 2004;128:669–678. doi: 10.1530/rep.1.00444. [DOI] [PubMed] [Google Scholar]
- 158.Renfree MB, Shaw G. Diapause. Annu. Rev. Physiol. 2000;62:353–375. doi: 10.1146/annurev.physiol.62.1.353. [DOI] [PubMed] [Google Scholar]
- 159.Hess AP, Nayak NR, Giudice LC. Oviduct and endometrium: cyclic changes in the primate oviduct and endometrium. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Vol. 1. Elsevier Academic Press; 2006. pp. 337–381. [Google Scholar]
- 160.Schlafke S, Enders AC. Cellular basis of interaction between trophoblast and uterus at implantation. Biol. Reprod. 1975;12:41–65. doi: 10.1095/biolreprod12.1.41. [DOI] [PubMed] [Google Scholar]
- 161.World Health Organization . Born Too Soon: The Global Action Report on Preterm Birth. World Health Organization; Geneva: 2012. [Google Scholar]
- 162.Roizen JD, Asada M, Tong M, Tai HH, Muglia LJ. Preterm birth without progesterone withdrawal in 15-hydroxyprostaglandin dehydrogenase hypomorphic mice. Mol. Endocrinol. 2008;22:105–112. doi: 10.1210/me.2007-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Park CB, DeMayo FJ, Lydon JP, Dufort D. NODAL in the uterus is necessary for proper placental development and maintenance of pregnancy. Biol. Reprod. 2012;86:194. doi: 10.1095/biolreprod.111.098277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hamilton S, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol. Reprod. 2011;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- 165.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of Toll-like receptor 4. Biol. Reprod. 2003;69:1957–1963. doi: 10.1095/biolreprod.103.019620. [DOI] [PubMed] [Google Scholar]
- 166.Döring B, et al. Ablation of connexin43 in uterine smooth muscle cells of the mouse causes delayed parturition. J. Cell Sci. 2006;119:1715–1722. doi: 10.1242/jcs.02892. [DOI] [PubMed] [Google Scholar]
- 167.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N. Engl. J. Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 168.Meis PJ, et al. Prevention of recurrent preterm delivery by 17 α-hydroxyprogesterone caproate. N. Engl. J. Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 169.Groom KM, Shennan AH, Jones BA, Seed P, Bennett PR. TOCOX–a randomised, double-blind, placebo-controlled trial of rofecoxib (a COX-2-specific prostaglandin inhibitor) for the prevention of preterm delivery in women at high risk. BJOG. 2005;112:725–730. doi: 10.1111/j.1471-0528.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 170.Kawagoe J, et al. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev. Cell. 2012;23:858–865. doi: 10.1016/j.devcel.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]