Abstract
Orexins are hypothalamic neuropeptides that have a documented role in mediating the acute stress response. However, their role in habituation to repeated stress, and the role of orexin receptors (OX1R and OX2R) in the stress response, has yet to be defined. Orexin neuronal activation and levels in the cerebrospinal fluid (CSF) were found to be stimulated with acute restraint, but were significantly reduced by day five of repeated restraint. As certain disease states such as panic disorder are associated with increased central orexin levels and failure to habituate to repeated stress, the effect of activating orexin signaling via Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) on the hypothalamic–pituitary–adrenal (HPA) response was evaluated after repeated restraint. While vehicle-treated rats displayed habituation of Adrenocorticotropic Hormone (ACTH) from day 1 to day 5 of restraint, stimulating orexins did not further increase ACTH beyond vehicle levels for either acute or repeated restraint. We delineated the roles of orexin receptors in acute and repeated stress using a selective OX2R antagonist (MK-1064). Pretreatment with MK-1064 reduced day 1 ACTH levels, but did not allow further habituation on day 5 compared with vehicle-treated rats, indicating that endogenous OX2R activity plays a role in acute stress, but not in habituation to repeated stress. However, in restrained rats with further stimulated orexins by DREADDs, MK-1064 decreased ACTH levels on day 5. Collectively, these results indicate that the OX2R plays a role in acute stress, and can prevent habituation to repeated stress under conditions of high orexin release.
Keywords: MK-1064, orexin receptor, hypocretin, stress, habituation, DREADDs
INTRODUCTION
Orexins (also called hypocretins) are peptides generated from the prepro-orexin precursor that is exclusively localized in cells of the lateral and posterior hypothalamic region (de Lecea et al., 1998; Sakurai et al., 1998). Prepro-orexin is cleaved into two highly structurally related and highly conserved peptides, orexin A and orexin B, which bind to two G-protein-coupled orexin receptors, orexin 1 and orexin 2 receptors (OX1R and OX2R, respectively) (de Lecea et al., 1998; Sakurai et al., 1998). Orexin A has nearly equal affinities for both receptors, while Orexin B has higher affinity for OX2R (Gotter et al., 2012b). While many brain regions express both receptors, some regions exhibit differential expression (Marcus et al., 2001). For example, the cingulate cortex and locus coeruleus selectively express OX1R, while the paraventricular nuclei of the hypothalamus (PVN) and shell neurons of nucleus accumbens preferentially express OX2R (Marcus et al., 2001).
Orexins are implicated in a wide variety of neuroendocrine and behavioral responses including arousal, food intake, cognitive function, autonomic responses, emotional memory, and the stress response (Sakurai, 2014). The most salient phenotype of orexin knockouts is reduced arousal resulting in narcolepsy-like behavior, which has led to the identification and development of OXR antagonists for the pharmacological treatment of insomnia (Gotter et al., 2012a). Conversely, i.c. v. administration of exogenous orexin A to rats or orexin receptor agonists to mice promote arousal and wakefulness (Hagan et al., 1999; Nagahara et al., 2015). Orexins are also important in regulating the neurobiological systems that respond to stressful stimuli. For example, orexins promote the hypothalamic–pituitary–adrenal (HPA) axis response (Winsky-Sommerer et al., 2004, 2005; Berridge et al., 2010; Johnson et al., 2012). Specifically, central orexin administration (either Orexin A or Orexin B) increases HPA hormones (Jászberényi et al., 2000; Kuru et al., 2000; Spinazzi et al., 2006). Conversely, orexin neurons are activated by corticotropin releasing hormone and respond to stressors such as forced swim stress (Winsky-Sommerer et al., 2005; Chang et al., 2007; Furlong et al., 2009; Chen et al., 2013). Both orexin 1 and 2 receptors have been implicated in acute stress; the role of each receptor appears dependent on the paradigm of stress used (Chang et al., 2007; Gozzi et al., 2013; Bonaventure et al., 2015).
While this evidence indicates a role for orexins in activating the HPA activity under conditions of acute stress, the role of orexin signaling in response to repeated stress is not known. With repeated exposure to moderately intense stressors, individuals typically habituate to that stress as indicated by decreasing responsivity in behavioral, HPA and autonomic measures (Grissom et al., 2008; Grissom and Bhatnagar, 2009). Failure to habituate to a stressor is a hallmark of stress-related illnesses such as post-traumatic stress disorder (PTSD) and panic disorder (Johnson et al., 2012). Moreover, patients with panic anxiety symptoms have higher levels of orexin in their CSF (Johnson et al., 2010). If involved in habituation to a stressor, orexins may be a good target for treatment for some of these stress-related psychiatric illnesses.
To determine the role of orexins in acute and repeated restraint, we first characterized orexin neuronal activation and levels in the CSF in both conditions. We found these measures to be increased with acute restraint, but they significantly decreased with repeated restraint. In order to increase orexin activation prior to each of five daily 30-min restraints (to model higher orexin levels as observed in panic disorder), we used Designers Receptors Exclusively Activated by Designer Drugs (DREADDs) driven by the orexin promoter. We found that even with additional orexin stimulation, HPA activation habituated by day 5 of restraint. However, we were able to delineate the roles of orexin receptors in acute and repeated stress using a selective OX2R-specific antagonist (MK-1064) (Roecker et al., 2014). Prior to this, the roles of orexin receptors in the stress response, whether acute or repeated, had not yet been fully characterized. As OX2R is abundant in the PVN and is known to play a role in arousal, it may mediate excitatory effects at this site where the HPA response is initiated (Shirasaka et al., 2001a; Gotter et al., 2012b). In support of this, we found that OX2R receptor promotes the acute stress response, and it partially prevents habituation to repeated stress only in conditions of high orexin release.
EXPERIMENTAL PROCEDURES
Animals
Adult male Sprague–Dawley rats (225–250 g) were obtained from Charles River Laboratories (Wilmington, MA, USA). Rats were singly housed in polycarbonate enclosures with standard bedding and with food and water available ad libitum. Animals were acclimated to a 12-h light–dark cycle with lights on at 06:15 and lights off at 18:15 in a temperature controlled vivarium for at least 5 days prior to administration of any stress protocols. All experiments took place during the inactive phase between 0800 and 1200 (Zeitgeber Time 01:45–05:45). Experiments followed the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Children’s Hospital of Philadelphia Research Institute’s Animal Care and Use Committee.
Stereotaxic delivery of DREADD-containing viruses
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) are constructs packaged into viruses that contain synthetic G-protein-coupled receptors (GPCRs) and can be activated by the otherwise pharmacologically inert ligand Clozapine-N-Oxide (CNO). We obtained the CMV-hM3Dq-mCitrine plasmid from Dr. Bryan Roth (University of North Carolina, Chapel Hill, NC, USA). Slice electrophysiology has demonstrated that CNO application to cells expressing this Gq-coupled designer receptor causes depolarization and increases firing rate (Alexander et al., 2009). We next obtained a 1295-bp promoter for human preproorexin gene (Ple112) from Addgene plasmid No. 29004 (gift of Dr. Elizabeth Simpson, Univ. of British Columbia). This promoter was subcloned upstream of the hM3Dq-mCitrine region to replace the construct’s CMV promoter to drive transgene expression specifically in orexin neurons. The fragment Ple112-hm3Dq-mCitrine was then subcloned between the Inverted Terminal Repeats (ITRs) of the AAV2 genome. In a separate study, we found AAV1 serotype displayed optimal tropism for Sprague–Dawley rat hypothalamic neurons when we delivered in vivo and compared to AAV5,8,9 expression of GFP reporters driven by common constitutively active promoters Synapsin and CB7. Based on this finding, The University of Pennsylvania Vector Core produced a recombinant adenovirus rAAV2/1-Ple112-hM3Dq-mCitrine (using AAV1 serotype capsid for optimal transduction in orexin neurons) for our use.
Surgeries were performed in aseptic conditions. Animals were anesthetized using a cocktail of ketamine, xylazine, and acepromazine. Using stereotaxic technique, DREADD-containing virus (109 titer, 1 μl bilaterally) was injected into the lateral hypothalamus (coordinates: 2.5 mm caudal to bregma, 1.8 mm from mid-line and 8 mm ventral to dura mater). After surgery, animals were injected with meloxicam (2 mg/kg). Animals recovered for 4 weeks while virus expressed before any further experiments were performed.
Immunofluorescence staining for visualizing the virus tag was conducted as follows. Rats were rapidly decapitated and brains were flash frozen in 2-methyl butane. Coronal sections for the lateral hypothalamus were cut on a cryostat into six serial sets of 20-um-thick sections directly onto the slide. Rostral-caudal coordinates (relative to bregma) for the lateral hypothalamic area analyzed were −2.12 mm to −3.60 mm. Tissue was fixed for 30 min in 4% paraformaldehyde (Electron Microscopy Sciences; Hatfield, PA, USA). Sections were washed several times with Phosphate-Buffered Saline (PBS; pH 7.4) with 0.3% Triton-X (Sigma–Aldrich; St Louis, MO, USA), and then incubated with primary antibodies for both Orexin A (1:250, sc-8070; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and GFP (1:500, ab290; AbCam, Cambridge, UK) in a solution of PBS with 5% Normal Donkey Serum (EMD Millipore; Billerica, MA, USA) and 0.3% Triton-X overnight. As the mCitrine tag on the DREADD-containing virus originates from Aquorea victoria jellyfish, GFP antibodies are known to react with these proteins (Le et al., 2006). Sections were rinsed several times in PBS, followed by the addition of AlexaFluor488 Donkey anti-goat and AlexaFluor647 Donkey anti-rabbit (1:200, A-11055 and A-31573; Life Technologies, Carlsbad, CA, USA). Sections were given a final set of washes and coverslipped with fluoromount. Images were acquired with a Leitz DMR microscope with a digital camera (Leica Microsystems; Buffalo Grove, IL, USA). The NIH Image J colocalization plugin was used to determine percent orexin cells transduced by the virus. Quantification revealed 71 ± 5.4% of neurons within the lateral hypothalamus exhibited the DREADDs tag. Roughly 1% of these cells were not dual labeled with orexin A antibody.
Drugs
Clozapine N-Oxide (CNO; Sigma–Aldrich; St Louis, MO, USA) was dissolved in saline and 8% DMSO and injected intraperitoneally at 2 mg/kg in a 5 mg/mL solution. This dose is in accordance with doses used in previous DREADDs studies in rats (Farrell and Roth, 2013). MK-1064, a selective OX2R antagonist, (Roecker et al., 2014) was dissolved overnight in 20% Vitamin E d-a-tocopherol polyethylene glycol 1000 succinate (Vit E-TPGS) (Sigma–Aldrich; St Louis, MO, USA). Rats were orally administered either 20% Vit E-TPGS as vehicle or 30 mg/kg MK-1064, both at 1 mL/kg for consistent volume. All animals were given a vehicle dose 1 day prior to the start of the restraint paradigm to habituate to the oral gavage procedure. Animals received injections of vehicle (20% Vit E TPGS, p.o.) or MK-1064 and vehicle (saline and 8% DMSO, i.p.) or CNO 90 min prior to the start of the 30-min restraint. This timing was chosen based on the fact that both MK-1064 and CNO have been shown to promote behavioral effects in the rat within 30 min of administration and effects last up to 4 h after administration (Alexander et al., 2009; Farrell and Roth, 2013; Hasegawa et al., 2014; Roecker et al., 2014). Additionally, CNO was given at the time of MK-1064 injection to minimize the effects of repeated handling prior to restraint.
Assaying orexin levels in the CSF
DREADD-expressing rats were injected IP with either vehicle or CNO 90 min prior to either CSF collection. In a separate experiment, DREADD-expressing rats were injected IP with vehicle or CNO 90 min prior to stress exposure. Specifically, rats were either exposed to 1 or 5 days of 30-min restraint (IP injections were given daily prior to each restraint for the 5-day group). Animals were restrained in Plexiglas restrainers (9 × 3.5 inches) made in the University of Pennsylvania Machine Shop. Plexiglas inserts (7.5 × 3.25 inches) were placed inside the restrainers appropriate for the size of each male rat so that they could not turn around in the restrainer. As orexins are known to exhibit a circadian rhythm (Taheri et al., 1999; Fujiki et al., 2001; Zeitzer, 2013), animals were restrained within 2 h after lights on, a time at which orexin levels reach a nadir. At 15 min into the 1st or 5th restraint, CSF was collected (rough 105 min post IP injection). Briefly, rats were anesthetized with a cocktail of ketamine and xylazine, placed in the ear bars of a stereotaxic apparatus with their nose pointing downward, and a 25-gauge needle was used to extract 100 μL of CSF from the cisterna magna. CSF was ejected into eppendorf tubes and frozen at −80 °C until radioimmunoassay. CSF levels of orexin were assayed with a Radioimmunoassay kit from Phoenix Pharmaceuticals (Burlingame, CA). The minimum levels of detection for orexin was 80 pg/ml. Intra-and interassay variability was 5–7% and 12–15%, respectively.
Assaying orexin neuronal activation
Two additional groups of rats were injected with the DREADDs’ construct and exposed to either 1 or 5 days of restraint with injections of vehicle or CNO administered as previously described. Brains were collected for one group of rats on Day 1 of restraint (30 min after restraint ended), while brains were not collected in the other cohort until Day 5 of restraint. The lateral hypothalamus of these brains were further analyzed for orexin neural activation. Specifically, sections of the lateral hypothalamus were cut and fixed as described above in DREADDs’ immunofluorescent staining. Sections were incubated with c-Fos antibody (1:1250, sc-52; Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by Biotin-SP-conjugated AffiniPure Donkey-Antirabbit IgG (1:200, Jackson Immunoresearch, West Grove, PA, USA), which was visualized with a 3′3′-diaminobenzidine reaction (Sigma– Aldrich, St Louis, MO, USA). Sections were then stained for orexin A (1:50, sc-8070; Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by biotinylated Horse Anti-Goat antibody (1:500, BA-9500; Vector Laboratories, Burlingame, CA, USA), which was visualized with a Nova Red reaction (SK-4800, Vector Laboratories, Burlingame, CA, USA). Sections were mounted with permount (Electron Microscopy Sciences, Fort Washington, PA, USA). Images were acquired with a Leitz DMR microscope with a digital camera (Leica Microsystems; Buffalo Grove, IL, USA). Four sections per animal (with rostral-caudal coordinates (relative to bregma) ranging from −2.04 to −3.24) were analyzed using NIH Image J. Specifically, a standardized box encompassed the lateral hypothalamus (calculated using Paxinos & Watson Rat Brain Atlas) and dual-labeled cells were counted by hand. All image analysis was performed with the experimenter blind to the treatment groups.
Assessing HPA reactivity to acute or repeated restraint with orexin manipulations
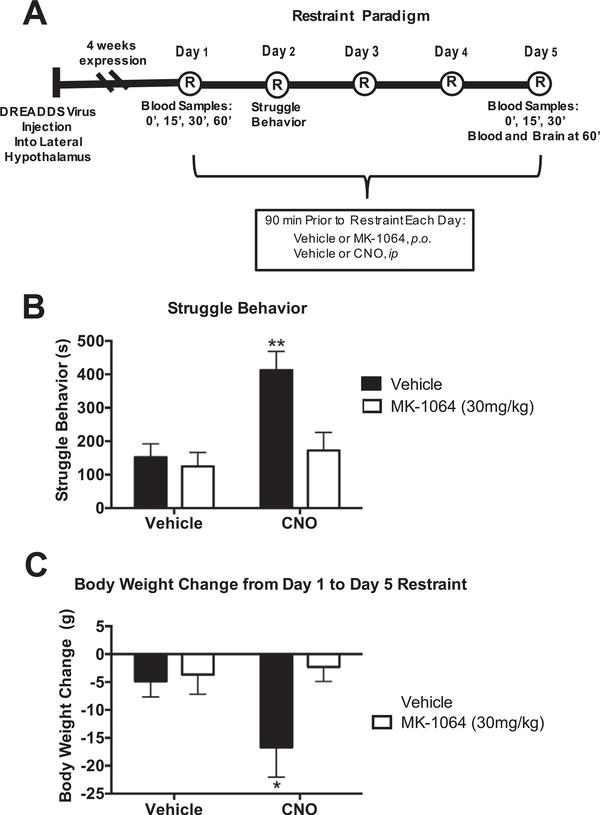
A naïve cohort of rats injected with DREADD-containing virus was exposed to either 1 or 5 consecutive days of restraint with injections of vehicle or CNO IP and either vehicle or MK-1064 administered PO 90 min prior to each restraint. See Fig. 3A for depiction of experimental paradigm. Animals were weighed on Day 1 and Day 5 of restraint. Video cameras were set up above the restrainers in order to record struggle behavior on day 2 of restraint. While rats are in the restrainer for 30 min, most of the struggle behavior occurs within the first 10 min. Therefore, we analyzed struggle behavior during this time period. A trained investigator blind to experimental groups hand scored struggle behavior – defined as attempts to escape, or intense movement of the animal while in the restrainer. Blood was collected on Day 1 and Day 5 of restraint to assess the HPA response to acute and repeated restraint, respectively. Briefly, on day 1, tail blood was taken at 0 min (prior to being placed in restraint), again at 15 min and 30 min (during restraint), and at 60 min (recovery time point in the home cage). Plasma corticosterone and Adrenocorticotropic Hormone (ACTH) were assayed with a Radioimmunoassay kit from MP Biomedical (Orangeburg, NY, USA). The minimum levels of detection for ACTH and corticosterone were 5.7 pg/ml and 0.6 μg/dl, respectively. Intra-and interassay variability was less than 10%.
Fig. 3.
OX2R underlies struggle behavior and body weight change during restraint stress (n = 7/group). (A) Timeline of experimental procedures. DREADD-containing viruses were injected into the lateral hypothalamus 4 weeks prior to the start of the restraint paradigm. MK-1064 and CNO were administered orally or subcutaneously, respectively, 90 min prior to each 30-min restraint for five consecutive days. Tail blood was collected at 0, 15, 30, and 60 min on day 1 of restraint for hormone assays. Struggle Behavior was quantified on Day 2 of restraint. Tail blood was collected at 0, 15, and 30 min on day 5 of restraint. Rats were euthanized at 60 min, with both blood and brain collected at this time. (B) Orexin activation by CNO increases struggle behavior (2-Way ANOVA, CNO: (F(1,22)= 9.8, p = 0.005; MK-1064: F(1,22) = 7.4, p = 0.013; Interaction: F (1,22) = 4.7, p = 0.042). (C) Body weight change after 5 days of restraint stress in rats differentially treated with CNO and/or MK-1064 (2-Way ANOVA, MK-1064: (F(1,23) = 4.3, p = 0.049; Interaction: F (1,23) = 3.1, p = 0.09). **p < 0.01.
Statistical analysis
Data are presented as the mean ± the standard error of the mean. For habituation of vehicle-treated animals, a 2-way ANOVA assessed Day and Time variables (Day 1 and 5 were compared, as well as the time point throughout restraint). A t-test was used to evaluate orexin neural activation and orexin levels in the CSF in control versus CNO treated animals on Day 1 and Day 5 of restraint. A 2-way ANOVA assessed Day and Drug variables for orexin neural activation and orexin A in the CSF throughout repeated restraint. For struggle behavior and body weight, a 2-way ANOVA assessed CNO and 2-SORA variables. For all other ACTH data, a 2-way ANOVA assessed Drug and Time variables on Day 1 and Day 5 separately. When warranted, Tukey’s corrected t-tests were performed. All hypothesis tests used α = 0.05 as the criterion level of significance. Statistical analysis was conducted with GraphPad Prism (GraphPad Software, La Jolla, CA, USA) in order to identify statistical differences.
RESULTS
Orexin levels habituate with repeated stress
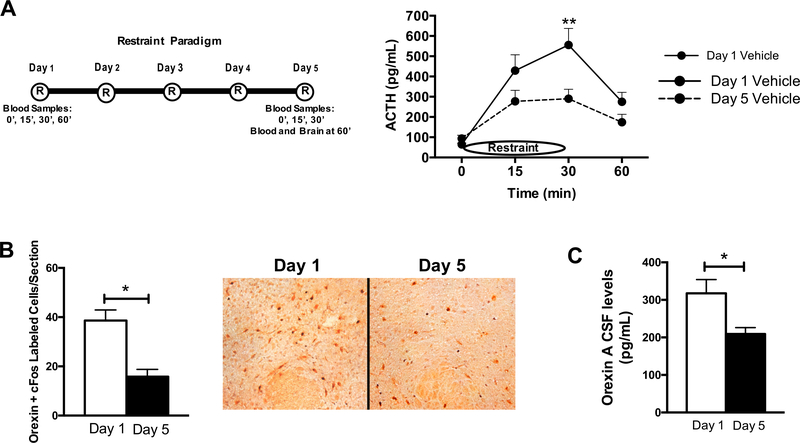
Habituation of the HPA response to 5 days of restraint stress was assessed by evaluating plasma ACTH levels prior to, during and after 30 min of restraint stress on day 1 and day 5 of restraint (paradigm and data in Fig. 1A). Consistent with previous results (Grissom and Bhatnagar, 2009), male rats in this paradigm habituated to 5 days of repeated restraint with regard to plasma ACTH levels. This effect was significant at 30 min of restraint. However, we did not observe habituation or group differences in our corticosterone assays, consistent with previous lab results that it takes 8–10 days of restraint to see corticosterone effects ((Grissom et al.,2008); data not shown). After demonstrating ACTH habituation to repeated restraint, we examined changes in orexins from one day to five days of daily 30-min restraint. To assess this, orexin neuronal activation and CSF levels of orexin were evaluated on both day 1 and day 5 of restraint. Representative images of the cFos/orexin dual stain on day 1 and day 5 of restraint are displayed in Fig. 1B. An average of 40 orexin cells per section were dual labeled with cFos after a single 30-min restraint, and this was significantly reduced to nearly half that amount by day 5 of restraint stress. In terms of percentages of total orexin neurons present, a single 30-min restraint activated 75% of orexin neurons, whereas only 40% were active on day 5 of restraint stress (data not shown). Orexin A levels in CSF showed a similar pattern to orexin neuronal activation, since orexin A levels in CSF habituated after 5 days of restraint stress (Fig. 1C). Specifically, orexin A levels were significantly reduced to approximately 66% of the day 1 value. In summary, both HPA and orexin activities decreased with repeated restraint stress.
Fig. 1.
Orexin levels habituate with repeated restraint stress (n = 10/group). (A) Left: Depiction of Restraint Paradigm and Blood collection. Right: Plasma ACTH data for Day 1 and Day 5 of restraint. Vehicle/Vehicle-treated animals display habituation over the 5 days of restraint stress, with decreased plasma ACTH values on day 5 compared to day 1 (2-Way ANOVA, Day: F(1,67) = 8.2, p = 0.006; Time: F(1,67) = 12.7, p < 0.0001). (B) Total orexin neurons dual labeled with cFos on day 1 versus day 5 of restraint stress (T test, p < 0.05). Right, representative 10x magnification images of the lateral hypothalamus with cFos (black) and orexin (red) dual stain on Day 1 and 5 of restraint. (C) Orexin levels in the CSF on day 1 and 5 of restraint stress (T test, p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Stimulation of orexins prior to each of five daily 30min restraints
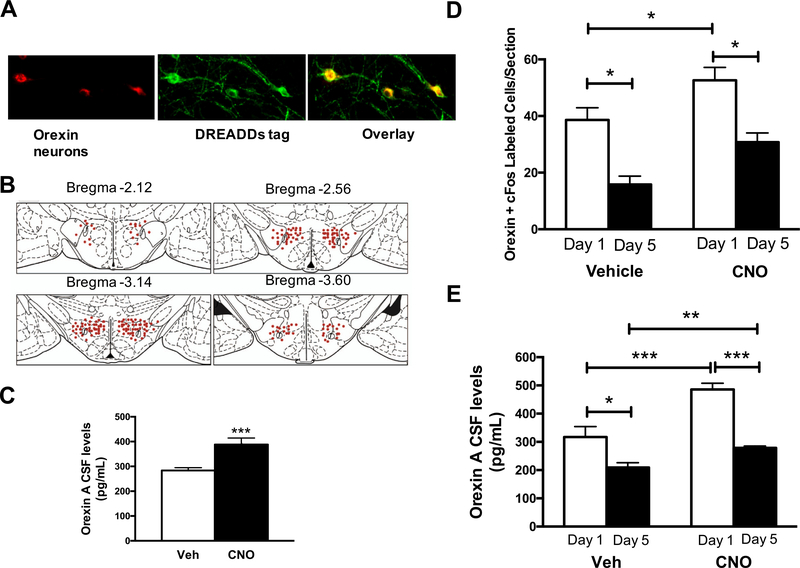
As certain disease states such as panic disorder are associated with increased central orexin levels and failure to habituate to repeated stress, we further stimulated orexins prior to each of five daily 30-min restraints. To do this, DREADDs were injected and expressed selectively in orexin neurons to activate these neurons. Representative fluorescent images of DREADDs expression at four weeks are displayed in Fig. 2A, along with a depiction of the spread of viral expression along four levels of Bregma (anterior to posterior) in Fig. 2B. Quantification revealed 71 ± 5.4% of neurons within the lateral hypothalamus exhibited the DREADDs’ tag (data not shown). Orexin levels in the CSF are quantified for vehicle- or CNO-treated DREADD-expressing rats in non-stress conditions in Fig. 2C. Relative to vehicle, CNO significantly increased the level of orexins in the CSF compared with vehicle-treated rats when evaluated 90 min after treatment.
Fig. 2.
Stimulation of orexins prior to each of five daily 30-min restraints (n = 10/group). (A) Representative images of DREADDs expression in the lateral hypothalamus at 20x magnification. Orexin Neurons = red, DREADDs’ tag = green, orexin neurons transfected with virus = yellow. (B) Viral expression along four levels of Bregma (anterior to posterior) in a cartoon using rat brain atlas images. Each red dot represents 10 cells expressing the viral tag. (C) Orexin A levels in CSF following vehicle or CNO treatment (non-restraint stress conditions; T test, p < 0.001). (D) Total orexin neurons dual labeled with cFos in acute and repeated restraint stress following either vehicle or CNO pretreatment (2-Way ANOVA, Day: (F (1,62) = 9.6, p < 0.001; CNO: F(1,62) = 4.1, p < 0.05, Interaction: F(1,62) = 0.01, p = 0.95). (E) Orexin A levels in the CSF following acute and repeated restraint stress with either vehicle or CNO pretreatment (2-Way ANOVA, Day: (F(1,37) = 26.47, CNO: p < 0.0001; Interaction: F(1,37) = 15.05, p = 0.0004; F(1,37) = 2.6, p = 0.11). *p < 0.05, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
These data validated the functionality of the DREADDs’ construct.
Moreover, orexin neuronal activation with DREADDs stimulation prior to each of five daily 30-min restraints was quantified (Fig 2D). Compared with vehicle treatment, CNO significantly increased the number of orexin neurons dual labeled with cFos (and hence neural activation) on day 1 of restraint. The level of CNO-induced orexin neuron recruitment on day 5, however, remained significantly below that seen on day 1 of restraint stress after CNO (just as was seen with vehicle-treated groups on day 1 compared with day 5).
Lastly, orexin levels in the CSF with DREADDs’ stimulation prior to each of five daily 30-min restraints were quantified (Fig 2E). Relative to vehicle-treated animals, CNO induced orexin A in CSF to significantly greater levels on day 1, verifying that DREADD-mediated CNO activation of orexin neurons resulted in augmented release of orexin A neuropeptide. While CNO treatment also increased orexin A levels on day 5 of restraint compared with vehicle treatment, CNO-treated animals exhibited significantly reduced orexin A levels on day 5 compared with day 1 of restraint, just as it did in vehicle-treated animals. Together, these data indicate that the DREADDs’ constructs increased orexin activation and levels in the CSF, but repeated restraint stress partially counteracts these effects by lowering orexin activity and levels in the CSF compared to day 1 of restraint.
OX2R underlies struggle behavior and body weight change during restraint stress
The contribution of orexin receptor signaling in both struggle behavior and body weight change in response to restraint stress was determined through use of a selective OX2R antagonist, MK-1064. Specifically, after DREADDs’ expression, rats underwent five consecutive days of 30-min restraint, with either vehicle or CNO to stimulate DREADDs prior to each restraint, and with either vehicle or MK-1064 to antagonize the orexin 2 receptor (paradigm depicted in Fig. 3A). Struggle behavior indicates the number of attempts to escape during restraint, which is an important component of the stress response and habituates with repeated restraint (Grissom et al., 2008). As demonstrated in Fig. 3B, the CNO/vehicle group spent significantly more time struggling than the other three treatment groups. Essentially, stimulating orexins with CNO increases struggle behavior, and blocking OX2R with MK-1064 reverses this effect. Coincident with changes observed in struggle behavior, body weight decreased from the first to the last day of restraint in levels of high orexin, but this was eliminated with MK-1064 pretreatment (Fig. 3C). Thus, while all rats lost weight over the 5 days of restraint stress, further stimulating orexins prior to each 30-min restraint exacerbated this effect and these results appear mediated through OX2R.
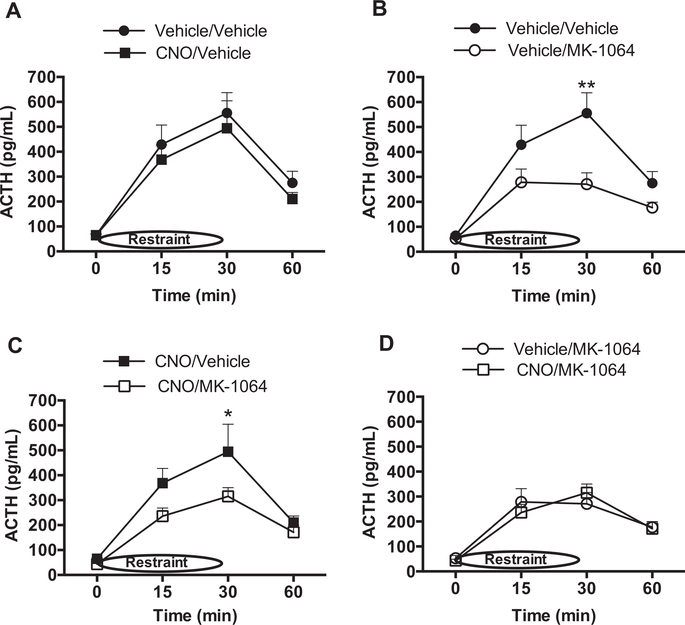
OX2R is involved in the ACTH response to acuterestraint stress
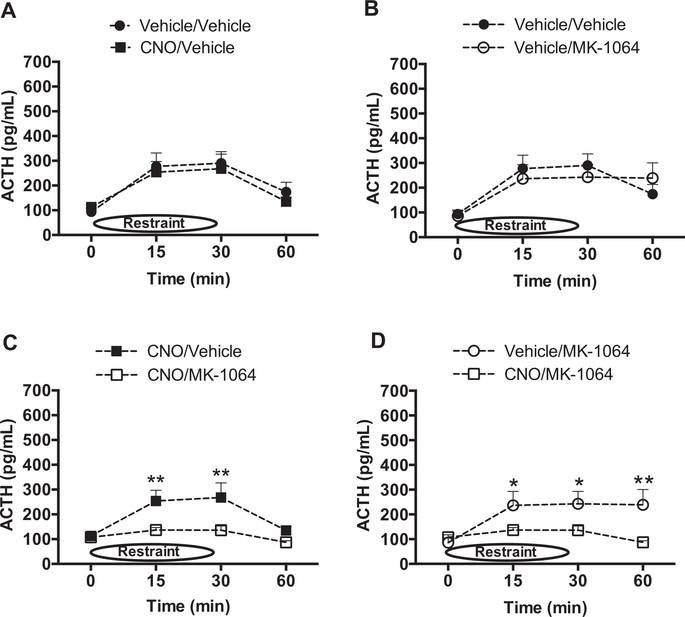
The role of orexin signaling in the HPA response to acute restraint stress was determined by measuring plasma ACTH levels in response to 30 min of restraint in DREADD-expressing rats pretreated with either vehicle or CNO in combination with either vehicle or the OX2R antagonist, MK-1064. CNO pretreatment did not increase ACTH levels compared with that from vehicle-treated rats (Fig. 4A). Pretreatment with MK-1064, however, significantly attenuated the ACTH response in combination with either vehicle (Fig. 4B), or CNO pre-treatment (Fig. 4C), indicating that OX2R is responsible for mediating at least part of the ACTH response to acute restraint. Importantly, the ACTH response was similarly attenuated by MK-1064 in either the presence or absence of CNO (Fig. 4D), indicating that activation of orexin signaling through mechanisms not involving OX2R contributes little to the ACTH response on this first day of restraint stress.
Fig. 4.
OX2R is involved in the ACTH response to acute restraint stress (n = 12/group). ACTH levels were evaluated on the first day of restraint stress following vehicle/vehicle versus CNO/ vehicle pretreatment to evaluate orexin neuron activation (A; no significance by ANOVA), vehicle/ vehicle versus vehicle/MK-1064 pretreatment to evaluate the role of endogenous OX2R (B; significant: 2-Way ANOVA, MK-1064: (F(1,63) = 9.3, p = 0.003), CNO/MK-1064 versus CNO/ vehicle pretreatment to assess the effect of exogenous OX2R activity induction (C; significant: 2Way ANOVA, Drug: F(1,71) = 4.6, p = 0.035), or Vehicle/MK-1064 versus CNO/MK-1064 pretreatment to understand the role of orexin-induced mechanisms other than OX2R (D; no significance by ANOVA). *p < 0.05, **p < 0.01.
Orexin signaling through OX2R and non-OX2R mechanisms have opposing influences on ACTH responses to repeated restraint stress
The role of orexin signaling in mediating the HPA response to repeated stress was evaluated in Fig. 5. Specifically, rats were evaluated for plasma ACTH levels on the 5th day of restraint stress following treatment with CNO and/or MK-1064. CNO treatment did not change ACTH levels on day 5 of restraint compared with vehicle-only controls (Fig. 5A). In the absence of CNO, blockade of endogenous OX2R activity by MK-1064 also did not further affect plasma levels of ACTH compared with vehicle (Fig. 5B), unlike that seen on the first day of restraint stress. However, in rats treated with CNO to induce orexin neuron activation in combination with MK-1064, the ACTH response was attenuated further (Fig. 5C). Because ACTH levels are elevated following stimulation of orexin neuron activation (CNO/Vehicle) relative to that following stimulation of orexin neuron activation plus antagonism of the OX2R (CNO/MK-1064), these results indicate that OX2R activity has the capacity to increase ACTH levels after 5 days of restraint stress. In other words, exogenous OX2R activity prevents the full habituation to repeated restraint. Indeed, ACTH responses are reduced by the CNO/ MK-1064 manipulation relative to the Vehicle/MK-1064 treatment (Fig. 5D). This comparison evaluates the influence of total orexin neuron activity minus that of OX2R, indicating that possibly OX1R, or other mechanisms induced by orexin neuron activity, has the capacity to further reduce ACTH responses to repeated restraint stress.
Fig. 5.
OX2R- and non-OX2R-mediated orexin mechanisms have opposing influences on ACTH responses to repeated restraint stress. (n = 12/group) ACTH levels were evaluated on the fifth day of restraint stress following vehicle/vehicle versus CNO/vehicle pretreatment to evaluate orexin neuron activation (A; no significance by ANOVA), vehicle/vehicle versus vehicle/MK-1064 pretreatment to evaluate the role of endogenous OX2R (B; no significance by ANOVA), CNO/MK1064 versus CNO/vehicle pretreatment to assess the effect of exogenous OX2R activity induction (C; significant: 2-Way ANOVA, Drug: F(1,45) = 13.1, p = 0.001), or Vehicle/MK-1064 versus CNO/MK-1064 pretreatment to understand the role of orexin-induced mechanisms other than OX2R (D; significant: 2-Way ANOVA, Drug: F(1,40) = 12.02, p < 0.01). *p < 0.05, **p < 0.01.
DISCUSSION
While orexins are known to play a role in the acute stress response, it was unclear how they mediated the response to repeated stressors, and the contribution of orexin receptors to both acute and repeated stress was undetermined. With repeated exposure to modest stressors, individuals typically habituate to that stress by decreasing responsivity, particularly in HPA measures (Grissom et al., 2008; Grissom and Bhatnagar, 2009). Failure to habituate is a hallmark of stress-related illnesses such as PTSD and panic disorder (Johnson et al., 2012). The present studies demonstrated HPA habituation to repeated restraint in male rodents, and determined that both orexin neuronal activation and levels in the CSF decrease after repeated restraint. As central orexins are augmented in some disease states such as panic disorder (where patients fail to habituate to repeated stress), high levels of orexin were stimulated via DREADDs prior to each of five daily 30-min restraints. Our data confirm that our DREADDs’ construct was efficacious in stimulating orexin neuronal activation and increasing orexin levels in the CSF in non-stress conditions, as well as in acute and repeated restraint. We were also able to discern the contribution of orexin receptor signaling in acute and repeated stress through use of a selective OX2R antagonist, MK-1064. We found that the OX2R contributed to struggle behavior and body weight decrease during repeated restraint. Moreover, the OX2R played a role in the plasma ACTH response to acute stress and repeated stress, although the latter was only in conditions of high orexin release.
Orexins and the HPA axis reciprocally interact. Specifically, central orexin increases corticosterone and ACTH, whereas CRH depolarizes orexin neurons (Russell et al., 2001; Samson et al., 2002; Winsky-Sommerer et al., 2004). The activation of orexin neurons during acute stress has been examined by a limited number of studies using a wide variety of stress paradigms (Chang et al., 2007; Furlong et al., 2009; Chen et al., 2013). As much of the previous literature has focused on the role of orexin A in stress (Ida et al., 2000; Russell et al., 2001; Sakamoto et al., 2004), our studies specifically examined orexin A activity both through dual labeling of orexin A neurons with cFos (a measure of neural activation) and orexin A levels in the CSF in acute and repeated stress. Importantly, our results indicate that acute restraint stress induces very high levels of orexin neuron activation (75%), accompanied by increased levels of orexin A in the CSF. We also show, for the first time, that orexin activity decreases with habituation to repeated stress. Thus, with repeated exposure to a moderately intense stressor, both the HPA and orexin responses diminish. When inducing additional orexin activation via DREADDs prior to each of five daily 30-min restraints, we were unable to increase orexin neuron activation to the levels seen with acute stress. Based on the literature, CNO does not appear to become less effective after repeated administration, so this is likely not the cause for these results (Kozorovitskiy et al., 2012). Moreover, this reduced responsiveness of orexin neurons does not appear due to depletion of available orexin neuropeptide stores, since DREADD-induced CSF levels of orexin A and orexin neuron activation as measured by c-fos expression were both attenuated during habituation. It is important to note that Orexin B also plays an important role in the stress response (Kuru et al., 2000; Shirasaka et al., 2001b; Spinazzi et al., 2006), so assessing orexin B levels in the future may shed more light on how the orexins are involved in repeated stress.
Struggle behavior and body weight changes are important components of the response to stressful stimuli. Our data show that exogenous orexin stimulation increased struggle behavior and decreased body weight. This was reversed by the OX2R antagonist, indicating that OX2R plays a role in these components of the stress response in conditions of high orexin release. This effect on struggle behavior is consistent with literature support for the OX2R in promoting arousal (Gotter et al., 2012b). Moreover, arousal is an important component of the stress response (Giardino and de Lecea, 2014), so it is likely that DREADD-induced orexin activation is increasing struggle behavior by increasing arousal. However, our data indicate that DREADDs differentially modulate the HPA response and struggling behavior, thus, mechanisms above and beyond those regulated by orexins may be involved. Although orexin antagonists are known to promote sleep (Gotter et al., 2016), they still allow arousability to salient stimuli (Tannenbaum et al., 2014, 2016). Thus, it is unlikely that reduction in struggle behavior is due to the sedative effects of the OX2R antagonist. However, as we did not directly measure sleep using telemetry devices, this possibility cannot be ruled out.
It is unclear where OX2Rs are acting to regulate struggle behavior and body weight gain. Previous literature has indicated that rats with high amygdala excitability show increased struggling behavior (Anisman et al., 1997; McIntyre et al., 1999). Given that OX2Rs are expressed in medial but not central amygdala, this subnuclei of the amygdala could be an important substrate for regulating struggle behavior (Marcus et al., 2001). Alternatively, both central and peripheral regions may play a role in OX2R mediation of body weight gain. For example, central orexins acting on hypothalamic and dopaminergic brain regions have been implicated in increasing spontaneous physical activity and nonexercise thermogenesis (Kotz et al., 2012). Moreover, orexins acting on white adipose tissue have been shown to promote lipolysis (Perez-Leighton et al., 2014). Thus, it is possible that the reduction in body weight observed with CNO treatment is a result of increased energy expenditure and lipolysis by animals in this treatment group.
There have been few studies delineating the contribution of each orexin receptor to the acute HPA response. One study reports that pretreatment with an OX2R antagonist prevents forced swim-induced increases in plasma ACTH and corticosterone (Chang et al., 2007). This is consistent with our findings that OX2R contributes to the HPA response induced by acute restraint stress. However, as MK-1064 pretreatment only reduced the HPA response by half, it is still possible that the other components of the stress response may play a role in regulating the acute HPA response to restraint. Possible components would not include the contribution of OX1R, however, as the ACTH response was similarly attenuated by MK-1064 in either the presence or absence of CNO. As OX2R is preferentially expressed (over OX1R) in the paraventricular nucleus of the hypothalamus, antagonizing OX2R (but not necessarily OX1R) would likely reduce the acute HPA response (Shirasaka et al., 2001a; Gotter et al., 2012b). However, other sites expressing OX2R such as the amygdala, paraventricular nucleus of the thalamus (PVT), and hippocampus may also be involved (Marcus et al., 2001).
We found that under endogenous orexin levels elicited by restraint alone, blocking OX2R had no effect on ACTH levels on day 5 of restraint. Thus, in these conditions, OX2R does not affect habituation. However, our data measuring orexin levels in the CSF revealed that compared with one day of 30-min restraint, orexin levels decreased by five days of daily 30-min restraint. Hence, blocking OX2R in low orexin conditions may not have an effect. However, when orexins are stimulated with DREADDs, blocking OX2R further attenuates ACTH responses. This indicates OX2R plays a role in repeated restraint stress only in conditions of high orexin release. This also indicates that OX2R desensitization does not appear to be involved in typical habituation. However, the ultimate effects of orexins throughout stress might be dependent on the balance of signaling from both orexin receptors (Scott et al., 2011). Though, not much is known about orexin receptor regulation after stress. While the OX2R activity promotes ACTH release and prevents habituation in high levels of orexin such as that seen during acute stress conditions, one possibility is that OX1R activated by orexin release could promote habituation, as CNO/MK-1064-treated animals have even lower plasma ACTH levels than Vehicle/MK-1064-treated animals after repeated restraint. Thus, one interpretation of the results is that OX1R underlies habituation. Direct tests of OX1R function are required to confirm this interpretation. Interestingly, previous data from our lab indicates that the OX1R in the posterior paraventricular thalamus is necessary in adapting to repeated swim stress, allowing facilitation to a novel restraint stressor (Heydendael et al., 2011). However, it is not clear whether orexins are involved in facilitation after habituating to repeated restraint. Overall, we believe the evidence suggests that orexin neuron has the capacity to influence habituation through opposing OX2R and non-OX2R mechanisms.
The results from this study improve our understanding of the role of orexin receptors in regulating the HPA response to both acute and repeated stress. We have confirmed the involvement of OX2R in acute stress, and have shown that it has the capacity to promote ACTH levels opposing habituation to repeated stress in conditions of exogenously elevated orexin signaling. Future studies should identify not only the neuronal pathways regulating the reduced responsiveness of orexin neurons during habituation, but also investigate the specific brain regions where each receptor may be acting to influence these changes in HPA activity, struggle behavior, and body weight gain. While there are high concentrations of OX2R in the PVN, the locus coeruleus preferentially expresses OX1R (Peyron et al., 1998; Marcus et al., 2001). Additionally, other stress regulatory regions with high concentrations of orexin input include the PVT and the amygdala. These experiments would allow for a greater understanding of mechanisms associated with hyperactivation of orexin signaling observed in psychiatric illness such as panic disorder.
Acknowledgments
This work was supported by Merck & Co., Inc.. LG was supported by award number T32NS007413 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is the sole responsibility of the authors and does not necessarily represent the official views of the NINDS of the National Institutes of Health. We gratefully acknowledge Lauren Wilson and Jane Dobkin for their help in coding struggle behavior, as well as Amanda Cornfeld for validating DREADDs expression by fluorescent labeling.
Footnotes
DISCLOSURES
ALG, CJW and JJR are employees of Merck & Co., Inc., Kenilworth, NJ (known as Merck Sharp & Dohme Corp. (MSD) outside the United States and Canada), have received salary and research support from the company, and may own stock/stock options in the company.
REFERENCES
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63:27–39. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2751885&tool= pmcentrez&rendertype=abstract [accessed 21.01.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Lu ZW, Song C, Kent P, McIntyre DC, Merali Z (1997) Influence of psychogenic and neurogenic stressors on endocrine and immune activity: differential effects in fast and slow seizing rat strains. Brain Behav Immun 11:63–74. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9193768 [accessed 07.01.2016]. [DOI] [PubMed] [Google Scholar]
- Berridge CW, España RA, Vittoz NM (2010) Hypocretin/orexin in arousal and stress. Brain Res 1314:91–102. Available at: www.sciencedirect.com/science/article/pii/S0006899309018939> [accessed 30.08.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman BT, Lebold TP, Nepomuceno D, Lord B, Wennerholm M, Shelton J, Carruthers N, Lovenberg T, Dugovic C (2015) A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther 352:590–601. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/25583879> [accessed 06.01.2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, Soya H (2007) Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci Res 57:462–466. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/17188385> [accessed 20.04.2015]. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, Vrontakis ME, Kirouac GJ (2013) Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct Funct. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/23955372> [accessed 28.01.2014]. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95:322–327. Available at: <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=18213&tool=pmcentrez&rendertype=abstract> [accessed 23. 01.2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Roth BL (2013) Pharmacosynthetics: reimagining the pharmacogenetic approach. Brain Res 1511:6–20. Available at: <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3562395&tool=pmcentrez&rendertype=abstract> [accessed 18.03.2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Ripley B, Honda K, Mignot E, Nishino S (2001) Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 hours and in response to food deprivation. NeuroReport 12:993–997. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/11303775> [accessed 25.02.2015]. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Vianna DML, Liu L, Carrive P (2009) Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 30:1603–1614. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/19811530> [accessed 16.02.2014]. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, de Lecea L (2014) Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol 29:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Roecker AJ, Hargreaves R, Coleman PJ, Winrow CJ, Renger JJ (2012a) Orexin receptors as therapeutic drug targets. Prog Brain Res 198:163–188. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/22813974> [accessed 18.03.2014]. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ (2012b) International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev 64:389–420. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/22759794> [accessed 18.03.2014]. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Forman MS, Harrell CM, Stevens J, Svetnik V, Yee KL, Li X, Roecker AJ, Fox SV, Tannenbaum PL, Garson SL, De Lepeleire I, Calder N, Rosen L, Struyk A, Coleman PJ, Herring WJ, Renger JJ, Winrow CJ (2016) Orexin 2 receptor antagonism is sufficient to promote NREM and REM sleep from mouse to man. Sci Rep 6:27147 Available at: <http://www.ncbi.nlm.nih.gov/pubmed/27256922> [accessed 13.02.2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Lepore S, Vicentini E, Merlo-Pich E, Bifone A (2013) Differential effect of orexin-1 and CRF-1 antagonism on stress circuits: a fMRI study in the rat with the pharmacological stressor Yohimbine. Neuropsychopharmacology 38:2120–2130. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/23736277> [accessed 28.01.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S (2009) Habituation to repeated stress: get used to it. Neurobiol Learn Mem 92:215–224. Available at: <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2773683&tool=pmcentrez&rendertype=abstract> [accessed 20.03.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Kerr W, Bhatnagar S (2008) Struggling behavior during restraint is regulated by stress experience. Behav Brain Res 191:219–226. Available at: <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2477735&tool=pmcentrez&rendertype=abstract> [accessed 20.03.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ et al. (1999) Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A 96:10911–10916. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/10485925> [accessed 08.09.2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E, Yanagisawa M, Sakurai T, Mieda M (2014) Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest 124:604–616. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3904620&tool=pmcentrez&rendertype=abstract> [accessed 31.03.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S (2011) Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152:4738–4752. Available at: <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3230061&tool=pmcentrez&rendertype=abstract>> [accessed 25.01.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N (2000) Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun 270:318–323. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/10733946> [accessed 11.08.2014]. [DOI] [PubMed] [Google Scholar]
- Jászberényi M, Bujdosó E, Pataki I, Telegdy G (2000) Effects of orexins on the hypothalamic–pituitary–adrenal system. J Neuroendocrinol 12:1174–1178. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/11106974> [accessed 11.08.2014]. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Träskman-Bendz L, Goddard AW, Brundin L, Shekhar A (2010) A key role for orexin in panic anxiety. Nat Med 16:111–115. Available at: <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2832844&tool=pmcentrez&rendertype=abstract> [accessed 03.08.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A (2012) Orexin, stress, and anxiety/panic states. Prog Brain Res 198:133–161. Available at: <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3665356&tool=pmcentrez&rendertype=abstract> [accessed 27.01.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C (2012) Brain orexin promotes obesity resistance. Ann N Y Acad Sci 1264:72–86. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/22803681> [accessed 30.08. 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL (2012) Recurrent network activity drives striatal synaptogenesis. Nature 485:646–650. Available at: <http://www.nature.com/doifinder/10.1038/nature11052> [accessed 05.01.2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H (2000) Centrally administered orexin/hypocretin activates HPA axis in rats. NeuroReport 11:1977–1980. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/10884055> [accessed 06.01.2016]. [DOI] [PubMed] [Google Scholar]
- Le T, Liang Z, Patel H, Yu MH, Sivasubramaniam G, Slovitt M, Tanentzapf G, Mohanty N, Paul SM, Wu VM, Beitel GJ (2006) A new family of Drosophila balancer chromosomes with a w-dfdGMR yellow fluorescent protein marker. Genetics 174:2255–2257. Available at: <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1698648&tool=pmcentrez&rendertype=abstract> [accessed 12.11.2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/11370008> [accessed 19.03.2014]. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Kent P, Hayley S, Merali Z, Anisman H (1999) Influence of psychogenic and neurogenic stressors on neuroendocrine and central monoamine activity in fast and slow kindling rats. Brain Res 840:65–74. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/10517953> [accessed 07.01.2016]. [DOI] [PubMed] [Google Scholar]
- Nagahara T, Saitoh T, Kutsumura N, Irukayama-Tomobe Y, Ogawa Y, Kuroda D, Gouda H, Kumagai H, Fujii H, Yanagisawa M, Nagase H (2015) Design and synthesis of non-peptide, selective orexin receptor 2 agonists. J Med Chem 58:7931–7937. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/26267383> [accessed 08.09.2016]. [DOI] [PubMed] [Google Scholar]
- Perez-Leighton CE, Billington CJ, Kotz CM (2014) Orexin modulation of adipose tissue. Biochim Biophys Acta 1842:440–445. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/23791983> [accessed 30.08.2016]. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/9822755> [accessed 11.08.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roecker AJ et al. (2014) Discovery of 500-chloro-N-[(5,6dimethoxypyridin-2-yl)methyl]-2,20:50,300-terpyridine-30carboxamide (MK-1064): a selective orexin 2 receptor antagonist (2-SORA) for the treatment of insomnia. ChemMedChem 9:311–322. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/24376006> [accessed 15.01.2015]. [DOI] [PubMed] [Google Scholar]
- Russell SH, Small CJ, Dakin CL, Abbott CR, Morgan DG, Ghatei MA, Bloom SR (2001) The central effects of orexin-A in the hypothalamic–pituitary–adrenal axis in vivo and in vitro in male rats. J Neuroendocrinol 13:561–566. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/11412343> [accessed 11.08.2014]. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y (2004) Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept 118:183–191. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/15003835> [accessed 15.02.2015]. [DOI] [PubMed] [Google Scholar]
- Sakurai T (2014) The role of orexin in motivated behaviours. Nat Rev Neurosci 15:719–731. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/25301357> [accessed 10.10.2014]. [DOI] [PubMed] [Google Scholar]
- Sakurai T et al. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. Available at: www.ncbi.nlm.nih.gov/pubmed/9491897> [accessed 15.02.2015]. [DOI] [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Follwell M, Ferguson AV (2002) Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept 104:97–103. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/11830283> [accessed 11.08.2014]. [DOI] [PubMed] [Google Scholar]
- Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, Nestler EJ, Elmquist JK, Lutter M (2011) Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res 222:289–294. Available at: <http://www. pubmedcentral.nih.gov/articlerender.fcgi?artid=3474296&tool=pmcentrez&rendertype=abstract> [accessed 30.10.2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H (2001a) Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol Regul Integr Comp Physiol 281:R1114–R1118. Available at: <http://ajpregu.physiology.org/content/281/4/R1114.abstract> [accessed 05.06. 2015]. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H (2001b) Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol Regul Integr Comp Physiol 281:R1114–R1118. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/11557617> [accessed 28.12.2015]. [DOI] [PubMed] [Google Scholar]
- Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG (2006) Orexins in the regulation of the hypothalamic–pituitary–adrenal axis. Pharmacol Rev 58:46–57. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/16507882> [accessed 05.08.2014]. [DOI] [PubMed] [Google Scholar]
- Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei MA, Bloom SR (1999) Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett 457:157–161. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/10486585> [accessed 30.10.2015]. [DOI] [PubMed] [Google Scholar]
- Tannenbaum PL, Stevens J, Binns J, Savitz AT, Garson SL, Fox SV, Coleman P, Kuduk SD, Gotter AL, Marino M, Tye SJ, Uslaner JM, Winrow CJ, Renger JJ (2014) Orexin receptor antagonist-induced sleep does not impair the ability to wake in response to emotionally salient acoustic stimuli in dogs. Front Behav Neurosci 8:182 Available at: <http://www.ncbi.nlm.nih.gov/pubmed/24904334> [accessed 15.02.2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum PL, Tye SJ, Stevens J, Gotter AL, Fox SV, Savitz AT, Coleman PJ, Uslaner JM, Kuduk SD, Hargreaves R, Winrow CJ, Renger JJ (2016) Inhibition of orexin signaling promotes sleep yet preserves salient arousability in monkeys. Sleep 39:603–612. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/26943466> [accessed 15.02.2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L (2004) Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci 24:11439–11448. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/15601950> [accessed 11.08.2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L (2005) Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol 32:285–294. Available at: <http://www.ncbi.nlm.nih.gov/pubmed/16385142> [accessed 15.02.2015]. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM (2013) Control of sleep and wakefulness in health and disease. Prog Mol Biol Transl Sci 119:137–154. Available at: <http://www.sciencedirect.com/science/article/pii/B9780123969712000063> [accessed 11.08.2015]. [DOI] [PubMed] [Google Scholar]