Abstract
RAR-related orphan receptor γ (RORγ) is a nuclear receptor that plays an essential role in the development of T helper 17 (Th17) cells of the adaptive immune system. The NLRP3 inflammasome is a component of the innate immune system that processes interleukin (IL)-1β into a mature cytokine. Elevated activity of the NLRP3 inflammasome contributes to the progression of an array of inflammatory diseases. Bone marrow–derived macrophages (BMDMs) isolated from RORγ-null mice displayed reduced capacity to secrete IL-1β, and they also displayed a reduction in Nlrp3 and Il1b gene expression. Examination of the promoters of the Il1b and Nlrp3 genes revealed multiple putative ROR response elements (ROREs) that were occupied by RORγ. RORγ inverse agonists were effective inhibitors of the inflammasome. RORγ inverse agonists suppressed lipopolysaccharide (LPS)/ATP-stimulated IL-1β secretion and expression of Il1b and Nlrp3 in BMDMs. Additionally, the ability of the RORγ inverse agonists to suppress IL-1β secretion was lost in Nlrp3-null macrophages. The potential for targeting the NLRP3 inflammasome in vivo using RORγ inverse agonists was examined in two models: LPS-induced sepsis and fulminant hepatitis. Pharmacological inhibition of RORγ activity reduced plasma IL-1β as well as IL-1β production by peritoneal macrophages in a model of LPS-induced sepsis. Additionally, RORγ inverse agonists reduced mortality in an LPS/d-galactosamine–induced fulminant hepatitis mouse model. These results illustrate a major role for RORγ in regulation of innate immunity via modulation of NLRP3 inflammasome activity. Furthermore, these data suggest that inhibiting the NLRP3 inflammasome with RORγ inverse agonists may be an effective method to treat NLRP3-associated diseases.
Keywords: nuclear receptor, interleukin 1 (IL-1), innate immunity, inflammasome, liver, liver injury, transcription factor, drug discovery, macrophage
Introduction
The NLRP3 inflammasome complex is a critical component of the innate immune system and functions as a central sensor of pathogen-associated and/or cellular damage–associated molecules. Upon activation by these pathogen/damage-associated molecules, the NLRP3 inflammasome facilitates processing and secretion of the pro-inflammatory cytokine IL-1β3 (1). Activation of the NLRP3 inflammasome requires two steps. First, the expression of genes encoding proteins critical for NLRP3 inflammasome function, such as Nlrp3 and Il1b, is induced by “priming” with a pro-inflammatory stimuli, such as lipopolysaccharide (LPS). Then damage- or pathogen-associated molecular patterns (DAMPs or PAMPs) act as secondary signals to trigger several pathways activating the assembly of the NLRP3 inflammasome and activation of caspase-1–dependent processing of proIL-1β to mature IL-1β. A wide range of DAMPs/PAMPs that activate the NLRP3 inflammasome have been identified, including cholesterol and urate crystals, reactive oxygen species, ATP, β-amyloid, and bacterial toxins (2–4). Thus, the function of the inflammasome is tightly regulated by multiple signals that limit unconstrained production of IL-1β. Inappropriate regulation of NLRP3 inflammasome activity is associated with significant pathology. Abnormally elevated NLRP3 inflammasome activity is fundamental in the development of IL-1β–driven diseases, including atherosclerosis, Alzheimer's disease, and rheumatoid arthritis, where accumulation of crystals of cholesterol, β-amyloid, or hydroxyapatite is perceived as an endogenous sterile stress (2, 5, 6). Thus, pharmacological suppression of the NLRP3 inflammasome may be a reasonable target for development of a new class of therapeutics to treat a number of diseases associated with elevated inflammation.
Nuclear receptors are a large family of ligand-dependent transcription factors involved in regulation of developmental, metabolic, and inflammatory processes. The retinoic acid receptor–related orphan receptors (RORs) are a subgroup of nuclear receptors that are well-known as regulators of the circadian rhythm, metabolism, and immune function (7–9). The ROR subfamily is composed of three members: RORα, RORβ, and RORγ. RORγ has been the focus of particularly intense investigation due to its role as a critical factor driving T-helper 17 (Th17) lymphocyte differentiation and function (10). Regulation of Th17 cell differentiation and function is particularly important with regard to autoimmune diseases, where the number and activity of these cells are elevated. We and others have demonstrated that targeting inhibition of RORγ activity with inverse agonists is an effective method for suppressing Th17 cell differentiation and function as well as treatment of autoimmune diseases in rodent models (7, 11–13). Several of these RORγ inverse agonists are now being evaluated in clinical trials to treat autoimmune diseases such as psoriasis (14). Beyond Th17 lymphocytes, RORγ is also expressed in innate immune cells, such as macrophages and natural killer cells, suggesting a wider role for RORγ beyond Th17 cell differentiation and function in regulation of immunity.
In this report, we describe the regulation of the NLRP3 inflammasome by RORγ via direct regulation of Nlrp3 and Il1b gene expression. Furthermore, we demonstrate that RORγ inverse agonists effectively inhibit NLRP3 inflammasome activity in vitro and in vivo, suggesting that this class of drug may have much wider utility in the treatment of inflammatory disorders than originally anticipated.
Results
RORγ is required for normal NLRP3 inflammasome activity in macrophages
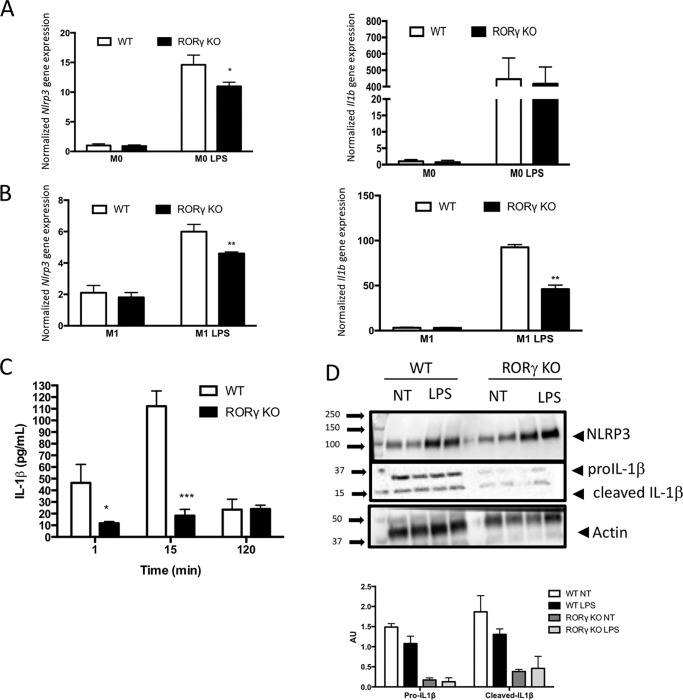
We recently demonstrated that the nuclear receptor REV-ERBα plays a role in regulation of the NLRP3 inflammasome (15). Bone marrow–derived macrophages (BMDMs) from Rev-erbα-null mice display elevated Nlpr3 and Il1b gene expression due to the inability of REV-ERB to suppress the expression of these genes (15). Given that RORγ often functions in a reciprocal manner to REV-ERB, we performed a similar experiment in BMDMs from RORγ-null mice. BMDMs were cultured for 6 days and then polarized (M1) or not (M0) with LPS (100 ng/ml) for 24 h. After treatment, M0 and M1 cells were treated with LPS (100 ng/ml) for 1 h to activate the NLRP3 inflammasome. As illustrated in Fig. 1B, Nlrp3 and Il1b expression was significantly reduced in M1 BMDMs from RORγ-null mice relative to WT M1 BMDM under LPS-stimulated conditions. In undifferentiated M0 BMDMs, we observed that Nlrp3 gene expression was reduced following LPS treatment in the RORγ-null cells, but Il1b expression was not changed (Fig. 1A). We then assessed the effect of RORγ deletion on IL-1β secretion. BMDMs were polarized for 24 h with LPS (100 ng/ml) followed by treatment with ATP for varying lengths of time (1, 15, or 120 min) to simulate IL-1β secretion. We observed an attenuation in Il-1β secretion from the RORγ knockout (KO) BMDMs compared with WT cells at the 1- and 15-min times post-ATP stimulation (Fig. 1C). Although we observed ∼25% decrease in Nlrp3 gene expression in M1 polarized BMDMs (Fig. 1B), NLRP3 protein expression was only suppressed ∼8% in the RORγ-null BMDMs (M1 polarized) (Fig. 1D). Even with the relatively small reduction in NLRP3 expression, intracellular IL-1β protein expression (proIL-1β and cleaved IL-1β) was substantially reduced in RORγ KO macrophages compared with WT macrophages (Fig. 1D). These data indicate that RORγ plays a role in the regulation of NLRP3 inflammasome activity. Loss of RORγ results in reduced responsiveness of the NLRP3 inflammasome, and our data suggest that RORγ is necessary for normal expression of Nlrp3 and Il1b expression.
Figure 1.
NLRP3 inflammasome activity is reduced in BMDMs from RORγ-null mice. A, Nlrp3 (left) and Il1b (right) gene expression in M1 BMDMs derived from WT and RORγ KO mice in response to no treatment or LPS treatment. B, Nlrp3 (left) and Il1b (right) gene expression in M0 BMDMs derived from WT and RORγ KO mice in response to no treatment or LPS treatment. C, IL-1β secreted from M1 BDMDs from WT or RORγ KO mice treated with LPS after 0, 15, or 120 min of ATP stimulation. D, NLRP3 and IL-1β protein expression from WT or RORγ KO M1 polarized BMDMs (18-h treatment with 100 ng/ml LPS) followed by a medium change with (LPS) or without (NT) an additional 24-h LPS treatment. The bottom panel provides normalization of the level of expression of NLRP3, proIL-1β, and cleaved IL-1β to actin expression. *, p < 0.05; **, p < 0.01; ***, p < 0.001 by Student's t test. Error bars, S.E.
RORγ inverse agonists SR2211 and SR1555 suppress Nlrp3 and Il1b gene expression
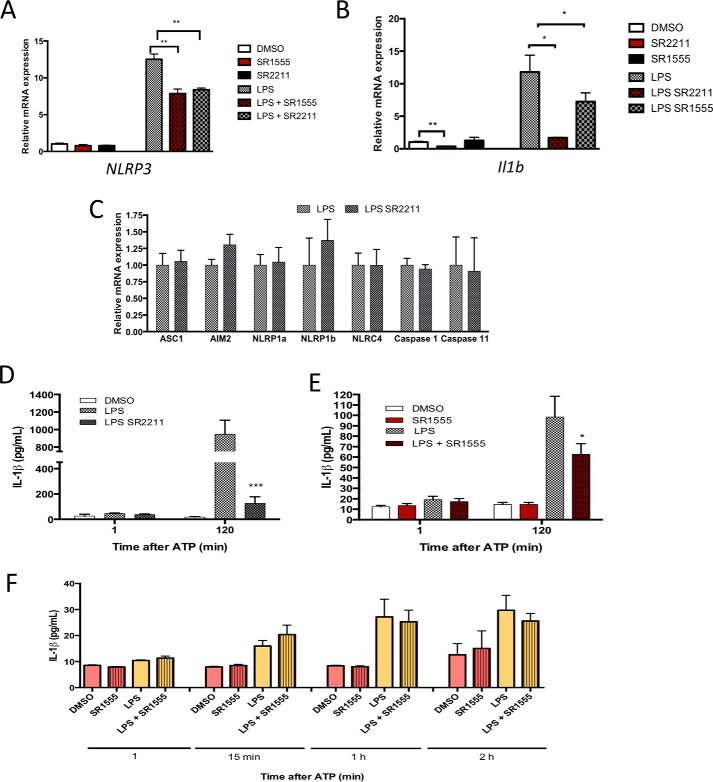
RORγ appears to be required for the response of the NLRP3 inflammasome to inflammatory stimuli, such as LPS/ATP. In light of this finding, we then examined the effect of RORγ-specific inverse agonists SR2211 and/or SR1555 (16, 17) on Nlrp3 and Il1b gene expression. BMDMs from WT animals differentiated for 7 days in culture and then treated for 24 h with LPS (100 ng/ml). Macrophages were then treated with DMSO, SR2211 (10 μm), LPS (100 ng/ml), or LPS plus SR2211 for 24 h prior to assessment of gene expression. Treatment with RORγ inverse agonist SR2211 decreased the expression of both Nlrp3 and Il-1β genes, in the presence or absence of LPS (Fig. 2A). The same results were observed with SR1555, another RORγ selective inverse agonist (Fig. 2B). The effects of the RORγ inverse agonist were selective to Nlrp3 and Il1b, as the expression of other inflammasome components (caspase-1, Asc, Aim2, Nlrp1a, Nlrp1b, Nlrc4, caspase-11) was unaffected by SR2211 treatment (Fig. 2C).
Figure 2.
RORγ inverse agonist reduce NLRP3 inflammasome activity. A, Nlrp3 (left) and Il1b (right) gene expression in M1 BMDMs (LPS or no treatment) treated with vehicle or RORγ inverse agonist SR2211 (10 μm). B, Nlrp3 (left) and Il1b (right) gene expression in M1 BMDMs (LPS or no treatment) treated with vehicle or RORγ inverse agonist SR1555 (10 μm). C, expression of various inflammasome/inflammasome-associated genes in response to treatment with SR1555. M1 BMDMs were treated with LPS and SR1555 as in B. D, IL-1β secretion by M1 BMDMs treated with vehicle, LPS, or LPS + SR2211 (10 μm) followed by ATP treatment (0, 15, or 120 min) after LPS treatment. E, IL-1β secretion by M1 BMDMs treated with vehicle, LPS, or LPS + SR1555 (10 μm) followed by ATP treatment (0, 15, or 120 min) after LPS treatment. F, IL-1β production from BMDM Nlrp3 KO treated with LPS (50 ng/ml) and SR1555 (10 μm) in the presence of ATP. *, p < 0.05; **, p < 0.01; ***, p < 0.001 by Student's t test. Error bars, S.E.
Given the clear effects of pharmacological inhibition of RORγ, we also assessed the effects of SR2211 and SR1555 on IL-1β secretion from BMDMs. Treatment with either RORγ inverse agonist induced a decrease of IL-1β secretion by the macrophages after 2 h of ATP stimulation (Fig. 2, D and E). We then studied the protein level of IL-1β and caspase-1 activity in response to SR2211 in macrophages.
To determine whether the effects of the RORγ inverse agonists on IL-1β secretion were dependent on targeting NLRP3 inflammasome activity, we tested the effect of a RORγ inverse agonist in Nlrp3-null BMDMs. The basal level of IL-1β secreted by these macrophages was low compared with WT macrophages, and most importantly, the RORγ inverse agonist SR1555 had no effect on IL-1β secretion in the Nlrp3-null macrophages, indicating that the effect of the RORγ targeted drug depended on the NLRP3 inflammasome (Fig. 2F). We also tested the specificity of SR1555 in RORγ KO BMDMs. Cells were treated with DMSO or SR1555 in the presence or absence of LPS. As expected, we did not see any effect of SR1555 on Nlrp3, Il1b gene expression, or IL-1β production (Fig. S1). Next, we sought to determine whether the effects we observed in the mouse are conserved in humans. We examined the effect of SR1555 in primary human macrophages in vitro in the presence of LPS. Treatment with SR1555 decreased NLRP3 and IL1B gene expression in human cells, suggesting that RORγ regulates this pathway in humans as well as mice (Fig. S2). These data clearly suggest that targeting RORγ with pharmacological agents may be an effective method to inhibit NLRP3 inflammasome activity.
RORγ response elements are located in the promoters of the Nlrp3 and Il1b genes
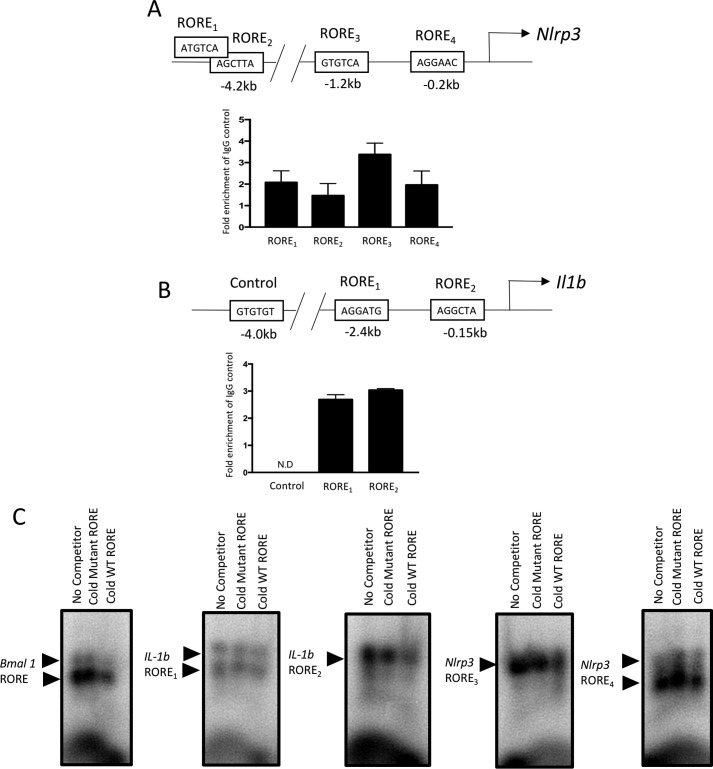
Based on our data indicating that RORγ is required for normal expression of Nlrp3 and Il1b as well as our recent study describing the direct regulation of these genes by REV-ERB, a nuclear receptor that typically recognizes similar or identical response elements as ROR (18), we sought to determine whether RORγ directly binds to response elements in the promoters of the Nlrp3 and Il1b genes. Two functional REV-ERB response elements were identified in the promoter of the Il1b gene and four were identified in the promoter of the Nlrp3 gene (15), and we focused on determining whether these were also bound by RORγ using ChIP (Fig. 3A). RAW267.4 macrophages were used and ChIP was performed using an antibody directed against RORγ, and the putative ROREs were analyzed by RT-qPCR. We observed enrichment of RORγ at all four sites within the Nlrp3 promoter, with the −1.2-kb site providing the greatest signal (Fig. 3A), and both sites within the Il1b promoter also displayed enriched RORγ binding (Fig. 3B). As a negative control, we assessed the potential binding of RORγ at a site 4 kb upstream of the transcription start site of the Il1b gene with no identifiable RORE. We observed no signal at this site (Fig. 3B). To further confirm direct binding of RORγ to these putative ROREs, we performed electrophoretic mobility shift assays. As shown in Fig. 3C, in vitro transcribed/translated RORγ protein induced a mobility shift of a radiolabeled RORE derived from the Bmal1 promoter (left panel, left lane). If a 10× molar excess of a mutated Bmal1 RORE was added as a competitor, there was no effect on RORγ binding to the labeled RORE (middle lane). However, if unlabeled WT Bmal1 RORE was used as a competitor, the binding of RORγ to the labeled RORE was significantly reduced. We performed identical analysis using the two putative ROREs derived from the proximal Nlrp3 promoter as well the two ROREs from the Il1b promoter and observed similar results. These data indicate that, like REV-ERBα, RORγ recognizes specific DNA sequences in the promoters of the Nlrp3 and Il1b genes, allowing direct regulation of expression of these genes.
Figure 3.
Identification of RORγ binding sites within the Nlrp3 and Il1b promoters by ChIP. A, a schematic of the Nlrp3 promoter is shown in the top panel with the putative ROREs. The bottom panel shows the results of ChIP/QPCR at the four putative ROREs, indicating enrichment of RORγ at each of these four sites. B, a schematic of the Il1b promoter is shown in the top panel with the putative ROREs. The bottom panel shows the results of ChIP/QPCR at the two putative ROREs, indicating enrichment of RORγ at each of these. A negative control was also used 4 kb upstream of the Il1b TSS that did not contain a putative RORE. C, EMSA demonstrating the ability of RORγ (in vitro transcribed/translated) to bind to the radiolabeled ROREs from Il1b and Nlrp3 promoters. In the left panel, a positive control (Bmal1 RORE) is used without an unlabeled competitor (left lane; no competitor), a 10× molar excess of unlabeled mutant competitor RORE (middle lane; cold mutant competitor), and a 10× molar excess of competitor (right lane; cold WT RORE). In the four right-hand panels of C, the two proximal RORE elements from the Nlrp3 promoter and the two ROREs within the Il1b promoter were examined in a similar manner as the Bmal1 RORE EMSA. Arrows, RORE–RORγ complexes. Error bars, S.E.
RORγ inverse agonists inhibit NLRP3 inflammasome activity in a mouse model of sepsis
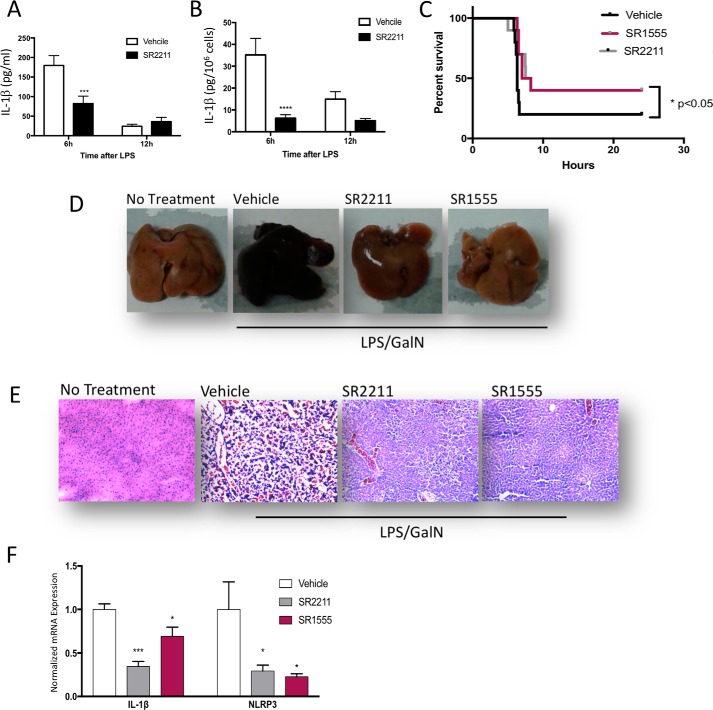
Given the effect of RORγ inverse agonists on suppression of NLRP3 inflammasome activity, we sought to determine whether these pharmacological agents may be effective inhibitors of the inflammasome in vivo. We initially utilized an acute model of sterile peritoneal inflammation that mimics many aspects of the inflammatory response in sepsis (15). Mice were pretreated with SR2211 or vehicle and challenged by intraperitoneal administration of LPS to specifically activate the NLRP3 signaling pathway (19). Animals were sacrificed 6 or 12 h after LPS challenge, and blood and peritoneal macrophages were collected. After 2 h in culture, supernatant from the macrophages was collected and analyzed with an IL-1β ELISA. As shown in Fig. 4A, plasma IL-1β levels were significantly reduced in SR2211-treated mice 6 h after LPS administration. The IL-1β levels were reduced at 12 h after LPS administration versus 6 h, at which time the SR2211 did not have an effect on IL-1β levels. IL-1β secretion from the isolated peritoneal macrophages was also suppressed by SR2211 treatment 6 h after LPS administration. Similar to the plasma data, IL-1β secretion was reduced at 12 h versus 6 h after LPS administration, and at this time, the SR2211 had lost its effect (Fig. 4B). Thus, in alignment with the in vitro macrophages data, these results suggest that pharmacological suppression of RORγ leads to suppression of IL-1β secretion consistent with inhibition of NLRP3 inflammasome activity.
Figure 4.
RORγ inverse agonist are effective in treatment of sepsis and fulminant hepatitis via targeting the NLRP3 inflammasome. A, plasma IL-1β level in mice treated with vehicle or SR2211 at 6 or 12 h after LPS administration (n = 6/group). B, IL-1β level secreted by peritoneal macrophages in culture collected from mice treated with vehicle or SR2211 at 6 or 12 h after LPS injection. C, Kaplan–Meier survival curve of mice in a fulminant hepatitis model challenged with LPS/GalN and pretreated or not with vehicle (black line, n = 10), SR2211 (gray line, n = 10/group), or SR1555 (red line, n = 10). D, representative appearance of livers from untreated (not LPS/GalN), vehicle-treated, SR2211-treated, and SR1555-treated animals 6 h after LPS + GaIN injection. WT liver from a nontreated animal is used as a healthy liver reference. E, representative H&E-stained liver from untreated (no LPS/GalN), vehicle-treated, SR2211-treated, and SR1555-treated animals 6 h after LPS + GaIN injection. WT liver from a nontreated animal (N.T.) is used as a healthy liver reference. F, RT-qPCR analysis of Nlrp3 and Il1b gene expression from vehicle-, SR2211-, and SR1555-treated animals 6 h after LPS + GaIN injection. *, p < 0.05; **, p < 0.01; ***, p < 0.001 by Student's t test. Error bars, S.E.
RORγ inverse agonists reduce mortality due to fulminant hepatitis
Acute liver failure, also known as fulminant hepatitis (FH), is a condition where rapid deterioration of liver function results in coagulopathy and can be fatal within hours. Excessive activation of immune cells, mainly liver macrophages, is involved in the development of the disease, leading to hepatocyte necrosis, systemic inflammation, and liver failure (20). Despite advances in treatment, FH carries a very high mortality, especially in young adults. The treatments for FH are very limited, and liver transplant remains one of the major treatments. Low doses of LPS in combination with the specific hepatotoxic agent d-galactosamine (GalN) promotes specific liver injury and recapitulates the clinical picture of acute liver injury in humans (21). LPS activates Toll-like receptors and their downstream signaling pathway, leading to the expression of pro-inflammatory cytokines, including Il-1β, whose secretion is governed by activation of the NLRP3 inflammasome via the combination of LPS and GaIN in the FH model. REV-ERB agonists are very effective in reducing NLRP3 inflammasome activity and reducing mortality in this mouse model of FH (15). Because our data indicate that RORγ regulates the NLRP3 inflammasome in a similar manner, we sought to determine whether RORγ inverse agonists also display efficacy in this model. We assessed the ability of two selective RORγ inverse agonists, SR1555 and SR2211, in this model. Three-month-old WT animals were treated with RORγ inverse agonist SR1555, SR2211, or vehicle both 13 h and 1 h before LPS-GaIN injection. Both RORγ inverse agonists displayed similar efficacy, more than doubling the survival rate of mice in the FH model (Fig. 4C). Because the RORγ inverse agonists were quite effective reducing mortality in this model, we decided to repeat the experiment but collected tissue 6 h after inducing the acute liver injury. As illustrated in Fig. 4D, the LPS/GalN treatment significantly altered liver morphology relative to nontreated (N.T.) control livers. The diseased livers were black and necrotic, whereas livers from mice treated with either SR2211 or SR1555 displayed much more normal coloration (Fig. 4C). Histologically, H&E-stained liver sections display considerable damage in the LPS/GalN-treated mice versus the nontreated mice (Fig. 4D). SR2211 and SR1555 treatment clearly had a protective effect against LPS/GaIN–induced liver damage as observed in the sections, but there were still signs of hepatocellular toxicity, albeit considerably reduced relative to vehicle-treated livers. Expression of Nlrp3 and Il1b was significantly reduced in the livers from mice treated with either SR1555 or SR2211 relative to the vehicle-treated mice. These data clearly indicate that RORγ inverse agonists are protective in the mouse model of FH and suggest that at least one clear mechanism underlying this protection is inhibition of NLRP3 inflammasome activity.
Discussion
Excessive NLRP3 inflammasome activity is responsible for chronic inflammation that drives pathological processes in multiple diseases, including atherosclerosis, Alzheimer's disease, fulminant hepatitis, and diabetes (2, 3, 20, 22). In fact, many of these disease processes are significantly attenuated in mice that lack NLRP3 inflammasome functionality (22–24). Thus, the NLRP3 inflammasome has emerged as a valid pharmacological target for the treatment of a range of diseases. The NLRP3 inflammasome is activated in response to PAMPs/DAMPs, and this activation up-regulates pro-inflammatory cytokine production, including IL-1β. Activation of the NLRP3 inflammasome requires a two-step mechanism as follows. 1) “Priming” induces the transcription of the key inflammasome/cytokine genes (such as LPS). 2) A distinct signal is required for inflammasome assembly and activation. A variety of secondary signals that drive NLRP3 inflammasome assembly and activity have now been described and include cholesterol crystals, ATP, or reactive oxygen species. Hence, the NLRP3 inflammasome is a key platform coordinating the inflammatory response after integration of information from various stimuli.
In this study, we found that RORγ binds to the promoters of the Nlrp3 and Il1b genes and drives the expression of these genes. Although a previous study showed that a RORγ inverse agonist inhibited Il-1β secretion from RAW264.7 cells, the mechanism of such an effect was not investigated (25). We observed that loss of RORγ leads to reduced Nlrp3 and Il1b expression in macrophages. Selective RORγ inverse agonists also suppress Nlrp3 and Il1b expression and reduce NLRP3 inflammasome activity, as evidenced by reduced IL-1β secretion both in cultured macrophages and in vivo. Suppression of IL-1β secretion by RORγ was clearly mediated by targeting the NLRP3 inflammasome because the effect of a RORγ inverse agonist was lost in macrophages derived from Nlrp3-null mice.
We also demonstrated that pharmacological targeting of RORγ is an effective method for reducing acute inflammation in disease models associated with excessive activation of the NLRP3 inflammasome, including sepsis (sterile) and FH. In FH, there is rapid and massive necrosis of the liver due to severe acute inflammation leading to coma and cerebral edema over a period of several days to several weeks. Symptoms of FH become severe very rapidly, and in many cases, the only effective treatment is liver transplant. Excessive NLRP3 inflammasome activity has been linked to the pathological effects of FH, and acetaminophen overdose, a leading cause of FH, has been associated with extreme NLRP3 inflammasome activation (26, 27). Although it is possible that additional RORγ-dependent mechanism may be important for the efficacy in treatment of FH, it is very likely that inhibiting the NLRP3 inflammasome is one important component.
Clearly, targeting inhibition of the NLRP3 inflammasome holds potential for treatment of FH as well as a range of other diseases associated with abnormal NLRP3 inflammasome activity, and RORγ inverse agonists appear to be effectively inhibiting its activity. Unlike other recently described NLRP3 inflammasome inhibitors that directly target the assembly of the NLRP3 inflammasome (such as MCC950) (27), RORγ targets the expression of a critical component of the inflammasome (the Nlrp3 gene) as well as the substrate for the inflammasome (the Il1b gene), providing a distinct mechanism for suppression of activity. Based on their distinct mechanisms of action, it may be possible to derive additional efficacy in targeting inflammation by using both of these classes of drugs.
We recently found that the nuclear receptor REV-ERBα regulates the NLRP3 inflammasome. Given that RORs and REV-ERBs recognize similar or, in many cases, identical DNA response elements, we were able to determine that these receptors are recognizing the same elements within the Nlrp3 and Il1b promoters (15). REV-ERBα is a repressor of transcription, whereas RORγ is an activator of transcription. The REV-ERBα agonists that drive active repression of gene transcription appear to be much more efficacious in reducing NLRP3 activity than the RORγ inverse agonists. The greater level of efficacy of REV-ERBα agonists is also conserved in the FH model, where the REV-ERBα agonist, SR9009, was able to protect against mortality to a much greater extent than the RORγ inverse agonists. We believe this is likely because the RORγ inverse agonists can only block the activator action of RORγ occupying the promoters, whereas the REV-ERB agonist is effective in recruiting corepressors to the promoters, driving inhibition of any level of activation of transcription of the promoter. Thus, the REV-ERB agonists should be quite effective in blocking any activation of Nlrp3 or Il1b transcription from any source.
A number of RORγ inverse agonists are under clinical development based on the ability of RORγ to suppress Th17 cell development and function (14). Our group, as well as the Littman group (13), were the first to identify RORγ inverse agonists and characterize their ability to suppress Th17 cell development/function and reduce the severity of autoimmune disease in rodent models (7). However, with the current study, we now understand that RORγ plays a much wider role in immune function, where it targets a critical component of the innate immune system, the NLRP3 inflammasome. With the progression of RORγ inverse agonists in clinical development, there should be examination of their potential to be used in situations where NLRP3 inflammasome activity is a target for disease treatment.
Experimental procedures
BMDMs
8–12-week-old C57BL/6 or RORγ KO mice were euthanized according to the animal protocols approved by Saint Louis University institutional animal care and use committee. Bone marrow hematopoietic stem cells were isolated from the tibia and femur and cultured during 1 week in Dulbecco's modified Eagle's medium, 20% L929-conditioned medium containing macrophage colony–stimulating factor and 10% fetal bovine serum. BMDMs were either pretreated with the RORγ inverse agonist and treated with LPS (100 ng/ml) or cultured with LPS and treated with RORγ inverse agonist (10 μm) or vehicle. For the ELISA, macrophages were primed with LPS (100 ng/ml) for 12 h and activated for 30 min to 2 h with ATP pH 7 (5 mmol/liter). The supernatants were collected, cleared by centrifugation, and analyzed by ELISA using the mouse Il-1β (R&D) kit according to the manufacturer's instructions.
Human primary macrophages
Cells were obtained from StemCell Technologies and treated with LPS (100 ng/ml) in the presence of SR1555 (5 μm) or DMSO for 24 h.
Quantitative real-time PCR
Total RNA was isolated from mouse tissues using PureLink RNA mini kit (Ambion). RNA was reverse-transcribed to make cDNA using the qScriptTM cDNA synthesis kit (Quanta Biosciences) according to the manufacturer's instructions. Real-time PCR was performed using a SYBR Green PCR master mix kit (Roche Applied Science). Primers were purchased from Integrated DNA Technologies.
Western blotting
Twenty-five μg of protein in Laemmli buffer were separated using SDS-PAGE and transferred to polyvinylidene difluoride membranes. After saturation in 10% skimmed milk, membranes were incubated with the primary antibody directed against IL-1β (1:1000; REF), NLRP3 (1:1000; AG-20B-0014, Adipogen), or actin (1:1000; sc-1616, Santa Cruz Biotechnology, Inc.) in Tris-buffered saline–bovine serum albumin solution (2.5%). Then appropriate horseradish peroxidase–coupled secondary antibody diluted (1:10,000) in 10% skimmed milk (1.15363.0500, Merck Millipore) was used.
ChIP
ChIP experiments were performed in RAW264.7 cells using Simple ChIP Plus Sonication ChIP Kit (catalog no. 57976, Cell Signaling) according to the manufacturer's instructions.
Electrophoretic mobility shift assay (EMSA)
Murine RORγ protein was obtained by in vitro transcription/translation (TNT kit, Promega). The different single-stranded oligonucleotides (forward) were [32P]ATP-labeled with T4 polynucleotide kinase (Promega) before annealing with their unlabeled antisense (reverse) oligonucleotide partners. Probes were purified and counted, and 20,000 cpm were used for each binding reaction. Unlabeled specific and nonspecific competitor probes were included at the indicated molar excess.
Animals and treatment
All procedures were approved and conducted in accordance with the Saint Louis University institutional animal care and use committee.
Fulminant hepatitis
10-week-old WT male mice were intraperitoneally injected with LPS (Sigma-Aldrich) and d-galactosamine hydrochloride. For SR2211 and SR1555 treatment, mice were pretreated with SR2211 or SR1555 or vehicle (10% DMSO, 10% Tween PBS). After 16 h, mice received another intraperitoneal injection of SR2211 or SR1555 or vehicle followed by intraperitoneal injection of LPS and d-galactosamine hydrochloride. To determine the survival rate, mice were monitored every 60 min after LPS/d-GalN injection for 48 h. In another experiment, mice were sacrificed 6 h after LPS/d-GalN administration, and blood and liver were harvested. Histology and gene expression were performed on liver.
Peritonitis
For peritonitis experiments, 10-week-old WT male mice were intraperitoneally injected with the SR2211 at ZT23 and with LPS 1 h after SR2211 injection at ZT0. Mice were sacrificed every 4 h after LPS injection and peritoneal lavage was performed with sterile PBS. PECs were cultured, and cell supernatants were collected after 2 h. IL-1β protein content was then quantified by ELISA.
Author contributions
C. B., M. H. M., and T. P. B. conceptualization; C. B. and M. H. M. formal analysis; C. B., M. H. M., and A. A. investigation; C. B., M. H. M., and T. P. B. writing-original draft; C. B., M. H. M., and T. P. B. writing-review and editing; A. A. and T. P. B. methodology; T. P. B. supervision; T. P. B. funding acquisition; T. P. B. project administration.
Supplementary Material
Acknowledgment
We thank Sherry Burris for performing the histological analysis.
This work was supported by National Institutes of Health Grant R01MH092769 (to T. P. B.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1 and S2.
- IL
- interleukin
- RORE
- RORγ response element
- ZT
- Zeitgeber time
- LPS
- lipopolysaccharide
- DAMP
- damage-associated molecular pattern
- PAMP
- pathogen-associated molecular pattern
- BMDM
- Bone marrow–derived macrophages
- KO
- knockout
- qPCR
- quantitative PCR
- FH
- fulminant hepatitis
- GalN
- d-galactosamine
- H&E
- hematoxylin and eosin
- EMSA
- electrophoretic mobility shift assay.
References
- 1. Pétrilli V., Dostert C., Muruve D. A., and Tschopp J. (2007) The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19, 615–622 10.1016/j.coi.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 2. Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nuñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K. A., Rock K. L., Moore K. J., et al. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T.-C., Gelpi E., Halle A., Korte M., Latz E., and Golenbock D. T. (2013) NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou R., Yazdi A. S., Menu P., and Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]
- 5. Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., and Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 10.1038/ni.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vande Walle L., Van Opdenbosch N., Jacques P., Fossoul A., Verheugen E., Vogel P., Beyaert R., Elewaut D., Kanneganti T.-D., van Loo G., and Lamkanfi M. (2014) Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73 10.1038/nature13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Solt L. A., Kumar N., Nuhant P., Wang Y., Lauer J. L., Liu J., Istrate M. A., Kamenecka T. M., Roush W. R., Vidović D., Schürer S. C., Xu J., Wagoner G., Drew P. D., Griffin P. R., and Burris T. P. (2011) Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472, 491–494 10.1038/nature10075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Billon C., Sitaula S., and Burris T. (2016) Inhibition of RORα/γ suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol. Metab. 5, 997–1005 10.1016/j.molmet.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jetten A. M. (2009) Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 7, e003 10.1621/nrs.07003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun Z., Unutmaz D., Zou Y.-R., Sunshine M. J., Pierani A., Brenner-Morton S., Mebius R. E., and Littman D. R. (2000) Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 10.1126/science.288.5475.2369 [DOI] [PubMed] [Google Scholar]
- 11. Cyr P., Bronner S. M., and Crawford J. J. (2016) Recent progress on nuclear receptor RORγ modulators. Bioorg. Med. Chem. Lett. 26, 4387–4393 10.1016/j.bmcl.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 12. Banerjee D., Zhao L., Wu L., Palanichamy A., Ergun A., Peng L., Quigley C., Hamann S., Dunstan R., Cullen P., Allaire N., Guertin K., Wang T., Chao J., Loh C., and Fontenot J. D. (2016) Small molecule mediated inhibition of ROR γ-dependent gene expression and autoimmune disease pathology in vivo. Immunology 147, 399–413 10.1111/imm.12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huh J. R., Leung M. W. L., Huang P., Ryan D. A., Krout M. R., Malapaka R. R. V., Chow J., Manel N., Ciofani M., Kim S. V., Cuesta A., Santori F. R., Lafaille J. J., Xu H. E., Gin D. Y., et al. (2011) Digoxin and its derivatives suppress Th17 cell differentiation by antagonizing RORγt activity. Nature 472, 486–490 10.1038/nature09978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bronner S. M., Zbieg J. R., and Crawford J. J. (2017) RORγ antagonists and inverse agonists: a patent review. Expert Opin. Ther. Pat. 27, 101–112 10.1080/13543776.2017.1236918 [DOI] [PubMed] [Google Scholar]
- 15. Pourcet B., Zecchin M., Ferri L., Beauchamp J., Sitaula S., Billon C., Delhaye S., Vanhoutte J., Mayeuf-Louchart A., Thorel Q., Haas J., Eeckhoute J., Dombrowicz D., Duhem C., Boulinguiez A., et al. (2018) Nuclear receptor subfamily 1 group D member 1 regulates circadian activity of NLRP3 inflammasome to reduce the severity of fulminant hepatitis in mice. Gastroenterology 154, 1449–1464.e20 10.1053/j.gastro.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar N., Lyda B., Chang M. R., Lauer J. L., Solt L. A., Burris T. P., Kamenecka T. M., and Griffin P. R. (2012) Identification of SR2211: a potent synthetic RORγ selective modulator. ACS Chem. Biol. 7, 672–677 10.1021/cb200496y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solt L. A., Kumar N., He Y., Kamenecka T. M., Griffin P. R., and Burris T. P. (2012) Identification of a selective RORγ ligand that suppresses T(H)17 cells and stimulates T regulatory cells. ACS Chem. Biol. 7, 1515–1519 10.1021/cb3002649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kojetin D. J., and Burris T. P. (2014) REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 13, 197–216 10.1038/nrd4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guarda G., Braun M., Staehli F., Tardivel A., Mattmann C., Förster I., Farlik M., Decker T., Du Pasquier R. A., Romero P., and Tschopp J. (2011) Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 10.1016/j.immuni.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 20. Kim S.-J., and Lee S.-M. (2013) NLRP3 inflammasome activation in d-galactosamine and lipopolysaccharide-induced acute liver failure: role of heme oxygenase-1. Free Radic. Biol. Med. 65, 997–1004 10.1016/j.freeradbiomed.2013.08.178 [DOI] [PubMed] [Google Scholar]
- 21. Furuya S., Kono H., Hara M., Hirayama K., Sun C., and Fujii H. (2015) Interleukin 17A plays a role in lipopolysaccharide/d-galactosamine-induced fulminant hepatic injury in mice. J. Surg. Res. 199, 487–493 10.1016/j.jss.2015.05.060 [DOI] [PubMed] [Google Scholar]
- 22. Vandanmagsar B., Youm Y. H., Ravussin A., Galgani J. E., Stadler K., Mynatt R. L., Ravussin E., Stephens J. M., and Dixit V. D. (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 10.1038/nm.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu C., Ding H., Li Y., Pearson J. A., Zhang X., Flavell R. A., Wong F. S., and Wen L. (2015) NLRP3 deficiency protects from type 1 diabetes through the regulation of chemotaxis into the pancreatic islets. Proc. Natl. Acad. Sci. U.S.A. 112, 11318–11323 10.1073/pnas.1513509112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Heijden T., Kritikou E., Venema W., van Duijn J., van Santbrink P. J., Slütter B., Foks A. C., Bot I., and Kuiper J. (2017) NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1457–1461 10.1161/ATVBAHA.117.309575 [DOI] [PubMed] [Google Scholar]
- 25. Chang M. R., Lyda B., Kamenecka T. M., and Griffin P. R. (2014) Pharmacological repression of RORγ is therapeutic in the collagen induced arthritis experimental model. Arthritis Rheumatol. 66, 579–588 10.1002/art.38272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klecha A. J., Genaro A. M., Gorelik G., Barreiro Arcos M. L., Silberman D. M., Schuman M., Garcia S. I., Pirola C., and Cremaschi G. A. (2006) Integrative study of hypothalamus-pituitary-thyroid-immune system interaction: thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J. Endocrinol. 189, 45–55 10.1677/joe.1.06137 [DOI] [PubMed] [Google Scholar]
- 27. Imaeda A. B., Watanabe A., Sohail M. A., Mahmood S., Mohamadnejad M., Sutterwala F. S., Flavell R. A., and Mehal W. Z. (2009) Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 119, 305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.