Abstract
The ribosomal maturation factor P (RimP) is a highly conserved protein in bacteria and has been shown to be important in ribosomal assembly in Escherichia coli. Because of its central importance in bacterial metabolism, RimP represents a good potential target for drug design to combat human pathogens such as Mycobacterium tuberculosis. However, to date, the only RimP structure available is the NMR structure of the ortholog in another bacterial pathogen, Streptococcus pneumoniae. Here, we report a 2.2 Å resolution crystal structure of MSMEG_2624, the RimP ortholog in the close M. tuberculosis relative Mycobacterium smegmatis, and using in vitro binding assays, we show that MSMEG_2624 interacts with the small ribosomal protein S12, also known as RpsL. Further analyses revealed that the conserved residues in the linker region between the N- and C-terminal domains of MSMEG_2624 are essential for binding to RpsL. However, neither of the two domains alone was sufficient to form strong interactions with RpsL. More importantly, the linker region was essential for in vivo ribosomal biogenesis. Our study provides critical mechanistic insights into the role of RimP in ribosome biogenesis. We anticipate that the MSMEG_2624 crystal structure has the potential to be used for drug design to manage M. tuberculosis infections.
Keywords: ribosome assembly, crystal structure, mycobacteria, protein-protein interaction, protein complex, RimP, ribosomal maturation factor P, ribosomal biogenesis, MSMEG 2624, Mycobacterium smegmatis, small ribosomal protein S12, RpsL, tuberculosis, bacterial translation
Introduction
Ribosomes account for a large portion of cell mass, and their synthesis can be highly energy-consuming. It is estimated that in rapidly growing Escherichia coli, around 90% of the energy consumption is for protein synthesis, and a significant amount is used for generating ribosomes (1). The complete 70S bacterial ribosome is composed of the 30S small subunit and the 50S large subunit. In general, the 30S small subunit consists of the 16S rRNA and 21 ribosomal proteins (S1–S21); the 50S large subunit consists of the 23S and 5S rRNAs and 36 ribosomal proteins (L1–L36). Although active ribosomes can be reconstituted in vitro using individually purified ribosomal proteins and rRNAs, the process occurs at a much slower rate and requires harsher conditions (2). By contrast, in vivo ribosomal biogenesis is assisted by various ribosomal cofactors, including helicase, chaperones, maturation factors, and GTPase, and hence needs a lower activation energy and produces fewer intermediates (3).
The ribosomal maturation factor P (RimP), also known as yhbC, is a highly conserved ribosomal cofactor in both Gram-negative and Gram-positive bacteria (Fig. S1, A–C). Null mutation of RimP in E. coli shows slower growth than WT at high temperatures (4). In the food-borne pathogen Salmonella enteritidis, the RimP mutant shows decreased growth rate and becomes more sensitive to both reactive oxygen and nitrogen intermediates but less virulent in vitro (5). In the Gram-positive pathogen Streptococcus pneumoniae, the null mutant is lethal (6). Overall, these phenotypic studies highlight the physiological importance of RimP in bacteria.
Several mechanistic studies have showed the functional association of RimP with ribosomal biogenesis. Wikström and co-workers (4) found that the RimP null mutant reduced the levels of polysome and mature 70S, whereas it increased the amounts of 30S and 50S in E. coli. Moreover, less accumulation of 30S than 50S was observed in polysome profiling (4). In their study, RimP was only found in the fractions of 30S subunit but not others in sucrose gradient centrifugation, and primer extension studies demonstrated that RimP knockout bacteria up-regulated the level of pre-16S rRNA but down-regulated mature 16S rRNA levels (4). Recently, quantitative MS studies on E. coli suggested that RimP can increase the binding kinetics of the S5 and S12 ribosomal proteins to the 5′ domain of rRNA in vitro (7). In addition, it was reported that the relative timing of the assembly of the 3′ domain and the formation of the central pseudoknot structure in the 16S rRNA depend on the presence of the assembly factor RimP (8). These in vivo and in vitro data indicate that RimP is essential for the biogenesis of the 30S small subunit in E. coli.
Despite extensive biochemical and biophysical study, the mechanism of RimP in ribosomal biogenesis is still not fully understood. The only available structure to date is the solution structure of SP14.3, an ortholog in S. pneumoniae, solved using NMR. It consists of a highly negatively charged N-terminal domain and a slightly positively charged C-terminal domain resembling the Sm fold (6). Because of the flexible linker between the two domains, their relative orientation is not defined. The structure, however, is limited in clarifying its mechanism in ribosomal biogenesis.
Mycobacterium tuberculosis is the causative agent of tuberculosis, which killed 1.7 million people in 2016 (9). Active M. tuberculosis infection leads to severe pulmonary and occasionally extrapulmonary symptoms, particularly in immunocompromised patients, such as those with HIV. Moreover, the emergence of multidrug-resistant strains confounds effective treatment, which typically involves prolonged use of multiple antibiotics. Therefore, the discovery of a druggable target in M. tuberculosis is of importance in future drug development. Here, we describe the crystal structure of RimP homolog MSMEG_2624 in Mycobacterium smegmatis, a model organism for studying M. tuberculosis. We demonstrate that the linker region of MSMEG_2624 plays an important physiological role in interacting with RpsL, the small ribosomal protein S12, thereby affecting ribosomal biogenesis. Because RimP is a highly conserved mycobacterial protein, the understanding of its mechanism will provide insight in studying ribosomal biogenesis and developing new drugs to target M. tuberculosis.
Results
The crystal structure of MSMEG_2624
MSMEG_2624 was purified as a monomer in solution (data not shown). The crystal structure of MSMEG_2624 was solved by single isomorphous replacement with anomalous scattering (SIRAS) phasing, and the final model was refined to 2.2 Å (Fig. 1A). Detailed statistics of data processing, phasing, structure refinement, and Ramachandran plot are shown in Table 1 and Fig. S2. Both N-terminal (aa4 1–93) and C-terminal (aa 94–181) domains of MSMEG_2624 are similar to that of SP14.3, with 2.1 and 2.8 Å Cα root mean square deviation, respectively. The C-terminal domains of MSMEG_2624 and SP14.3 resemble the Sm fold, which is composed of six β sheets. The electron density of the loop between α2 and β3 is missing, which indicates a disordered structure (Fig. S3). The major difference between MSMEG_2624 and SP14.3, however, lies in the additional α-helix (α2′), connected by the long loop to the last β sheet β6′ (aa 158–165) on the C terminus of MSMEG_2624, which forms the handle of the barrel-like Sm fold (Fig. 1, B and C).
Figure 1.
The crystal structure of MSMEG_2624. A, the cartoon colors the structure of MS- MEG_2624 by secondary structure. B and C, comparison between the C-terminal domain of SP14.3 (B) and that of MSMEG_2624 (C). The additional α helix and the long connecting loop in MSMEG_2624 are highlighted in red. D, electron density of the interdomain linker, at 2.0σ level. E and F, residues participate in the interdomain interaction. Hydrogen bond interactions are highlighted using yellow dashed lines.
Table 1.
Crystal data processing, phasing and structure refinement statistics
In the two column results, the left column gives the raw count, right column gives the percentage.

Despite the high resemblance between the crystal structure of MSMEG_2624 and the solution structure of SP14.3 at the individual domain level, they differ in the interdomain orientation. Whereas the solution structure of SP14.3 did not reveal a rigid orientation from the NOE data, our crystal structure of MSMEG_2624 shows strong electron density around the interdomain linker, indicating a well-defined clamplike orientation in the crystal (Fig. 1D). Under this orientation, the handle-like structure from the C-terminal domain interacts with the N-terminal domain through 1) hydrophobic interaction between the side chains of Phe-161 and Val-50 and 2) the hydrogen bond network formed among the Ser-162, Asp-35, and Val-36 (Fig. 1, E and F). These interactions are absent in the structure of SP14.3 and might be involved in the stabilization of the interdomain orientation, resulting in a more rigid structure. It is unclear whether the interdomain orientation is also present in solution, as we observed that both domains of MSMEG_2624 participated in crystal contact with the neighboring molecule (Fig. S4), which is expected for a protein that consists of only two domains.
The interdomain linker of MSEMG_2624 is essential for the coordinated binding with RpsL
To study the function of each domain of MSEMG_2624 in ribosome biogenesis, we first tested their interaction with binding partners. In previous interaction network studies of E. coli and Helicobacter pylori, RimP was reported to interact with S5, S7, and S12 of the small ribosomal subunit (10). In M. smegmatis, we detected the interaction between MSMEG_2624 and RpsL (S12) by co-immunoprecipitation followed by tandem MS (Fig. S5) and further validated it using a tandem affinity purification assay with recombinant proteins (Fig. 2A). We found that neither the C- nor the N-terminal domain of MSMEG_2624 alone is sufficient to bind RpsL (Fig. 2, B and C), which suggested that the two domains of MSEMG_2624 cooperatively interact with RpsL. In addition, the first 25 residues of RpsL were dispensable for the binding to MSMEG_2624 (Fig. S6).
Figure 2.
Tandem affinity purification maps the MSMEG_2624–RpsL interaction. His-MS- MEG_2624 (A) or His-tagged individual MSMEG_2624 domains (B and C) and GST-RpsL were recombinantly co-expressed and purified through Ni2+ and then GST affinity purification. The fractions are labeled at the top of the corresponding lanes (FT, flow-through; GST-FT/wash/elution, GST affinity chromatography of fractions after the first His elution; GST-elution only, elution using the GST affinity chromatography without the Ni2+ affinity chromatography). Red arrows mark where the corresponding proteins are expected.
We hypothesized that the linker region was essential for the coordination between the two domains of MSMEG_2624 to bind RpsL (Fig. 3A). To test this hypothesis, we mutated residues in this region and tested their effects on binding with RpsL. The charged residues, Asp-93 and Arg-94, were either deleted or reversed/neutralized; residues with more (Pro-95) or less (Gly-91) restricted torsion angles were swapped (Fig. 3A and Fig. S7). As shown in Fig. 3B, both linker deletion mutants significantly reduced the binding efficiency to RpsL, suggesting the importance of the linker region. The swapping of glycine to proline at position 91 but not 95 abolished the binding. We speculate that this distinct effect might be explained by the fact that G91P mutant disrupted the interdomain orientation by steric hindrance, whereas P95G merely increased the flexibility of the backbone. Alternatively, the residue Gly-91 might directly interact with RpsL. For mutations on the charged residues, R94D/A but not D93R/A greatly reduced the strength of the binding. In addition, we noticed that R94D showed a much stronger effect than R94A, suggesting that the positive charge of Arg-94 might directly participate in binding with RpsL through electrostatic interaction. To test whether the decreased binding efficiency of the mutants was due to protein denaturation or aggregation, we used size-exclusion chromatography to measure their molecular size. We found that all mutants had similar elution profiles as the WT, suggesting their proper folding (Fig. S8). The linker region is significantly enriched for highly conserved residues (p = 0.0005, Fisher exact test), suggesting its functional importance. Strikingly, we observed that all mutants that affected conserved residues showed a decrease in binding efficiency with RpsL, which suggested that these sites were under purifying selections. Taken together, we demonstrated that the linker of MSMEG_2624 was essential in coordinating the two domains to bind with RpsL.
Figure 3.

Site-directed mutagenesis on the linker region of MSMEG_2624 and the effects on MSMEG_2624–RpsL interaction. A, crystal structure of the linker region of MSMEG_2624. Evolutionarily conserved residues are marked by an asterisk. B, pulldown assay determines the binding efficiency between GST-RpsL and His-tagged MSMEG_2624 and its mutants. Top, Western blot (anti-His antibody) detects the input amount of His-MSMEG_2624 after the first Ni2+ affinity chromatography. Middle, Coomassie staining detects the input amount of GST-RpsL after the second GST affinity chromatography. Bottom, Western blot (anti-His antibody) detects the amount of eluted His-MSMEG_2624 after the second GST affinity chromatography. Asterisks mark the evolutionarily conserved residues. Cyan rectangular boxes mark where the gels/blots are cropped from the original ones. Images of cropped blots (top and bottom) are aligned by the same loading control of the purified His-MSMEG_2624 protein (not shown), whereas those of cropped gels (middle) are aligned by the protein ladder (not shown).
The interdomain linker of MSMEG_2624 is essential for ribosomal biogenesis
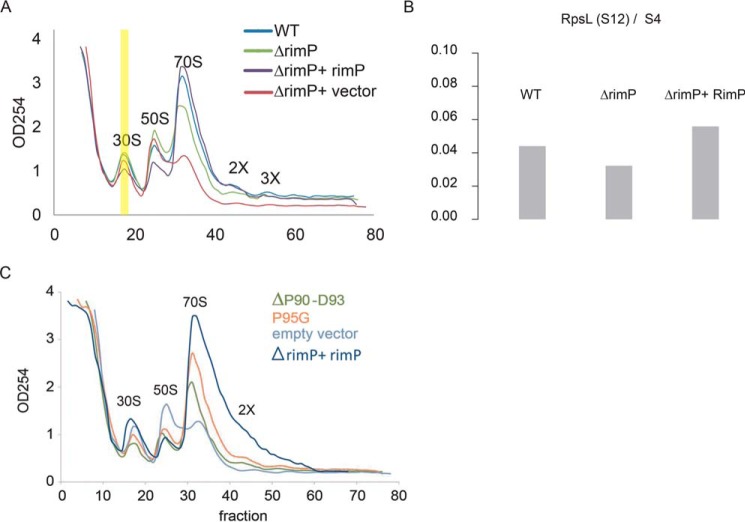
Although previous literature suggested that RimP was involved in the maturation of the 30S ribosome by stabilizing the pseudoknot structure and facilitating the incorporation of late binder ribosomal proteins, a detailed mechanism is still lacking. Having shown the importance of interdomain linker of MSMEG_2624 in binding RpsL, we analyzed the functions of RimP in ribosomal biogenesis in terms of its interaction with RpsL. Similarly to RimP in E. coli, MSMEG_2624 knockout in M. smegmatis showed reduction in polysomes and 70S ribosomes and concomitantly increased free 30S and 50S ribosomal subunits (Fig. 4A). The phenotype can be rescued by complementation of RimP in the knockout strain (Fig. 4A). In addition, we confirmed using MS that MSMEG_2624 was essential for the efficient recruitment of RpsL to the maturing 30S subunit (Fig. 4B). To specifically characterize the in vivo effects of the linker region of MSMEG_2624 on ribosomal biogenesis, we analyzed the ribosomal profile of the MSMEG_2624 knockout strains of M. smegmatis, complemented by two MSMEG_2624 mutants. We compared one that significantly abolished the interaction with RpsL (ΔP90–D93), with another that did not (P95G). The knockout strain complemented by the ΔP90–D93 mutant showed a significantly lower level of 70S ribosome than that complemented by P95G, suggesting that the interdomain linker of MSMEG_2624 is essential for ribosome biogenesis via its interaction with RpsL (Fig. 4C). Taken together, we propose a model of ribosomal biogenesis in which the two domains of RimP cooperatively bind with S12 through the linker region, whereby it facilities efficient maturation of the complete 70S ribosome.
Figure 4.
The efficiency of in vivo ribosomal biogenesis of MSMEG_2624 mutants. A, sucrose gradient centrifugation of the WT M. smegmatis, the MSMEG_2624 knockout strain, the MSMEG_2624 knockout with the vector that expresses MSMEG_2624, and the MSMEG_2624 knockout that carries the empty vector. The vertical yellow bar marks the fraction further characterized using MS in B. B, The bar plot shows the ratio of S12/S4 in the 30S subunit of WT, the MSMEG_2624 deletion mutant strain, and the MSMEG_2624 knock-out strain with the vector that expresses MSMEG_2624. C, sucrose gradient centrifugation shows the ribosome profile of the MSMEG_2624 mutants which affects binding with RpsL (ΔP90-D93, shown in green), and which does not (P95G, shown in red). The controls of empty vector and empty vector + WT are shown in cyan and purple.
Discussion
In this study, we have reported a high-resolution crystal structure of RimP homolog in M. smegmatis that revealed a well-defined interdomain orientation. Moreover, we have provided a mechanistic view of RimP in ribosomal biogenesis. Our binding assays demonstrate that the two domains of RimP cooperatively bind with the small ribosomal protein RpsL through its linker region. More importantly, this linker region is essential for ribosomal biogenesis. Sashital et al. (8) reported that in the absence of RimP, the central pseudoknot structure of rRNA was unstable, resulting in the accumulation of intermediates. These intermediate products were depleted of S5 and S12, which are both pseudoknot-interacting ribosomal proteins. We propose that the linker region of RimP forms a platform for recruiting S12 and facilitating the rRNA binding. Indeed, this hypothesis could explain the functional importance of the evolutionarily conserved residues in this linker region.
In the structure of MSMEG_2624 and SP14.3, the C-terminal domain has an Sm fold that is also observed in Hfq, an RNA chaperone that mediates the interaction between sRNAs and their mRNA targets. Hfq is a hexamer that efficiently binds RNA, whereas RimP remains as a monomer in solution and does not appear to show RNA-binding activity. Indeed, the amino acid sequence between Hfq and RimP diverges significantly. This observation indicates that Hfq and the C-terminal domain of RimP are functionally divergent, even though they are structurally similar.
RimP is highly conserved in prokaryotes, and its functional importance in ribosomal biogenesis has been reported in multiple bacterial species. As no homolog protein has been identified in mammals, it has the potential of becoming a drug target for therapeutic purposes to combat bacterial infections, such as tuberculosis. This makes our structure and biochemical study of particular importance for future drug design.
Experimental procedures
Bacterial strains and plasmids
The bacterial strains and vectors used in this work are summarized in Table S1.
Protein expression, purification, and crystallization
Recombinant His-tagged MSMEG_2624 was expressed in E. coli BL21 (DE3) cells, and purified using nickel-nitrilotriacetic acid affinity chromatography. The His tag was cleaved, and the protein was further purified by size-exclusion chromatography (10 mm Tris-HCl, pH 7.4, 250 mm NaCl). MSMEG_2624 crystals were grown under 20% PEG 3350, 6% acetonitrile, 0.2 m sodium citrate (pH 8.0) by the hanging-drop vapor-diffusion method at 16 °C. The native crystals were cryoprotected by quickly passing through the crystallization buffer containing 12% glycerol before flash freezing. Heavy atom soaking was performed by directly adding 40 mm K2PtCl4 water solution to crystals in the mother liquor twice with a 15-min interval between, to a final concentration of ∼20 mm. The crystals were soaked in the dark overnight and back-soaked for 5 min in cryoprotectant buffer containing 8.8 mm K2PtCl4 and 12% glycerol before data collection.
Structure determination
Data sets were processed using the CCP4 suite (11). Specifically, data were integrated using iMosflm version 1.5 and merged and scaled using SCALA. Scailit was used to scale across the data sets after they were combined using CAD. Heavy atom sites were searched using Afro/Crunch2 followed by refining/phasing using Bp3 using the CRANK suite (12). The initial map was built by the automated model building software Buccaneer (13), followed by iterative local noncrystallographic symmetry (NCS) phased refinement using DM (14) and Refmac (15). Local NCS operator was only used in early stages of refinement to help identify the initial phase. After the refinement statistics converged in the Refmac program, we used Phenix refinement (16) and manual building using the molecular graphics program COOT (17), without the use of NCS. Structures were visualized using PyMOL (18). Statistics of the quality of the structure were calculated using MolProbity (19). Statistics of crystal contacts were calculated using Pisa analysis (20). The structure was deposited to the Protein Data Bank (entry 5GL6).
Construction of MSMEG_2624 knockout strain (ΔrimP)
The rimP null mutant strain (ΔrimP) was constructed using homologous recombination to replace the rimP gene with the hygromycin selection marker as described previously (21). Briefly, a DNA substrate for allelic replacement of rimP (MSMEG_2624) was generated by cloning 500-bp upstream and downstream regions of the rimP gene to 5′ and 3′ ends of the hygromycin (hyg) gene, respectively, and transformed into the Msmeg mc2155 strain harboring a pJV53 vector, which can produce recombinase for efficient recombination. The successful knockout strain ΔrimP::hyg was selected and confirmed by sequencing and Western blot analysis.
In vitro binding of recombinant RimP (MSMEG_2624) and RpsL
Plasmids pGEX-6P-1-RpsL and pRsfDuet-1-RimP were co-transformed to E. coli BL21(DE3). The bacteria were grown in 3 liters of Luria–Bertani medium at 37 °C until A600 ∼0.6. Protein expression was induced by the addition of 1 mm isopropyl 1-thio-β-d-galactopyranoside at 16 °C overnight. Bacteria were harvested and resuspended in 1× PBS and 0.5% Triton X-100. Bacteria were lysed by sonication, and cell debris and precipitants were removed by centrifugation. The supernatant was mixed with TALON Cobalt beads (Clontech) for 1.5 h at 4 °C followed by 10 column bed volumes of 1× PBS washing. Proteins were then eluted with 20 mm Tris-HCl, 500 mm NaCl, 250 mm imidazole. The eluted protein was incubated with GSH column (GE Healthcare) for 1.5 h at 4 °C and washed with 10 column bed volumes of 1× PBS. Proteins were eluted with 20 mm Tris-HCl, 150 mm NaCl, and 10 mm reduced GSH.
Polysome profiling
The strains WT, ΔrimP, ΔrimP+rimP, and ΔrimP+vector were cultured in 7H9 medium, and the cells were harvested at early log phase (A600 ∼1). The cell pellets were lysed as described previously, and the ribosome was extracted according to the method reported previously (22).
Mass spectrometry study of ribosomal fractions
Sample preparation
The fraction was precipitated in acetone with a 1:4 ratio at −20 °C overnight. The protein pellet was dissolved in urea buffer (8 m urea in 50 mm ammonium bicarbonate). Protein concentration was measured by a Bradford assay. 10 μg of protein were diluted to 1 m urea by 50 mm ammonium bicarbonate and digested overnight at 37 °C using sequencing grade trypsin (Promega) with an enzyme/substrate ratio of 1:50. 5 μg of peptide were desalted by C18 Ziptip (Millipore). The sample pellet was reconstituted in formic acid and injected into MS.
LC-MS/MS experiment
The obtained peptides were reconstituted in 12 μl of 0.1% formic acid, and 2 μl was injected into an Easy-nLC 1200 (Thermo). Peptides were separated on a reverse phase C18 column (75-μm inner diameter × 15 cm, 3-μm particle size) and analyzed by an Orbitrap mass spectrometer (Thermo). The mobile phase buffer consisted of 0.1% formic acid in ultrapure water (buffer A) with an eluting buffer of 0.1% formic acid in 80% (v/v) acetonitrile (buffer B) run with a linear 50-min gradient of 7–25% buffer B at a flow rate of 250 nl/min. The mass spectrometer was operated in positive ion mode acquiring a survey mass spectrum with a mass resolution of 120,000, m/z = 350–1800 using an automatic gain control (AGC) target of 3 × 106. The 12 most intense ions were selected for higher-energy collisional dissociation fragmentation (normalized collision energy 27), and MS/MS spectra were generated with an AGC target of 1 × 105 at a resolution of 30,000. The dynamic exclusion time was set to 30 s.
Database analysis
The raw data from MS/MS spectra were searched against the M. smegmatis strain mc2 155 (downloaded on August 20, 2018) using the Sequest HT node integrated within the Proteome Discoverer (PD) software (version 2.2, Thermo). The precursor and fragment mass tolerances were set to 10 ppm and 0.02 Da, respectively. A maximum of two missed cleavages was allowed for trypsin digestion. Cysteine carbamidomethylation was set as static modification, whereas methionine oxidation and N-terminal acetylation were set as variable modifications. False discovery rates of peptide spectrum matches and identified peptides were determined using the Percolator algorithm at 1% based on q value. For quantification, the precursor ion areas in a node-based processing and consensus workflow in PD 2.2 were used. The areas of the ions in the MS1 scan were calculated using the Minora Feature alignment and feature mapping. The abundance values of proteins were obtained via a label-free quantification method using LC-MS/MS.
Author contributions
T. C. and T. C. K. L. conceptualization; T. C., X. W., C. O. K. L., H.-K. K., J. L., S. L., H. Q. P., R. W., L. Z., and R. Y. T. K. data curation; T. C. and T. C. K. L. formal analysis; T. C. visualization; T. C., X. W., and C. O. K. L. methodology; T. C. writing-original draft; T. C. and T. C. K. L. writing-review and editing; X. W., C. O. K. L., and J. C. K. N. investigation; K.-F. L., J. C. K. N., and T. C. K. L. resources; J. C. K. N. and T. C. K. L. supervision; T. C. K. L. validation; T. C. solving the crystal structure.
Supplementary Material
Acknowledgments
We thank Professor Yibei Xiao for discussion of structural analysis and Lauren A. Choate, Dr. Wendy Kwok, and Paul R. Munn for helpful discussion of the manuscript.
This work was supported by General Research Fund (GRF) 11101518, 9042616, and 21101917 and Health and Medical Research Fund (HMRF) Grant 15140342. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Table S1 and Figs. S1–S8.
The atomic coordinates and structure factors (code 5GL6) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- aa
- amino acids
- NCS
- noncrystallographic symmetry
- AGC
- automatic gain control.
References
- 1. Neidhardt F. C., Ingraham J. L., and Schaechter M. (1990) Physiology of the Bacterial Cell: A Molecular Approach, p. 520, Sinauer Associates Inc., Sunderland, MA [Google Scholar]
- 2. Nierhaus K. H., and Dohme F. (1974) Total reconstitution of functionally active 50S ribosomal subunits from Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 71, 4713–4717 10.1073/pnas.71.12.4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Champney W. S. (1977) Kinetics of ribosome synthesis during a nutritional shift-up in Escherichia coli K-12. MGG Mol. Gen. Genet. 152, 259–266 10.1007/BF00693079 [DOI] [PubMed] [Google Scholar]
- 4. Nord S., Bylund G. O., Lövgren J. M., and Wikström P. M. (2009) The RimP protein is important for maturation of the 30S ribosomal subunit. J. Mol. Biol. 386, 742–753 10.1016/j.jmb.2008.12.076 [DOI] [PubMed] [Google Scholar]
- 5. Chang J., Pang E., He H., and Kwang J. (2008) Identification of novel attenuated Salmonella enteritidis mutants. FEMS Immunol. Med. Microbiol. 53, 26–34 10.1111/j.1574-695X.2008.00394.x [DOI] [PubMed] [Google Scholar]
- 6. Yu L., Gunasekera A. H., Mack J., Olejniczak E. T., Chovan L. E., Ruan X., Towne D. L., Lerner C. G., and Fesik S. W. (2001) Solution structure and function of a conserved protein SP14.3 encoded by an essential Streptococcus pneumoniae gene. J. Mol. Biol. 311, 593–604 10.1006/jmbi.2001.4894 [DOI] [PubMed] [Google Scholar]
- 7. Bunner A. E., Nord S., Wikström P. M., and Williamson J. R. (2010) The effect of ribosome assembly cofactors on in vitro 30S subunit reconstitution. J. Mol. Biol. 398, 1–7 10.1016/j.jmb.2010.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sashital D. G., Greeman C. A., Lyumkis D., Potter C. S., Carragher B., and Williamson J. R. (2014) A combined quantitative mass spectrometry and electron microscopy analysis of ribosomal 30S subunit assembly in E. coli. Elife 3, 10.7554/eLife.04491 10.7554/eLife.04491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization (2016) WHO Global Tuberculosis Report 2016, World Health Organization, Geneva [Google Scholar]
- 10. Kerrien S., Aranda B., Breuza L., Bridge A., Broackes-Carter F., Chen C., Duesbury M., Dumousseau M., Feuermann M., Hinz U., Jandrasits C., Jimenez R. C., Khadake J., Mahadevan U., Masson P., et al. (2012) The IntAct molecular interaction database in 2012. Nucleic Acids Res. 40, D841–D846 10.1093/nar/gkr1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G. W., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 10.1107/S0907444910045749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ness S. R., de Graaff R. A. G., Abrahams J. P., and Pannu N. S. (2004) Crank: new methods for automated macromolecular crystal structure solution. Structure 12, 1753–1761 10.1016/j.str.2004.07.018 [DOI] [PubMed] [Google Scholar]
- 13. Cowtan K. (2006) The Buccaneer software for automated model building. Acta Crystallogr. D Biol. Crystallogr. 62, 1002–1011 10.1107/S0907444906022116 [DOI] [PubMed] [Google Scholar]
- 14. Cowtan K. (1999) Error estimation and bias correction in phase-improvement calculations. Acta Crystallogr. D Biol. Crystallogr. 55, 1555–1567 10.1107/S0907444999007416 [DOI] [PubMed] [Google Scholar]
- 15. Murshudov G. N., Vagin A. A., and Dodson E. J. (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 10.1107/S0907444996012255 [DOI] [PubMed] [Google Scholar]
- 16. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 18. DeLano W. L. (2015) The PyMOL Molecular Graphics System, version 1.8, Schrödinger LLC, New York [Google Scholar]
- 19. Chen V. B., Arendall W. B. 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., and Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 10.1107/S0907444909042073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krissinel E., and Henrick K. (2007) Inference of macromolecular asssemblies from crystalline state. J. Mol. Biol. 372, 774–797 10.1016/j.jmb.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 21. van Kessel J. C., and Hatfull G. F. (2007) Recombineering in Mycobacterium tuberculosis. Nat. Methods 4, 147–152 10.1038/nmeth996 [DOI] [PubMed] [Google Scholar]
- 22. Bylund G. O., Wipemo L. C., Lundberg L. A. C., and Wikström P. M. (1998) RimM and RbfA are essential for efficient processing of 16S rRNA in Escherichia coli. J. Bacteriol. 180, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.