ABSTRACT
Plants react to environmental cues by altering their growth and development, which can include organ tropic responses. These differential growth responses are triggered by the hormone auxin, and AUXIN RESPONSE FACTORs (ARFs) have been implicated in numerous organ tropisms in Arabidopsis thaliana. Surprisingly, despite being critical for light capture and overall plant morphology, inflorescence stem tropic responses remain relatively understudied, with presumed direct links to ARF function yet to be established. Here, we show that the expression patterns of ARF5/MONOPTEROS and ARF7/NONPHOTOTROPIC HYPOCOTYL4 are consistent with roles in inflorescence stem tropisms. Mutation of these factors does not alter inflorescence stem responses to gravity or unilateral auxin application, meaning their participation in these processes is presumably masked by functional redundancies. Future resolution of these redundancies will likely require higher order arf mutant combinations, guided by detailed expression analyses of ARFs in the inflorescence stem.
KEYWORDS: Arabidopsis, auxin response factor (ARF), development, functional redundancy, inflorescence stem, tropisms
Abbreviations
- ARF
Auxin Response Factor
- DAG
days after germination
- DEX
dexamethasone
- GR
glucocorticoid receptor
- MP
MONOPTEROS
- NPH4
NONPHOTOTROPIC HYPOCOTYL4
- PIN
PIN-FORMED
As sessile organisms, higher plants need to continuously assess their surroundings to optimize their growth and development. This includes tropic (or differential) growth of organs in response to environmental cues such as light and gravity. Plant stems generally exhibit positive phototropism and negative gravitropism (upward growth) to facilitate the harvesting of light for photosynthesis, while roots display positive gravitropism (downward growth) to acquire nutrients and water and to anchor the plant.1
Distinct stages of growth tropisms have been identified.1,2 For instance, gravitropism begins with gravity perception within specific tissues that harbor starch-rich plastids termed amyloplasts. Amyloplasts are assumed to function as statoliths, which sediment in response to gravity and provide information regarding the optimal direction of organ growth.3 In stems, the endodermis is an amyloplast-containing cell layer believed to be critical in gravitropism. This notion is supported by mutants defective in endodermis formation, which produce stems with abnormal gravitropic responses.4 The second stage of gravitropism involves the transduction of information provided by statolith sedimentation into a signal, namely the asymmetric lateral distribution of the phytohormone auxin across the stem or root. This asymmetric auxin localization results in the third stage of the tropic response, organ bending, which occurs via differential cell elongation.3
Genetic analyses of Arabidopsis thaliana have identified various factors that mediate tropic growth, including those involved in the movement of and response to auxin. For instance, mutations of various genes belonging to the PIN-FORMED (PIN) family of membrane efflux facilitators and the AUXIN RESPONSE FACTOR (ARF) family of transcriptional regulators result in abnormal tropic responses in hypocotyls and roots.5 In contrast, roles for these genes in the differential growth of the inflorescence stem have not been well established, reflecting a general lag in our understanding of the tropic responses of this organ. This knowledge gap is surprising given that inflorescence stem tropisms are critical for dictating overall plant shape and for optimizing processes like photosynthesis. Even so, ARF participation in this tropic response is to be expected, and is indirectly suggested by dominant mutations of the Aux/IAA gene IAA7/AUXIN RESISTANT2 (AXR2), which result in pleiotropic auxin-related defects including abnormal inflorescence stem tropisms.6 These types of gain-of-function mutations stabilize Aux/IAA protein products, which then antagonize the activity of transcriptional activating ARFs.7
We therefore sought to identify ARFs that participate in inflorescence stem tropic responses, and reasoned that those known to modulate tropisms in other plant organs would be promising candidates. ARF5/MONOPTEROS (MP) and ARF7/NONPHOTOTROPIC HYPOCOTYL4 (NPH4) are 2 closely related genes that have been shown to redundantly function in root gravitropism.8 Furthermore, nph4 hypocotyls are defective in both phototropic and gravitropic responses.9,10 Finally, both ARF5 and ARF7 physically interact with IAA7 at the protein level,11 interactions which could, in part, underlie defective inflorescence stem tropisms in dominant iaa7/axr2 mutants. Thus, to investigate roles for these ARFs in differential stem growth, we determined expression patterns of MP and NPH4 in inflorescence stems and analyzed tropic responses in corresponding mutant backgrounds.
Expression of ARFs that facilitate auxin-mediated stem tropisms would be expected in cell layers that contribute to such responses, including auxin-rich source tissues (vasculature), gravity sensing layers (endodermis), or outer cells that undergo differential growth. Cross-sections of inflorescence stems expressing a functional MP::MP-GUS translational reporter showed strong MP levels in vascular bundles (Fig. 1A), in agreement with MP expression at the RNA level.12 It is therefore possible that MP facilitates differential organ growth by promoting the lateral distribution of auxin from central tissues to the stem periphery. Interestingly, we have previously shown that PIN3 is a direct target of MP,8 raising the possibility that MP-dependent expression of PIN3, a gene implicated in both root and hypocotyl gravitropisms,13,14 mediates auxin redistribution during inflorescence stem tropic responses. Additionally, MP is required for floral bud initiation and vascular strand continuity,15 indicating that MP may also indirectly contribute to inflorescence stem tropisms by facilitating the production and long-distance transport of auxin from apical source tissues. In contrast to MP expression, an ARF7::GFP transcriptional reporter was strongly expressed in the stem periphery, including in the area of the epidermis and outer cortex (Fig. 1B and C). NPH4 would be a logical factor involved in mediating differential inflorescence stem elongation in peripheral cells, as it is central to auxin-dependent differential growth responses in numerous other tissues.16
Figure 1.
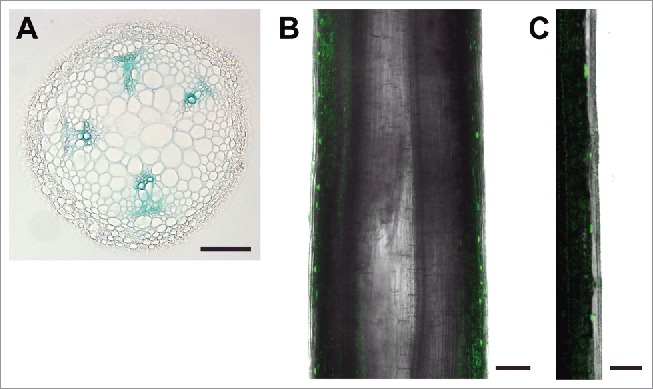
Expression of ARF5/MP and ARF7/NPH4 in Arabidopsis inflorescence stems. (A) Stem cross-section showing MP::MP-GUS expression localized to vascular bundles. (B and C) Confocal images of lateral hand-sections of stems displaying ARF7::GFP expression in peripheral cell layers. GFP confocal images are overlayed on brightfield images. (C) Close-up of peripheral cell layers (epidermis on right) showing distribution of the nuclear-localized GFP reporter. Bars: (A and B) 100 μm; (C) 50 μm.
Since both ARFs are suitably positioned to contribute to inflorescence stem tropisms, we assessed whether mutation of MP and NPH4 affected such processes. We reasoned that testing the inflorescence stems of mp nph4 double mutants would reveal any defects present in either single mutant. Moreover, although non-overlapping expression patterns of MP and NPH4 argue against functional redundancy in the stem, analysis of mp nph4 would unmask any synthetic or synergistic interactions between the 2 mutants. Unfortunately, however, mp nph4 mutants do not form a hypocotyl or embryonic root, and exhibit an enlarged shoot apical meristem initially devoid of lateral organs (Fig. 2A and B).8,17 With time, these meristems initiate radialized lateral outgrowths (Fig. 2C and D) that ultimately transition into highly disorganized masses of atypical structures, a subset of which loosely resemble pin-like inflorescence stems (Fig. 2E-G). Overall, however, this severely compromised meristem activity precludes the formation of organized inflorescence stems.
Figure 2.
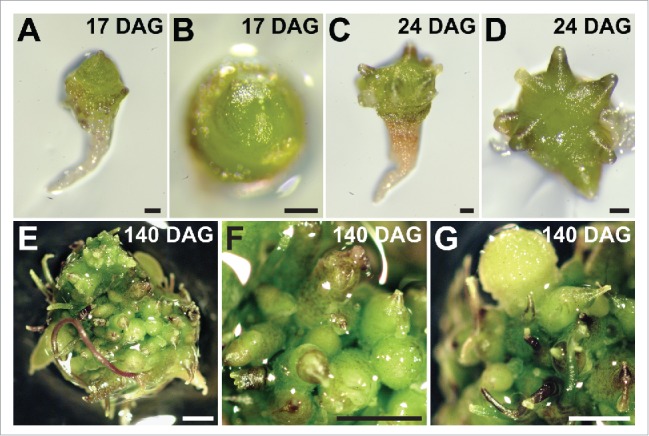
Shoot apical meristem proliferation of untreated mp nph4 MP-GR seedlings. (A and C) Lateral and (B and D) apical views of mp nph4 MP-GR seedlings. Leaves are absent at 17 d after germination (DAG) (A and B) while lateral outgrowths become apparent by 24 DAG (C and D). (E-G) Prolonged growth of the shoot apical meristem (140 DAG) results in the proliferation of disorganized structures that bear little resemblance to normal plant organs. Bars: 200 μm.
To overcome this hurdle, we transiently activated MP using a dexamethasone (DEX)-inducible MP::MP-GR transgene (MP-GR in the following) during early mp nph4 development to obtain normally patterned seedlings.8 Subsequent growth in the absence of DEX results in the tapering off of MP-GR activity and leads to phenotypic reversion to mp nph4. This allows the assessment of ARF contribution to tropic growth of organs, such as stems, that would otherwise not form in this genetic background. In a similar manner, we recently determined that, unlike either single mutant, mp nph4 primary roots are agravitropic.8
We first performed an assay on mp MP-GR individuals to confirm that activation of MP early in development later tapers off upon DEX removal. Plants were germinated and grown on different concentrations of DEX for varying amounts of time, followed by transfer to DEX-free growth conditions (Fig. 3). MP-mediated phenotypic rescue was apparent by the formation of flowers and in turn seed-bearing siliques, which do not form in mp mutants due to defects in lateral organ initiation during reproductive growth.15 The number of siliques produced was positively correlated with both the concentration and duration of DEX treatment (Fig. 3A). Upon DEX removal, inflorescence stems of all treatments invariably lost the ability to produce flowers and seed-bearing siliques, reverting back to the mp mutant condition (Fig. 3A and B). We were therefore confident that we had established a protocol that allowed us to trigger the initiation of inflorescence stems that could be used to test the effect of mp nph4 mutation on tropic responses.
Figure 3.
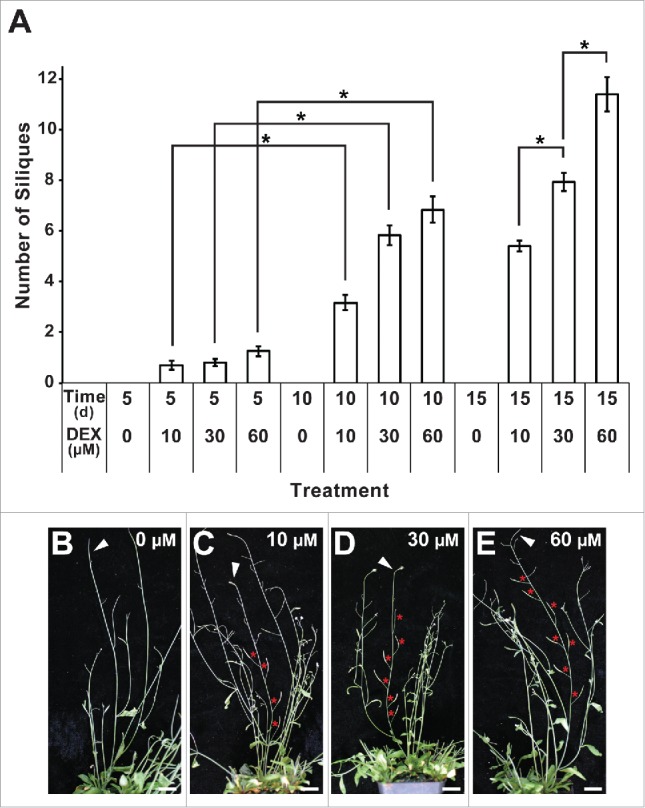
Effect of concentration and duration of DEX treatment on MP activation in mp MP-GR inflorescence stems. (A) Seedlings were germinated and grown on 0, 10, 30 or 60 μM DEX for 5, 10 or 15 d, and numbers of seed-bearing siliques on the primary inflorescence stems produced from each treatment were recorded. Columns represent mean values +/− SEM. Statistical significance of treatment comparisons was determined by Student t test analysis (*, P < 0.0005). The results show an increase in silique number with increasing duration of DEX treatment (see 5 d vs 10 d values) and increasing DEX concentration (see 15 d values). (B-E) Representative plants treated for 10 d on varying concentrations of DEX. The primary inflorescence stem of each plant is indicated (arrowhead), while seed-bearing siliques are designated with asterisks. In each case, at the depicted stage of development, the inflorescence stem has lost the ability to initiate functional flowers/siliques. Bars: 2 cm.
To generate organized mp nph4 inflorescence stems, mp nph4 MP-GR individuals were treated with DEX during embryogenesis and for part of their vegetative growth. This transient treatment sufficiently normalized the overall bodyplan to allow inflorescence stem initiation. However, the pin-like nature of these stems indicated that the effect of MP activation had worn off by the time of assessment (Fig. 4). To analyze stem gravitropism, plants of reproductive age were placed horizontally in the dark (to abrogate possible phototropic interference) for 24 h, a time sufficient to assess gravity-induced differential growth.4 The inflorescence stems of wild type and 2 mp alleles (cultivated via adventitious rooting) showed normal negative gravitropism (Fig. 4A-C). Similarly, mp nph4 double mutant stems also responded properly to the gravity stimulus (Fig. 4D). These results indicate that MP and NPH4 are not strictly required for inflorescence stem gravitropism.
Figure 4.

Tropic responses of Arabidopsis inflorescence stems. (A, E, I, and M) Wild type (WT), (B, F, J, and N) mpG12, (C, G, K, and O) mpBS1354 and (D, H, L, and P) mp nph4 MP-GR plants are depicted. (A-D) Inflorescence stem gravitropic responses. Potted plants (with initially upright inflorescence stems) were turned 90° onto their sides and placed in the dark for 24 h. Images depict plants at the conclusion of the assay, with the gravity vector downward. (E-P) Unilateral auxin application. Lanolin paste (pink) containing 0, 1 or 10 mM indole-3-acetic acid was applied near the apices of inflorescence stems. Inflorescence stem bending was assessed 24 h after application. Ratios indicate the fraction of treated inflorescence stems resembling the depicted image with respect to degree of bending. Bars: (A-D) 2 cm; (E-P) 5 mm.
To test for a general role of ARFs in auxin-mediated tropic growth, we next unilaterally applied auxin (as a lanolin paste) to the apical regions of inflorescence stems of these same genetic backgrounds. Auxin treatment of wild type stems resulted in differential growth away from the side of application, the extent of which correlated with the concentration of auxin used Fig. 4E, I, M. Inflorescence stems of mp mutants also bent upon auxin treatment Fig. 4F, G, J, K, N, O, consistent with previous observations,18 indicating that mp inflorescence stems are not entirely unresponsive to auxin application. Again, mp nph4 stems responded to auxin treatment normally, bending to a degree that reflected the amount of auxin applied Fig. 4H, L, and P, and therefore demonstrating that these 2 ARFs are not absolutely necessary for this response.
Since auxin signal transduction is intimately tied to differential organ growth, ARF participation in inflorescence stem tropic responses is to be expected, even though such links have yet to be established. Many lines of evidence suggest involvement of MP and NPH4 specifically, including gene expression patterns in relevant stem tissues, their confirmed roles in the tropic responses of other organs, and their physical interaction with the one canonical auxin-signaling component (IAA7/AXR2) implicated in inflorescence stem tropisms by its mutation. In this context, it may seem surprising that mp and nph4 mutant backgrounds display normal stem tropisms. However, ARFs belong to a large multi-gene family with many reported functional redundancies between members.7 Therefore, it is entirely plausible that overlapping functions between ARFs have prevented mutational analyses from establishing a direct link between ARFs and inflorescence stem tropisms. Although MP and NPH4 act redundantly in multiple processes (including embryonic and vascular patterning),17 their complementary expression patterns in inflorescence stems indicate that their participation in stem tropic responses is likely masked by other ARF members. This may include ARF19, which functions redundantly with NPH4 in root gravitropism, lateral root formation and auxin-inducible gene regulation.19-21 More detailed investigations into the expression domains of ARF19 and other ARFs will be required to tease apart these genetic relationships, as the high-resolution stem expression patterns described here for MP and NPH4 are lacking for other members of this gene family. In the future, a complete ARF expression map will predict the arf mutant combinations most likely to be compromised in inflorescence stem tropisms. Such relationships are completely predictable, given widespread roles for ARFs in the differential growth of other organs.
Materials and methods
Plant growth conditions and treatments, as well as Arabidopsis mutant and transgenic lines, have been described previously,8 along with the ARF7::GFP line which uses a nuclear localized GFP.22 Brightfield microscopy of MP::MP-GUS sections was performed using an Olympus BX61 compound microscope, while ARF7::GFP distribution was visualized with an Olympus Fluoview FV1200 Laser Scanning Confocal microscope.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest, financial or otherwise.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank T. Berleth and B. Chow for helpful comments on the manuscript.
Funding
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R15GM114733 to N.T.K.
References
- 1.Kaufman PB, Wu LL, Brock TG, Kim D. Hormones and the orientation of growth In: Davies PJ, editor. Plant Hormones. Dordrecht(Netherlands: ): Kluwer Academic Publishers; 1995. p. 547-71; http://dx.doi.org/ 10.1007/978-94-011-0473-9 [DOI] [Google Scholar]
- 2.Feldmann LJ. Root gravitropism. Physiol Plant 1985; 65:341-4; PMID:11540851; http://dx.doi.org/ 10.1111/j.1399-3054.1985.tb02405.x [DOI] [PubMed] [Google Scholar]
- 3.Hashiguchi Y, Tasaka M, Morita MT. Mechanism of higher plant gravity sensing. Am J Bot 2013; 100:91-100; PMID:23115136; http://dx.doi.org/ 10.3732/ajb.1200315 [DOI] [PubMed] [Google Scholar]
- 4.Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 1998; 14:425-30; PMID:9670559; http://dx.doi.org/ 10.1046/j.1365-313X.1998.00137.x [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci 2006; 63:2738-54; PMID:17013565; http://dx.doi.org/ 10.1007/s00018-006-6116-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato A, Sasaki S, Matsuzaki J, Yamamoto KT. Light-dependent gravitropism and negative phototropism of inflorescence stems in a dominant Aux/IAA mutant of Arabidopsis thaliana, axr2. J Plant Res 2014; 127:627-39; PMID:24938853; http://dx.doi.org/ 10.1007/s10265-014-0643-1 [DOI] [PubMed] [Google Scholar]
- 7.Chapman EJ, Estelle M. Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet 2009; 43:265-85; PMID:19686081; http://dx.doi.org/ 10.1146/annurev-genet-102108-134148 [DOI] [PubMed] [Google Scholar]
- 8.Krogan NT, Marcos D, Weiner AI, Berleth T. The auxin response factor MONOPTEROS controls meristem function and organogenesis in both the shoot and root through the direct regulation of PIN genes. New Phytol 2016; 212:42-50; PMID:27441727; http://dx.doi.org/ 10.1111/nph.14107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liscum E, Briggs WR. Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 1995; 7:473-85; PMID:7773019; http://dx.doi.org/ 10.1105/tpc.7.4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liscum E, Briggs WR. Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol 1996; 112:291-6; PMID:8819327; http://dx.doi.org/ 10.1104/pp.112.1.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Larrieu A, Wells D, Guedon Y, et al.. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol 2011; 7:508; PMID:21734647; http://dx.doi.org/ 10.1038/msb.2011.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 1998; 17:1405-11; PMID:9482737; http://dx.doi.org/ 10.1093/emboj/17.5.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002; 415:806-9; PMID:11845211; http://dx.doi.org/ 10.1038/415806a [DOI] [PubMed] [Google Scholar]
- 14.Rakusova H, Gallego-Bartolome J, Vanstraelen M, Robert HS, Alabadi D, Blazquez MA, Benkova E, Friml J. Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J 2011; 67:817-26; PMID:21569134; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04636.x [DOI] [PubMed] [Google Scholar]
- 15.Przemeck GK, Mattsson J, Hardtke CS, Sung ZR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 1996; 200:229-37; PMID:8904808; http://dx.doi.org/ 10.1007/BF00208313 [DOI] [PubMed] [Google Scholar]
- 16.Stowe-Evans EL, Harper RM, Motchoulski AV, Liscum E. NPH4, a conditional modulator of auxin-dependent differential growth response in Arabidopsis. Plant Physiol 1998; 118:1265-75; PMID:9847100; http://dx.doi.org/ 10.1104/pp.118.4.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardtke CS, Ckurshumova W, Vidaurre DP, Singh SA, Stamatiou G, Tiwari SB, Hagen G, Guilfoyle TJ, Berleth T. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL4. Development 2004; 131:1089-100; PMID:14973283; http://dx.doi.org/ 10.1242/dev.00925 [DOI] [PubMed] [Google Scholar]
- 18.Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. Regulation of phyllotaxis by polar auxin transport. Nature 2003; 426:255-60; PMID:14628043; http://dx.doi.org/ 10.1038/nature02081 [DOI] [PubMed] [Google Scholar]
- 19.Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al.. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 2005; 17:444-63; PMID:15659631; http://dx.doi.org/ 10.1105/tpc.104.028316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weijers D, Benkova E, Jager KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jurgens G. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J 2005; 24:1874-85; PMID:15889151; http://dx.doi.org/ 10.1038/sj.emboj.7600659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 2005; 43:118-30; PMID:15960621; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02432.x [DOI] [PubMed] [Google Scholar]
- 22.Rademacher EH, Moller B, Lokerse AS, Llavata-Peris CI, van den Berg W, Weijers D. A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J 2011; 68:597-606; PMID:21831209; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04710.x [DOI] [PubMed] [Google Scholar]


