Abstract
In the presences of additives it has been demonstrated that the critical micelle concentration of amphiphiles can be modified in two ways: through specific interactions with the amphiphile and/or by changing the nature of the solvent. This project focused on the attempt to determine the effects on the critical micelle concentration of hexadecylphospholcholine brought upon by the addition of cavitands (vase-shaped molecular receptors). These effects were measured using various methods for measuring the critical micelle concentration of a surfactant. These methods include: measurements via surface tension using a surface tensiometer, surface tension measurements via the micropipette method, and dye micellization via fluorescent spectrophotometer. Consistent with literature values, the CMC of HePC occurred at 17.8 µM for the Wilhelmy plate method, the capillary height method and the filter paper method. The addition of 50 µM of cavitand caused the CMC to increase to 31.6 µM in the Wilhelmy plate method, the filter paper method and the capillary height method. The CMC of HePC using the dye micellization method occurred around 17.8 µM, however, experiments performed with the addition of cavitands proved to be inconclusive at determining the CMC value. We envision that cavitands may serve as small molecule membrane receptors, our efforts towards studying their effect on critical micelle concentration is a cornerstone of predicting cavitand behavior in more complex lipid bilayer systems, including mammalian cells.
Introduction/Background
Amphiphilic molecules such as surfactants have the ability to spontaneously self-organize into various structures e.g. micelles and bilayers (Evans & Wennerstrom, 1999). This is because the amiphiphilic characteristics of surfactants are partially soluble in water but also have the ability to form a monolayer at the air-water interface (Evans & Wennerstrom, 1999). It is important to note that the process of micellization (formation of micelles) is recognized as the critical micelle concentration (CMC). The CMC occurs by two competing factors: the movement of the hydrophobic chain into the oil-like micelle interior (micellization), and the repulsion of head groups as they come together, where the latter opposes the former (Evans & Wennerstrom, 1999). Therefore, at concentrations below the CMC amphiphilic molecules are essentially soluble in water, while at concentrations above the CMC micelles are found throughout the solution.
The critical micelle concentration of amphiphiles can be determined by using various methods. One commonly used method is measuring the surface tension of a solution. When using this method, the surface tension tends to decrease from 72 mN/m for pure water to a constant value once the CMC is reached for an amphiphile (Evans & Wennerstrom, 1999). The decrease in surface tension occurs because the surface tension of water is higher than that of the hydrocarbon molecule. Above the CMC there is little to no change in the chemical potential of the surfactant and therefore no surface tension change occurs.
Dye micellization is another method used in determining the CMC of surfactants. Certain dyes can be used to determine the CMC of surfactants by one of two ways: through a shift in the wavelength maximum in the presences of micelles or by absorbance at a set wavelength to measure the extent of dye uptake by the surfactant (Patist, 2000).
In the presences of additives it has been demonstrated that the critical micelle concentration of amphiphiles can be modified in two ways: through specific interactions with the amphiphile and/or by altering the characteristics of the solvent (Alam, Naqvi, & Kabir-ud-Din, 2007). Research studies have consistently shown that the majority of additives will cause the CMC to decrease (Alam et al., 2007). Additives such as glycine cause a CMC decrease in MEGA-10 (Ruiz, Hierrezuelo, & Molina-Bolivar, 2008) and certain organic molecules cause a CMC decrease of sodium caprylate, sodium laurate, sodium palmitate and sodium stearate (Akhter & Alawi, 2000). However, there are certain alcohols such as methanol and ethanol that have shown a CMC increase in sodium caprylate, sodium laurate, sodium palmitate, and sodium stearate (Akhter & Alawi, 2000). The CMC of these surfactants increased due to the alcohol interactions with the hydrocarbon chains that caused their solubility to increase in an aqueous medium.
The structure of hexadecylphosphocholine (HePC) can be broken down into two parts: a hydrophobic chain of 16 carbons and a hydrophilic head of phosphocholine. These unique properties of HePC have allowed this surfactant to be used in various areas of study. Current uses of HePC are as an antitumoral agent in the treatment of cutaneous metastases from breast cancer (Unger, Peukert, Sindennann, & Hilgard 1992; Hilgard, Klemmer, Stekar, & Unger, 1993) and as an oral medication treatment for visceral leishmaniasis (Sundar, More, Singh, Sharma, Makhavia, Kumar, & Murray, 2000). Though HePC has been showen to be affective in interacting with the cell membrane, the actual mechanism of action is still not known.
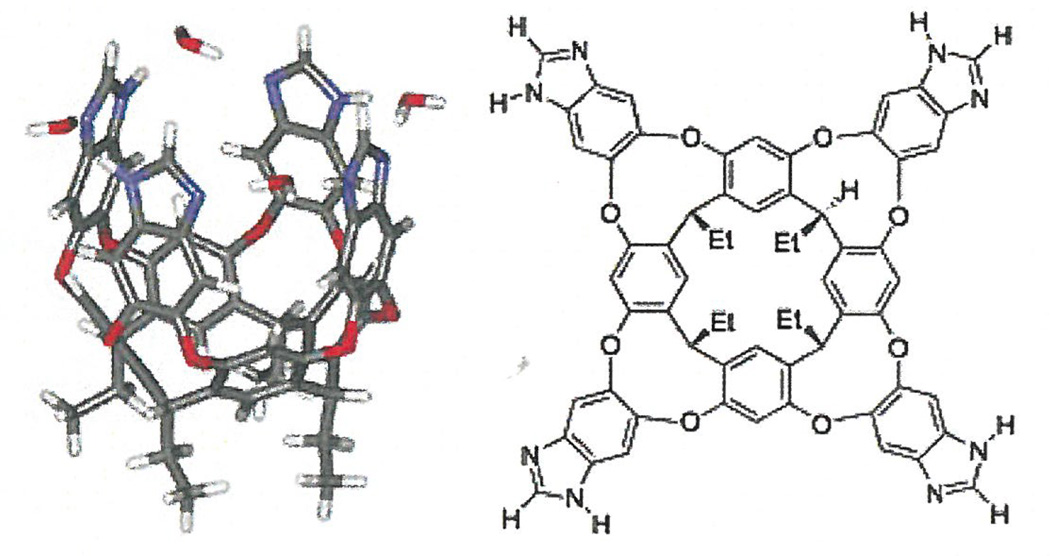
For this study, the additive under investigation was an ethyl footed resorcin[4]arene imidazole cavitand (vase-shaped molecular receptor) (scheme 1). Cavitands can serve as a small-molecule host that are capable of guest selectivity, slow guest exchange, acceleration, and catalysis of reactions (Schramm, Hooley, & Rebeck, 2007). In addition, some cavitands have the capability of imbedding within micelles through a host-guest interaction, where the cavitand is a guest within the micelle. Our work aimed to determine the effects on the critical micelle concentration of hexadecylphospholcholine brought upon by the addition \ cavitands. We hypothesized that cavitands may one day serve as small molecule membrane receptors, and studying their effect on critical , micelle concentration of HePC is a key concept in understanding the mechanism of membrane interaction in the presence of additives, as well as in predicting cavitand behavior in more complex systems (e.g., vesicles, lipid bilayers, cell membranes).
Scheme 1.
Three dimensional (left) and chemical (right) presentations of an ethyl footed resorcin[4]arene imidazole cavitand (vase-shaped molecular receptor).
Materials and Methods
Hexadecylphosphocholine (HePC) was purchased from Avanti Polar Lipids. Cavitand was prepared according to literature procedure (Schramm et al., 2007). 8-Anilino-1-naphthalenesulfonic acid ammonium salt was purchased from SIGMA Life Science. Two stock solutions of HePC were prepared in de-ionized water: 0.001 M and 0.02 M. These stock solutions were used for serial dilutions and titration studies to give the final concentrations indicated. We compared three methods, capillary height method, the Wilhelmy plate method and the dye micellization.
The capillary height method (micropipette method) uses the relationship between the meniscus, the capillary rise, the capillary radius and the contact angle to determine the surface tension of the solution. The surface tension measurement results from the interfacial properties of the liquid interacting with the solid walls of the capillary tube. The procedure used to perform this experiment was taken from the Anatrace Tech Bulletin 105. A 15 µL pipette was used to measure the height of the rising solution. Series of titrations were done using 0.001 M and 0.02 M HePC stock solutions to acquire 9 different surfactant concentrations. Height measurements were recorded for each concentration. This experiment was performed in triplicate and the mean peak heights were plotted against concentration. The CMC was taken as the intersection point of the two slopes.
The Wilhelmy plate is a thin platinum plate that is attached to a force balance and is brought into contact with a fluid. The force balance then measures the weight change brought upon by the contact of the plate with the meniscus. In turn, the surface pressure measured can then be used to calculate the surface tension of the solution (Evans & Wennerstrom, 1999). For this experiment we used a KRÜSS tensiometer. Initial experiments were done using serial dilutions and were later altered to titrations due to the sensitivity of the tensiometer. The surface pressure readings obtained from the tensiometer are used the following equation to obtain surface tension: π = γ - γo. Were π is the surface pressure read from the tensiometer, γ is the surface tension without HePC (DI water is 72 mN/m) and γo is the surface tension with HePC present. Values calculated for γo, were then plotted against concentration. The CMC was determined to occur at the intersection of the two slopes (Evans & Wennerstrom, 1999).
For the filter paper method, the same concept was used as in the Wilhelmy plate method, expect a 2×1 cm filter paper was used in place of the Wilhelmy plate. For this experiment, the serial dilution method was used and new filter paper was used for every concentration. Surface pressure readings from the tensiometer where converted and plotted following the method described above.
For the dye micellization method, it has been reported that certain dyes can be used to determine the CMC of surfactants by one of two ways: through a shift in the wavelength maximum in the presence of micelles or by absorbance at a set wavelength to measure the extent of dye uptake by the surfactant (Patist, Bhagwat, Penfield, & Aikens, 2000). For nonionic surfactants, graphing a range of concentrations against their absorbance at a set wavelength will give an increase in absorbance then eventually flatten again as the dye fully moves into the to the micelle interior depleting the continuous-phase dye. The procedure used to perform this experiment was taken from the Anatrace Tech Bulletin 104. Single scan readings were taken using a fluorescent spectrophotometer.
Excitation and emission for 8-Anilino-1-naphthalenesulfonic acid ammonium salt were set at 410 nm and 490 nm respectively. Emission at 490 nm was then graphed against concentration and the CMC was defined as the intersecting point of the two lines.
Results
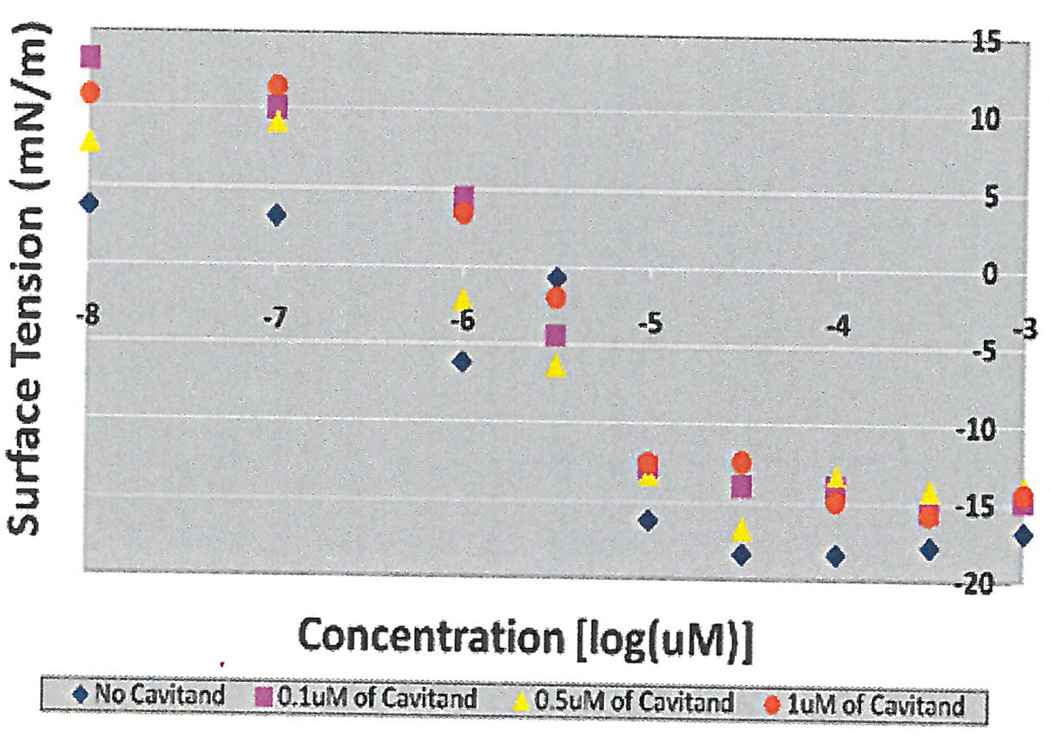
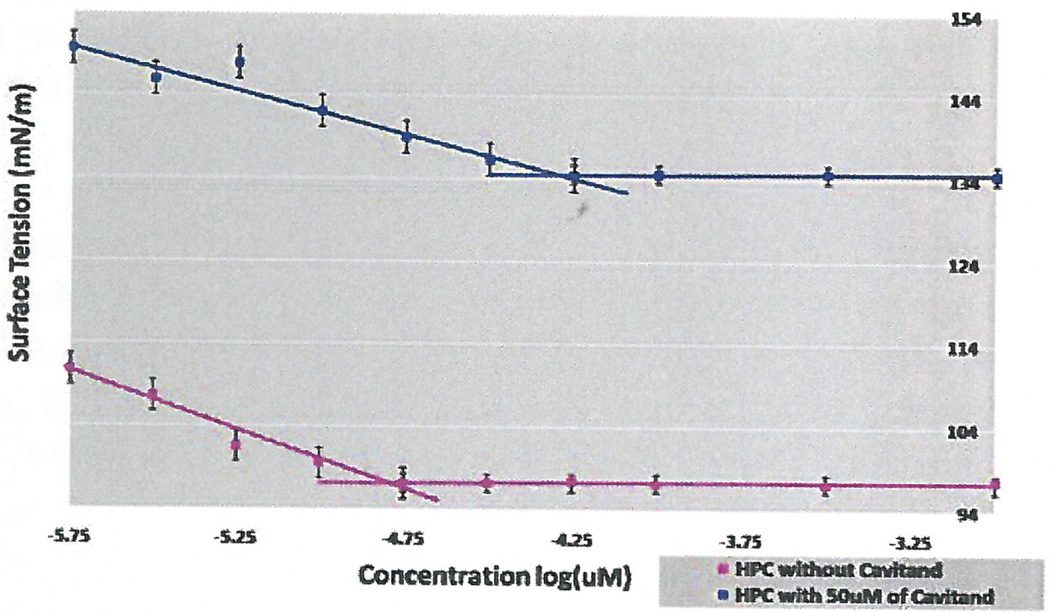
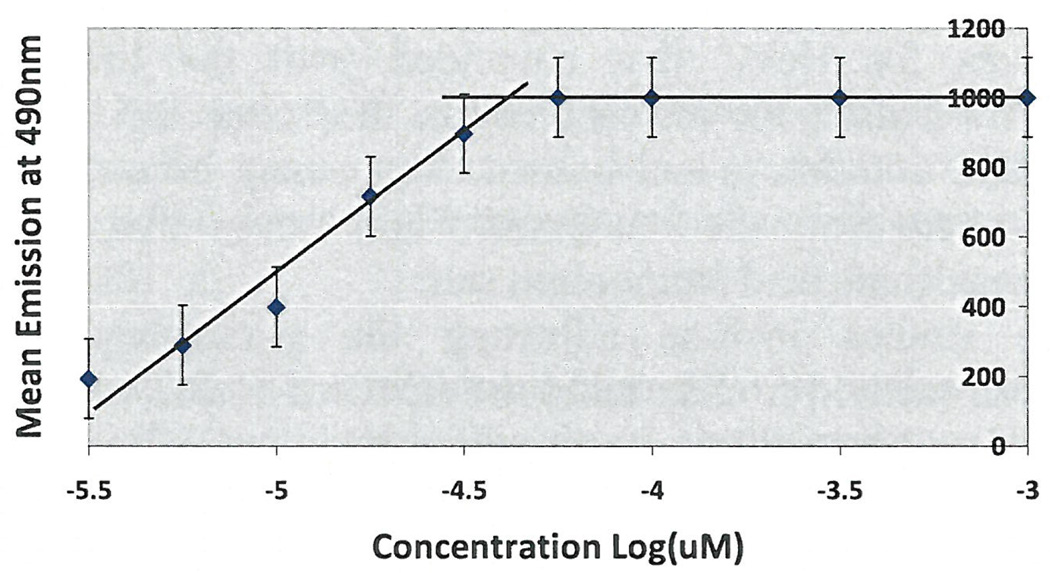
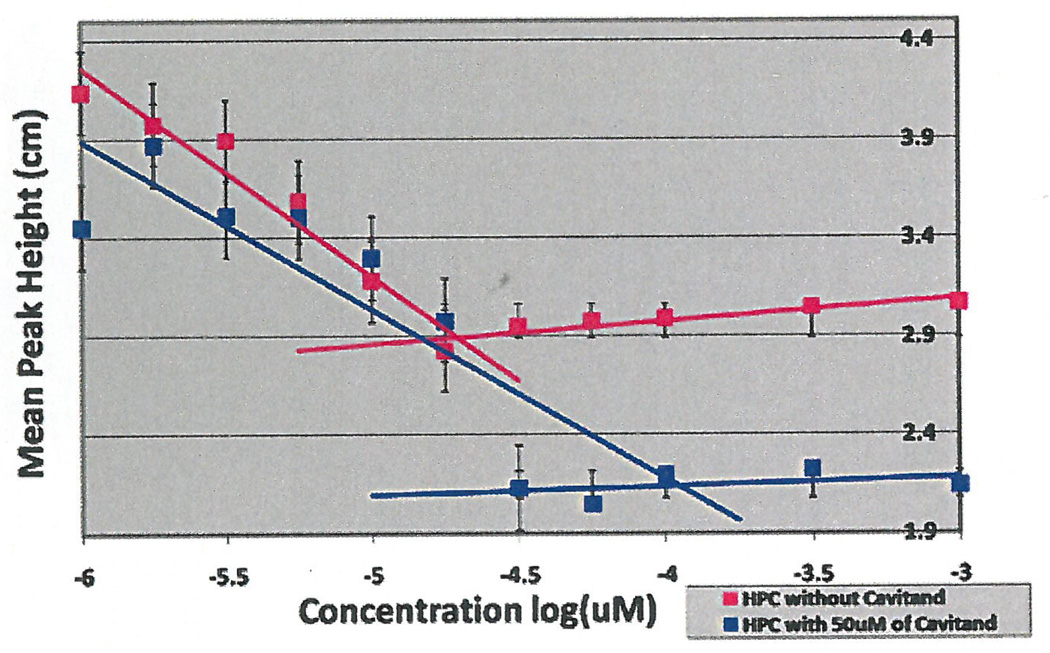
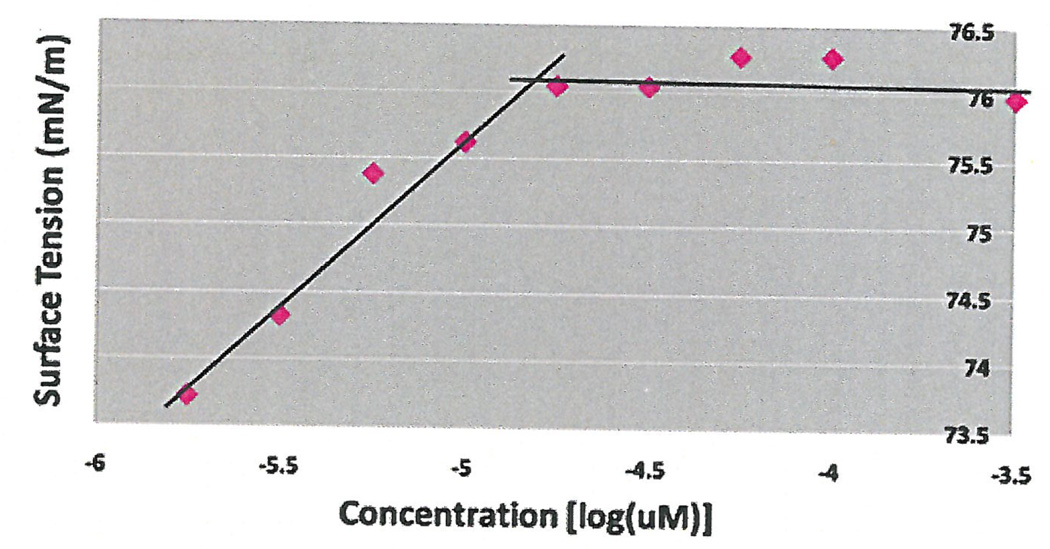
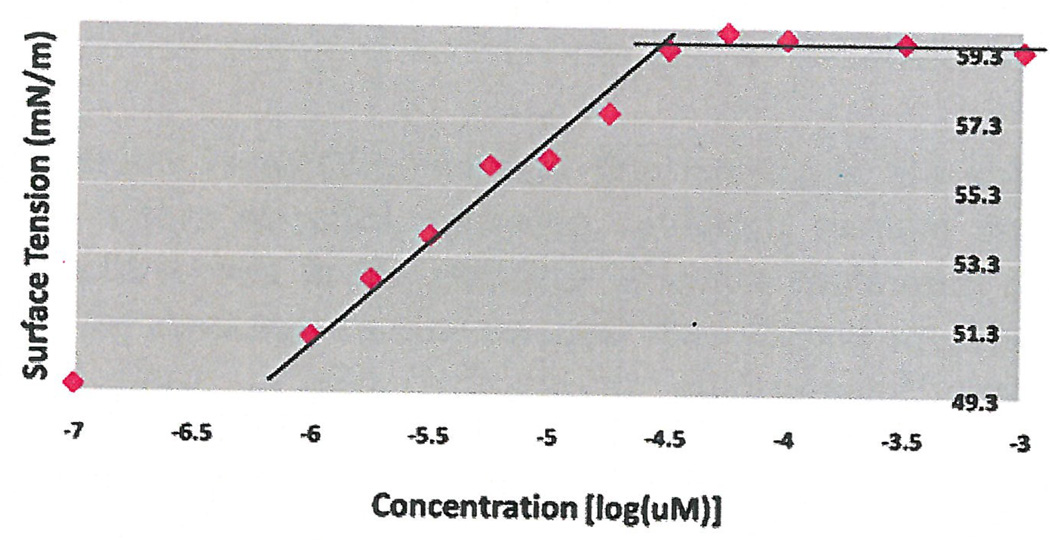
Initial experiments were done using 3 concentrations of cavitand 0.1 µM, 0.5 µM and 1 µM using surface tension measurements by the filter paper method. As seen in Figure 1, the CMC occurred at 10 µM without additives, which coincided with research studies that concluded the CMC of HePC to occur between 5 µM to 12 µM (Lukac, Lacko, & Devinsky, 2008; Rakotomango, Loiseau, & Saint-Pierre-Chazalet, 2004; Dittrich, Haftendorn, & Ulbrich-Hofmann 1998; Yassen, Wang, Su, & Lu, 2005). However, a notable CMC change could not be determined for the experiments preformed with the addition of cavitand (Figure 1). Revisions to the HePC concentrations were made to ensure enough data points around the literature CMC values of HePC, as well as an increase in cavitand concentration to 50 µM. The experiment was repeated using Wilhelmy plate method, the capillary height method, the filter paper method and the dye micellization method (Figure 2–5). Without the addition of cavitand, the CMC of HePC occurred at 17.8 µM for the Wilhelmy plate method, the capillary height method and the filter paper method, which is consistent with literature values (Figure 2–4). Upon the addition of 50 µM of cavitand, the CMC increased to 31.6 µM in the Wilhelmy plate method, the filter paper method and the capillary height method (Figure 2–4). The CMC of HePC without the addition of cavitands using the dye micellization method occurred around 17.8 µM, which is consistent with the literature values. However, initial experiments preformed with the addition of cavitands proved to be inconclusive at determining the CMC value, due to fluorescence interference from the cavitand.
Figure 1.
Determination of CMC with and without addition of cavitand using the filter paper method and serial dilutions. Three concentrations of Cavitand were used 0.1 µM, 0.5 µM and 1 µM. CMC with and without cavitand additive occurred at 101 µM.
Figure 2.
CMC determination of HePC with and without the addition of 50 µM of cavitand using surface tension filter paper method and serial dilutions. CMC of HePC without addition of cavitand occurred at 17.8 µM (pink) and with cavitand at 31.6 µM (blue).
Figure 5.
CMC determination of HePC using dye micellization and titrations. CMC of HePC without addition of cavitand occurred around 17.8 µM and with cavitand addition could not be determined.
Figure 4.
CMC determination of HePC with and without the addition of 50 µM of cavitand using Capillary height method and titrations. CMC of HePC without addition of cavitand occurred at 17.8 µM (pink) and with cavitand addition at 31.6 µM.
Discussion
Previous studies have demonstrated that cavitands are able to embed themselves within micelles, allowing cavitands to take on their vase-shaped structure even in the presence of water (Schramm et al., 2007). The ability for cavitands to take on their vase-shaped structure is thought to occur because of the hydrophobic environment created by the hydrocarbon chains of the surfactant. The CMC increase observed in the micropipette method, the Wilhelmy plate method, and the filter paper method is believed to occur because of the cavitand interacting with the hydrophobic carbon chains causing the hydrocarbon chains of HePC to increase their solubility in water. In addition to the property changes in the surfactant tails, the size and hydrophobic property of the cavitand may cause the increase in CMC because more individual HePC molecules may be needed to form the micelle to accommodate this additional hydrophobic surface.
For the dye micellization method, the controlled experiment reported CMC values for HePC that coincided with the literature values. However, the reason for the inability to determine the CMC of HePC with cavitand addition is not known. There may be some interactions occurring between the cavitand and the dye, 8-Anilino-1-naphthalenesulfonie acid ammonium salt.
Future studies include adjusting the parameters of the dye micellization method to determine the CMC of HePC with the addition of cavitands. As well as a comparative investigation between the hydrophobic cavitand used for this study and a water soluble cavitand to determine if their effect on the CMC of surfactants is similar. Moreover, the investigation of cavitand activity in micelles will one day be used in developing methods to predict cavitand behavior in more complex systems (e.g., vesicles, lipid bilayers, cell membranes).
Figure 3a.
CMC determination of HePC without the addition of 50 µM of cavitand using the Wilhelmy plate method and tritrations. CMC of HePC without addition of cavitand occurred at 17.8 µM.
Figure 3b.
CMC determination of HePC with addition of 50 µM of cavitand using the Wilhelmy plate method and tritrations. CMC of HePC with addition of cavitand occurred at 31.6 µM.
Acknowledgements
We would like to thank Dr. Michael P. Schramm for being an excellent ) professor and mentor. Also, Dr. Krzysztof Slowinski for allowing us to use the KRÜSS tensiometer in his lab, and in particular, William Hammond for teaching us how to use the instrument. Dr. Paul Weers for reaching and allowing us to use the fluorescent spectrophotometer in his lab. Lastly, a special thanks to the McNair Scholars Program for its support throughout the project.
Contributor Information
Massiel Trujillo, Department of Biological Sciences.
Dr. Michael P. Schramm, Department of Chemistry and Biochemistry
References
- Akhter SM, Alawi SM. The effect of organic additives on critical micelle concentration of non-aqueous micellar solutions. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2000;17:311–320. [Google Scholar]
- Alam S, Naqvi AZ, Kabir-ud-Din Surface and micellar properties of some amphiphilic drugs in the presence of additives. Journal of Chemical and Engineering Data. 2007;52(4):1326–1331. [Google Scholar]
- Dittrich N, Haftendorn R, Ulbrich-Hofmann R. Hexadecylphosphocholine and 2-modified 1.2-diacylglycerols as effectors of phospholipase D. Biochimica Et Biophysica Acta. 1998;1391:256–272. doi: 10.1016/s0005-2760(97)00208-7. [DOI] [PubMed] [Google Scholar]
- Evans F, Wennerstrom H. The Colloidal Domain Where Physics, Chemistry, Biology, and Technology Meet. New York, New York: Wiley-VCH; 1999. [Google Scholar]
- Hilgard P, Klemmer T, Stekar J, Unger C. Alkylphosphocholines: A new class of membrane-active anticancer agents. Cancer Chemotherapy Pharmacology. 1993;32(2):90–95. doi: 10.1007/BF00685608. [DOI] [PubMed] [Google Scholar]
- Lukac M, Lacko I, Devinsky F. Interaction between hexadecylphosphocholine and cetyltrimethylammonium bromide. Acta Facultatis Pharmaceuticae Universitatis Comenianae, LV. 2008:142–151. [Google Scholar]
- Patist A, Bhagwat SS, Penfield KW, Aikens P. On the measurement of critical micelle concentrations and pure and technical-grade nonionic surfactants. Journal of Surfactants and Detergents. 2000;3(1):53–58. [Google Scholar]
- Rakotomanga M, Loiseau PM, Saint-Pierre-Chazalet M. Hexadecylphosphocholine interaction with lipid monolayers. Biochimica Et Biophysica Acta. 2004;1661:212–218. doi: 10.1016/j.bbamem.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Ruiz CC, Hierrezuelo JM, Molina-Bolivar JA. Effect of glycine on the surface activity and micellar properties of N-decanoyl-N-methylglycamide. Colloid Polym Sci. 2008;286:1281–1289. [Google Scholar]
- Schramm MP, Hooley RJ, Rebeck JJ. Guest recognition with micelle-bound cavitands. Journal of American Chemical Society. 2007;129:9773–9779. doi: 10.1021/ja0723378. [DOI] [PubMed] [Google Scholar]
- Sundar S, More DK, Singh VP, Sharma S, Makhavia A, Kumar PCK, Murray HW. Failure of pentavalent antimony in visceral leishmaniasis in India. Report from the center of the Indian epidemic Clincal Infection. 2002;31:1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- Unger C, Peukert H, Sindermann P, Hilgard P. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Exp. Tumor Res. 1992;34:153–159. doi: 10.1159/000420840. [DOI] [PubMed] [Google Scholar]
- Yaseen A, Wang Y, Su T, Lu J. Surface adsorption of zwitterionic surfactants: N-alkyl phosphocholines characterized by surface tensiometry and neutron reflection. Journal of Colloid and Interface Science, 2005;288(2):361–370. doi: 10.1016/j.jcis.2005.03.024. [DOI] [PubMed] [Google Scholar]