Abstract
Invariant natural killer T (iNKT) cells are innate T cells that express a semi-invariant T cell receptor (TCR) and recognize lipid antigens presented by CD1d molecules. As part of innate immunity, iNKT cells rapidly produce large amounts of cytokines after activation and regulate the function of innate and adaptive immune cells in antimicrobial immunity, tumor rejection and inflammatory diseases. Global transcriptional profiling has advanced our understanding of all aspects of iNKT cell biology. In this review, we discuss transcriptional analyses of iNKT cell development, functional subsets of iNKT cells, and global comparisons of iNKT cells to other innate and adaptive immune cells. Global transcriptional analysis revealed that iNKT cells have a transcriptional profile distinct from NK cells and MHC-restricted T cells, both during thymic development and in the periphery. The transcription factors EGR2 and PLZF (and microRNA like miR-150) are key regulators of the iNKT cell transcriptome during development. PLZF is one of several factors that control the homing and maintenance of organ-specific iNKT cell populations. As in MHC-restricted T cells, specific transcription factors are characteristic of functional subsets of iNKT cells, such as the transcription factor T-bet in the NKT1 subset. Exciting future directions for global transcriptional analyses include iNKT cells in disease models, diverse NKT cells and human studies.
Keywords: NKT cell development, NKT cell subset, Global transcriptome profiling, Global transcriptional profiling, PLZF
1. Introduction
Invariant natural killer T (iNKT) cells, also known as type I or classical NKT cells, are innate αβ T cells expressing a semi-invariant T cell receptor (TCR) that recognizes lipid antigens presented by CD1d [1]. As part of the innate immune system, iNKT cells rapidly produce large amounts of cytokines just minutes after activation. These cytokines profoundly modulate the innate and adaptive arms of the immune system in infection, tumor rejection and inflammatory disease. In humans, iNKT cells generally express an invariant Vα24-Jα18 TCR α chain paired with Vβ11. In mice, iNKT cells generally express a semi-invariant TCR of Vα14-Jα18 paired with a limited Vβ repertoire (usually Vβ2, Vβ7, or Vβ8.2). iNKT cells are a well-studied representative of the growing family of innate T lymphocytes, and comparisons of iNKT cells with MHC-restricted T cells illuminate the principles that govern the interaction of the innate and adaptive immune systems. Global transcriptional profiling (via RNA microarray or RNA-sequencing [RNA-Seq]) has revolutionized research into the unique combination of innate and effector memory-like functions in iNKT cells. With a global view of innate and adaptive programs, transcriptional analyses address the question, “What is an iNKT cell?” To answer this question, we will review the transcriptional analyses of iNKT cell development, the transcriptional programs that regulate functional subsets of iNKT cells in the periphery, and the innate (“NK cell-like”) character of the iNKT cell transcriptome. We conclude with future directions for global transcriptional analyses of iNKT cells.
2. Transcription factors and microRNA in iNKT cell development
2.1. Distinct transcriptional profile for iNKT cells in development
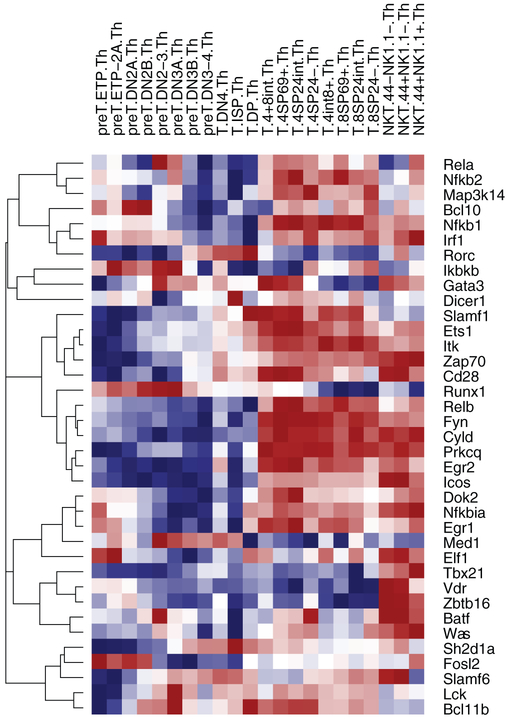
Global transcriptional analyses have advanced our understanding of the transcription factors involved in iNKT cell development. iNKT cells develop in the thymus, where the first iNKT cell precursors arrive at post-natal day 5 [2]. iNKT cells diverge from MHC-restricted T cells in the double positive (DP, CD4+CD8+) thymyocyte pool [3]. A subset of DP thymocytes express the transcription factor retinoic acid receptor-related Orphan Receptor γ (RORγt) and rearrange their Vα14 and Jα18 segments to form the invariant TCRα chain. The semi-invariant T cell receptor on iNKT cell precursors recognizes lipid antigens presented by CD1d, a homologue of major histocompatibility complex (MHC) [4]. These cells then down-regulate CD8 and enter stage 0 (CD44−NK1.1−CD4+). iNKT cells proceed through three subsequent stages of development in the thymus. Our laboratory performed global transcriptional analysis of thymic iNKT cell subsets in C57BL/6J mice as part of the Immunological Genome Project (Imm-Gen, www.immgen.org) [5]. ImmGen is a multi-center consortium of immunologists and computational biologists characterizing the immune system transcriptome using a systematic approach. By microarray analysis, the transition from DP to stage 1 (CD44− NKT1.1−) changed expression in 1850 genes; the transition from stage 1 to stage 2 (CD44+ NKT1.1−), 155 genes; and the transition from stage 2 to stage 3 (CD44+ NKT1.1+), 697 genes [5]. Focusing on genes known to regulate the development of iNKT cells, our data demonstrated that thymic iNKT cells had a distinctly different profile than MHC-restricted thymic T cells (Fig. 1). For example, Zbtb16, Tbx21, and Gata3 showed opposite patterns of expression across all iNKT cell thymic subsets compared to all MHC-restricted T cell thymic subsets. Other transcription factors, such as Egr2 or Slamf6, showed a mixed pattern of expression in NKT cell and MHC-restricted T cell subsets. We will focus on two transcription factors in iNKT cell development, EGR2 and PLZF.
Fig. 1.
Expression profile of thymic T cell subsets: selected genes involved in iNKT cell development. T cell precursors, MHC-restricted T cells and stage 1, 2, and 3 iNKT cells were sorted by flow cytometry from the thymi of C57BL/6J mice, and global transcriptional analysis was performed by RNA microarray.
2.2. EGR2
The transcription factor EGR2 is an important regulator of iNKT cell development. Studies on Egr2 are elegant examples of global transcriptional analysis paired with chromatin immunoprecipitation with deep sequencing (ChIP-seq). Calcineurin B1 and NFAT signaling drive transcription of the Egr family (Egr1, Egr2, and Egr3). In the Egr family, only Egr2-deficient mice have a significant reduction in thymic and peripheral iNKT cell numbers compared to wild-type mice, although studies show some subtle defects in Egr1-deficiency [6,7]. Egr2-deficient mice are not viable due to defective brain development and so are studied as chimeras. Egr2-deficient fetal liver chimeric mice had impaired progression from stage 1 (CD44− NK1.1−) to stage 2 (CD44+ NK1.1−) [6] while Egr2-deficient bone marrow chimeric mice showed arrest at stage 2 [7]. In contrast, MHC-restricted T cells have grossly normal development in Egr2-deficient chimeric mice. To screen for targets of EGR2, Seiler et al. used four criteria [7]: (1) upregulation in the global transcriptome of thymic NKT cells, as compared to CD4+ single-positive thymocytes in our ImmGen dataset. There were 614–983 genes with increased relative expression in each iNKT stage 1, 2 or 3; (2) binding by EGR2 in the ChIP-seq of thymic NKT cells from Vα14 transgenic mice. There were 33–43 genes in iNKT stages 1–3; (3) binding by EGR2 in the ChIP-seq of thymocytes from wild-type mice treated with anti-TCRβ antibody. There were 13–17 genes in iNKT stages 1–3; (4) conservation of the binding site in human and mouse. There were 5–7 genes in iNKT stages 1–3. The nine genes that met all four criteria were Zbtb16, Il2rb, Fasl, Ccnd2 (cyclin D2), Slc9a9 (ion exchange protein in late endosome), Ptprv (transmembrane tyrosine phosphatase involved in cell cycle), Hivep3 (transcription factor that may bind the recombination signal sequences flanking the V, D, and J regions of T cell receptor), Tbkbp1 (adaptor protein likely involved in TNF and NFκB signaling), and Fam46a. Electrophoretic mobility-shift assays and in vivo studies confirmed EGR2 transactivation of Il2rb and, notably, Zbtb16, which encodes the key transcriptional factor PLZF [7].
2.3. PLZF
The discovery that iNKT cells highly express promyelocytic leukemia zinc finger (PLZF, encoded by the gene Zbtb16) was a key insight into iNKT cell development and function [8,9]. PLZF is a member of the BTB-POZ-ZF (broad complex, tramtrack, bric-a-brac or poxvirus and zinc finger) family of transcription factors that control many functions of the immune system. Initially thought to be specific for iNKT cells, PLZF is expressed in other innate T cell subsets (i.e., mucosal-associated invariant T (MAIT) cells and a population of γδ T cells [10,11]) and innate lymphoid precursor cells in the fetal liver and bone marrow [12]. PLZF is induced immediately after positive selection of iNKT cells [8,9] (Fig. 1) and is required for both iNKT cell development and effector functions. In the absence of PLZF, the number of iNKT cells is dramatically reduced to approximately 5–10% of normal levels. In addition, the trafficking of iNKT cells to tissues depends on PLZF. In PLZF-deficient mice, iNKT cells accumulated in the lymph nodes, rather than peripheral organs, likely due to high expression of CD62L compared to wild-type iNKT cells [9]. Mature iNKT cells require PLZF for normal effector function. After activation, PLZF-deficient iNKT cells failed to secrete high levels of IFNγ and IL-4 and lacked an activated or memory phenotype [8,9]. Ectopic expression of PLZF was sufficient to confer an iNKT-like phenotype to MHC-restricted T cells [8,9]. More generally, PLZF is linked to innate effector functions in several cell types besides iNKT cells.
To identify downstream effects of PLZF, Kreslavsky and colleagues compared gene expression profiles of iNKT cells from WT and PLZF-deficient mice. PLZF controlled key aspects of development, survival, homeostasis and trafficking [13]. PLZF regulated ThPOK (encoded by Zbtb7b), which is required for iNKT cell development [14]. PLZF also regulated Id2, which is critical for iNKT cell survival in the liver [15]. PLZF also regulated the expression of genes coding for chemokine receptors (Ccr2 and Ccr10) and cytokine receptors (Il12rb1 and Il18r1) [13]. To explain the molecular mechanism of PLZF, Mathew et al. used immunoprecipitation to show that PLZF associates with the E3 ubiquitin ligase cullin 3 (CUL3) [16]. PLZF transports CUL3 to the nucleus, where they both associate with a chromatin-modifying complex. The ability of PLZF to drive the ubiquitination of key nuclear proteins helps to explain its diverse effects on the iNKT cell transcriptome. These effects of PLZF on the transcriptome help explain the profound NKT cell abnormalities in PLZF-deficient mice [8,9].
2.4. MicroRNA
MicroRNA (miRNA) are 22 nucleotide, single-stranded RNA that modulate the stability of RNA post-transcription. Global transcriptional analysis is a natural fit for studies of miRNA, since miRNA can have wide-ranging effects on the transcriptome. For example, ectopic expression of miRNA Lin28 in bone marrow hematopoetic stem/progenitor cells (HSPC) reprograms HSPC to skew toward the innate lymphocyte pathway and increased production of iNKT cells [17]. In mice with the conditional deletion of Dicer (Dicerflox/flox × Cd4-Cre mice), DP thymocytes can still express mature miRNAs, but single positive thymocytes have a profound reduction in functional miRNA [18]. This conditional deletion of Dicer reduced thymic iNKT cell number by over 90%, with similar decreases in peripheral iNKT cell number. Thymic iNKT cells had a distinct miRNA profile (by miRNA microarray) when compared to thymic MHC-restricted T cells. Of 70 miRNA detected, thymic iNKT cells differently expressed 17 miRNA compared to MHC-restricted thymic T cells. Real-time PCR confirmed that thymic iNKT cells had higher expression of miR-21 and lower expression of let-7g, miR-106a, miR-34b-3p, miR-17, miR-30c, miR-106b, miR-16, miR-467a, miR-467b, miR-690, miR-15b, miR-669f and miR-150 (in order of increasing magnitude of fold-difference). The expression patterns of miRNA in iNKT cell subsets is unknown and may be informative. For example, miR-150 was down-regulated in iNKT cells (compared to MHC-restricted T cells) in the thymus. One might conclude that miR-150 is less important in development. However, the miRNA microarray was done in pooled thymic iNKT cells. The pooling masked that miR-150 expression increased significantly from thymic stage 1 to stage 3. Correspondingly, miR-150-deficient mice have a defect in later stages of iNKT cell differentiation [18,19].
Global transcriptional analysis greatly facilitates assessment of the down-stream effects of miRNA. For example, mice deficient in miR-181a1b1 have a nearly complete loss of iNKT cells at the DP stage. Global transcriptional profiling of double-positive thymocytes by RNA-Seq showed more than 80% of genes had lower expression in miR-181a1b1-deficient mice as compared to wild-type mice. These down-regulated genes included several metabolic pathways (e.g., glycolytic, pentose phosphate and nucleotide synthesis) [20]. The 146 upregulated genes included Pten, a negative regulator of the PI3K pathway, a key regulator of metabolism. PTEN may drive the broad metabolic dysregulation in developing iNKT cells from miR-181a1b1-deficient mice, as inhibition of Pten expression rescued iNKT cell development [20]. Global transcriptome analyses of both miRNA and mRNA have illuminated iNKT cell development in the thymus.
3. iNKT cell subsets
3.1. Tissue-specific subsets of iNKT cells
iNKT cells leave the thymus and move to the periphery at stage 2 (CD44+ NKT1.1−) or stage 3 (CD44+ NKT1.1+) of development. At baseline, iNKT cells are most abundant in the liver and spleen, but iNKT cells can dramatically increase their numbers in a range of organs during inflammation or infection, such as the lung in asthma and chronic bronchitis [21,22], the pancreas in type I diabetes [23,24] and in other situations. From organ to organ, iNKT cells have varying numbers and phenotypes, even different frequencies of TCR Vβ chain usage [25]. Organ-specific differences in iNKT cells likely derive from both characteristics acquired before entering the organ and the effects of the organ environment on iNKT cells. Organ-specific chemo-attractants and integrins select for specific subsets of iNKT cells, and these interactions help generate organ-specific iNKT cell populations. For example, splenic iNKT cells home to the spleen in part because of their response to BCA-1 (CXCL13), a chemokine that mediates B and T cell homing to follicular regions of lymphoid tissue [26]. In contrast, hepatic iNKT cells do not respond to BCA-1. For hepatic iNKT cells, expression of LFA-1 is necessary to retain these iNKT cells in the liver sinusoids, but LFA-1-deficient mice have normal numbers of iNKT cells in the spleen and other organs [27,28]. One regulator of organ-specific differences is the transcription factor PLZF. When PLZF was over-expressed in MHC-restricted CD4+ T cells, these cells up-regulated LFA-1 and accumulated in the liver [27]. PLZF-deficient mice had altered homing and increased numbers of iNKT cells in lymph nodes [9].
Other factors besides homing regulate organ-specific populations of iNKT cells. The steady-state equilibrium reflects homing and proliferation balanced by recirculation and cell death. iNKT cells have very different patterns of recirculation compared to other T cells. Parabiotic congenic mice demonstrated that iNKT cells have markedly reduced recirculation in tissues compared to recirculation by other T cells, as first shown by the Bendelac lab in liver, lung and spleen [27] and by our laboratory in adipose tissue [29]. Within two weeks of parabiosis, B cells and MHC-restricted T cells achieved almost complete chimerism in the liver, lung, spleen, and adipose tissue of parabiotic mice. In contrast, iNKT cells had minimal recirculation, and 90–95% of organ-resident iNKT cells in these tissues were endogenous and not derived from the parabiotic partner mouse [29]. With reduced recirculation, local regulation of iNKT cell survival takes on increased importance. Multiple signals from the organ environment regulate iNKT survival. Mice with a hepatocyte-specific deficiency in expression of 1L-7 had a significant decrease of iNKT cells in the liver (but normal numbers in thymus and spleen) [30]. In another example, intra-vital microscopy revealed that CXCR6 is not necessary for normal recruitment and patrolling behavior. CXCR6 is required, however, for a normal number of iNKT cells in the liver sinusoids, and so may enhance survival of iNKT cells in the liver [31].
In addition to cell number, the phenotype of iNKT cells varies from organ to organ. Antigen presenting cells (APCs) regulate many of these organ-specific phenotypes. In one demonstration, both thymic and splenic iNKT cells had increased production of IFNγ after incubation ex vivo with splenic APCs, as compared to after incubation with thymic APCs [32]. The co-stimulatory molecules on APCs may mediate their organ-specific influences. For example, expression of SLAM on DCs skews NKT cells toward a “Th-2-like” phenotype [33], and different genetic haplotypes at the Slam locus correlate with different iNKT behavior in several mouse strains [34,35]. In theory, APCs in different organs could present different endogenous antigens to skew NKT cell behavior; however, this mechanism has not been clearly demonstrated in vivo.
To examine how iNKT cells subsets differ from organ to organ, our laboratory performed a global transcriptional analysis that compared CD4+ and CD4− iNKT cells from liver, spleen and lung of naïve C57BL/6J mice. iNKT cell gene expression patterns showed significantly more variability in CD4+ versus CD4− iNKT subsets in lung than liver (261 differently expressed genes in lung, 159 in spleen, 17 in liver) [5]. Underscoring the value of an unbiased approach, the differently regulated genes have a broad range of functions, from NK receptors (e.g., Klra3) and chemokine receptors (e.g., Ccr7 or Ccr9) to genes of uncertain significance (e.g., Hist1h2bc or Snord1c). The interaction of iNKT cell subsets with different organ environments is a key topic for further study.
3.2. iNKT cells have distinct phenotypic and functional subsets
Despite having a limited TCR repertoire, “invariant” NKT cell subsets are quite heterogeneous in phenotype and function. In mice, cell surface markers, such as CD4, NK1.1 and IL-17RB, can help to distinguish functional subsets [2,36–38]. For example, NK1.1– iNKT cells and CD4+ iNKT cells tend to produce IL-4 and little IFNγ, while NK1.1+ iNKT cells and CD4– iNKT cells tend to skew in the opposite direction [37]. Alternatively, one can sub-divide populations by expression of CD4 and IL-17RB. IL17RB– iNKT cells produce IFNγ; CD4+IL17RB+ iNKT cells produce Th1, Th9 and Th17 cytokines in response to IL-25, and CD4–IL17RB+ iNKT cells also express RORγt and produce Il-17 in response to IL-23 [38]. One problem for some surface receptors is their strain dependence. For example, Balb/c mice do not express NK1.1 [39]. Instead, programs of gene expression may more accurately identify iNKT cell subsets. Analogous to helper T cells, iNKT cells can be divided into NKT1, NKT2, NKT17, NKT-Follicular Helper (NKTFH), and NKT10 subsets based on transcription factors and cytokine production [40]. The Hogquist lab proposed an intracellular staining protocol for transcription factor expression to classify Th1-like, Th2-like, and Th17-like iNKT cell subsets [39]. Interestingly, the “key regulators” of the NKT1, NKT2, NKT17, NKTFH, and NKT10 subsets are analogous to equivalent MHC-restricted T cell subsets.
3.3. NKT1
NKT1 cells, as their name suggests, primarily produce the Helper T helper type 1 (Th1) cytokine IFNγ, but NKT1 cells also produce IL-4 in some situations [39]. NKT1 cells express high levels of T-bet, the Th-1-associated transcription factor, but also express low levels of GATA3 [39,41]. NKT1 cells lack IL17RB expression, generally express NK1.1 (in C57BL/6 mice), and require IL-15 for homeostasis [38]. In C57BL/6 mice, the most abundant subset is NKT1 cells; NKT1 cells are much reduced in Balb/c mice. In terms of location, the NKT1 subset dominates in the liver, even in Balb/c mice. The enrichment of the NKT1 subset in the liver may account for the enhanced cytotoxic function of CD4– iNKT cells in the liver compared to iNKT cells elsewhere [42].
3.4. NKT2
NKT2 cells produce IL-4 and little to no IFNg when stimulated with the potent lipid ligand α-galactosylceramide (α-GalCer) in vivo. NKT2 cells and NKT17 cells both express high levels of T helper type 2 (Th2)-like transcription factors GATA3 and IRF4. GATA-3 is a zinc-finger transcription factor that plays an essential role in T cell development and Th2 cell differentiation [43,44]. ChIP-Seq analysis in T cell subsets identified genome-wide GATA3 binding sites. Some GATA-3 binding sites, such as the Ctla4 and Icos genes, are widely shared among T cell subsets. However, some GATA-3 binding motifs and target genes are specific to particular T cell subsets [41]. In most T cells, WGATAA was the top binding motif, and the Runx motif was the top secondary binding motif. However, in iNKT cells, TCF11 was the top secondary binding motif [41]. This difference in secondary-motifs partially explains the different activity of GATA-3 in different T cell subsets. In mice, NKT2 cells are the most predominant subset in the thymus and spleen of Balb/c mice. Overall, NKT2 cells are approximately eight times more abundant in the Balb/c strain compared to C57BL/6 [39]. This difference in NKT2 cell number may contribute to differences between the Balb/c and C57BL/6 strains in some experimental models, such as allergic asthma. In humans, CD4 expression on iNKT cells may predict Th2-like iNKT cells; and the relative abundance of Th2-like iNKT cells varies widely in humans [36,37].
3.5. NKT17
NKT17 cells predominantly produce Th17 cytokines such as IL-17 and IL-22. NKT17 cells were discovered as a subset of hepatic NK1.1− iNKT cells producing abundant IL-17 (and less IL-4 and IFNγ) after stimulation by α-GalCer or a microbial lipid antigen [45]. The transcription factor RORγt drives development of NKT17 cells in the thymus [45]. Global transcriptional profiling revealed that the transcription factor Th-POK represses the development of NKT17 cells. Thus, in Th-POK deficiency, RORγt expression is increased, and iNKT cells produce IL-17 [14]. In mice, NKT17 cells are abundant in the lymph nodes, lungs, and skin and have proven important in several disease models, such as airway neutrophilia induced by α-GalCer [45].
3.6. NKTFH
Like MHC-restricted T Follicular Helper (TFH) cells, a distinct population of iNKTFH cells can provide cognate help to antigen-specific B cells [46–48]. iNKTFH cells provide help to lipid-reactive B cells that internalize lipid antigens via their B cell receptor. iNKTFH cells can initiate germinal center reactions faster than MHC-restricted T cells, but iNKTFH cells did not generate long-lived plasma cells. As in MHC-restricted cells TFH, BCL-6 was the transcription regulator of iNKTFH cells and induced expression of PD-1 and CXCR5 [49]. Also like MHC-restricted TFH cells, NKTFH cells produced IL-21, which drove B cell help. 80% of NKTFH cells expressed CD4 and were generally NK1.1−. NKTFH cells are another example of NKT cell heterogeneity driven by a key transcription factor influencing localization and function.
3.7. NKT10
A population of regulatory iNKT cells has recently been described [38,50–52] with characteristic production of IL-10. IL-10 is a regulatory cytokine that can downregulate inflammatory responses. Motomura et al. reported that the transcription factor E4BP4, encoded by Nfil3, is responsible for IL-10 production in T helper cells and iNKT cells [50]. Following stimulation with γ-GalCer, a small proportion of splenic iNKT cells upregulated E4BP4 and produced IL-10. Moreover, iNKT cells from E4BP4−/− mice did not produce IL-10. IL-10 producing iNKT cells were in the CD4+IL-17RB+ subset of iNKT cells [38]. Il-17RB is a receptor of IL-25, and treating cells with IL-25 induced E4BP4 and IL-10 production, among other cytokines, including IL-13 [38,50]. Our laboratory has found that regulatory IL-10 producing iNKT cells are enriched in adipose tissue [51]. Adipose iNKT cells are protective in obesity induced inflammation and metabolic disorder [51,53–55]. The protective role of adipose iNKT cells is likely due to a regulatory phenotype and production of IL-10, which dampened inflammation in macrophages (and possibly in adipocytes themselves) [51,54]. Using IL-10 reporter mice, Sag et al. recently confirmed the enrichment of IL-10 producing iNKT cells in adipose tissue. The “NKT10” cell subset is also a naturally occurring population in human peripheral blood mononuclear cells (PBMCs) [52]. Further studies are required to determine whether iNKT cells produce IL-10 after TCR-driven stimulation and represent a post-activation or anergy-like state; or whether IL-10 producing E4BP4+ iNKT cells are a thymically determined subset that traffics to adipose tissue.
4. What is an iNKT cell?
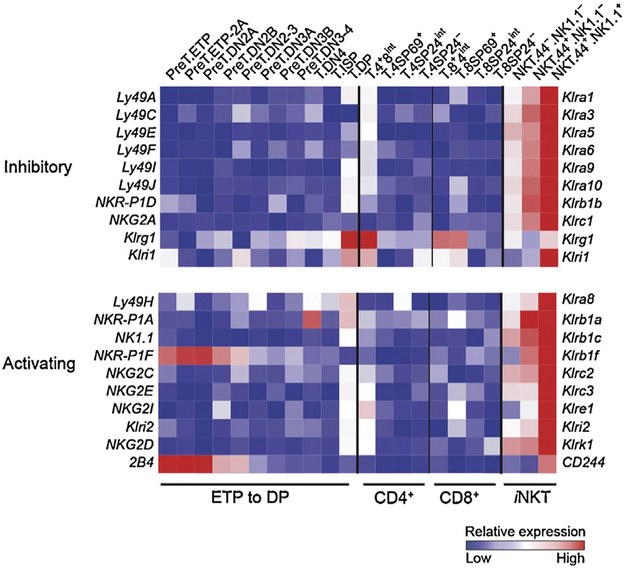
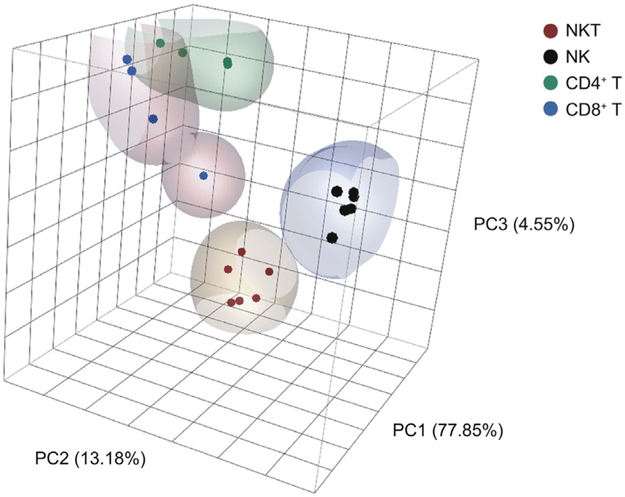
Since the term “natural killer” in the moniker “iNKT cell” is a historical term based on expression of NK1.1, it was open to question whether NKT cells functionally deserved the description as “NK cell-like.” Global transcriptional analysis revealed that iNKT cells occupy a distinct space “in between” MHC-restricted T cells and NK cells. Our laboratory performed global transcriptional profiling of thymic iNKT cell subsets through ImmGen, and our analysis showed that iNKT cells progressively acquire NK cell-like character as the iNKT cells diverge from MHC-restricted T cells during development. For example, in thymic stages 2 and 3, maturing iNKT cells coordinately upregulate a broad spectrum of NK cell inhibitory and activating receptors (Fig. 2). To assess the “innate” and “adaptive” character of iNKT cells, we compared the global transcriptome of our iNKT subsets (from spleen, liver and lung) to NK cell subsets (from spleen and liver) and naïve or memory MHC-restricted T cell subsets (from spleen or lymph node) (Fig. 3). Principal component analysis placed iNKT cells in a unique transcriptional space, separate from other innate and adaptive lymphocytes.
Fig. 2.
Expression profile of thymic T cell subsets: Natural Killer Receptor (NKR) genes. T cell precursors, MHC-restricted T cells and stage 1, 2, and 3 iNKT cells were sorted by flow cytometry from the thymi of C57BL/6J mice, and global transcriptional analysis by RNA microarray was performed (Fig. 1E from [5]).
Fig. 3.
Principal component analysis of iNKT, NK and MHC-restricted T cell subsets. iNKT cells subsets (CD4+/−) from spleen, liver and lung; NK cell subsets (CD49c+/−, CD49H+/−) from spleen and liver; and MHC-restricted T cellsr in naïve CD4+ and CD8+ subsets (CD25LoCD44LoCD62LHi), memory CD4+ subsets (CD25−CD44HiCD122Lo) and CD8+ subsets (CD25−CD44HiCD122Hi)from spleen and lymph nodes were sorted by flow cytometry from C57BL/6J mice. Global transcriptional analysis was performed by RNA microarray and transformed by principal component analysis.
Recent work extends our analysis by comparing iNKT cells to specific subsets of NK cells. Daussy et al. focused on Eomesoder-min (Eomes)-negative NK cells. T-bet drives the development of Eomes− NK cells in the liver. Global transcriptional profiles of liver iNKT cells, Eomes+ NK cells and Eomes− NK cells demonstrated that liver iNKT cells, which lack Eomes exression in mice, more closely resemble Eomes− NK cells [56]. iNKT cells and Eomes− NK cells share similar expression patterns for integrins (increased Itga1, Itgav, Itgb3; decreased Itga2, Itgam, Itgb7), metallo-proteineases (increased Mmp9, Adam13), and chemokine receptors (highly increased Cxcr3 and Cxcr6; decreased Cx3cr1). This analysis suggests that NKT cells and the Eomes− NK cells may have functional similarities, such as in trafficking. The work of our laboratory and others reinforce the idea that the “natural killer” moniker in NKT cells is indeed quite meaningful. Studies on additional gene expression datasets have been informative. For example, Vahl et al. explored whether conditional activation or deletion of the alpha chain of the semi-invariant T cell receptor in iNKT cells (Vα1414i-TCR) confers “NKT cell phenotype”. To assess the “NKT cell phenotype” of CD8+ T cells expressing transgenic Vα14i-TCR, Vahl et al. measured expression of selected transcription factors (i.e., PLZF, EGR2, ThPOK) and seven NK cell receptors/markers (e.g., NKG2D, NKG2A). Based on these selected genes, they concluded that transgenic expression of Vα14i-TCR does not confer “NKT cell identity”. Similarly, they concluded that deletion of the Vα14i-TCR in NKT cells does not remove their “NKT cell identity” because the cells could still rapidly produce cytokines. However, these analyses could be extended with a global transcriptional analysis to characterize the acquisition or loss of “NKT cell identity”.
5. Future directions
In just the past decade, investigators have greatly advanced our understanding of the NKT cell transcriptome. They have identified key transcription factors and characterized their global effects on development, function and NKT cell subsets. Global transcriptome analyses revealed that, in line with their unique functions, iNKT cells have a unique transcriptome distinct from that of NK cells and MHC-restricted T cells. A promising future direction is the NKT transcriptome in dynamic situations, such as inflammation, infection, and models of human disease. With a comprehensive database of the NKT cell transcriptome during dynamic situations, the NKT community could perform a bioinformatic analysis similar to that done by Novershtern et al. on 38 human hematopoietic lineages, which identified hundreds of “transcriptional modules” and their cis-regulatory circuits [57]. A second promising future direction is the transcriptome of immune cells regulated by NKT cells. NKT cells are powerful transactivators of the immune system and control the activation of neutrophils, NK cells, γδ T cells, macrophages, dendritic cells, B cells and MHC-restricted T cells [1]. However, we lack global transcriptome analyses of cells after transactivation by NKT cells. The literature holds just a few examples, such as dendritic cells after co-culture with NKT cells [58,59]. It would be invaluable to have a systematic analysis of the global transcriptome of neutrophils, NK cells, γδ T cells and macrophages after transactivation by iNKT cells.
This review focuses on invariant NKT cells in mouse models. The transcriptomes of diverse NKT cells and human NKT cells are additional important areas for the next decade. Diverse NKT cells, also known as type II NKT cells, have a more diverse repertoire of TCRs and are not limited to the Vα14 TCR alpha chain used by iNKT cells. Diverse NKT cells do not recognize the lipid antigen α-GalCer, the canonical lipid antigen for invariant NKT cells [1]. Therefore, CD1d tetramer loaded with α-GalCer identifies invariant NKT cells but does not bind to diverse NKT cells. With heterogeneous antigen specificity, diverse NKT cells often cannot be identified in vivo and are challenging to isolate for transcriptional analysis. Human studies have their own challenges, such as the low numbers of iNKT cells in human peripheral blood (i.e., often less than 0.1% [60]). New technologies, such as single cell transcriptional profiling by RNA-Seq, may help meet these challenges in diverse NKT cell and human studies. Human studies will also benefit from creative approaches to translational science. For example, Crawford et al. used a mouse model of a human mutation in a “bedside-to-bench” study [61]. DOCK8 is a guanine nucleotide exchange factor for CDC42 [62]. Human patients with a rare congenital defect in DOCK8 have an 80% decrease in peripheral NKT cell number, which may contribute to their susceptibility to infection and malignancy [61]. To investigate this clinical presentation, Crawford et al. studied DOCK8-deficient mice that mimicked the human phenotype. Global transcriptome analysis of thymic iNKT cells identified a candidate gene, Itgae (integrin αE, CD103) for continued study. This study is just one example of the creative possibilities in marrying murine and human immunology. Clearly, the transcriptional space of NKT cells is a space that we have just begun to explore.
Acknowledgements
We thank the ImmGen Project Consortium for their support, and the U.S. National Institutes of Health Tetramer Core Facility (contract HHSN272201300006C) for CD1d tetramers. P.J.B. thanks the American Academy of Asthma, Allergy and Immunology AR Trust and NIH (AI102945) for their support.
Abbreviations:
- α-GalCer
α-galactosylceramide
- BTB-POZ-ZF
broad complex, tramtrack, bric-a-brac or poxvirus and zinc finger
- CCR2
C-C chemokine receptor type 2
- CCR10
C-C chemokine receptor type 10
- ChIP-seq
chromatin immunopre-cipitation with deep sequencing
- CUL3
cullin 3
- DOCK8
dedicator of cytokinesis 8
- DP
double positive
- EGR2
early growth response protein 2
- Eomes
eomesoder-min
- ImmGen
Immunological Genome Project
- iNKT
invariant natural killer T cell
- iNKTFH
invariant natural killer cell follicular helper
- IFNγ
interferon-gamma
- IL-4
interleukin-4
- IL-17RB
interleukin-17 receptor B
- IRF4
interferon regulatory factor 4
- LFA-1
lymphocyte function-associated antigen 1
- MAIT
mucosal-associated invariant T
- MHC
major histocompatibility complex
- miRNA
microRNA
- NFIL3
nuclear factor, interleukin-3 regulated
- NF-B
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
natural killer
- NKG
natural killer cell group
- PBMC
peripheral blood mononuclear cell
- PLZF
promyelocytic leukemia zinc finger
- RNA-Seq
RNA-sequencing
- RORγt
retinoic acid receptor-related orphan receptor γ
- T-bet
T-box expressed in T cells
- TCR
T cell receptor
- TFH
T follicular helper
- Th1
T helper type 1
- Th2
T helper type 2
- TNF
tumor necrosis factor
References
- [1].Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol 2009;102:1–94. [DOI] [PubMed] [Google Scholar]
- [2].Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J Exp Med 2002;195(7):835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc Natl Acad Sci U S A 2005;102(14):5114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol 2001;2(10):971–8. [DOI] [PubMed] [Google Scholar]
- [5].Cohen NR, Brennan PJ, Shay T, Watts GF, Brigl M, Kang J, et al. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nat Immunol 2013;14(1):90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hu T, Gimferrer I, Simmons A, Wiest D, Alberola-Ila J. The Ras/MAPK pathway is required for generation of iNKT cells. PLoS ONE 2011;6(5):e19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seiler MP, Mathew R, Liszewski MK, Spooner CJ, Barr K, Meng F, et al. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat Immunol 2012;13(3):264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol 2008;9(9):1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 2008;29(3):391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, et al. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proc Natl Acad Sci U S A 2009;106(30):12453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, et al. Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3. J Immunol 2010;184(3):1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature 2014;508(7496):397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gleimer M, von Boehmer H, Kreslavsky T. PLZF controls the expression of a limited number of genes essential for NKT cell function. Front Immunol 2012;3:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Engel I, Zhao M, Kappes D, Taniuchi I, Kronenberg M. The transcription factor Th-POK negatively regulates Th17 differentiation in Valpha14i NKT cells. Blood 2012;120(23):4524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Monticelli LA, Yang Y, Knell J, D’Cruz LM, Cannarile MA, Engel I, et al. Transcriptional regulator Id2 controls survival of hepatic NKT cells. Proc Natl Acad Sci U S A 2009;106(46):19461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mathew R, Seiler MP, Scanlon ST, Mao AP, Constantinides MG, Bertozzi-Villa C, et al. BTB-ZF factors recruit the E3 ligase cullin 3 to regulate lymphoid effector programs. Nature 2012;491(7425):618–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 2012;335(6073):1195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fedeli M, Napolitano A, Wong MP, Marcais A, de Lalla C, Colucci F, et al. Dicer-dependent microRNA pathway controls invariant NKT cell development. J Immunol 2009;183(4):2506–12. [DOI] [PubMed] [Google Scholar]
- [19].Zheng Q Zhou L, Mi QS. MicroRNA miR-150 is involved in Valpha14 invariant NKT cell development and function. J Immunol 2012;188(5):2118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limon P, Kaech SM, et al. The microRNA miR-181 is acritical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity 2013;38(5):984–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med 2003;9(5):582–8. [DOI] [PubMed] [Google Scholar]
- [22].Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, et al. Persistent activation of an innate immune response translates respirator viral infection into chronic lung disease. Nat Med 2008;14(6):633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med 2001;7(9):1052–6. [DOI] [PubMed] [Google Scholar]
- [24].Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med 2001;7(9):1057–62. [DOI] [PubMed] [Google Scholar]
- [25].Masuda K, Makino Y, Cui J, Ito T, Tokuhisa T, Takahama Y, et al. Phenotypes and invariant alpha beta TCR expression of peripheral V alpha 14+ NKT cells. J Immunol 1997;158(5):2076–82. [PubMed] [Google Scholar]
- [26].Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol 2003;171(6):2960–9. [DOI] [PubMed] [Google Scholar]
- [27].Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med 2011;208(6):1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ohteki T, Maki C, Koyasu S, Mak TW, Ohashi PS. Cutting edge: LFA-1 is required for liver NK1.1 + TCR alpha beta+ cell development: evidence that liver NK1.1 + TCR alpha beta+ cells originate from multiple pathways. J Immunol 1999;162(7):3753–6. [PubMed] [Google Scholar]
- [29].Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat Immunol 2015;16(1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liang B, Hara T, Wagatsuma K, Zhang J, Maki K, Miyachi H, et al. Role of hepatocyte-derived IL-7 in maintenance of intrahepatic NKT cells and T cells and development of B cells in fetal liver. J Immunol 2012;189(9): 4444–50. [DOI] [PubMed] [Google Scholar]
- [31].Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 2005;3(4):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang Y, Ueno A, Bao M, Wang Z, Im JS, Porcelli S, et al. Control of NKT cell differentiation by tissue-specific microenvironments. J Immunol 2003;171(11):5913–20. [DOI] [PubMed] [Google Scholar]
- [33].Baev DV, Caielli S, Ronchi F, Coccia M, Facciotti F, Nichols KE, et al. Impaired SLAM-SLAM homotypic interaction between invariant NKT cells and dendritic cells affects differentiation of IL-4/IL-10-secreting NKT2 cells in nonobese diabetic mice.J Immunol 2008;181(2):869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aktan I, Chant A, Borg ZD, Damby DE, Leenstra PC, Lilley GW, et al. Slam haplo-types modulate the response to lipopolysaccharide in vivo through control of NKT cell number and function. J Immunol 2010;185(1):144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jordan MA, Fletcher JM, Pellicci D, Baxter AG. Slamf1: the NKT cell control gene Nkt1.J Immunol 2007;178(3):1618–27. [DOI] [PubMed] [Google Scholar]
- [36].Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med 2002;195(5):625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med 2002;195(5):637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, et al. Development and function of invariant natura killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol 2012;10(2):e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol 2013;14(11):1146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol 2013;25(2):161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wei G, Abraham BJ, Yagi R, Jothi R, Cui K, Sharma S, et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 2011;35(2):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med 2005;202(9):1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4+ T cells. Cell 1997;89(4):587–96. [DOI] [PubMed] [Google Scholar]
- [44].Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci U S A 2004;101(7):1993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med 2007;204(5):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A 2008;105(24):8345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes.J Exp Med 2003;197(8):1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].King IL, Fortier A, Tighe M, Dibble J, Watts GF, Veerapen N, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol 2012;13(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol 2012;13(1):35–43. [DOI] [PubMed] [Google Scholar]
- [50].Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol 2011;12(5):450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012;37(3):574–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest 2014;124:3725–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest 2012;122(9):3343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, et al. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem 2012;287(17):13561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Huh JY, Kim JI, Park YJ, Hwang IJ, Lee YS, Sohn JH, et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol 2013;33(2):328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med 2014;211(3):563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Novershtern N, Subramanian A, Lawton LN, Mak RH, Haining WN, McConkey ME, et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 2011;144(2):296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Caielli S, Conforti-Andreoni C, Di Pietro C, Usuelli V, Badami E, Malosio ML, et al. On/off TLR signaling decides proinflammatory or tolerogenic dendritic cell maturation upon CD1d-mediated interaction with invariant NKT cells. J Immunol 2010;185(12):7317–29. [DOI] [PubMed] [Google Scholar]
- [59].Globisch T, Steiner N, Fulle L, Lukacs-Kornek V, Degrandi D, Dresing P, et al. Cytokine-dependen regulation of dendriti cell differentiation in the splenic microenvironment. Eur J Immunol 2014;44(2):500–10. [DOI] [PubMed] [Google Scholar]
- [60].Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest 2002;110(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Crawford G, Enders A, Gileadi U, Stankovic S, Zhang Q, Lambe T, et al. DOCK8 is critical for the survival and function of NKT cells. Blood 2013;122(12):2052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 2012;119(19):4451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]