Abstract
The field of neuropathology has a long histology-based tradition of glioma classification. Recent advances in molecular analysis and genome sequencing have prompted a paradigm shift in traditional neuropathology. We discuss the discovery and clinical relevance of molecular biomarkers in diffuse gliomas in adult patients and how these biomarkers led to the revision of the 4th edition of the World Health Organization (WHO) classification of these tumors. We relate the progress in clinical classification to an overview of studies using molecular profiling to study gene expression and DNA methylation to categorize diffuse gliomas in adults and issues dealing with intratumoral heterogeneity. Together, these efforts can be expected to further refine the taxonomy of diffuse gliomas, to facilitate selection of more appropriate treatment regimens, and to ultimately improve the lives of the patients that suffer from these tumors.
Keywords: diffuse gliomas, astrocytoma, oligodendroglioma, IDH mutations, 1p/19q codeletion
Evolution of the WHO classification of Tumors of the Central Nervous System
Gliomas are tumors of the central nervous system (CNS) whose neoplastic cells microscopically resemble non-tumorous glial cells. Gliomas account for the great majority of tumors originating in the brain parenchyma.1 The term “glioma” was introduced by the German pathologist Rudolf Virchow in the 1850’s (Figure 1), and in 1925 Cushing and Bailey introduced the entity “glioblastoma multiforme” to describe a high-grade malignant glioma showing a wide range of histological features (hence 'multiforme').2,3
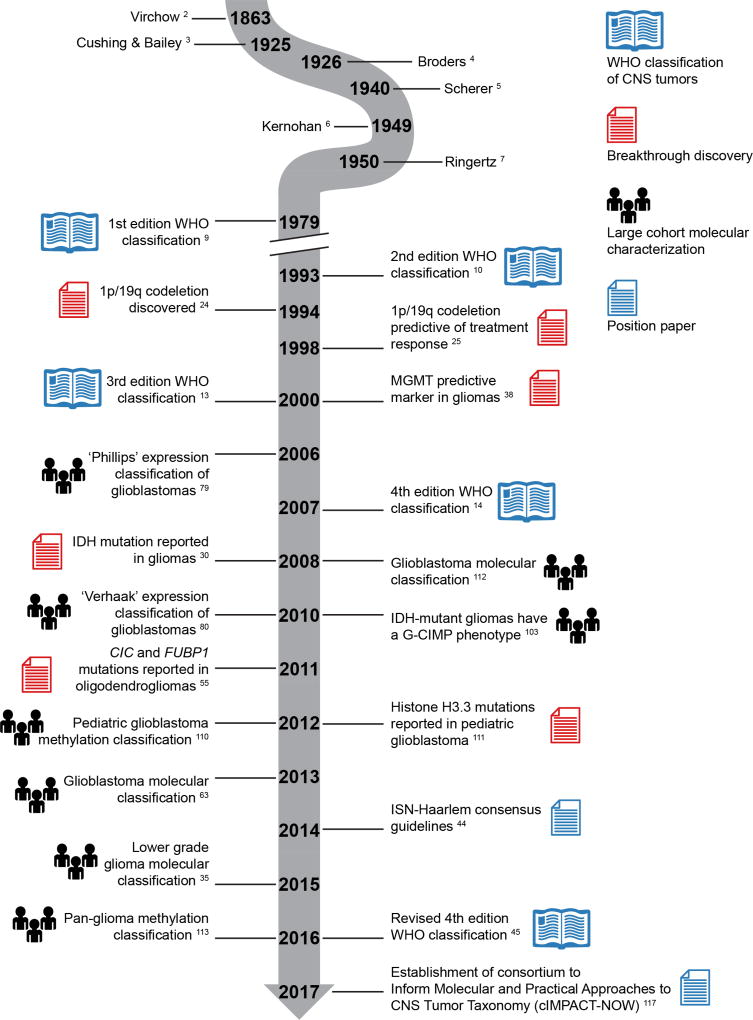
Figure 1. A timeline of events in the history of molecular neuropathology.
This selection of events is categorized into breakthrough discoveries, large cohort molecular characterizations, position papers and different editions of the WHO classification of CNS tumors. ISN = International Society of Neuropathology.
Later on, (neuro)pathologists like Ringertz, Scherer, Broders and Kernohan provided important next building blocks for systematic histopathological classification of gliomas.4–8 Especially among adults, the vast majority of these tumors are diffuse gliomas, characterized by growth of tumor cells over long distances in the surrounding brain parenchyma ('diffuse infiltrative growth'). Traditionally, these diffuse gliomas were classified according to their microscopic similarities with (precursors) of glial cells and then designated as astrocytomas, oligodendrogliomas, or mixed diffuse glioma/oligoastrocytoma. Additionally, a malignancy grade (ranging from low-grade to high-grade) was assigned to these gliomas based on the presence or absence of particular histological features.
Even after publication of the first edition of the World Health Organization (WHO) classification of tumors of the CNS in 1979, different schemes for typing and grading of diffuse gliomas were used in parallel.9 However, the second edition of the WHO classification (published in 1993) was much more universally accepted as the standard for glioma classification.10,11 For grading of astrocytomas, this latter classification incorporated elements of the St. Anne-Mayo grading approach in which absence or presence of mitotic activity, microvascular proliferation, and necrosis were used to assign a malignancy grade.12
The third and fourth edition of the WHO classification of CNS tumors (published in respectively 2000 and 2007) were built on essentially the same approach of histopathology-based diagnosis of diffuse gliomas, in some situations supported by the use of immunohistochemical markers.13–17 However, despite being the time-honored diagnostic gold standard, it was increasingly clear that histopathologic classification of diffuse gliomas suffers from considerable inter- and intraobserver variability, even among expert-(neuro)pathologists, and that the use of molecular markers had great potential to substantially improve the unequivocal discrimination of clinically relevant diffuse glioma subgroups.18–23
In the 1990s, the discovery that gliomas with a combined deletion of chromosome arms 1p and 19q (1p/19q codeletion) were associated with significantly improved survival and increased sensitivity to procarbazine, lomustine and vincristine (PCV) chemotherapy paved the way for molecular neuropathology of CNS tumors.24–28 Typically, 1p/19q-codeleted tumors showed oligodendroglial histology, but the codeletion was reported to occur across different types and grades of diffuse gliomas with its favorable prognostic and predictive impact.25 Of note, the apparent chemosensitivity of oligodendrogliomas was previously reported in 1988, years before a connection was made to the 1p/19q codeletion in 1998.29 The fact that 1p/19q-codeleted tumors responded relatively well to PCV treatment led to introduction of 1p/19q-testing in clinical practice before this molecular marker became a part of the WHO diagnostic criteria for a subset of diffuse gliomas.28
Another finding with a major impact on the molecular neuropathology of diffuse gliomas is mutations of the isocitrate dehydrogenase 1 (IDH1) gene and the related IDH2 gene.30–32 IDH-mutant and IDH-wildtype astrocytomas are clinically different tumors despite overlapping histological appearances. IDH mutations were first reported in glioblastomas (GBM) and later on discovered to be much more prevalent among lower grade (WHO grade II and III) gliomas.31 IDH mutations contribute to gliomagenesis by activating alternative transcriptional programs via the genome-wide disruption of DNA methylation.33 It has been proposed that this stochastic process is subject to careful selection in an effort to prioritize epigenetic changes that promote tumorigenesis.34 Using modern genomic sequencing technologies, several groups independently reported that histologically similar gliomas could be subdivided into distinct tumor entities based on mutations in IDH and the 1p/19q codeletion, providing further fuel to the notion that a reclassification of these tumors is needed.35–37
Although the methylation status of the DNA repair enzyme O(6)-methylguanine-DNA methyltransferase (MGMT) does not distinguish between particular molecular subtypes of glioma, it is an important predictive biomarker. As was first reported in 2000, epigenetic silencing of the MGMT gene by methylation of the promoter appears to compromise conventional DNA repair mechanisms and thereby increases sensitivity to conventional chemotherapy using alkylating agents such as temozolomide.38–41 Especially in elderly patients with glioblastoma lacking MGMT promoter methylation (i.e. MGMT unmethylated), the benefit of temozolomide treatment often does not seem to outweigh the negative side-effects.42,43
Due to increasing insights into the diagnostic potential of molecular markers in glial and other CNS tumors, a meeting was organized in Haarlem, Netherlands in 2014 under the sponsorship of the International Society of Neuropathology (ISN) to focus on how molecular information could be optimally incorporated into a next WHO classification. The paper that resulted from this meeting provided the basis for the design of an integrated histo-molecular classification of especially glial and embryonal CNS tumors as represented in the 2016 revised fourth edition of the WHO classification.44–46 Indeed, state-of-the-art diagnosis of diffuse gliomas now requires assessment of presence or absence of IDH mutation and 1p/19q codeletion. Meanwhile, a “Not Otherwise Specified” (NOS) category was created for cases were information on these defining molecular features is lacking, e.g. because molecular testing was not available, not informative, or not performed.
Glioma Subclassification based on Molecular Features
With the addition of molecular features to the 2016 WHO classification scheme, IDH mutation and 1p/19q codeletion status have become glioma subtype-defining features. Diffuse gliomas that are both IDH-mutant and 1p/19q-codeleted are classified as oligodendroglioma, whereas tumors lacking codeletion of these chromosome arms are classified as astrocytoma and can be further separated based on IDH-status (Figure 2). Because of this, the ambivalent oligoastrocytoma diagnosis can be expected to largely disappear in favor of distinct molecularly-defined subtypes, barring rare cases that contain a mixture of glioma cells carrying the codeletion with oligodendroglial appearance as well as astrocytic cells with retained 1p/19q.47 In addition to subtype defining molecular changes, gliomas are characterized by somatic mutations or copy number changes in a number of genes from various pathways, substantiating the hypothesis that the subtypes follow different gliomagenic trajectories and represent different biologies.
Figure 2. Groups of diffuse gliomas classified according to IDH mutation and 1p/19q codeletion status demonstrate a distinct landscape of molecular features.
Gene pathways are indicated. Genes in orange indicate genes preferentially targeted by gain-of-function (such as hotspot) mutational events or amplification events. Genes marked in blue indicate genes commonly affected by loss-of-function mutation (such as frameshift) or deletion events. Only features with frequencies greater than five percent are shown. Frequencies were derived from a recent publication113. TPM = TERT promoter mutation, RTK = receptor tyrosine kinase, PI3K = phosphoinositide-3 kinase, TMM = telomere maintenance mechanism.
On average, IDH-mutant astrocytomas and GBMs are diagnosed at a median age of 38 years and demonstrate a median survival after diagnosis of 75 months overall, although this varies across WHO grades. IDH-mutant astrocytomas show frequent loss-of-function mutations or deletions in Tumor Protein 53 (TP53) and Alpha Thalassemia/Mental Retardation Syndrome X-Linked (ATRX). TP53 is well known as the “guardian of the genome” or “cellular gatekeeper” and functions to prevent cancer growth by activating a cascade leading to cell cycle arrest, senescence, and apoptosis.48–50 Inactivation of the ATP-dependent helicase ATRX has been linked to recombination-driven alternative telomere maintenance mechanisms and may provide glioma cells with unlimited proliferative capacity.51–53 This molecular information may be helpful for diagnosing astrocytomas, even when molecular testing is not possible. For instance, a diffuse glioma that on immunohistochemical analysis lacks staining of tumor cell nuclei for ATRX and shows extensive and strong p53 staining of these nuclei (most likely representing the presence of respectively deactivating ATRX and TP53 mutation) is highly indicative for an IDH-mutant, 1p/19q-non-codeleted astrocytoma. Obviously, acknowledging that about 90% of IDH-mutant diffuse gliomas carry the IDH1 R132H mutation, immunohistochemistry for the IDH1 R132 mutant protein is a more direct way to test the IDH status of these tumors.54
With a median survival time of 116 months, IDH-mutant and 1p/19q-codeleted oligodendrogliomas demonstrate the highest survival rate amongst diffuse gliomas. With a median age at diagnosis of 46 years, patients with these tumors are generally older compared to those with IDH-mutant astrocytomas. IDH-mutant, 1p/19q-codeleted oligodendrogliomas commonly contain point mutations in 1p gene Capicua Transcriptional Repressor (CIC) and 19q gene Far Upstream Element Binding Protein 1 (FUBP1). Nearly 100% of gliomas in this category harbor mutations in the Telomerase Reverse Transcriptase (TERT) promoter. The mechanism for CIC and FUBP1 mutations remains unknown, although FUBP1 mutant tumors are associated with poorer outcomes.37,55,56 TERT promoter mutations result in telomere maintenance and replicative immortality by constitutively activating the transcription of the enzymatic component of telomerase.57–59
In adults, IDH-wildtype diffuse gliomas are more frequently diagnosed in older patients (median age at diagnosis 59 years) and show the least favorable prognosis with a median survival of 14.0 months. These IDH-wildtype tumors demonstrate higher mutational load than both IDH-mutant tumor types. Common events include TERT promoter mutations, loss-of-function mutations or deletions in phosphoinositide 3-kinase (PI3K), phosphatase and tensin homolog (PTEN), and cell cycle regulator cyclin dependent kinase Inhibitor 2A (CDKN2A), as well as gain-of-function mutations and/or amplifications in receptor tyrosine kinases (RTKs) epidermal growth factor receptor (EGFR) and platelet derived growth factor receptor alpha (PDGFRA). Deletions or loss-of-function mutations of the tumor suppressor PTEN are thought to promote tumorigenesis by antagonizing the suppression of RTKs es including EGFR and PDGFRA.60–62 Amplifications or gain-of-function mutations of the latter genes promote tumorigenesis by stimulating cellular proliferation through increased growth factor signaling.63–66 Deletions of CDKN2A make tumor cells insensitive to growth-inhibitory signals and allow cells to bypass cellular senescence.67–69
Transcriptome Profiling and Intratumoral Heterogeneity
Tumor subtyping is a field that is of interest to clinicians and scientists alike because it has enabled the discovery of novel tumor entities that are biologically and clinically distinct. With the advent of array-based gene expression profiling in the late 1990s, many clinician-scientists were quick to employ these methods across their in-house datasets. Expression subtyping was one of the earliest techniques that rapidly gained traction in the community. Microarray technologies such as the AffyMetrix U133 genechip were at the forefront of the field and allowed one to simultaneously query the expression of thousands of genes in any given sample. The formation of large scientist-run and government-supported consortia such as the Cancer Genome Atlas (TCGA) facilitated researchers from around the world to collaborate, share their data, and work with large and costly datasets otherwise outside the reach of single laboratories.
Using supervised statistical learning approaches in the early 2000s, several groups demonstrated that gene expression microarray analysis of glioma samples can reproduce histological classification at high accuracy.70–72 Moreover, they found that supervised statistical learning approaches were often better able to distinguish poor versus favorable outcome groups compared to conventional histopathology.73,74 Studying the origin of genes associated with distinct clinical phenotypes, it was found that progression from low-grade to high grade was associated with upregulation of cell cycle genes and that treatment resistance was associated with a self-renewal signature.75,76 Despite frequent difficulties in histologic classification, tumor samples from different broad histological categories may demonstrate distinct transcriptional profiles.77,78
Building upon this work researchers set out to use unsupervised learning approaches, where instead of trying to fit the molecular data to conform to a known histological classification, they employed techniques such as principal component analysis and hierarchical clustering to group samples independent of prior information and instead based on how similar they are to one another. Using these techniques, between 2006 and 2010, several groups were able to identify expression subtypes characterized by the activation of distinct transcriptional pathways, such as a “mesenchymal” subtype enriched in angiogenesis and inflammatory genes, a “classical” subtype enriched in stem cell and cell cycle genes, a “proneural” subtype enriched in neurodevelopmental genes, and a “neural” subtype enriched in adult neural markers.79,80 Importantly, these groups driven by gene expression were found to be of independent prognostic importance beyond the existing histopathological tumor groups, in part due to enrichment of the relatively favorable outcome IDH-mutant GBM in the proneural group.
With the growing popularity of expression subtyping, tumor heterogeneity has become an increasingly important concern. Several multisector sequencing studies demonstrated that multiple samples from the same tumor can be classified according to different transcriptomic subtypes.81–83 One study took multiple samples from a set of ten tumors and found that in 60% of tumors multiple fragments from the same tumor were classified into at least two different expression subtypes.81 A second study collected biopsies from the tumor core and tumor margin and reported that at the tumor margin samples generally classified as the neural subtype, whereas core samples were enriched in the three remaining subtypes.82
In a recent (2017) revision to the glioma subtypes, unsupervised learning was used to classify IDH-wildtype tumors after discarding a set of genes enriched in non-tumor tissue and the previously described neural subtype was no longer detectable, suggesting this subtype was not a glioma-intrinsic subtype and reflected contamination from non-tumor tissue.84 Indeed, because of their diffuse infiltrative growth pattern, one can expect that diffuse gliomas contain a variable amount of 'contamination' of non-neoplastic parenchymal cells.85–87 In addition, data suggests that the mesenchymal subtype is both a tumor-intrinsic phenotype and is further enriched by tumor-associated cells in the tumor microenvironment such as immune cells and cellular components of the tumor microvasculature, some of which also contribute mesenchymal expression traits.84 It has been shown that tumors can be highly immunogenic and recruit various immune effectors.88–90 Similarly, it has been shown that tumor cells can activate angiogenic pathways and encourage vascular proliferation.90–92 These studies underline the role of the microenvironment in gliomagenesis and its impact on determining transcriptional subtypes. Altogether, these studies highlight the importance of tumor heterogeneity in the transcriptional profiling in gliomas, and the need to take into account contributions from individual cell types (Figure 3).
Figure 3. Cell type abundance is an important contributor to transcriptional subtypes.
Tumor cells in diffuse gliomas can have a proneural, classical, or mesenchymal transcriptional profile. The now defunct neural subtype appeared to be the result of substantial admixture of non-tumor cells in especially less cellular/more peripheral parts of diffuse gliomas. Cells from the tumor microenvironment including microvascular and immune cells contribute to the mesenchymal subtype.
Transcriptomic studies from 2014 – 2017 have shown that individual cells from the same tumor may activate different transcriptional subtypes, highlighting heterogeneity at a single-cell level.93–95 In a study using single-cell sequencing approaches to study oligodendrogliomas, researchers found cancer cells of oligodendroglial lineage, astrocytic lineage, as well as a relatively limited population of neural stem cells to be present within the same tumor.94 In a follow-up study, data suggested that more aggressive tumors can be characterized by a larger population of stem cells.95 A recent study confirmed these findings using orthogonal approaches, identifying populations of slow-cycling stem-like cells, rapidly-cycling progenitor cells, and a large population of growth arrested cells in glioblastoma xenograft models.96 These studies reveal that in a 'bulk' diffuse glioma sample, in addition to heterogeneity driven by the infiltration of immune and stromal cells and heterogeneity incurred from neighboring non-neoplastic brain tissue, there is considerable clonal heterogeneity within the population of cancer cells themselves with several distinct tumor clones at different stages of differentiation.
Methylation Profiling and the Identification of DNA Methylation-Based Subgroups
DNA methylation profiling has emerged as another method capable of distinguishing gliomas by tumor type and provides complementary molecular information to transcriptional profiles. DNA methylation refers to the covalent addition of a methyl group to the fifth position carbon of a cytosine nucleotide resulting in 5-methylcytosine. This epigenetic modification occurs primarily in the context of nucleotide-pairs in which cytosine is followed by guanine (i.e., CpG dinucleotides) and is a critical regulator of gene expression.97 Importantly, altered DNA methylation is a frequent event across cancers, including glioma.98 Early studies of DNA methylation and glioma biology in the 1990s primarily focused on the methylation of single candidate genes such as MGMT.38–40 In the early 2000s, studies extended the search for candidate genes differentially methylated across histologically distinct glioma subtypes using real time polymerase chain reaction methods, and reported that glioma tumor types demonstrated differential methylation patterns.99,100 Cohort-level studies of glioma DNA methylation were transformed in the late 2000s by the introduction of methylation microarrays such as the Illumina GoldenGate cancer panel assaying 1,505 CpG sites and the Illumina 27K array investigating roughly 27,000 CpGs sites.101 These research tools enabled a more agnostic and expanded approach to studying disrupted genome-wide DNA methylation patterns. In an early analysis of methylation microarray data, a supervised clustering approach of 87 GBMs was the first study to reveal extensive glioma DNA methylation heterogeneity with some tumors exhibiting high levels of methylation while others predominantly displaying low levels of methylation.102 Genome-wide assessment of 272 GBMs in the TCGA dataset later identified that a subset of GBMs harbor a distinctive Glioma CpG Island Methylator Phenotype (G-CIMP), which reflected genome-wide patterns of promoter hypermethylation.103 The authors also discovered that the occurrence of G-CIMP was tightly associated with IDH1 mutations and the proneural expression subtype, and that those patients with G-CIMP positive tumors without IDH1 mutations had a younger age at diagnosis.103 In this same study, a small number of primary-recurrent IDH-mutant GBM pairs (n=8) all retained their G-CIMP classification highlighting the stability of the phenotype.103 The presence of a homogeneous G-CIMP DNA methylation profile across both IDH-mutant GBMs and lower-grade gliomas was subsequently validated in a separate cohort.104 These seminal contributions led to mechanistic follow-up studies, eventually establishing that IDH1 mutations directly lead to genome-wide hypermethylation via the production of the oncometabolite 2-hydroxyglutarate and its inhibitory effect on the DNA demethylating enzyme ten-eleven translocation methylcytosine dioxygenase 1 (TET1).105–108
Glioma methylation subtypes display characteristic genetic features that are indicative of distinct biological entities. In 2012, Sturm et al. applied the more comprehensive Illumina 450K microarray to 151 GBMs and integrated these profiles with an expanded catalogue of genetic alterations.109,110 The authors then leveraged an unsupervised statistical learning method to cluster DNA methylomes based upon the most variable methylation sites across the genome.110 The resulting adult GBM classification revealed four methylation clusters including: IDH, Receptor Tyrosine Kinase (RTK) I, Mesenchymal, and RTK II. Diffuse gliomas in children clustered in different categories that are enriched in mutations in the H3 Histone Family Member 3A (H3F3A) gene.111 These methylation based-clusters were so named because of their association with the previously described GBM driver events.112 For example, tumors in the “IDH” cluster carried IDH1 mutations, displayed G-CIMP, and had a more favorable prognosis; “RTK I” tumors significantly more often harbored PDGFRA amplification; “Mesenchymal” tumors had methylation profiles most similar to normal brain tissue despite substantial copy number changes and were enriched for the mesenchymal gene expression cluster; and “RTK II” tumors were characterized by high frequency of chromosome 7 gain and chromosome 10 loss.110 In 2016, the ultimate TCGA panglioma analysis analyzed 932 glioma samples (516 lower grade/WHO grade II and III gliomas and 129 GBMs with methylation data) to better define biologically distinct glioma subgroups.113 Using the TCGA data (Illumina microarrays), our group selected 1,300 tumor-specific methylated CpGs to define six clusters across gliomas, which we referred to as LGm1–6. LGm1–3 tumors were primarily IDH1 or IDH2 mutant tumors (99% of the tumors in these clusters combined) and were heavily enriched for low-grade gliomas (93% of tumors in these clusters). This largest glioma methylation study to date permitted the further subdivision of the “IDH” group identified in Sturm et al. into a 1p/19q codeleted cluster with elevated methylation (LGm3), a G-CIMP-high IDH-mutant non-codeleleted cluster (LGm2), and a newly identified G-CIMP-low cluster with reduced methylation leading to gene expression changes and genetic aberrations in cell-cycle genes (LGm1). Notably, the G-CIMP-low tumors demonstrated poorer overall survival when compared with the other IDH-mutant subgroups. The remaining DNA methylation clusters represented IDH-wildtype tumors. For example, LGm4 was described as “Classic-like” similar to the Sturm RTK II classification, LGm5 was “Mesenchymal-like” similar to the Sturm RTK I classification, and LGm6 was a subtype sharing epigenomic and genomic features with pilocytic astrocytoma (PA) despite a histological diagnosis of diffuse glioma. Interestingly, the lower-grade gliomas of LGm6 had near euploid copy number profiles and low frequency of typically observed glioblastoma alterations (e.g., EGFR, CDKN2A, and PTEN). Thus, the low-grade gliomas in LGm6 were referred to as PA-like, while the glioblastomas in this group were best described as LGm6-GBM. Finally, the study demonstrated that DNA methylation subtypes provided prognostic value independent of age and WHO grade that was assigned to these tumors.113
DNA methylation profiles of longitudinally collected samples support epigenetic reprogramming as a driver of glioma recurrence.113–116 In 2015, using 450K array data and multi-sector sampling, researchers demonstrated that IDH-mutant tumors tend to have homogeneous subtypes within a given tumor.116 Yet, when examined over time the methylation profiles showed concerted changes as hypomethylation events led to an increase in the expression of cell cycle genes. Interestingly, in brain tumors where there was no observed loss of methylation at cell cycle genes these tumors displayed genetic alterations in these pathways suggesting the convergence of these disparate mechanisms on aberrant cell cycle activation.116 It was recently estimated that between 10–40% of G-CIMP-high tumors recur with a G-CIMP-low phenotype, indicating that the G-CIMP-high to –low transition may be a marker of progression.113,114 An improvement in both sample number and genomic coverage of CpG sites was achieved in a 2017 study that measured 112 IDH-wildtype and 13 IDH-mutant primary-recurrent tumor pairs with reduced representation bisulfite sequencing (RRBS).115 Use of a high coverage sequencing approach such as RRBS allowed for a between 3–5 fold increase in coverage for most samples under study. Similar to previous studies of primary-recurrent glioma samples, the authors discovered that relative hypomethylation was a striking feature at recurrence, and specifically that demethylation of Wnt signaling gene promoters was associated with worse prognosis.115 Gliomas have been shown to display high levels of heterogeneity with subpopulations harboring distinct genetic and epigenetic alterations.83,116 The authors applied the single-allele methylation resolution of the RRBS technology to quantify the epigenetic heterogeneity of each tumor sample and found extensive epigenetic heterogeneity across individual tumors.
Concluding Remarks
The integration of molecular features with neuropathological assessment has yielded invaluable insights into the underpinnings of diffuse gliomas. Past studies have helped to identify glioma-initiating events, revealed potentially targetable oncogenic proteins, defined co-occurrences of molecular alterations in specific tumor subgroups, demonstrated that epigenetic inactivation of genes is predictive of therapeutic response, and that there exists a dynamic molecular landscape in glioma progression. The resulting molecular evidence has enabled the further refinement of tumor subgroups that would have previously been indiscernible using histopathological assessment alone.
The ability to separate tumors based on molecular alterations possesses clear clinical relevance. The unsupervised learning approaches applied to sequencing data do not suffer from the inter-observer variability that can hinder histologically defined diagnostic entities. Indeed, diagnostic entities that were once histologically ambiguous can now often be accurately classified into molecular subgroups that display different clinical behavior. Tumor classification improvements can be made even to well-understood diagnostic entities such IDH-mutant tumors. For example, combining multiple molecular data, such as mutations and DNA methylation, further parsed IDH-mutant tumors into subgroups with unique clinical and molecular characteristics. As sequencing costs drop and more tumors are comprehensively profiled, the sensitivity to detect new molecular subtypes increases. In response to the rapid changes to tumor classifications the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) has been launched to evaluate the value of molecular markers for CNS tumor classification with greater frequency than allowed by the current WHO timeline.117 In parallel, efforts utilizing DNA methylation profiling to characterize tumors such as the online platform for next generation neuropathology “MolecularNeuropathology.org” are at the forefront of innovation in molecular neuropathology.
Future advancement in glioma classification will require additional research to more fully understand the contribution of diverse molecular alterations that may exist within different regions of a tumor mass. In this regard, recent results from multi-sector, longitudinal, and single-cell experiments are challenging the notion that a tumor can be represented by single molecular subtype. These studies have made it clear that a given tumor often encompasses multiple molecular subtypes over space and time. Thus, it is likely that future clinically relevant biomarkers will need to provide information on not only the classification of the tumor as a whole, but also on the proportions of molecularly different single tumor cell types within a glioma. Thus, additional high-resolution modeling of genetic, transcriptomic, and epigenetic intratumoral heterogeneity are needed to facilitate the development of yet more precise biomarkers.118 The further characterization of diffuse gliomas in space and time will serve to improve therapeutic results in individual patients through a more comprehensive understanding of their unique disease.
KEY CONCEPTS.
-
–
Diffuse gliomas that are both IDH-mutant and 1p/19q-codeleted are classified as oligodendroglioma, whereas tumors lacking codeletion of these chromosome arms are classified as astrocytoma and can be further separated based on IDH-status.
-
–
Diffuse midline glioma, H3 K27M-mutant, was added in the WHO 2016 classification as a separate entity. These highly aggressive tumors generally occur in children and are located in the 'midline' of the CNS (brainstem, thalamus, cerebellum and spinal cord).
-
–
GBMs can be classified into expression subtypes characterized by activation of distinct transcriptional pathways: “mesenchymal” subtype enriched in angiogenesis and inflammatory genes, a “classical” subtype enriched in stem cell and cell cycle genes, and a “proneural” subtype enriched in neurodevelopmental genes. Recent data suggests that the previously defined “neural” subtype is not a glioma-intrinsic subtype and may reflect contamination from non-tumor tissue.
-
–
GBMs can also be classified by methylation subtypes: IDH (which carry IDH1 mutations, display G-CIMP, and have a more favorable prognosis), RTK I (which frequently harbor PDGFRA amplification), Mesenchymal (methylation profiles most similar to normal brain tissue despite substantial copy number changes and were enriched for the mesenchymal gene expression cluster), and RTK II (characterized by high frequency of chromosome 7 gain and chromosome 10 loss).
Acknowledgments
We thank members of the Verhaak laboratory for insightful discussion and valuable input. We thank Zoë Reifsnyder of the Jackson Laboratory creative team for artwork. This work was supported by grants from the National Institutes of Health R01 CA190121 and P30CA034196, the National Brain Tumor Society Oligo Research Fund and DefeatGBM Initiative, and grant 11026 from the Dutch Cancer Society KWF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: We have no disclosures to make.
References
- 1.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-oncology. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virchow R. Die krankhaften Geschwülste. Dreissig Vorlesungen, gehalten während des Wintersemesters 1862–1863 an Der Universität Zu Berlin. Berlin, Germany: A. Hirschwald; 1863. [Google Scholar]
- 3.Bailey P, Cushing H. Microchemical Color Reactions as an Aid to the Identification and Classification of Brain Tumors. Proceedings of the National Academy of Sciences of the United States of America. 1925;11(1):82–84. doi: 10.1073/pnas.11.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broders AC. Carcinoma: Grading the malignancy of carcinoma, grading and practical application. Arch Path (Lab Med) 1926;2:376. [Google Scholar]
- 5.Scherer HJ. A Critical Review: The Pathology of Cerebral Gliomas. Journal of Neurology and Psychiatry. 1940;31 doi: 10.1136/jnnp.3.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kernohan JW, Mabon RF, et al. A simplified classification of the gliomas. Proceedings of the staff meetings Mayo Clinic. 1949;24(3):71–75. [PubMed] [Google Scholar]
- 7.Ringertz N. Grading of gliomas. Acta pathologica et microbiologica Scandinavica. 1950;27(1):51–64. [PubMed] [Google Scholar]
- 8.Louis DN, von Deimling A. Grading of diffuse astrocytic gliomas: Broders, Kernohan, Zulch, the WHO… and Shakespeare. Acta neuropathologica. 2017 doi: 10.1007/s00401-017-1765-z. [DOI] [PubMed] [Google Scholar]
- 9.Zülch K. Histologic typing of tumours of the central nervous system. Geneva, Switzerland: World Health Organization; 1979. [Google Scholar]
- 10.Kleihues P, Burger P, Scheithauer B. Histological typing of tumours of the central nervous system. World Health Organization international histological classification of tumours. 2. Berlin, Heidelberg: Springer Verlag; 1993. [Google Scholar]
- 11.Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain pathology (Zurich, Switzerland) 1993;3(3):255–268. doi: 10.1111/j.1750-3639.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 12.Daumas-Duport C, Scheithauer B, O'Fallon J, Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer. 1988;62(10):2152–2165. doi: 10.1002/1097-0142(19881115)62:10<2152::aid-cncr2820621015>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Kleihues P, Cavenee W. World Health Organisation classification of tumours: Pathology and genetics of tumours of the nervous system. Lyon, France: IARC Press; 2000. [Google Scholar]
- 14.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000;88(12):2887. doi: 10.1002/1097-0142(20000615)88:12<2887::aid-cncr32>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheithauer BW. Development of the WHO classification of tumors of the central nervous system: a historical perspective. Brain Pathol. 2009;19(4):551–564. doi: 10.1111/j.1750-3639.2008.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta neuropathologica. 2010;120(3):297–304. doi: 10.1007/s00401-010-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79(7):1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Aldape K, Simmons ML, Davis RL, et al. Discrepancies in diagnoses of neuroepithelial neoplasms: the San Francisco Bay Area Adult Glioma Study. Cancer. 2000;88(10):2342–2349. [PubMed] [Google Scholar]
- 21.Kros JM, Troost D, van Eden CG, van der Werf AJ, Uylings HB. Oligodendroglioma. A comparison of two grading systems. Cancer. 1988;61(11):2251–2259. doi: 10.1002/1097-0142(19880601)61:11<2251::aid-cncr2820611120>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Kros JM, Gorlia T, Kouwenhoven MC, et al. Panel review of anaplastic oligodendroglioma from European Organization For Research and Treatment of Cancer Trial 26951: assessment of consensus in diagnosis, influence of 1p/19q loss, and correlations with outcome. Journal of neuropathology and experimental neurology. 2007;66(6):545–551. doi: 10.1097/01.jnen.0000263869.84188.72. [DOI] [PubMed] [Google Scholar]
- 23.Kros JM. Grading of gliomas: the road from eminence to evidence. Journal of neuropathology and experimental neurology. 2011;70(2):101–109. doi: 10.1097/NEN.0b013e31820681aa. [DOI] [PubMed] [Google Scholar]
- 24.Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. The American journal of pathology. 1994;145(5):1175–1190. [PMC free article] [PubMed] [Google Scholar]
- 25.Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. Journal of the National Cancer Institute. 1998;90(19):1473–1479. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- 26.Kraus JA, Koopmann J, Kaskel P, et al. Shared allelic losses on chromosomes 1p and 19q suggest a common origin of oligodendroglioma and oligoastrocytoma. Journal of neuropathology and experimental neurology. 1995;54(1):91–95. doi: 10.1097/00005072-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 27.von Deimling A, Louis DN, von Ammon K, Petersen I, Wiestler OD, Seizinger BR. Evidence for a tumor suppressor gene on chromosome 19q associated with human astrocytomas, oligodendrogliomas, and mixed gliomas. Cancer research. 1992;52(15):4277–4279. [PubMed] [Google Scholar]
- 28.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cairncross JG, Macdonald DR. Successful chemotherapy for recurrent malignant oligodendroglioma. Ann Neurol. 1988;23(4):360–364. doi: 10.1002/ana.410230408. [DOI] [PubMed] [Google Scholar]
- 30.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science (New York, NY) 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. The American journal of pathology. 2009;174(4):1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. The New England journal of medicine. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flavahan WA, Drier Y, Liau BB, et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529(7584):110–114. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357(6348):eaal2380. doi: 10.1126/science.aal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Network CGAR. Brat DJ, Verhaak RGW, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. The New England journal of medicine. 2015;372(26):2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, TERT Promoter Mutations in Tumors. The New England journal of medicine. 2015;372(26):2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. The New England journal of medicine. 2000;343(19):1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 39.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer research. 1999;59(4):793–797. [PubMed] [Google Scholar]
- 40.Costello JF, Futscher BW, Tano K, Graunke DM, Pieper RO. Graded methylation in the promoter and body of the O6-methylguanine DNA methyltransferase (MGMT) gene correlates with MGMT expression in human glioma cells. The Journal of biological chemistry. 1994;269(25):17228–17237. [PubMed] [Google Scholar]
- 41.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England journal of medicine. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 42.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 43.Wick W, Weller M, van den Bent M, et al. MGMT testing-the challenges for biomarker-based glioma treatment. Nature reviews Neurology. 2014;10(7):372–385. doi: 10.1038/nrneurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 44.Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–435. doi: 10.1111/bpa.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC Press; 2016. [Google Scholar]
- 46.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 47.Barresi V, Lionti S, Valori L, Gallina G, Caffo M, Rossi S. Dual-Genotype Diffuse Low-Grade Glioma: Is It Really Time to Abandon Oligoastrocytoma As a Distinct Entity? Journal of neuropathology and experimental neurology. 2017;76(5):342–346. doi: 10.1093/jnen/nlx024. [DOI] [PubMed] [Google Scholar]
- 48.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24(17):2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 49.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 50.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 51.Barthel FP, Wei W, Tang M, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nature genetics. 2017;49(3):349–357. doi: 10.1038/ng.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clynes D, Jelinska C, Xella B, et al. Suppression of the alternative lengthening of telomere pathway by the chromatin remodelling factor ATRX. Nature communications. 2015;6:7538. doi: 10.1038/ncomms8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reuss DE, Sahm F, Schrimpf D, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta neuropathologica. 2015;129(1):133–146. doi: 10.1007/s00401-014-1370-3. [DOI] [PubMed] [Google Scholar]
- 55.Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 Contribute to Human Oligodendroglioma. Science. 2011;333(6048):1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu X, Martinez-Ledesma E, Zheng S, et al. Multigene signature for predicting prognosis of patients with 1p19q co-deletion diffuse glioma. Neuro-oncology. 2017;19(6):786–795. doi: 10.1093/neuonc/now285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiba K, Lorbeer FK, Shain AH, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science. 2017 doi: 10.1126/science.aao0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bell RJA, Rube HT, Kreig A, et al. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science (New York, NY) 2015;348(6238):1036–1039. doi: 10.1126/science.aab0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science (New York, NY) 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 61.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nature genetics. 1997;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 62.Pennisi E. New tumor suppressor found--twice. Science. 1997;275(5308):1876–1878. doi: 10.1126/science.275.5308.1876. [DOI] [PubMed] [Google Scholar]
- 63.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(16):7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF) Developmental biology. 1965;12(3):394–407. doi: 10.1016/0012-1606(65)90005-9. [DOI] [PubMed] [Google Scholar]
- 66.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes & development. 2008;22(10):1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer research. 2002;62(19):5551–5558. [PubMed] [Google Scholar]
- 68.Uhrbom L, Nister M, Westermark B. Induction of senescence in human malignant glioma cells by p16INK4A. Oncogene. 1997;15(5):505–514. doi: 10.1038/sj.onc.1201227. [DOI] [PubMed] [Google Scholar]
- 69.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 70.Rickman DS, Bobek MP, Misek DE, et al. Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer research. 2001;61(18):6885–6891. [PubMed] [Google Scholar]
- 71.Godard S, Getz G, Delorenzi M, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer research. 2003;63(20):6613–6625. [PubMed] [Google Scholar]
- 72.van den Boom J, Wolter M, Kuick R, et al. Characterization of gene expression profiles associated with glioma progression using oligonucleotide-based microarray analysis and real-time reverse transcription-polymerase chain reaction. The American journal of pathology. 2003;163(3):1033–1043. doi: 10.1016/S0002-9440(10)63463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nutt CL, Mani DR, Betensky RA, et al. Gene expression-based classification of malignant gliomas correlates better with survival than histological classification. Cancer research. 2003;63(7):1602–1607. [PubMed] [Google Scholar]
- 74.Freije WA, Castro-Vargas FE, Fang Z, et al. Gene expression profiling of gliomas strongly predicts survival. Cancer research. 2004;64(18):6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- 75.Tso CL, Freije WA, Day A, et al. Distinct transcription profiles of primary and secondary glioblastoma subgroups. Cancer research. 2006;66(1):159–167. doi: 10.1158/0008-5472.CAN-05-0077. [DOI] [PubMed] [Google Scholar]
- 76.Murat A, Migliavacca E, Gorlia T, et al. Stem cell-related "self-renewal" signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(18):3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 77.Shai R, Shi T, Kremen TJ, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22(31):4918–4923. doi: 10.1038/sj.onc.1206753. [DOI] [PubMed] [Google Scholar]
- 78.Liang Y, Diehn M, Watson N, et al. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(16):5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 80.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gill BJ, Pisapia DJ, Malone HR, et al. MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(34):12550–12555. doi: 10.1073/pnas.1405839111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H, Zheng S, Amini SS, et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome research. 2015;25(3):316–327. doi: 10.1101/gr.180612.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Q, Hu B, Hu X, et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer cell. 2017;32(1):42–56. e46. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Loo P, Campbell PJ. ABSOLUTE cancer genomics. Nat Biotech. 2012;30(7):620–621. doi: 10.1038/nbt.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carter SL, Cibulskis K, Helman E, et al. Absolute quantification of somatic DNA alterations in human cancer. Nature biotechnology. 2012;30(5):413–421. doi: 10.1038/nbt.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aran D, Sirota M, Butte AJ. Systematic pan-cancer analysis of tumour purity. Nature communications. 2015;6:8971. doi: 10.1038/ncomms9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 89.Yoshihara K, Shahmoradgoli M, Martinez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nature communications. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 91.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 92.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nature reviews Neuroscience. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 93.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tirosh I, Venteicher AS, Hebert C, et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 2016;539(7628):309–313. doi: 10.1038/nature20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Venteicher AS, Tirosh I, Hebert C, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355:6332. doi: 10.1126/science.aai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lan X, Jorg DJ, Cavalli FMG, et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature. 2017 doi: 10.1038/nature23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes & development. 2011;25(10):1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stone AR, Bobo W, Brat DJ, Devi NS, Van Meir EG, Vertino PM. Aberrant methylation and down-regulation of TMS1/ASC in human glioblastoma. Am J Pathol. 2004;165(4):1151–1161. doi: 10.1016/S0002-9440(10)63376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uhlmann K, Rohde K, Zeller C, et al. Distinct methylation profiles of glioma subtypes. Int J Cancer. 2003;106(1):52–59. doi: 10.1002/ijc.11175. [DOI] [PubMed] [Google Scholar]
- 101.Bibikova M, Le J, Barnes B, et al. Genome-wide DNA methylation profiling using Infinium(R) assay. Epigenomics. 2009;1(1):177–200. doi: 10.2217/epi.09.14. [DOI] [PubMed] [Google Scholar]
- 102.Martinez R, Martin-Subero JI, Rohde V, et al. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics. 2009;4(4):255–264. doi: 10.4161/epi.9130. [DOI] [PubMed] [Google Scholar]
- 103.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Christensen BC, Smith AA, Zheng S, et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. Journal of the National Cancer Institute. 2011;103(2):143–153. doi: 10.1093/jnci/djq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer cell. 2011;19(1):17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McCarthy N. Metabolism: unmasking an oncometabolite. Nature reviews Cancer. 2012;12(4):229. doi: 10.1038/nrc3248. [DOI] [PubMed] [Google Scholar]
- 108.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sandoval J, Heyn H, Moran S, et al. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics. 2011;6(6):692–702. doi: 10.4161/epi.6.6.16196. [DOI] [PubMed] [Google Scholar]
- 110.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 111.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 112.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ceccarelli M, Barthel FP, Malta TM, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016;164(3):550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de Souza CF, Sabedot TS, Malta TM, et al. Distinct epigenetic shift in a subset of Glioma CpG island methylator phenotype (G-CIMP) during tumor recurrence. bioRxiv. 2017 doi: 10.1016/j.celrep.2018.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klughammer J, Kiesel B, Roetzer T, et al. The DNA methylation landscape of glioblastoma disease progression shows extensive heterogeneity in time and space. bioRxiv. 2017 doi: 10.1038/s41591-018-0156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mazor T, Pankov A, Johnson BE, et al. DNA Methylation and Somatic Mutations Converge on the Cell Cycle and Define Similar Evolutionary Histories in Brain Tumors. Cancer cell. 2015;28(3):307–317. doi: 10.1016/j.ccell.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Louis DN, Aldape K, Brat DJ, et al. Announcing cIMPACT-NOW: the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy. Acta neuropathologica. 2017;133(1):1–3. doi: 10.1007/s00401-016-1646-x. [DOI] [PubMed] [Google Scholar]
- 118.Smallwood SA, Lee HJ, Angermueller C, et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods. 2014;11(8):817–820. doi: 10.1038/nmeth.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]