Abstract
Amphibian metamorphosis has long been used as model to study postembryonic development in vertebrates, a period around birth in mammals when many organs/tissues mature into their adult forms and is characterized by peak levels of plasma thyroid hormone (T3). Of particular interest is the remodeling of the intestine during metamorphosis. In the highly-related anurans Xenopus laevis and Xenopus tropicalis, this remodeling process involves larval epithelial cell death and de novo formation of adult stem cells via dedifferentiation of some larval cells under the induction of T3, making it a valuable system to investigate how adult organ-specific stem cells are formed during vertebrate development. Here, we will review some studies by us and others on how T3 regulates the formation of the intestinal stem cells during metamorphosis. We will highlight the involvement of nucleosome removal and a positive feedback mechanism involving the histone methyltransferases in gene regulation by T3 receptor (TR) during this process.
Keywords: Thyroid hormone receptor, adult stem cell, amphibian metamorphosis, Xenopus laevis, Xenopus tropicalis, histone methylation, histone acetylation, intestinal remodeling
Introduction
Adult organ-specific stem cells are critical for tissue homeostasis, repair, and regeneration in vertebrates. These stem cells are often developed as organs mature into their adult forms. For many organs, this takes place during postembryonic development in mammals, a period about 4 months before to several months after birth in human when plasma thyroid hormone (T3) concentration is high. Among such organs is the intestine. The adult mammalian intestine has a self-renewing epithelial system, where the stem cells reside in the crypts while most of the differentiated cells are located in the villi. The offspring of the stem cells migrate along the crypt-villus axis as they gradually differentiate into different types of epithelial cells and undergoes apoptosis, mostly at the tip of the villus, to complete the self-renewal cycle (MacDonald et al. 1964; Toner et al. 1971; van der Flier and Clevers 2009; Shi et al. 2011). Early studies have shown that the mouse intestine matures into the adult form during the first three weeks or so after birth as the plasma thyroid hormone (T3) level rises to a peak level (Matsuda and Shi 2010; Harper et al. 2011; Muncan et al. 2011; Sun and Shi 2012). In particular, the neonatal mouse intestine after birth lacks any crypts. The crypts are formed during the first few weeks after birth to establish the self-renewing adult epithelium. Increasing evidence suggest that T3 play a critical role in the formation and/or function of adult intestinal stem cells in mammals (Plateroti et al. 1999; Plateroti et al. 2001; Flamant et al. 2002; Plateroti et al. 2006; Kress et al. 2009; Yakut et al. 2011; Bochukova et al. 2012; van Mullem et al. 2012; Moran and Chatterjee 2015; Sun et al. 2016). On the other hand, it is difficult to alter T3 levels in the uterus-enclosed mammalian embryos and reduce T3 levels in the neonates since T3 synthesis begins during embryogenesis and neonates are dependent on maternal supply of nutrients for survival and development. Thus, how T3 affects adult intestinal stem cell development in mammals remains to be determined.
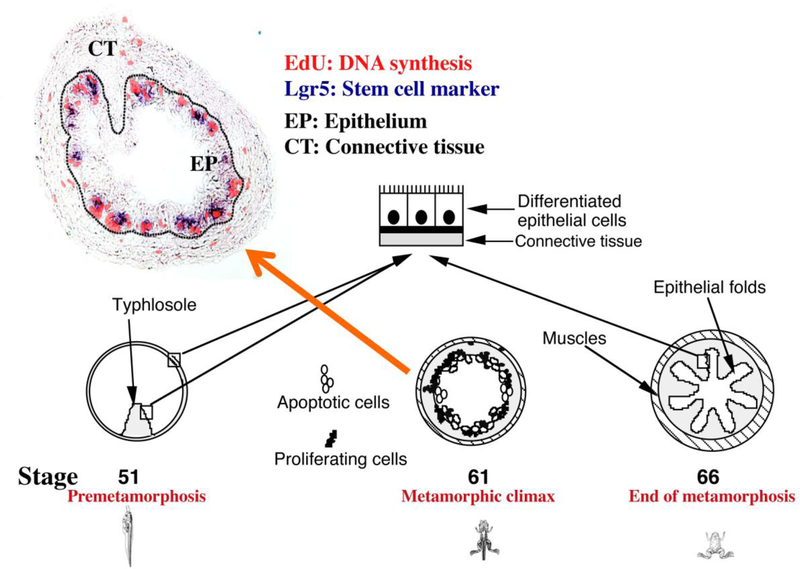
Intestinal remodeling during amphibian metamorphosis offers an opportunity to study how T3 regulates adult intestinal stem cell development. The adult Xenopus intestine resembles adult mammalian intestine with a self-renewing system in the form of epithelial folds, with the stem cells localized in the trough of the fold, similar to the crypt-villus structure in mammals (Fig. 1) (Shi and Ishizuya-Oka 1996; Sterling et al. 2012). The tadpole intestine, however, has only a single epithelial fold, the typhlosole, and the epithelium is surrounded by thin layers of connective tissue and muscles (Fig. 1). During metamorphosis, the larval epithelial cells undergo programmed cell death while adult progenitor/stem cells are developed de novo and exist as clusters of proliferating cells (also referred to as cell nests or islet cells) (Fig. 1) (Shi and Ishizuya-Oka 1996). These cells subsequently give rise to the adult epithelium, with concurrent development of the connective tissue and muscles. Like any other processes during amphibian metamorphosis, intestinal remodeling is totally dependent on T3 and can even be reproduced in tadpole intestinal organ cultures with T3 treatment (Ishizuya-Oka and Shimozawa 1991; Shi and Ishizuya-Oka 1996), making it a unique system to study the development of adult organ-specific stem cells.
Fig. 1.
Xenopus intestinal metamorphosis serves as a model for studying adult organ-specific stem cell development in vertebrates. In premetamorphic tadpoles (e.g., at stage 51), the intestine has only a single fold, the typhlosole, where connective tissue is abundant, and is structurally similar to the mammalian embryonic intestine. At the metamorphic climax around stage 61, the vast majority of the larval epithelial cells undergo apoptosis (the open circles). A small fraction of the larval epithelial cells undergo dedifferentiation into cells that rapidly proliferate (EdU positive) and express the adult stem cell marker Lgr5 (black dots in the stage 61 diagram). By stage 66 (the end of metamorphosis), these cells differentiate to form a multiply folded epithelium surrounded by elaborate connective tissue and thick muscle layers. See (Okada et al. 2015) for EdU labeling and Lgr5 in situ hybridization.
T3-induced formation of adult intestinal stem cells during metamorphosis
Earlier studies have failed to identify any progenitor or adult stem cells in the premetamorphic Xenopus intestinal epithelium (McAvoy and Dixon 1977; Shi and Ishizuya-Oka 1996). The tadpole epithelium consists of a monolayer of differentiated cells that are yet mitotically active. During metamorphosis, the larval epithelial cells undergo apoptosis. Organ culture and primary cell culture studies have shown that during metamorphosis, T3 induces the larval epithelial cell death via two distinct mechanisms, directly inducing apoptosis within the epithelial cells or indirectly through T3 action in the underlying non-epithelial tissues, in part by activating the expression of matrix metalloproteinases to degrade/modify the extracellular matrix (Ishizuya-Oka and Shimozawa 1992b; Su et al. 1997a; Su et al. 1997b; Fu et al. 2007; Ishizuya-Oka et al. 2009; Mathew et al. 2009; Ishizuya-Oka et al. 2010; Mathew et al. 2010; Hasebe et al. 2011). The adult epithelial progenitor/stem cells are formed de novo, organ-autonomously in response to T3 (Ishizuya-Oka and Shimozawa 1992b). Recombinant organ culture studies with wild type and transgenic-GFP expressing tadpoles have revealed that the adult stem cells originate from the larval epithelium (Ishizuya-Oka et al. 2009), suggesting that some larval epithelial cells are induced by T3 to undergo dedifferentiation to become the adult epithelial stem cells.
T3 can act via both genomic and non-genomic pathways, with the latter through the binding of T3 to cell surface or cytoplasmic proteins. Molecular and transgenic studies in Xenopus laevis have shown that the metamorphic effects of T3 are due to transcriptional regulation of gene expression through nuclear T3 receptors (TRs) (Shi 1994; Sachs et al. 2000; Schreiber et al. 2001; Buchholz et al. 2003; Nakajima and Yaoita 2003; Buchholz et al. 2004; Buchholz et al. 2006; Brown and Cai 2007; Bagamasbad et al. 2008; Denver et al. 2009; Schreiber et al. 2009; Shi 2009; Shi et al. 2012). More recently, gene knockout studies have provided direct evidence for the important role of TRs during Xenopus tropicalis development (Choi et al. 2015; Sachs 2015; Wen and Shi 2015; Yen 2015; Wen and Shi 2016; Choi et al. 2017; Wen et al. 2017b; Buchholz and Shi 2018; Nakajima et al. 2018; Sakane et al. 2018). To determine if T3 induces the formation of the adult stem cells tissue-autonomously, we have used recombinant organ-cultures made of tissues from wild type and transgenic animals expressing a dominant positive TR (dpTR) (Buchholz et al. 2004; Hasebe et al. 2011). The dpTR functioned as a T3-bound TR but without a need to actually bind to T3 and was placed under the control of a heat shock-inducible promoter. Heat shock-treatment of the organ cultures showed that the expression of the dpTR in all tissues of the intestine in the absence of T3 was sufficient to induce intestinal metamorphosis, showing that T3 action through TR is sufficient for intestinal metamorphosis, including stem cell formation (Hasebe et al. 2011). More importantly, such experiments also revealed that the formation of adult stem cells requires T3 action not only in the epithelium but also in the underlying non-epithelial tissues. In particular, the expression of dpTR in the larval epithelium alone was able to induce the dedifferentiation of larval epithelial cells and upregulate sonic hedgehog gene, which is highly expressed in the proliferating adult epithelial progenitor/stem cells, in these cells. However, such cells failed to express markers of adult intestinal stem cells. In addition, the expression of dpTR in only the non-epithelial tissues lead to only epithelial cell death without the formation of any adult stem cells. Thus, the formation of the adult stem cells requires both T3 action in the tadpole epithelial cells and T3-induced cell-cell interactions between the epithelium and non-epithelial tissues (Hasebe et al. 2011), in agreement with previous findings for a role of cell-cell interaction during intestinal metamorphosis (Ishizuya-Oka and Shimozawa 1992a; Schreiber et al. 2005; Schreiber et al. 2009).
Chromatin remodeling and histone modification by TR during intestinal metamorphosis
TR is a T3-dependent, DNA binding transcription factor. TR can form a heterodimer with 9-cis retinoic acid receptor (RXR), which is also a member of the nuclear hormone receptor superfamily (Lazar 1993; Tsai and O’Malley 1994; Mangelsdorf et al. 1995; Yen 2001; Laudet and Gronemeyer 2002). TR/RXR heterodimers bind to T3-response elements (TREs) in T3-inducible genes both in the presence and absence of T3 in chromatin (Lazar 1993; Tsai and O’Malley 1994; Mangelsdorf et al. 1995; Wong et al. 1995; Wong et al. 1997; Wong et al. 1998; Yen 2001; Shi 2009). This binding leads to repression or activation of the T3-inducible genes in the absence or presence of T3 via the recruitment of corepressor or coactivator complexes, respectively. Interestingly, the corepressor complexes contain histone deacetylases (HDACs) while coactivator complexes contain histone acetyltransferases, histone methyltransferases, and/or chromatin remodeling enzymes, implicating a role of chromatin remodeling and histone modification in gene regulation by TR.
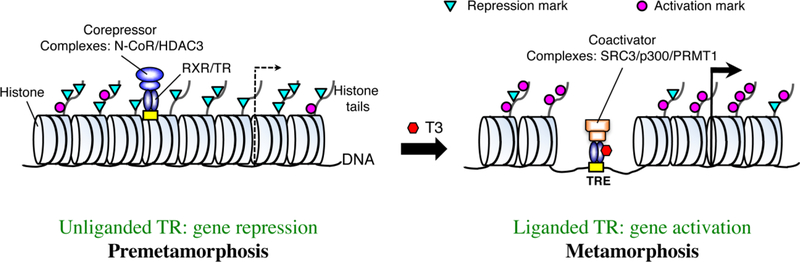
Indeed, chromatin immunoprecipitation (ChIP) studies on the intestine and tail during metamorphosis have shown that T3 activation of gene expression involves increased levels of so-called activation histone marks, i.e., those histone modifications associated with high levels of mRNA expression, and reduction in repression histone marks, i.e., those histone modifications associated with repressed genes (Fig. 2) (Sachs and Shi 2000; Sachs et al. 2002; Havis et al. 2003; Tomita et al. 2004; Paul et al. 2005a; Paul et al. 2005b; Paul et al. 2007; Matsuda et al. 2009; Bilesimo et al. 2011; Matsuura et al. 2012b; Shi et al. 2012; Grimaldi et al. 2013). In addition, ChIP analyses of total histones at the TR binding sites in target genes have revealed that liganded TR causes the removal of approximately 2 nucleosomes in each TR binding region during metamorphosis (Matsuura et al. 2012b), in agreement with earlier studies using T3-responsive reporter plasmid minichromosomes in the reconstituted frog oocyte transcription system (Wong and Shi 1995; Wong et al. 1995; Wong et al. 1997; Hsia and Shi 2002). These findings suggest that in the premetamorphic tadpole intestine, TR/RXR heterodimers bind to TREs in chromatin and recruit HDAC-containing corepressors to remove activation histone marks and add repression histone marks, leading to gene repression (Fig. 2). During metamorphosis, T3 binds to TR and leads to the removal of the corepressor complexes and recruitment of the coactivator complexes. Such complexes cause the removal of up to two nucleosomes near the TR binding region, increasing activation histone marks, and reducing in repression histone marks, and eventually lead to gene activation and induce the intestinal remodeling process.
Fig. 2. Regulation of T3-inducible genes by TR during Xenopus development.
In premetamorphic tadpoles, there is little T3 and TR is unliganded. The unliganded TR/RXR heterodimer binds to TREs in the target genes and recruits corepressor complexes such as the N-CoR-HDAC3 complex, resulting in the reduction in the levels of activation histone marks and increase of repression marks, and consequently gene repression. During metamorphosis, high levels of T3 leads to T3-binding to TR. Liganded TR/RXR recruits coactivator complexes such as SRC complexes as shown to disrupt chromatin and modify histones, leading to increased levels of activation histone marks and gene activation. N-CoR: nuclear corepressor, HDAC: histone deacetylase, SRC3: steroid receptor coactivator 3 (a histone acetyltransferase), p300: a histone acetyltransferase, PRMT1: protein arginine methyltransferase 1.
A positive feedback loop involving histone methyltransferases in gene regulation by TR during intestinal stem cell development
The studies as reviewed above have provided a detailed understanding on how TR regulates gene expression in the context of chromatin in vivo and revealed a requirement of T3-induced gene expression changes in both the epithelium and non-epithelial tissues for the formation of adult stem cells during intestinal metamorphosis. The next key issue toward understanding the development of the stem cells is thus to identify and functionally characterize the genes that are regulated by T3 in different intestinal tissues during metamorphosis. A lot of efforts have been made in this regard and many genes have been identified and found to be involved in adult stem cell formation/proliferation (Shi and Brown 1993; Amano and Yoshizato 1998; Ishizuya-Oka et al. 2001; Buchholz et al. 2007; Das et al. 2009; Heimeier et al. 2010; Luu et al. 2013; Miller et al. 2013; Sun et al. 2013; Fu et al. 2017; Okada et al. 2017; Okada and Shi 2018).
Of particular interest among the genes are two encoding histone methyltransferases, protein arginine methyltransferase (PRMT) 1 and Dot1L (Dot1-like) (Matsuda et al. 2009; Matsuura et al. 2012b). PRMT1 is a histone H4R3 methyltransferase and is a well-known TR coactivator (Chen et al. 1999; Matsuda et al. 2009). PRMT1 is induced by T3 during intestinal metamorphosis in Xenopus laevis. Mechanistically, T3 appears to activate the transcription factor cMyc7 in the developing stem cells and cMyc in turns activate the PRMT1 promoter (Fujimoto et al. 2012; Okada et al. 2017). These findings suggest that T3 induces the expression of PRMT1, although indirectly, and PRMT1 in turn feeds back positively on T3 action by functioning as a TR coactivator to further enhance T3 signaling to promote intestinal stem cell development.
Consistently, PRMT1 has little expression in premetamorphic intestine and is upregulated in the developing stem cells but not the dying larval epithelial cells during metamorphosis, just like cMyc, which activates PRMT1 promoter (Fujimoto et al. 2012; Okada et al. 2017). More importantly, transgenic overexpression of PRMT1 enhances the activation of T3 response genes by T3 and increases the number of stem cells during metamorphosis (Matsuda and Shi 2010). Conversely, knockdown the expression of PRMT1 in the tadpole intestine results in reduced number of stem cells during metamorphosis (Matsuda and Shi 2010). These findings support a role of PRMT1 in adult stem cell formation and/or proliferation during intestinal remodeling.
The other histone methyltransferase, Dot1L, is the only enzyme that can methylate histone H3K79 in vitro (Nguyen and Zhang 2011) and is directly regulated by T3 at the transcription level through the binding of TR to a TRE in its promoter (Matsuura et al. 2012a). Importantly, H3K79 methylation at TREs of T3 response genes is increased during metamorphosis in the intestine, raising the possibility that Dot1L is a TR coactivator that functions through H3K79 methylation, a known activation histone mark (Matsuura et al. 2012b). Indeed, transcription studies in the reconstituted frog oocyte system showed that Dot1L could enhance transcriptional activation by TR (Wen et al. 2017a). This might be in part due to increased TR binding to the TRE when Dot1L was overexpressed in the oocyte (Wen et al. 2017a). No recruitment of Dot1L to the TRE of the reporter plasmid minichromosome was detected in the frog oocyte, although it remains possible that Dot1L was weakly/transiently recruited by liganded TR to the TRE (Wen et al. 2017a).
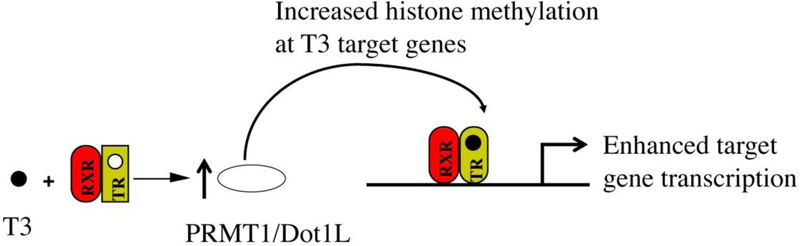
In addition, Dot1L overexpression also enhances T3-induction of gene expression in frog cell lines as well as in transgenic tadpoles (Wen et al. 2017a). Finally, a TALEN-nuclease was found to be able to cause up to 90% mutation in feeding stage tadpoles upon injecting its mRNAs into fertilized Xenopus tropicalis eggs and caused a correspondingly 80–90% reduction in H3K79 methylation of total H3 in the tadpoles, suggesting that in the developing tadpoles, Dot1L is the only methyltransferase for H3K79 (Wen et al. 2015a). Importantly, upon T3 treatment of such knockdown tadpoles, the induction of endogenous T3 target genes were found to be reduced, indicating that endogenous Dot1L contributes to gene activation by TR. Collectively, these findings support a role of Dot1L as a TR coactivator during intestinal remodeling and adult stem cell development via a positive feedback mechanism (Fig. 3).
Fig. 3. A positive feedback mechanism to enhance T3 activation of gene transcription through histone methylation.
T3 induces the expression of Dot1L directly at the transcription level (Matsuura et al. 2012a) and PRMT1 indirectly via transcriptional activation of cMyc by TR in the developing stem cells (Fujimoto et al. 2012; Okada et al. 2017). Dot1L and PRMT1 in turn function as TR coactivators to increase local histone methylations to enhance transcription (Fujimoto et al. 2012; Wen et al. 2017a). It is worth pointing out that there has been no direct evidence for the recruitment of Dot1L to TREs, although PRMT1 has been shown to be recruited by TR to TREs in the presence of T3 during metamorphosis (Matsuda et al. 2009).
Conclusion
Intestinal remodeling during amphibian metamorphosis offers a unique opportunity to study the development of adult organ-specific stem cells and the mechanism of gene regulation by TR in vivo. Studies on this model system have revealed that during intestinal stem cell development and subsequent formation of the adult epithelium, T3 activates the expression of genes in a number of pathways that are known to be important for stem cells and cell proliferation, including the hedgehog, Notch, BMP, and Wnt signal pathways, in the development and/or proliferation of adult intestinal stem cells during this T3-dependent process (Stolow and Shi 1995; Ishizuya-Oka et al. 2001; Ishizuya-Oka et al. 2006; Hasebe et al. 2008; Hasebe et al. 2012; Ishizuya-Oka and Hasebe 2013; Ishizuya-Oka et al. 2014; Wen et al. 2015b; Hasebe et al. 2016; Hasebe et al. 2017). Thus, these pathways are involved both in the formation of adult stem cells and their subsequent function and/or maintenance in the adult. While some of the T3-regulated genes in these pathways, such as sonic hedgehog (Stolow and Shi 1995), appear to be directly regulated by TR at the transcription level, others are likely regulated by T3 indirectly through the regulation of other genes by TR.
Like most DNA-binding transcription factors, TR functions by recruiting cofactor complexes upon binding to specific DNA elements in the target genes. In vitro and cell culture studies have identified many TR-cofactor complexes containing histone modifying enzymes and/or chromatin remodelers. Studies on Xenopus intestinal remodeling have provided one of the few pieces of in vivo evidence supporting a role of epigenetic modifications in gene regulation by TR during vertebrate development. First, most, although not all, histone marks analyzed so far correlate with gene regulation by TR during Xenopus intestinal remodeling as well as tail resorption (Bilesimo et al. 2011; Matsuura et al. 2012b; Grimaldi et al. 2013). That is, the levels of activation histone marks are increased at target genes during metamorphosis when T3 is present, supporting that TR utilizes such epigenetic modifications to control gene expression during adult stem cell development in the intestine. Second, liganded TR causes drastic chromatin remodeling, leading to the loss of two nucleosomes around the TR/RXR binding region. Finally and perhaps most interestingly, T3 activates the expression of at least two histone modifying enzymes, PRMT1 and Dot1L, that can also function as TR co-activators, demonstrating the existence of positive feedback mechanisms involving histone modifications to further enhance T3 action during metamorphosis (Fig. 3). Such mechanisms are likely important to ensure spatiotemporal coordination of the transformations of different tissues during metamorphosis. While a lot have been learnt, most of the studies so far have been correlative. Functional analysis, especially by using gene editing technologies (Young et al. 2011; Lei et al. 2012; Lei et al. 2013), are required to determine roles of the endogenous histone modifying enzymes and chromatin remodelers in chromatin remodeling and histone modification in regulating gene expression and adult stem cell development.
Highlights:
Thyroid hormone induces adult intestinal stem cell formation via dedifferentiation of larval epithelial cells.
Unliganded thyroid hormone receptor recruits histone deacetylase-containing complexes to target genes to establish a repressive state.
Liganded thyroid hormone receptor causes the loss of nucleosomes at target genes in tadpole.
Liganded thyroid hormone receptor recruits histone acetyltransferase and methyltransferase-containing complexes to target genes to add activation histone marks.
Thyroid hormone upregulates the expression of two histone methyltransferases, which in turn feedback positively to enhance thyroid hormone receptor function via histone methylation.
Acknowledgement
This work was supported by the intramural Research Program of NICHD, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
None.
References
- Amano T, Yoshizato K. 1998. Isolation of genes involved in intestinal remodeling during anuran metamorphosis. Wound Repair Regen 6: 302–313. [DOI] [PubMed] [Google Scholar]
- Bagamasbad P, Howdeshell KL, Sachs LM, Demeneix BA, Denver RJ. 2008. A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor beta. J Biol Chem 283: 2275–2285. [DOI] [PubMed] [Google Scholar]
- Bilesimo P, Jolivet P, Alfama G, Buisine N, Le Mevel S, Havis E, Demeneix BA, Sachs LM. 2011. Specific Histone Lysine 4 Methylation Patterns Define TR-Binding Capacity and Differentiate Direct T3 Responses. Mol Endocrinol 25: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M et al. 2012. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med 366: 243–249. [DOI] [PubMed] [Google Scholar]
- Brown DD, Cai L. 2007. Amphibian metamorphosis. Dev Biol 306: 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B. 2007. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol 303: 576–590. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Hsia VS-C, Fu L, Shi Y-B. 2003. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol 23: 6750–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Fu L, Shi YB. 2006. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 145: 1–19. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Shi YB. 2018. Dual function model revised by thyroid hormone receptor alpha knockout frogs. Gen Comp Endocrinol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Tomita A, Fu L, Paul BD, Shi Y-B. 2004. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol 24: 9026–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. 1999. Regulation of transcription by a protein methyltransferase. Science 284: 2174–2177. [DOI] [PubMed] [Google Scholar]
- Choi J, Ishizuya-Oka A, Buchholz DR. 2017. Growth, development, and intestinal remodeling occurs in the absence of thyroid hormone receptor alpha in tadpoles of Xenopus tropicalis. Endocrinology 158: 1623–1633. [DOI] [PubMed] [Google Scholar]
- Choi J, Suzuki KI, Sakuma T, Shewade L, Yamamoto T, Buchholz DR. 2015. Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression as revealed by gene disruption in Xenopus tropicalis. Endocrinology 156: 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Heimeier RA, Buchholz DR, Shi YB. 2009. Identification of direct thyroid hormone response genes reveals the earliest gene regulation programs during frog metamorphosis. J Biol Chem 284: 34167–34178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver RJ, Hu F, Scanlan TS, Furlow JD. 2009. Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev Biol 326: 155–168. [DOI] [PubMed] [Google Scholar]
- Flamant F, Poguet AL, Plateroti M, Chassande O, Gauthier K, Streichenberger N, Mansouri A, Samarut J. 2002. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRalpha gene. Mol Endocrinol 16: 24–32. [DOI] [PubMed] [Google Scholar]
- Fu L, Das B, Matsuura K, Fujimoto K, Heimeier RA, Shi YB. 2017. Genome-wide identification of thyroid hormone receptor targets in the remodeling intestine during Xenopus tropicalis metamorphosis. Sci Rep 7: 6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Hasebe T, Ishizuya-Oka A, Shi Y-B. 2007. Roles of matrix metalloproteinases and ECM remodeling during thyroid hormone-dependent intestinal metamorphosis in Xenopus laevis. Organogenesis 3: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Matsuura K, Hu-Wang E, Lu R, Shi YB. 2012. Thyroid Hormone Activates Protein Arginine Methyltransferase 1 Expression by Directly Inducing c-Myc Transcription during Xenopus Intestinal Stem Cell Development. J Biol Chem 287: 10039–10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi A, Buisine N, Miller T, Shi YB, Sachs LM. 2013. Mechanisms of thyroid hormone receptor action during development: lessons from amphibian studies. Biochim Biophys Acta 1830: 3882–3892. [DOI] [PubMed] [Google Scholar]
- Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. 2011. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci U S A 108: 10585–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Buchholz DR, Shi YB, Ishizuya-Oka A. 2011. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells 29: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Fujimoto K, Kajita M, Fu L, Shi YB, Ishizuya-Oka A. 2017. Thyroid Hormone-Induced Activation of Notch Signaling is Required for Adult Intestinal Stem Cell Development During Xenopus Laevis Metamorphosis. Stem Cells 35: 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Fujimoto K, Kajita M, Ishizuya-Oka A. 2016. Thyroid hormone activates Wnt/beta-catenin signaling involved in adult epithelial development during intestinal remodeling in Xenopus laevis. Cell Tissue Res 365: 309–318. [DOI] [PubMed] [Google Scholar]
- Hasebe T, Kajita M, Fu L, Shi YB, Ishizuya-Oka A. 2012. Thyroid hormone-induced sonic hedgehog signal up-regulates its own pathway in a paracrine manner in the Xenopus laevis intestine during metamorphosis. Dev Dyn 241: 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Kajita M, Shi YB, Ishizuya-Oka A. 2008. Thyroid hormone-up-regulated hedgehog interacting protein is involved in larval-to-adult intestinal remodeling by regulating sonic hedgehog signaling pathway in Xenopus laevis. Dev Dyn 237: 3006–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havis E, Sachs LM, Demeneix BA. 2003. Metamorphic T3-response genes have specific co-regulator requirements. EMBO Reports 4: 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimeier RA, Das B, Buchholz DR, Fiorentino M, Shi YB. 2010. Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol 11: R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia VS-C, Shi Y-B. 2002. Chromatin Disruption and Histone Acetylation in the Regulation of HIV-LTR by Thyroid Hormone Receptor. Mol Cell Biol 22: 4043–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Hasebe T. 2013. Establishment of intestinal stem cell niche during amphibian metamorphosis. Curr Top Dev Biol 103: 305–327. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi YB. 2009. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. Faseb J 23: 2568–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Hasebe T, Shi YB. 2010. Apoptosis in amphibian organs during metamorphosis. Apoptosis 15: 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Hasebe T, Shimizu K, Suzuki K, Ueda S. 2006. Shh/BMP-4 signaling pathway is essential for intestinal epithelial development during Xenopus larval-to-adult remodeling. Dev Dyn 235: 3240–3249. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Kajita M, Hasebe T. 2014. Thyroid hormone-regulated Wnt5a/Ror2 signaling is essential for dedifferentiation of larval epithelial cells into adult stem cells in the Xenopus laevis intestine. PLoS One 9: e107611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. 1991. Induction of metamorphosis by thyroid hormone in anuran small intestine cultured organotypically in vitro. In Vitro Cell Dev Biol 27A: 853–857. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. 1992a. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Roux’s Arch Dev Biol 201: 322–329. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. 1992b. Programmed cell death and heterolysis of larval epithelial cells by macrophage-like cells in the anuran small intestine in vivo and in vitro. J Morphol 213: 185–195. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Ueda S, Inokuchi T, Amano T, Damjanovski S, Stolow M, Shi Y-B. 2001. Thyroid hormone-induced expression of Sonic hedgehog correlates with adult epithelial development during remodeling of the Xenopus stomach and intestine. Differentiation 69: 27–37. [DOI] [PubMed] [Google Scholar]
- Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. 2009. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem 284: 1234–1241. [DOI] [PubMed] [Google Scholar]
- Laudet V, Gronemeyer H. 2002. The nuclear receptor FactsBook Academic Press, San Diego. [Google Scholar]
- Lazar MA. 1993. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14: 184–193. [DOI] [PubMed] [Google Scholar]
- Lei Y, Guo X, Deng Y, Chen Y, Zhao H. 2013. Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell Biosci 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Guo X, Liu Y, Cao Y, Deng Y, Chen X, Cheng CH, Dawid IB, Chen Y, Zhao H. 2012. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs). Proc Natl Acad Sci U S A 109: 17484–17489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu N, Wen L, Fu L, Fujimoto K, Shi YB, Sun G. 2013. Differential regulation of two histidine ammonia-lyase genes during Xenopus development implicates distinct functions during thyroid hormone-induced formation of adult stem cells. Cell Biosci 3: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald WC, Trier JS, Everett NB. 1964. Cell proliferation and migration in the stomach, duodenum, and rectum of man: Radioautographic studies. Gastroenterology 46: 405–417. [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. 1995. The nuclear receptor superfamily: the second decade. Cell 83: 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S, Fu L, Fiorentino M, Matsuda H, Das B, Shi Y-B. 2009. Differential regulation of cell type specific apoptosis by stromelysin-3: A potential mechanism via the cleavage of the laminin receptor during tail resorption in Xenopus laevis. J Biol Chem 284: 18545–18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S, Fu L, Hasebe T, Ishizuya-Oka A, Shi YB. 2010. Tissue-dependent induction of apoptosis by matrix metalloproteinase stromelysin-3 during amphibian metamorphosis. Birth Defects Res C Embryo Today 90: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Hasebe T, Shi Y-B. 2009. Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol Cell Biol 29: 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Shi YB. 2010. An essential and evolutionarily conserved role of protein arginine methyltransferase 1 for adult intestinal stem cells during postembryonic development. Stem Cells 28: 2073–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Fujimoto K, Das B, Fu L, Lu CD, Shi YB. 2012a. Histone H3K79 methyltransferase Dot1L is directly activated by thyroid hormone receptor during Xenopus metamorphosis. Cell Biosci 2: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Fujimoto K, Fu L, Shi Y-B. 2012b. Liganded thyroid hormone receptor induces nucleosome removal and histone modifications to activate transcription during larval intestinal cell death and adult stem cell development. Endocrinology 153: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy JW, Dixon KE. 1977. Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool 202: 129–138. [Google Scholar]
- Miller TC, Sun G, Hasebe T, Fu L, Heimeier RA, Das B, Ishizuya-Oka A, Shi YB. 2013. Tissue-specific upregulation of MDS/EVI gene transcripts in the intestine by thyroid hormone during Xenopus metamorphosis. PLoS One 8: e55585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C, Chatterjee K. 2015. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract Res Clin Endocrinol Metab 29: 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncan V, Heijmans J, Krasinski SD, Buller NV, Wildenberg ME, Meisner S, Radonjic M, Stapleton KA, Lamers WH, Biemond I et al. 2011. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun 2: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tazawa I, Yaoita Y. 2018. Thyroid Hormone Receptor alpha- and beta-Knockout Xenopus tropicalis Tadpoles Reveal Subtype-Specific Roles During Development. Endocrinology 159: 733–743. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Yaoita Y. 2003. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn 227: 246–255. [DOI] [PubMed] [Google Scholar]
- Nguyen AT, Zhang Y. 2011. The diverse functions of Dot1 and H3K79 methylation. Genes Dev 25: 1345–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Miller TC, Wen L, Shi YB. 2017. A balance of Mad and Myc expression dictates larval cell apoptosis and adult stem cell development during Xenopus intestinal metamorphosis. Cell death & disease 8: e2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Shi YB. 2018. EVI and MDS/EVI are required for adult intestinal stem cell formation during postembryonic vertebrate development. FASEB J 32: 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Wen L, Miller TC, Su D, Shi YB. 2015. Molecular and cytological analyses reveal distinct transformations of intestinal epithelial cells during Xenopus metamorphosis. Cell Biosci 5: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, Buchholz DR, Fu L, Shi Y-B. 2005a. Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. . J Biol Chem 280: 27165–27172. [DOI] [PubMed] [Google Scholar]
- Paul BD, Buchholz DR, Fu L, Shi Y-B. 2007. SRC-p300 coactivator complex is required for thyroid hormone induced amphibian metamorphosis. J Biol Chem 282: 7472–7481. [DOI] [PubMed] [Google Scholar]
- Paul BD, Fu L, Buchholz DR, Shi Y-B. 2005b. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol Cell Biol 25: 5712–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateroti M, Chassande O, Fraichard A, Gauthier K, Freund JN, Samarut J, Kedinger M. 1999. Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology 116: 1367–1378. [DOI] [PubMed] [Google Scholar]
- Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O. 2001. Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol Cell Biol 21: 4761–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateroti M, Kress E, Mori JI, Samarut J. 2006. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol 26: 3204–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM. 2015. Unliganded thyroid hormone receptor function: amphibian metamorphosis got TALENs. Endocrinology 156: 409–410. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Damjanovski S, Jones PL, Li Q, Amano T, Ueda S, Shi YB, Ishizuya-Oka A. 2000. Dual functions of thyroid hormone receptors during Xenopus development. Comp Biochem Physiol B Biochem Mol Biol 126: 199–211. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, Shi Y-B. 2002. N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol Cell Biol 22: 8527–8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM, Shi Y-B. 2000. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. PNAS 97: 13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane Y, Iida M, Hasebe T, Fujii S, Buchholz DR, Ishizuya-Oka A, Yamamoto T, Suzuki KT. 2018. Functional analysis of thyroid hormone receptor beta in Xenopus tropicalis founders using CRISPR-Cas. Biol Open 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Cai L, Brown DD. 2005. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci U S A 102: 3720–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. 2001. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. PNAS 98: 10739–10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Mukhi S, Brown DD. 2009. Cell-cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol 331: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-B. 1994. Molecular biology of amphibian metamorphosis: A new approach to an old problem. Trends Endocrinol Metab 5: 14–20. [DOI] [PubMed] [Google Scholar]
- Shi Y-B. 2009. Dual functions of thyroid hormone receptors in vertebrate development: the roles of histone-modifying cofactor complexes. Thyroid 19: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-B, Brown DD. 1993. The earliest changes in gene expression in tadpole intestine induced by thyroid hormone. J Biol Chem 268: 20312–20317. [PubMed] [Google Scholar]
- Shi Y-B, Ishizuya-Oka A. 1996. Biphasic intestinal development in amphibians: Embryogensis and remodeling during metamorphosis. Current Topics in Develop Biol 32: 205–235. [DOI] [PubMed] [Google Scholar]
- Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. 2011. The development of the adult intestinal stem cells: Insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci 1: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YB, Matsuura K, Fujimoto K, Wen L, Fu L. 2012. Thyroid hormone receptor actions on transcription in amphibia: The roles of histone modification and chromatin disruption. Cell Biosci 2: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling J, Fu L, Matsuura K, Shi Y-B. 2012. Cytological and morphological analyses reveal distinct features of intestinal development during Xenopus tropicalis metamorphosis. PLoS One 7: e47407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolow MA, Shi YB. 1995. Xenopus sonic hedgehog as a potential morphogen during embryogenesis and thyroid hormone-dependent metamorphosis. Nucleic Acids Res 23: 2555–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Shi Y, Shi Y-B. 1997a. Cyclosporin A But not FK506 Inhibits Thyroid Hormone-Induced Apoptosis in Xenopus Tadpole Intestinal Epithelium. FASEB J 11: 559–565. [DOI] [PubMed] [Google Scholar]
- Su Y, Shi Y, Stolow M, Shi Y-B. 1997b. Thyroid hormone induces apoptosis in primary cell cultures of tadpole intestine: cell type specificity and effects of extracellular matrix. J Cell Biol 139: 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Heimeier RA, Fu L, Hasebe T, Das B, Ishizuya-Oka A, Shi Y-B. 2013. Expression Profiling of Intestinal Tissues Implicates Tissue-Specific Genes and Pathways Essential for Thyroid Hormone-Induced Adult Stem Cell Development. Endocrinology 154: 4396–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Roediger J, Shi YB. 2016. Thyroid hormone regulation of adult intestinal stem cells: Implications on intestinal development and homeostasis. Rev Endocr Metab Disord 17: 559–569. [DOI] [PubMed] [Google Scholar]
- Sun G, Shi Y-B. 2012. Thyroid hormone regulation of adult intestinal stem cell development: Mechanisms and evolutionary conservations. Int J Biol Sci 8: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, Shi Y-B. 2004. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol 24: 3337–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner PG, Carr KE, Wyburn GM. 1971. The Digestive System: An Ultrastructural Atlas and Review Butterworth, London. [Google Scholar]
- Tsai MJ, O’Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem 63: 451–486. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. 2009. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu Rev Physiol 71: 241–260. [DOI] [PubMed] [Google Scholar]
- van Mullem A, van Heerebeek R, Chrysis D, Visser E, Medici M, Andrikoula M, Tsatsoulis A, Peeters R, Visser TJ. 2012. Clinical phenotype and mutant TRalpha1. N Engl J Med 366: 1451–1453. [DOI] [PubMed] [Google Scholar]
- Wen L, Fu L, Guo X, Chen Y, Shi YB. 2015a. Histone methyltransferase Dot1L plays a role in postembryonic development in Xenopus tropicalis. FASEB J 29: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Fu L, Shi YB. 2017a. Histone methyltransferase Dot1L is a coactivator for thyroid hormone receptor during Xenopus development. FASEB J 31: 4821–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Hasebe T, Miller TC, Ishizuya-Oka A, Shi YB. 2015b. A requirement for hedgehog signaling in thyroid hormone-induced postembryonic intestinal remodeling. Cell Biosci 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Shi YB. 2015. Unliganded thyroid hormone receptor alpha controls developmental timing in Xenopus tropicalis. Endocrinology 156: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Shi YB. 2016. Regulation of growth rate and developmental timing by Xenopus thyroid hormone receptor alpha. Dev Growth Differ 58: 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Shibata Y, Su D, Fu L, Luu N, Shi Y-B. 2017b. Thyroid hormone receptor αcontrols developmental timing and regulates the rate and coordination of tissue specific metamorphosis in Xenopus tropicalis. Endocrinology 158: 1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Patterton D, Imhof D, Guschin D, Shi Y-B, Wolffe AP. 1998. Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBO J 17: 520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Shi Y-B. 1995. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem 270: 18479–18483. [DOI] [PubMed] [Google Scholar]
- Wong J, Shi Y-B, Wolffe AP. 1997. Determinants of chromatin disruption and transcriptional regulation instigated by the thyroid hormone receptor: hormone-regulated chromatin disruption is not sufficient for transcriptinal activation. EMBO J 16: 3158–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Shi YB, Wolffe AP. 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TR beta A gene by the thyroid hormone receptor. Genes Dev 9: 2696–2711. [DOI] [PubMed] [Google Scholar]
- Yakut M, Üstün Y, Kabacan G, Soykan I. 2011. Thyroid Disorders in Patients with Inflammatory Bowel Diseases. International Journal of Clinical Medicine 2. [Google Scholar]
- Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81: 1097–1142. [DOI] [PubMed] [Google Scholar]
- Yen PM. 2015. Unliganded TRs regulate growth and developmental timing during early embryogenesis: evidence for a dual function mechanism of TR action. Cell Biosci 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Cherone JM, Doyon Y, Ankoudinova I, Faraji FM, Lee AH, Ngo C, Guschin DY, Paschon DE, Miller JC et al. 2011. Efficient targeted gene disruption in the soma and germ line of the frog Xenopus tropicalis using engineered zinc-finger nucleases. Proc Natl Acad Sci U S A 108: 7052–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]