Abstract
Platelets play a critical role in the pathophysiology of peripheral arterial disease (PAD). The mechanisms by which muscle ischemia regulates aggregation of platelets are poorly understood. We have recently identified the Nod-like receptor nucleotide-binding domain leucine rich repeat containing protein 3 (NLRP3) expressed by platelets as a critical regulator of platelet activation and aggregation, which may be triggered by activation of toll-like receptor 4 (TLR4). In this study, we performed femoral artery ligation (FAL) in transgenic mice with platelet-specific ablation of TLR4 (TLR4 PF4) and in NLRP3 knockout (NLRP3−/−) mice. NLRP3 inflammasome activity of circulating platelets, as monitored by activation of caspase-1 and cleavage of interleukin-1β (IL-1β), was upregulated in mice subjected to FAL. Genetic ablation of TLR4 in platelets led to decreased platelet caspase 1 activation and platelet aggregation, which was reversed by the NLRP3 activator Nigericin. Two weeks after the induction of FAL, ischemic limb perfusion was increased in TLR4 PF4 and NLRP3−/− mice as compared to control mice. Hence, activation of platelet TLR4/NLRP3 signaling plays a critical role in upregulating platelet aggregation and interfering with perfusion recovery in muscle ischemia and may represent a therapeutic target to improve limb salvage.
Keywords: platelets, NLRP3, hindlimb ischemia, peripheral arterial disease
Introduction
Peripheral arterial disease (PAD) is an advanced stage of atherosclerosis that may result in life-threatening conditions such as critical limb ischemia. Millions of Americans are affected by PAD, with a worldwide prevalence of over 200 million [1-2]. Failure of revascularization with surgical or endovascular procedures is associated with a significant risk of limb loss [2]; 6 to 12% of patients with revascularization failure die within 30 days of amputation [3].
Platelets play a critical role in the development and progression of PAD [4]. Activation and aggregation levels of circulating platelets are elevated in patients with PAD [5-6] and correlate with disease severity [7]. Current guidelines recommend aspirin as first-line therapy in the prevention of atherothrombotic events in patients with PAD [8]. Various other antiplatelet agents such as ADP receptor antagonists [9] and thromboxane inhibitors [10] have been investigated in patients with symptomatic PAD. Moreover, novel oral anticoagulants, such as low dose rivaroxaban in combination with aspirin, have recently shown promising results in patients with PAD [11]. However, treatment options for PAD are still limited, which is explained in part by an incomplete understanding of the underlying pathophysiological mechanisms.
Recent evidence proposes a critical role of inflammatory processes in the pathophysiology of PAD [12-15]. Platelets employ pattern recognition receptors toll-like receptor 4 (TLR4) and nucleotide-binding domain leucine rich repeat containing protein 3 (NLRP3) [16-19]. NLRP3 may become activated by TLR4 signaling [20-21], resulting in the formation of intracellular inflammasome complexes with the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1-dependent cleavage and secretion of interleukin 1β (IL-1β) [22]. In platelets, activation of TLR4 and NLRP3 promotes platelet activation/aggregation and thrombus formation [18-19, 23-24]. Moreover, activation of platelet TLR4 is involved in regulating limb perfusion and muscle necrosis after hindlimb ischemia [25]. The role of the platelet NLRP3 inflammasome in muscle ischemia, however, remains unknown. In this study, we hypothesized that the platelet NLRP3 inflammasome would be upregulated in a murine model of hindlimb ischemia via TLR4 and regulate platelet aggregation.
Materials and Methods
Animals and femoral artery ligation (FAL) model
The animal protocol complied with the regulation regarding the care and use of experimental animals published by the NIH and was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh (protocol number 16037594). C57/BL6 wild-type (WT) mice (male; 8-10 weeks) were purchased from Jackson Laboratories (Bar Harbor, ME). NLRP3−/− mice were obtained from Millennium Pharmaceuticals (Cambridge, MA) and were on a C57BL/6 background [26]. TLR4loxP/loxP (TLR4 Flox) control mice were generated as previously described [27]. Platelet specific TLR4 knockout (TLR4 PF4) mice were generated by crossing TLR4 Flox mice with Pf4-Cre transgenic mice as described [28]. Breeding of both NLRP3−/− and TLR4 PF4 mice were performed in the laboratory of Dr. Timothy Billiar (Department of Surgery, University of Pittsburgh Medical Center).
For FAL, mice were anesthetized with pentobarbital (0.1 cc/g intraperitoneal). After hair removal and preparation with iodine solution, transverse incisions were made in each groin and the femoral structures were isolated. On the right, the external iliac and femoral veins and arteries and all visible branches were ligated with 6-0 silk [29]. Care was taken to preserve the femoral nerve. On the left, the femoral vessels were exposed but not ligated. Animals were kept warm with a heating lamp during the surgical procedure. Mice were euthanized by an overdose of inhaled isoflurane followed by cervical dislocation at designated time points.
Platelet caspase-1 activity assay
Blood from mice was obtained 6 hours after surgery via cardiac puncture [30]. Mice that underwent surgery in the absence of FAL were used as sham controls. Murine platelets were isolated as previously described [18]. For certain experiments, isolated platelets from WT mice were incubated for 30 minutes with an inhibitor against NLRP3 (MCC950; 100 nM, Cayman Chemical, Ann Arbor, MI) [31] or caspase-1 (YVAD; 100 nM, Calbiochem, Darmstadt, Germany) [32]. DMSO was used as a control. In other experiments, isolated platelets derived from TLR4 PF4 or TLR4 Flox control mice were treated for 30 minutes with the NLRP3 inflammasome activator Nigericin (10 μM, Cayman Chemical) [33]. Activation of caspase-1 in isolated platelets was measured using the FAM-FLICA Caspase-1 Assay Kit according to the manufacturer’s protocol (Immunochemistry Technologies, Bloomington, MN). Platelets were analyzed in a black 96-well microtiter plate using a plate reader for relative fluorescence units (RFUs).
Detection of IL-1β in platelet supernatants
Platelets isolated from WT mice subjected to FAL or sham surgery were kept untreated or treated with MCC950 (100 nM) for 30 minutes. Platelets were lysed with Pierce IP Lysis Buffer (ThermoFisher Scientific; Pittsburgh, PA) and protein concentrations were determined with the Bradford Concentration Assay (ThermoFisher Scientific). 50 μg of protein was resolved by 8.5% SDS-polyarcylamide gel electrophoresis. Western Blotting onto nitrocellulose membranes (Bio Rad, Hercules, CA) was performed using the Criterion Blotter system (Bio Rad). Membranes were incubated overnight with anti-IL-1β polyclonal antibody (1:2000, rabbit IgG; abcam, Cambridge, MA). Anti-tubulin monoclonal antibody (1:2000, mouse IgM; BD Pharmingen/Biosciences; San Jose, CA) was used as a loading control. Antibody binding was detected with corresponding secondary fluorescence-labeled antibodies, HRP-conjugated Clarity Western Substrate (Bio Rad), and a SRX-101a Film processer (Konica, Cleveland, OH).
Platelet aggregometry
Blood from mice was obtained 6 hours after surgery via cardiac puncture. Platelet aggregation was evaluated using whole blood impedance aggregometry (Model 700, ChronoLog, Havertown, PA) as described previously [18]. Collagen (2 μg/ml; ChronoLog) was used as platelet agonist. Aggregation was measured for 6 minutes at 37°C with a stir speed of 1,200 rpm. Analysis was performed using the Aggrolink-8 software (ChronoLog). Data are reported as area under the curve (AUC), which incorporates the slope and amplitude of the aggregation curves.
Laser Doppler Perfusion Imaging (LDPI)
LDPI was performed as described previously [25]. In brief, animals were anesthetized with inhaled isoflurane and kept warm on a heating pad for the duration of the procedure. Similar ambient lighting was used for each study for standardization. After preparing each hindlimb with depilatory cream, the blood flow to both hindlimbs was measured using a laser doppler blood flow meter (Perimed, Stockholm, Sweden). Three sequential images of the entire hindlimb were obtained and averaged. Perfusion was expressed as a ratio of the ischemic to nonischemic limb at each time point to confirm adequacy of ligation. Laser doppler perfusion imaging was performed before and 1, 7, and 14 days after FAL.
Statistical analysis
Data show mean ± SD. Results are reported from at least three independent experiments. Two-way factorial ANOVA with post hoc Bonferroni correction was used as appropriate. All statistical analyses were performed using Graph Pad Prism software (GraphPad, San Diego CA).
Results
The platelet NLRP3 inflammasome is upregulated following FAL via TLR4
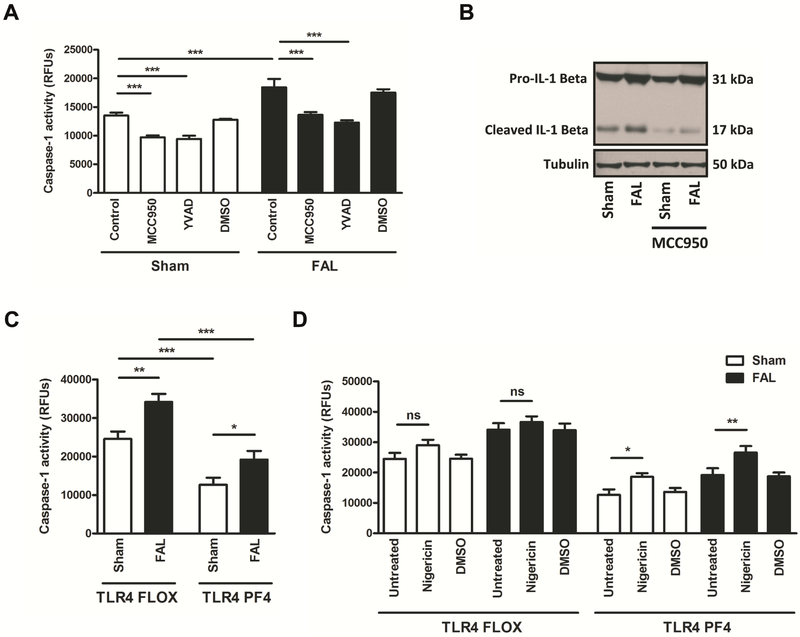
We studied activation of the platelet NLRP3 inflammasome in a hindlimb ischemia mouse model (FAL). Caspase-1 activity was significantly elevated in circulating platelets from WT mice subjected to FAL as compared to sham controls (Fig. 1A). Incubation of platelets with the NLRP3 inhibitor MCC950 significantly suppressed caspase-1 activation in platelets derived from FAL and sham mice. The caspase-1 inhibitor YVAD served as a positive control and significantly downregulated caspase-1 activation. We also studied cleavage of IL-1β in platelets, which allows monitoring of caspase-1 activity [18]. Platelet expression of cleaved IL-1β was increased in mice subjected to FAL and decreased in the presence of the NLRP3 inhibitor (Fig. 1B).
Figure 1. The platelet NLRP3 inflammasome is upregulated following FAL via TLR4.
(A) Platelet caspase-1 activity (measured by FLICA assay) is upregulated in mice subjected to FAL. Platelet caspase-1 activity is suppressed in the presence of the NLRP3 inhibitor MCC950 and the caspase-1 inhibitor YVAD. (B) Expression of cleaved IL-1β in platelets is increased in mice subjected to FAL and decreased in the presence of the NLRP3 inhibitor MCC950. (C) Platelet caspase-1 activity in the presence or absence of FAL is downregulated in TLR4 PF4 mice as compared to TLR4 Flox controls. (D) Downregulated platelet caspase-1 activity in TLR4 PF4 mice is restored by the NLRP3 activator Nigericin,which does not occur in platelets derived from TLR4 Flox controls. Data are presented as mean ±SD for N≥3 and at least three separate experiments in all studies. *p< 0.05, **p < 0.01,***p < 0.001 (2-way ANOVA with Bonferroni post-hoc test in A, C, D).
TLR4 signaling is involved in activating the NLRP3 inflammasome in immune cells and platelets [20-21, 23]. Hence, we next investigated whether FAL-induced activation of the platelet NLRP3 inflammasome depends on platelet TLR4. FAL-induced upregulation of platelet caspase-1 activity was significantly suppressed in mice whose platelets were lacking TLR4 (TLR4 PF4) as compared to platelets from TLR4 Flox control mice (Fig. 1C). Platelet caspase-1 activity at baseline (sham) also was significantly decreased in TLR4 PF4 mice as compared to control mice. Downregulated platelet caspase-1 activity in platelet-specific TLR4 knockout mice was significantly reversed by the NLRP3 activator Nigericin, which did not occur in platelets derived from TLR4 Flox controls (Fig. 1D), indicating that activation of platelet NLRP3 is mediated via TLR4.
Platelet TLR4/NLRP3 signaling promotes platelet aggregation in FAL
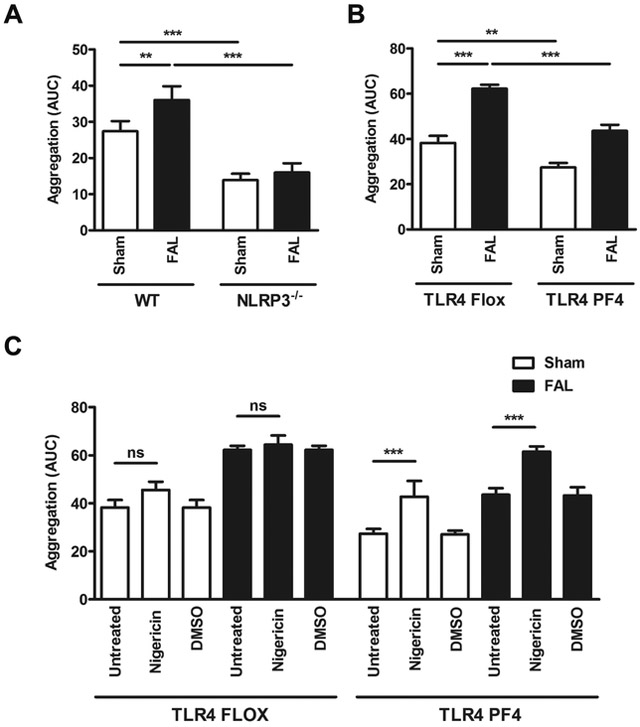
Next, we investigated the effect of FAL-induced TLR4-dependent platelet NLRP3 inflammasome activation on aggregation of platelets. Platelet aggregation was significantly increased in WT (Fig. 2A) and TLR4 Flox control mice (Fig. 2B) after FAL. Aggregation of platelets in the presence or absence of FAL was significantly suppressed in NLRP3−/− mice (Fig. 2A) and TLR4 PF4 mice (Fig. 2B). Downregulated platelet aggregation in mice with platelet-specific TLR4 knockout was significantly restored by the NLRP3 activator Nigericin, which did not occur in platelets derived from TLR4 Flox control mice, indicating a functionally active link between platelet TLR4 and NLRP3 regulating platelet aggregation (Fig. 2C).
Figure 2. Platelet TLR4/NLRP3 signaling promotes platelet aggregation in FAL.
(A) Collagen-induced platelet aggregation is increased when WT mice are subjected to FAL and decreased in NLRP3−/− mice. (B) Collagen-induced platelet aggregation is increased when TLR4 Flox control mice mice are subjected to FAL and decreased in TLR4 PF4 mice. (C) Downregulated platelet aggregation in TLR4 PF4 mice is restored by the NLRP3 activator Nigericin, which does not occur in platelets derived from TLR4 Flox controls. Data are presented as mean ± SD for N≥3 and at least three separate experiments in all studies. **p < 0.01, ***p < 0.001 (2-way ANOVA with Bonferroni post-hoc test in A-C).
Absence of platelet TLR4 and NLRP3 improves perfusion recovery two weeks after FAL
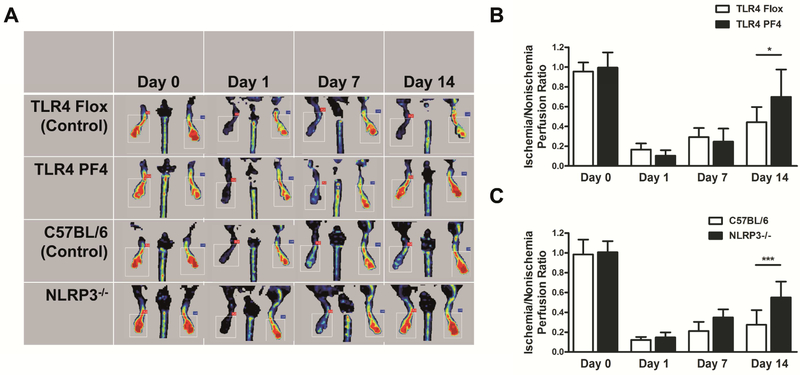
We tested the effect of platelet TLR4 and NLRP3 ablation on perfusion recovery following FAL. Perfusion of the femoral artery subjected to ligation and that of the contralateral nonligated femoral artery was evaluated by LDPI at baseline (day 0), shortly after FAL induction (day 1), and on days 7 and 14 (Fig. 3A). Two weeks after the induction of FAL, the ischemia/nonischemia perfusion ratio was significantly increased in mice whose platelets were lacking TLR4 (TLR4 PF4) as compared with that in TLR4 Flox control mice (Fig. 3B). Similar results were obtained in NLRP3−/− mice (Fig. 3C).
Figure 3. Absence of platelet TLR4 and NLRP3 improves perfusion recovery two weeks after FAL.
(A) Perfusion of the femoral artery subjected to ligation and that of the contralateral nonligated femoral artery is evaluated by LDPI in TLR4 Flox, TLR4 PF4, WT (C57BL/6), and NLRP3−/− mice at baseline (day 0), shortly after FAL induction (day 1), and on days 7 and 14. (B) Two weeks after the induction of FAL, the ischemia/nonischemia perfusion ratio is increased in TLR4 PF4 mice as compared with that in TLR4 Flox control mice. (C) Two weeks after the induction of FAL, the ischemia/nonischemia perfusion ratio is increased in NLRP3−/− mice as compared with that in WT (C57BL/6) control mice. Data are presented as mean ± SD for N≥3 and at least three separate experiments in all studies. *p < 0.05, ***p < 0.001 (2-way ANOVA with Bonferroni post-hoc test in B, C).
Discussion
In this study, we show that hindlimb ischemia following FAL induces activation of the platelet NLRP3 inflammasome via TLR4. We further identify a critical role of platelet TLR4/NLRP3 signaling in upregulating platelet aggregation and interfering with perfusion recovery after FAL.
While revascularization procedures may restore large vessel patency in PAD, upregulation of platelet aggregation and accumulation of platelet aggregates in the microvasculature are critical determinants of perfusion recovery and ongoing muscle injury [4, 34]. Hyperreactive platelets may form aggregates with immune cells, cause platelet microembolism, and significantly impair reperfusion therapy outcome, exacerbating vascular and tissue damage [7, 34-35]. Upon activation, platelets upregulate an arsenal of potent thromboinflammatory mechanisms, resulting in surface expression or release of adhesion proteins, growth factors, cytokines, chemokines, and coagulation factors into the local microenvironment [35-37].
The NLRP3 inflammasome is a critical thromboinflammatory mechanism employed by immune cells, which was described in platelets for the first time in the context of dengue fever [17]. In this study, the dengue virus triggered activation of the platelet NLRP3 inflammasome, which resulted in platelet shedding of IL-1β-rich microparticles and endothelial dysfunction. We have demonstrated recently that activation of platelets by collagen or thrombin also upregulates platelet NLRP3 inflammasome activity [18]. In another recent study, we show that the NLRP3 inflammasome in platelets is upregulated in sickle cell disease, which was mediated via the damage-associated molecular pattern molecule high-mobility group box 1 (HMGB1) and TLR4, promoting aggregation of circulating platelets [23]. We and others have shown that activation of platelet TLR4 and NLRP3 promotes platelet activation/aggregation and thrombus formation, proposing that upregulation of these pattern recognition receptor signals in platelets plays a critical role in disease states associated with abnormal coagulation and inflammation [18-19, 23-24].
In this study, the platelet NLRP3 inflammasome was upregulated in mice subjected to hindlimb ischemia, which was mediated via platelet TLR4 and resulted in elevated platelet aggregation levels. These findings were associated with improved perfusion in the muscle tissue of TLR4 PF4 and NLRP3−/− mice two weeks after FAL, indicating possible involvement of platelet TLR4/NLRP3 signaling in regulating perfusion recovery. The potential role of TLR4/NLRP3-mediated platelet aggregation in interfering with perfusion recovery from muscle ischemia, however, is currently unknown. Recent evidence supports a critical role of the NLRP3 inflammasome in immune cells in regulating progression of atherosclerosis [38]. It is possible that elevated platelet aggregation levels mediated by platelet TLR4/NLRP3 signaling increase microvascular thrombus formation and further exacerbate tissue damage as part of a vicious cycle occurring in chronic muscle ischemia, which is currently under investigation. Further studies are needed to investigate the role of platelet NLRP3 inflammasome activation in PAD and study this mechanism as a potential therapeutic target.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (K08HL103899 and R01 HL136556 to Dr. Ulka Sachdev). The work for this article was performed while Dr. Sebastian Vogel was at University of Pittsburgh. The opinions expressed in this article are the authors' own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. The authors declare no conflict of interest.
Abbreviations:
- ASC
apoptosis-associated speck-like protein containing a CARD
- FAL
femoral artery ligation
- IL-1β
interleukin-1β
- NLRP3
nucleotide-binding domain leucine rich repeat containing protein 3
- PAD
peripheral arterial disease
- TLR4
toll-like receptor 4
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Marston WA, Davies SW, Armstrong B, Farber MA, Mendes RC, Fulton JJ, Keagy BA, Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization, J Vasc Surg, 44 (2006) 108–114. [DOI] [PubMed] [Google Scholar]
- [2].Selvin E, Erlinger TP, Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000, Circulation, 110 (2004) 738–743. [DOI] [PubMed] [Google Scholar]
- [3].Nelson MT, Greenblatt DY, Soma G, Rajimanickam V, Greenberg CC, Kent KC, Preoperative factors predict mortality after major lower-extremity amputation, Surgery, 152 (2012) 685–694; discussion 694–686. [DOI] [PubMed] [Google Scholar]
- [4].Cassar K, Bachoo P, Brittenden J, The role of platelets in peripheral vascular disease, Eur J Vasc Endovasc Surg, 25 (2003) 6–15. [DOI] [PubMed] [Google Scholar]
- [5].Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J, Platelet activation is increased in peripheral arterial disease, J Vasc Surg, 38 (2003) 99–103. [DOI] [PubMed] [Google Scholar]
- [6].Ward AS, Porter N, Preston FE, Morris-Jones W, Platelet aggregation in patients with peripheral vascular disease, Atherosclerosis, 29 (1978) 63–68. [DOI] [PubMed] [Google Scholar]
- [7].Rajagopalan S, McKay I, Ford I, Bachoo P, Greaves M, Brittenden J, Platelet activation increases with the severity of peripheral arterial disease: implications for clinical management, J Vasc Surg, 46 (2007) 485–490. [DOI] [PubMed] [Google Scholar]
- [8].Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE, 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery, J Vasc Surg, 54 (2011) e32–58. [DOI] [PubMed] [Google Scholar]
- [9].Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, Han Z, Tan KS, Ratanakorn D, Chollate P, Zhao Y, Koh A, Hao Q, Markus HS, Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial, Lancet Neurol, 9 (2010) 489–497. [DOI] [PubMed] [Google Scholar]
- [10].Balsano F, Violi F, Effect of picotamide on the clinical progression of peripheral vascular disease. A double-blind placebo-controlled study. The ADEP Group, Circulation, 87 (1993) 1563–1569. [DOI] [PubMed] [Google Scholar]
- [11].Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Stork S, Zhu J, Lopez-Jaramillo P, O'Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD, Vanassche T, Avezum AA, Chen E, Branch K, Leong DP, Bangdiwala SI, Hart RG, Yusuf S, Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial, Lancet, 391 (2018) 219–229. [DOI] [PubMed] [Google Scholar]
- [12].Ozkaramanli Gur D, Guzel S, Akyuz A, Alpsoy S, Guler N, The role of novel cytokines in inflammation: Defining peripheral artery disease among patients with coronary artery disease, Vasc Med, 23 (2018) 428–436. [DOI] [PubMed] [Google Scholar]
- [13].Meyer A, Weithaeuser A, Steffens D, Bobbert P, Hassanein A, Ayral Y, Schultheiss HP, Rauch U, Inhibition of platelet function with clopidogrel is associated with a reduction of inflammation in patients with peripheral artery disease, Cardiovasc Revasc Med, 17 (2016) 169–175. [DOI] [PubMed] [Google Scholar]
- [14].Rein P, Saely CH, Silbernagel G, Vonbank A, Mathies R, Drexel H, Baumgartner I, Systemic inflammation is higher in peripheral artery disease than in stable coronary artery disease, Atherosclerosis, 239 (2015) 299–303. [DOI] [PubMed] [Google Scholar]
- [15].Brevetti G, Giugliano G, Brevetti L, Hiatt WR, Inflammation in peripheral artery disease, Circulation, 122 (2010) 1862–1875. [DOI] [PubMed] [Google Scholar]
- [16].Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW, Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo, Blood, 107 (2006) 637–641. [DOI] [PubMed] [Google Scholar]
- [17].Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza FA, Bozza PT, Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation, Blood, 122 (2013) 3405–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Murthy P, Durco F, Miller-Ocuin JL, Takedai T, Shankar S, Liang X, Liu X, Cui X, Sachdev U, Rath D, Lotze MT, Zeh HJ 3rd, Gawaz M, Weber AN, Vogel S, The NLRP3 inflammasome and bruton's tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in vitro thrombus formation, Biochem Biophys Res Commun, 483 (2017) 230–236. [DOI] [PubMed] [Google Scholar]
- [19].Qiao J, Wu X, Luo Q, Wei G, Xu M, Wu Y, Liu Y, Li X, Zi J, Ju W, Fu L, Chen C, Wu Q, Zhu S, Qi K, Li D, Li Z, Andrews RK, Zeng L, Gardiner EE, Xu K, NLRP3 regulates platelet integrin alphaIIbbeta3 outside-in signaling, hemostasis and arterial thrombosis, Haematologica, 103 (2018) 1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chi W, Li F, Chen H, Wang Y, Zhu Y, Yang X, Zhu J, Wu F, Ouyang H, Ge J, Weinreb RN, Zhang K, Zhuo Y, Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma, Proc Natl Acad Sci U S A, 111 (2014) 11181–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martin-Rodriguez S, Caballo C, Gutierrez G, Vera M, Cruzado JM, Cases A, Escolar G, Diaz-Ricart M, TLR4 and NALP3 inflammasome in the development of endothelial dysfunction in uraemia, Eur J Clin Invest, 45 (2015) 160–169. [DOI] [PubMed] [Google Scholar]
- [22].Strowig T, Henao-Mejia J, Elinav E, Flavell R, Inflammasomes in health and disease, Nature, 481 (2012) 278–286. [DOI] [PubMed] [Google Scholar]
- [23].Vogel S, Arora T, Wang X, Mendelsohn L, Nichols J, Allen D, Shet AS, Combs CA, Quezado ZMN, Thein SL, The platelet NLRP3 inflammasome is upregulated in sickle cell disease via HMGB1/TLR4 and Bruton tyrosine kinase, Blood Adv, 2 (2018) 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vogel S, Bodenstein R, Chen Q, Feil S, Feil R, Rheinlaender J, Schaffer TE, Bohn E, Frick JS, Borst O, Munzer P, Walker B, Markel J, Csanyi G, Pagano PJ, Loughran P, Jessup ME, Watkins SC, Bullock GC, Sperry JL, Zuckerbraun BS, Billiar TR, Lotze MT, Gawaz M, Neal MD, Platelet-derived HMGB1 is a critical mediator of thrombosis, J Clin Invest, 125 (2015) 4638–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sachdev U, Cui X, Tzeng E, HMGB1 and TLR4 mediate skeletal muscle recovery in a murine model of hindlimb ischemia, J Vasc Surg, 58 (2013) 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP, Koller BH, NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice, J Immunol, 189 (2012) 2006–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T Jr., Branca M, Russo A, Gribar SC, Ma C, Hackam DJ, Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis, Gastroenterology, 138 (2010) 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bauer EM, Chanthaphavong RS, Sodhi CP, Hackam DJ, Billiar TR, Bauer PM, Genetic deletion of toll-like receptor 4 on platelets attenuates experimental pulmonary hypertension, Circ Res, 114 (2014) 1596–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Messina LM, Brevetti LS, Chang DS, Paek R, Sarkar R, Therapeutic angiogenesis for critical limb ischemia: invited commentary, J Control Release, 78 (2002) 285–294. [DOI] [PubMed] [Google Scholar]
- [30].Sachdev U, Cui X, Hong G, Namkoong S, Karlsson JM, Baty CJ, Tzeng E, High mobility group box 1 promotes endothelial cell angiogenic behavior in vitro and improves muscle perfusion in vivo in response to ischemic injury, J Vasc Surg, 55 (2012) 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA, A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases, Nat Med, 21 (2015) 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. , A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes, Nature, 356 (1992) 768–774. [DOI] [PubMed] [Google Scholar]
- [33].Perregaux D, Barberia J, Lanzetti AJ, Geoghegan KF, Carty TJ, Gabel CA, IL-1 beta maturation: evidence that mature cytokine formation can be induced specifically by nigericin, J Immunol, 149 (1992) 1294–1303. [PubMed] [Google Scholar]
- [34].Blaisdell FW, The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review, Cardiovasc Surg, 10 (2002) 620–630. [DOI] [PubMed] [Google Scholar]
- [35].Gawaz M, Langer H, May AE, Platelets in inflammation and atherogenesis, J Clin Invest, 115 (2005) 3378–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gawaz M, Vogel S, Platelets in tissue repair: control of apoptosis and interactions with regenerative cells, Blood, 122 (2013) 2550–2554. [DOI] [PubMed] [Google Scholar]
- [37].Vogel S, Thein SL, Platelets at the crossroads of thrombosis, inflammation and haemolysis, Br J Haematol, 180 (2018) 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Karasawa T, Takahashi M, Role of NLRP3 Inflammasomes in Atherosclerosis, J Atheroscler Thromb, 24 (2017) 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.