Abstract
Background:
Hemostasis requires a balance between pro- and anti-coagulant factors. Hemophiliacs bleed due to a procoagulant deficiency. The targeted reduction in the activity of endogenous anticoagulant pathways is currently being investigated as a means of improving hemostasis in hemophilia. Protein Z (PZ) is a co-factor that serves as a catalyst for PZ-dependent protease inhibitor (ZPI) inactivation of factor (F)Xa at phospholipid surfaces.
Objectives:
Evaluate the effects of 1) PZ or ZPI gene-deletion in hemophilia mice and 2) blocking PZ in human hemophilic plasma.
Methods:
1) A Tail Vein Re-Bleeding assay (TVRB) was developed based on the serial disruption of clots forming over 15 minutes following a tail vein laceration in an anesthetized mouse. Wild type (WT)/FVIIIKO, PZKO/FVIIIKO and ZPIKO/FVIIIKO mice were evaluated in this model and their plasmas tested in thrombin generation assays. 2) A monoclonal antibody (Mab) against PZ was evaluated in human hemophilic plasma thrombin generation assays.
Results:
1) Clot formations (mean ± SEM) in the TVRB were: 4.0 ± 0.9 for WT/FVIIIKO mice; 23.8 ± 1.1 for WT/FVIIIKO mice replaced with 100% FVIII; 15.2 ± 1.1 for PZKO/FVIIIKO mice; and 14.7 ± 1.2 for ZPIKO/FVIIIKO mice. Thrombin generation in PZKO/FVIIIKO and ZPIKO/FVIIIKO mouse plasmas was similar to FVIIIKO plasma replaced with ~15% rFVIII, 2) A Mab against PZ added to human hemophilia plasma enhanced thrombin generation to an extent similar to the addition of ~15% FVIII.
Conclusions:
Blockade of the PZ/ZPI system may be sufficient to ameliorate the phenotype of severe hemophilia.
Keywords: Blood coagulation, factor VIII, hemophilia A, hemostasis, mice
INTRODUCTION
Ongoing studies are evaluating the therapeutic potential of suppressing specific natural anticoagulant processes to ameliorate the bleeding in individuals with hemophilia. Targeted anticoagulants include TFPI, antithrombin, activated protein C (PC), and protein S (PS) [1–4]. This approach appears most apropos for prophylactic therapy with a goal of improving the clinical hemophilia phenotype from severe (<1% factor (F)VIII), which is associated with spontaneous bleeding, to mild (5–40% FVIII) in which hemorrhage is uncommon in the absence of trauma or surgical procedures. Such non-FVIII replacement based therapy 1) would not be associated with the development of FVIII inhibitors, 2) could provide prophylactic treatment for patients with or without FVIII inhibitors, and 3) would greatly facilitate prophylactic treatment if agents with prolonged effects and subcutaneous delivery were shown to be efficacious and of low thrombotic risk.
Another endogenous anticoagulant pathway involves Protein Z (PZ) and PZ-dependent proteinase inhibitor (ZPI). PZ is a 62 kDa vitamin K-dependent cofactor [5] that serves as a catalyst [6] to dramatically enhance FXa inhibition by the 72 kDa serpin ZPI (SerpinA10) at phospholipid surfaces [7,8]. The PZ-dependent amplification of the inhibition of FXa by ZPI involves the binding of the C-terminal pseudo-catalytic domain of PZ to ZPI and the binding of the N-terminal γ-carboxyglutamic acid (Gla) domain of PZ and the Gla domain of FXa at the phospholipid surface [6,9–11]. Following the interaction of ZPI with FXa, PZ is released from the inhibitory complex. In the absence of PZ, ZPI also inhibits FXIa [12] and kinetic studies suggest it is the most potent known inhibitor of FXIa in plasma [13]. In normal individuals the ranges of PZ (15–233%) and ZPI (33–191%) are broad with mean levels of ~2 ug/mL (32 nM) and ~4 ug/mL (56 nM), respectively [14]. PZ circulates bound to ZPI in plasma [15].
The in vivo role of the PZ/ZPI anticoagulant pathway appears to be less than that of the other endogenous anticoagulant pathways. In people without hemophilia, modest deficiency of TFPI, antithrombin, PC or PS is associated with an increased risk of thrombosis, whereas a role for PZ or ZPI deficiency in human thrombosis has not been established [14,16–19], In mice with normal levels of FVIII, gene-deletion of TFPI, antithrombin, PC or PS causes death in utero or in the perinatal period from thrombosis, disseminated intravascular coagulation and a consumptive coagulopathy [20–24]. Concomitant hemophilia does not rescue the TFPI [25] or antithrombin [26] deficient mice, but does rescue PS deficient mice [4]. The PSKO/FVIIIKO mice, however, develop vascular thrombosis and disseminated intravascular coagulation when FVIII replacement therapy is administered [4]. PZ and ZPI gene-deleted mice with normal levels of FVIII are healthy.
Here, in proof-of-concept studies, we examine the effects of inhibition of the PZ/ZPI anticoagulant pathway on hemostasis in a mouse hemophilia model and thrombin generation in mouse and human hemophilia plasma.
STUDY DESIGN
Mice.
C57Bl/6N (B6) mice were from Charles River and B6;129S-F8tm1Kaz/J (FVIIIKO) mice in a C57Bl/6J-129S4/SvJae genetic background were from Jackson Laboratories, Bar Harbor, ME, USA. PZ and ZPI gene-deleted mice [27,28] had been backcrossed onto the B6 background (N>10). After appropriate breeding, offspring from PZ(+/−)/FVIIIKO matings and ZPI(+/−)/FVIIIKO matings were tested. Results obtained in PZ(+/+)/FVIIIKO and ZPI(+/+)/FVIIIKO mice did not detectably differ and these offspring are referred to collectively as WT/FVIIIKO mice. The Washington University Animal Studies Committee approved all mouse experiments.
Tail vein re-bleeding assay (TVRB).
The investigator preforming the assay is blinded as to genotype and treatments of the mice. A mouse is anesthetized with a 5 uL/gm intra-peritoneal injection of a solution containing ketamine (10 mg/mL) and dexmedetomidine (0.1 mg/mL) and placed on a 37°C warming plate. At the tail diameter of 2.25 mm a #11 scalpel (Medi-Cut, Dynarex Corp. Orangeburg, NY, USA) is used to make a horizontal incision across a lateral tail vein to the depth of the sharpened bevel (~1 mm) and a timer started. The position of the mouse is adjusted to immerse the tail in 37°C 0.85% NaCl in a hand-held test tube. The test tube is manipulated and replaced as needed to prevent the shed blood from obscuring the incision site. When bleeding stops the incision is wiped firmly with a tissue to disrupt the clot and reintroduce bleeding, and the tail once again immersed. This is repeated for 15 min., recording the times between and the total number of clot disruptions. The anesthetized mice are sacrificed following completion of the assay. Recombinant human FVIII (rFVIII) (Hexilate FS, CSL Behring LLC, King of Prussia, PA, USA), in 0.1 M NaCl, 0.02 M Hepes, 2 mg/mL bovine serum albumin, pH 7.4 (HSA), was administered at various concentrations to mice by retro-orbital infusion (2.5 uL/gm) 3 min. before testing. Plasma rFVIII levels following infusion were estimated assuming a murine plasma volume of 50 mL/kg. Male and female mice from 20.1 gm to 30.7 gm in size were used for the TVRB experiments.
Survival following tail transection.
The mouse is anesthetized short-term with isoflurane when its tail is transected 10 mm from the tip using a #11 scalpel. The mouse awakes in its cage and is monitored carefully for 16 hours. The investigator is blinded as to mouse genotype. Any mouse demonstrating substantial inactivity, hunched posture, ruffled hair, or inability to right itself was sacrificed under anesthesia and recorded as a non-survivor.
Proteins/plasmas.
PZ and FXa were from Enzyme Research Laboratories, South Bend, IN, USA. ZPI was isolated as previously described [7]. Anti-human PZ Mab2306.BF12.1 (anti-PZ Mab) was produced and purified using standard methods [15]. This IgG antibody recognizes an epitope in the C-terminal pseudo-catalytic domain of PZ [29]. Mouse tissue factor (mTF) was prepared as a saline extract of brain. An anesthetized male B6 mouse was perfused with 0.1 M NaCl, 0.02 M Hepes, pH 7.4 (HS), and the brain removed and rinsed with HS. Following homogenization in 5 mL HS (Tekmar Co., Mason, OH, USA), the mixture was allowed to settle in a test tube at 37°C for 10 min., the supernatant was harvested and stored frozen at −80°C in small aliquots. The mTF concentration in the preparation was 2.1 nM (Mouse Tissue Factor Elisa, Abcam, Cambridge, UK). A sample was thawed and briefly sonicated prior to use in thrombin generation assays. Mouse blood (360 uL) was drawn from the inferior vena cava of an anesthetized mouse into 40 uL of 3.8% sodium citrate; 20 uL of 1 mg/mL corn trypsin inhibitor (CTI, Enzyme Research Laboratories) was added; and plasma was obtained by centrifugation (5000 g x 15 min.) and stored at −80°C until used. Human hemophilia plasma with <1% FVIII activity was from George King Bio-Medical, Inc., Overland Park, KS, USA. The source of hTF was Dade Innovin (Siemens Healthcare, Tarrytown, NY, USA) and assumed to be at a concentration of 6 nM when prepared as recommended.
Anti-PZ Mab inhibition of PZ/ZPI inactivation of FXa.
The ability of anti-PZ Mab to inhibit the function of PZ was tested in a purified system as previously described [7]. A solution (30 uL) containing 32 nM PZ and 56 nM ZPI in HSA buffer (100 mM NaCl, 2 mg/mL bovine serum albumin, 20 mM Hepes, pH 7.2) was incubated at room temperature for 15 min. in wells of a microtiter plate to permit the PZ-ZPI complex to form [15]. Next, 20 uL of various concentrations of anti-PZ Mab in HSA was added. After a 30 min. incubation, 10 uL of rabbit brain cephalin (150 ug/mL, Pentapharm, Aesch, Switzerland) in HSA with 25 mM CaCl2 was added and then 20 uL FXa (10 nM) in HSA. After a 3-min. incubation, remaining FXa activity was determined in a kinetic assay by the addition of 20 uL chromogenic substrate CS11(65) (1.75 mM, Aniara, West Chester, OH, USA) and monitoring the change in A405 in a SpectraMax340 microtiter plate reader (Molecular Devices, San Jose, CA, USA). The extent of anti-PZ Mab blockade of PZ-dependent inhibition of FXa was determined from a PZ standard curve generated using reactions containing ZPI (56 nM) and variable concentrations of PZ (0 – 32 nM) in the assay in the absence of anti-PZ Mab.
Thrombin generation assay (TGA).
Assays were performed in a 96 well micro-titer plate in final volumes of 120 uL/well. Data were collected every 30 sec. for 90 min. in a Fluoroskan Ascent instrument (Thermo Electron Corp., Vantaa, Finland) set at 37°C. Data were analyzed using an in-house program developed as described [30].
Mouse Assay.
To 20 uL of plasma with CTI (collected as described above) was added 20 uL of HSA buffer containing no or serial 2-fold dilutions of rFVIII (starting at 2 U/mL). As trigger, 60 uL of 0.8 pM mTF containing 20 ug/mL rabbit brain cephalin in HSA was added. The reaction was started by adding 20 uL substrate solution [75 uM Z-GGR-AMC (Sigma-Aldrich, St. Louis, MO, USA), 120 mM CaCl2 in HSA].
Human Assay.
To human hemophilic plasmas was added 1/20th volume of 1 mg/mL CTI. To 30 uL of this plasma was added 30 uL of HSA alone, or with serial 4-fold dilutions of anti-PZ Mab (starting at 2 mg/mL) or with serial 2-fold dilutions of rFVIII (starting at 2 U/mL). Following a 30 min. incubation, 40 uL of 3 pM hTF containing 30 ug/mL rabbit brain cephalin in HSA was added and the reaction started by adding 20 uL of the substrate solution. A control IgG Mab did not affect the TGA in human plasma (data not shown).
Statistics.
One-way analysis of variance (ANOVA) with the Student-Newman-Keuls post-test was used for multiple comparisons and the two-sided Fisher’s Exact Test was used for paired comparisons.
RESULTS
Tail vein re-bleeding assay (TVRB).
The mouse has been used extensively to develop models of human disease. Tail tip transection with the measurement of bleeding time or blood loss has frequently been utilized as a method to assess hemostasis in these murine models and to determine the hemorrhagic effect of pharmaceutical agents. Due to the wide spread of results found with the tail transection method in both normal and gene-disrupted (e.g. hemophiliac) mice [31], a tail vein bleeding time (TVBT) model was introduced in 2001 in which laceration of a lateral tail vein, rather than tail transection, was used to induce bleeding [32]. The TVBT is sensitive to murine genetic background, aspirin and heparin treatment, αIIbβ3 deficiency [32], and other platelet and coagulation inhibitors [33,34], but not vascular smooth muscle cell tissue factor deficiency [35].
An important observation in the original TVBT report was that recurrence of hemorrhage consistently develops in factor VIII and factor IX deficient mice after the initial cessation of bleeding in the TVBT assay [32]. This re-bleeding phenomenon occurred predominantly when the mice awoke from the anesthetic, became active, and began attending to the tail wound. Recurrent bleeding was not detected in factor XI or carboxypeptidase B2 gene-deleted animals. Consequently, the incidence of re-bleeding, survival or delayed blood loss of hemophiliac mice following tail vein transection has been used to assess the efficacy of coagulation factor replacement products in murine preclinical studies [36–39].
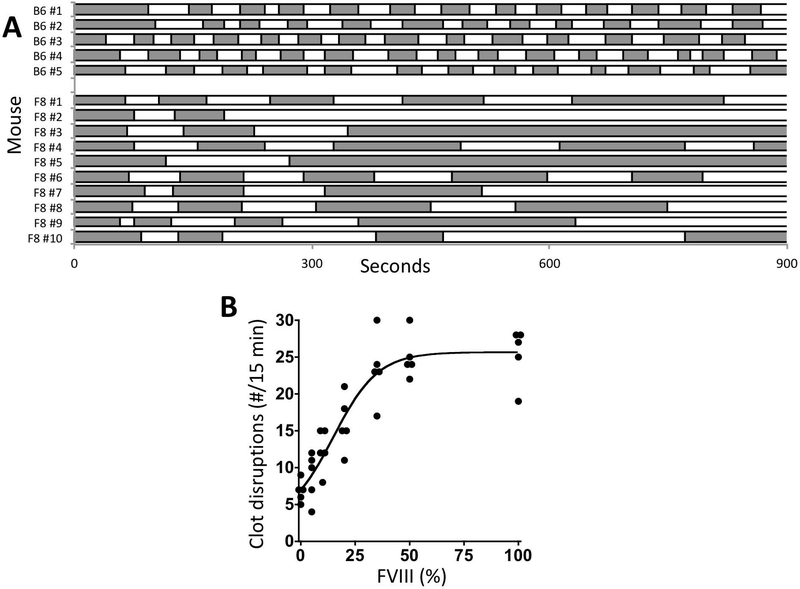
In 2008, Whinna developed a saphenous vein injury model to measure re-bleeding in the mouse [40]. Clots that formed at the wound site were serially disrupted over a 30 min. period and the total number of clot disruptions recorded [40,41]. In the current work, the serial disruption approach was applied to the original tail-vein bleeding time assay [32] to produce a tail vein re-bleeding assay (TVRB). In the TVRB, the number of clot disruptions (mean ± SEM) was 25.8 ± 0.7 for B6 control mice and 6.8±0.7 for FVIIIKO mice (Figure 1A). B6 control mice had relatively consistent bleeding times following each clot disruption. In contrast, bleeding times in FVIIIKO mice tended to prolong with successive disruptions and frequently bleeding simply failed to stop after a few disruptions. To validate the TVRB a FVIII dose-response experiment was performed in FVIIIKO mice and demonstrated a significant relationship (p<0.0001) between estimated plasma rFVIII levels and the number of disruptions in the TVRB (Figure 1B).
Figure 1. The abnormal TVRB in hemophilia mice is corrected by FVIII treatment.
A. Bleeding intervals in B6 and FVIIIKO mice differ in the TVRB. F8 refers to FVIIIKO mice. The changes in shading denote points of clot disruption. Note persistent bleeding following few disruptions in F8 mice #2, #3 and #5. B. Dose-response of rFVIII treatment in FVIIIKO mice in the TVRB. The number of clot disruptions in 15 min is plotted versus the estimated plasma rFVIII levels following infusion (p<0.0001). Statistical comparison of mouse groups treated to various FVIII levels versus FVIIIKO mice receiving the buffer control (0%): 5%, not significant; 10%, p<0.05; 20%, 35%, 50%, and 100%, p<0.001.
PZ or ZPI deletion improves hemostasis and plasma thrombin generation in hemophilia mice.
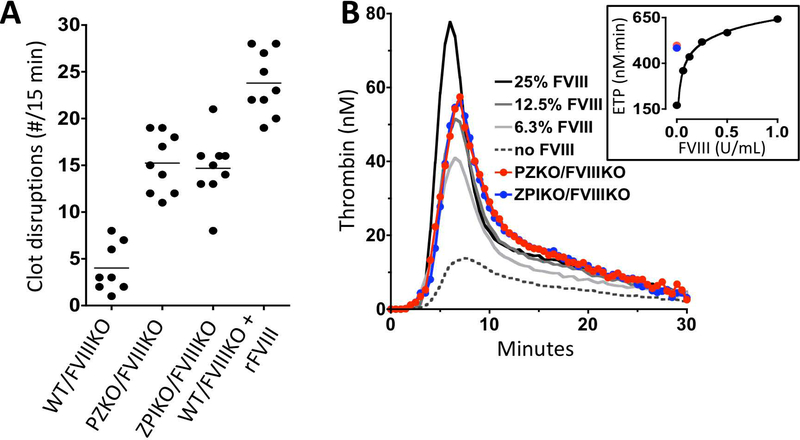
In the TVRB, the number of clot disruptions (mean ± SEM) for littermate mice were: WT/FVIIIKO 4.0 ± 0.9; PZKO/FVIIIKO 15.2 ± 1.1; ZPIKO/FVIIIKO 14.7 ± 1.2 and WT/FVIIIKO replaced with 100% FVIII 23.8 ± 1.1 (Figure 2A). The differences between all groups were statistically significant (p<0.001) except PZKO/FVIIIKO versus ZPIKO/FVIIIKO mice. The deletion of PZ or ZPI produced results in the TVRB similar to ~15% rFVIII in hemophilia mice (Figure 1B). To confirm the effect of PZ or ZPI deficiency on bleeding in hemophilia mice detected in the TVRB, the survival of WT/FVIIIKO (1/18) mice 16 hours following 10 mm tail transection was compared to that of PZKO/FVIIIKO mice (6/12, p = 0.009) and ZPIKO/FVIIIKO mice (12/22, p = 0.002).
Figure 2. PZ or ZPI deletion reduces bleeding in hemophilia mice.
A. Effects of PZ or ZPI gene-deletion in hemophilia mice on the TVRB. The number of clot disruptions in 15 min. are shown. All comparisons are significantly different (p<0.001) except that between the PZKO/FVIIIKO and ZPIKO/FVIIIKO animals. B. Effects of PZ or ZPI gene-deletion in FVIIIKO mice on thrombin generation. Shown are mouse TF-induced thrombin generation assays for PZKO/FVIIIKO mouse plasma (red), ZPIKO/FVIIIKO mouse plasma (blue), and WT/FVIIIKO mouse plasma supplemented with rFVIII to 0% (dotted line), 6% (light gray), 13% (dark gray), or 25% (black). The inset shows the endogenous thrombin potential (ETP) for the TGA assays; PZKO/FVIIIKO mouse plasma (red solid circle), ZPIKO/FVIIIKO mouse plasma (blue solid circle) and WT/FVIIIKO plasma supplemented with rFVIII where 1 U/mL = 100% FVIII.
Pooled plasma (n=5) from PZKO/FVIIIKO, ZPIKO/FVIIIKO, and WT/FVIIIKO mice and the latter supplemented with 0 – 100% rFVIII was evaluated using the thrombin generation assay (Figure 2B). Peak thrombin generation and the endogenous thrombin potential (ETP) in PZKO/FVIIIKO and ZPIKO/FVIIIKO plasmas produced results that were similar to 15% and 20% FVIII supplementation in WT/FVIIIKO plasma, respectively. These results are consistent with the in vivo effects of PZ and ZPI gene-deletion on the bleeding of FVIIIKO mice in the TVRB.
PZ inhibition improves thrombin generation in human hemophilia plasma.
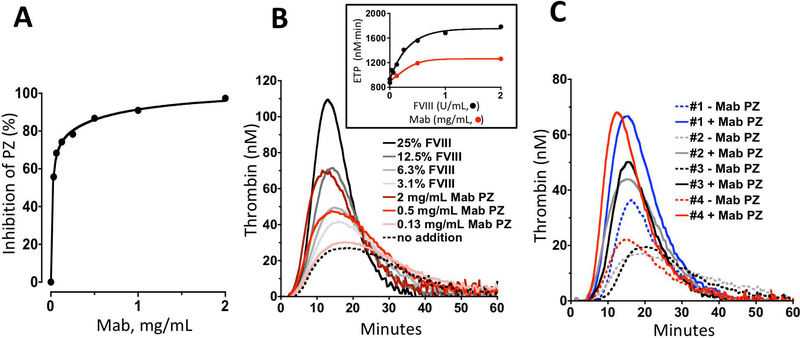
In the absence of a functional assay for PZ in plasma, the ability of the anti-PZ Mab to inhibit the inactivation of FXa by PZ/ZPI in the presence of phospholipids and calcium ions was tested in a system using purified components (Figure 3A). At 2 mg/mL the anti-PZ Mab produced 98% inhibition of PZ activity. The effect of the anti-PZ Mab was compared to various levels of FVIII supplementation in pooled hemophilia plasma (n=4) and the four individual hemophilia plasmas using the thrombin generation assay (Figures 3B and 3C). Under the test conditions, the anti-PZ Mab at 2 mg/mL increased the peak thrombin generation and the ETP to a degree equivalent to 12% and 20% rFVIII, respectively.
Figure 3. PZ inhibition enhances thrombin generation in human hemophilia plasma.
A. Effects of the anti-PZ Mab (2506.B12.1) on PZ-dependent, ZPI inhibition of FXa in a system using purified components. B. Effects of the anti-PZ Mab on pooled human hemophilic plasma thrombin generation. Shown are human TF-induced thrombin generation assays for pooled (n=4) human hemophilia plasma supplemented without (dotted line) or with anti-PZ Mab at 0.125 mg/mL (light red), 0.5 mg/mL (red) or 2.0 mg/mL (dark red) or with rFVIII at 3% (light gray), 6% (gray), 13% (dark gray) or 25% (black). The inset shows the ETP for the anti-PZ TGA data (gray circles) and FVIII replacement TGA data for supplementation with up to 200% rFVIII [1 U/mL=100% (black circles)]. C. Effects of anti-PZ Mab on TF-induced thrombin generation in the four individual human hemophilia plasmas: without (dotted lines) or with 2 mg/mL anti-PZ Mab (solid lines).
DISCUSSION
This work demonstrates that complete (by gene deletion) or near complete (by anti-PZ Mab treatment) suppression of the PZ/ZPI anticoagulant pathway, enhances coagulation in hemophilia A. The effect of extensive reduction in PZ/ZPI-dependent inhibition of FXa, however, is modest and limited, but nevertheless sufficient to significantly improve hemostasis in hemophilia mice. The results of the plasma thrombin generation assays in PZ and ZPI gene-deleted hemophilia mice and in human hemophilic plasma after PZ inhibition by the anti-PZ Mab were comparable to that produced by ~15% FVIII. It is not established, however, whether achieving the same enhancement in in vitro thrombin generation assays by distinct mechanisms (i.e. suppression of an anticoagulant pathway versus FVIII replacement) will necessarily translate into the same in vivo hemostatic effect. In this regard, however, it is notable that PZ or ZPI deletion in hemophilia mice was as effective as ~15% FVIII replacement in both the in vitro thrombin generation and the in vivo TVRB. A level of 15% FVIII is generally associated with a mild phenotype in hemophilia patients, though this relationship is not absolute [42,43].
The anti-PZ Mab used in our experiments recognizes an epitope in the C-terminal pseudo-catalytic domain of PZ [29]. This PZ domain plays a critical role in the tight binding (Kd=0.6 nM) of PZ to ZPI and its circulation in plasma in a complex with ZPI [6,9–11]. Thus, the low potency of this Mab likely in part reflects the requirement for it to compete with ZPI for PZ binding. Of the vitamin K-dependent coagulation proteins, PZ is unique in that it associates (and dissociates) much more slowly with phospholipid surfaces (Kd=170 nM) in the presence of Ca++ ions [44]. This slow interaction of PZ with phospholipids is rate limiting for both the interaction of PZ alone and the interaction of the PZ/ZPI complex with FXa on phospholipid surfaces [6,7]. Thus, an agent designed to interfere with the binding of PZ and the PZ/ZPI complex with phospholipid surfaces may be a considerably more potent inhibitor of PZ function than the monoclonal antibody used in our studies. An alterative approach, of course, would be through siRNA depression of PZ levels.
Clinical studies have not established low levels of PZ or ZPI as a thrombotic risk factor and PZ and ZPI gene-deleted mice (with normal levels of FVIII) are healthy. Nevertheless, prothrombotic effects of PZ and ZPI gene-deletion can be detected in certain mouse models with that of ZPI deficiency greater than that of PZ deficiency [27,28]. This presumably reflects the fact that ZPI directly inhibits FXIa as well as producing PZ-dependent inhibition of FXa [12,13]. Therefore, at least in regard to reducing the risk of thrombosis with FVIII replacement therapy for breakthrough bleeding in hemophilia, targeting PZ, rather than ZPI, for prophylactic therapy may be more appropriate.
In sum, these studies in mice and in human plasma suggest that the modest increase in coagulation produced by inhibition of the PZ/ZPI anticoagulant pathway may be sufficient to improve the phenotype of severe hemophilia A (and presumably hemophilia B). Additional preclinical studies in other animal hemophilia models and ultimately human clinical trials would be required to confirm the beneficial hemostatic effect seen in hemophilia mice. Clinical studies would also be required to determine whether the risk of thrombosis following FVIII rescue therapy for breakthrough bleeding in hemophilia patients receiving prophylactic treatment may be less with inhibition of the PZ/ZPI pathway than with inhibition of other natural anticoagulant pathways.
Essentials.
Protein Z (PZ) catalyzes PZ-dependent proteinase inhibitor (ZPI) inactivation of factor (F)Xa.
Gene-deletion of PZ or ZPI improves coagulation in hemophilia (FVIII knockout) mice.
A PZ blocking antibody enhances thrombin generation in human hemophilia plasma.
Suppression of the PZ/ZPI pathway may ameliorate the phenotype of severe hemophilia.
Acknowledgements
This work was supported by the National Institutes of Health grants HL077193 and HL112303 to G.J. Broze, Jr. and, in part, by a grant from Bayer Pharmaceuticals to T. Girard.
Footnotes
Addendum
T. Girard, N. Lasky, K. Grunz and G. J. Broze, Jr. did the experiments; T. Girard and G. J. Broze, Jr. wrote the manuscript.
Disclosure of Conflict of Interest
G. J. Broze, Jr. has served as a consultant for Bayer, Pfizer and Portola. The other author declare that they have no conflict of interest.
REFERENCES
- 1.Chowdary P, Lethagen S, Friedrich U, Brand B, Hay C, Abdul Karim F, Klamroth R, Knoebl P, Laffan M, Mahlangu J, Miesbach W, Dalsgaard Nielsen J, Martín-Salces M, Angchaisuksiri P. Safety and pharmacokinetics of anti-TFPI antibody (concizumab) in healthy volunteers and patients with hemophilia: a randomized first human dose trial. J Thromb Haemost 2015;13:743–54. [DOI] [PubMed] [Google Scholar]
- 2.Sehgal A, Barros S, Ivanciu L, Cooley B, Qin J, Racie T, Hettinger J, Carioto M, Jiang Y, Brodsky J, Prabhala H, Zhang X, Attarwala H, Hutabarat R, Foster D, Milstein S, Charisse K, Kuchimanchi S, Maier MA, Nechev L, et al. An RNAi therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophilia. Nat Med 2015;21:492–7. [DOI] [PubMed] [Google Scholar]
- 3.Polderdijk SG, Adams TE, Ivanciu L, Camire RM, Baglin TP, Huntington JA. Design and characterization of an APC-specific serpin for the treatment of hemophilia. Blood 2017;129:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince R, Bologna L, Manetti M, Melchiorre D, Rosa I, Dewarrat N, Suardi S, Amini P, Fernández JA, Burnier L, Quarroz C, Reina Caro MD, Matsumura Y, Kremer Hovinga JA, Griffin JH, Simon HU, Ibba-Manneschi L, Saller F, Calzavarini S, Angelillo-Scherrer A Targeting anticoagulant protein S to improve hemostasis in hemophilia. Blood 2018;131:1360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broze GJ Jr, Miletich JP. Human protein Z. J Clin Invest 1984;73:933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang X, Swanson R, Broze GJ Jr, Olson ST. Kinetic characterization of the protein Z-dependent protease inhibitor reaction with blood coagulation factor Xa. J Biol Chem 2008;283:29770–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Fiehler R, Broze GJ Jr. Isolation of a protein Z-dependent plasma protease inhibitor. Proc Natl Acad Sci USA 1998;95:9250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han X, Huang Z-F, Fiehler R, Broze GJ Jr. The protein Z-dependent protease inhibitor is a serpin. Biochemistry 1999;38:11073–8. [DOI] [PubMed] [Google Scholar]
- 9.Rezaie AR, Bae J-S, Manithody C, Qureshi SH, Yang L. Protein Z-dependent protease inhibitor binds to the C-terminal domain of protein Z. J Biol Chem 2008;283:19922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Z, Yan Y, Carrell RW, Zhou A. Crystal structure of protein Z-dependent inhibitor complex shows how protein Z functions as a cofactor in the membrane inhibition of factor X. Blood 2009;114:3662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Dementiev A, Olson ST, Gettings PG. Basis for the specificity and activation of the serpin protein Z-dependent proteinase inhibitor (ZPI) as an inhibitor of membrane-associated factor Xa. J Biol Chem 2010;285:20399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han X, Fiehler R, Broze GJ Jr. Characterization of the protein Z-dependent protease inhibitor. Blood 2000;96:3049–55. [PubMed] [Google Scholar]
- 13.Rezaie AR, Sun M-F, Gailani D. Contributions of basic amino acids in the autolysis loop of factor XIa to serpin specificity. Biochemistry 2006;45:9427–33. [DOI] [PubMed] [Google Scholar]
- 14.Al-Shanqeeti A, van Hycklama A, Berntorp E, Rosendaal FR, Broze GJ Jr. Protein Z and protein Z-dependent protease inhibitor: determinants of levels and risk of venous thrombosis. Thromb Haemost 2005;93:411–3. [DOI] [PubMed] [Google Scholar]
- 15.Tabatabai A, Fiehler R, Broze GJ Jr. Protein Z circulates in plasma in a complex with protein Z-dependent protease inhibitor. Thromb Haemost 2001;85: 655–60. [PubMed] [Google Scholar]
- 16.Refaai MA, Ahn C, Lu L, Wu K, Broze GJ Jr. Protein Z and ZPI levels and cardiovascular events. J Thromb Haemost 2006;4:1628–9. [DOI] [PubMed] [Google Scholar]
- 17.Corral J, Ganzalez-Conejero R, Hernandez-Espinosa D, Vicente V. Protein Z/Z-dependent protease inhibitor (PZ/ZPI) anticoagulant system and thrombosis. Brit J Haematol 2007;137:99–108. [DOI] [PubMed] [Google Scholar]
- 18.Tran HA, Eikelboom JW. Role of protein Z in stroke. Curr Treat Options Cardiovasc Med 2007;9:191–7. [DOI] [PubMed] [Google Scholar]
- 19.Bafunno V, Santacroce R, Margaglione M. The risk of occurrence of venous thrombosis: focus on protein Z. Thromb Res 2011;128:508–15. [DOI] [PubMed] [Google Scholar]
- 20.Huang ZF, Higuchi D, Lasky N, Broze GJ Jr. Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood 1997;90:944–51. [PubMed] [Google Scholar]
- 21.Ishiguro K, Kojima T, Kadomatsu K, Nakayama Y, Takagi A, Suzuki M, Takeda N, Ito M, Yamamoto K, Matsushita T, Kusugami K, Muramatsu T, Saito H. Complete antithrombin deficiency in mice results in embryonic lethality. J Clin Invest 2000;106:873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalbert LR, Rosen ED, Moons L, Chan JC, Carmeliet P, Collen D, Castellino FJ. Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest 1998;102:1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burstyn-Cohen T, Heeb MJ, Lemke G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J Clin Invest 2009;119:2942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saller F, Brisset AC, Tchaikovski SN, Azevedo M, Chrast R, Fernández JA, Schapira M, Hackeng TM, Griffin JH, Angelillo-Scherrer A. Generation and phenotypic analysis of protein S-deficient mice. Blood 2009;114:2307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Nielsen LV, Johansen PB, Hermit MB, Petersen LC, Mast AE. Absence of hematopoietic tissue factor pathway inhibitor mitigates bleeding in mice with hemophilia. Proc Natl Acad Sci USA 2012;109:3927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolliger D, Szlam F, Suzuki N, Matsushita T, Tanaka KA. Heterozygous antithrombin deficiency improves in vivo haemostasis in factor VIII-deficient mice. Thromb Haemost 2010;103:1233–8. [DOI] [PubMed] [Google Scholar]
- 27.Yin Z-F, Huang Z-F, Cui J, Fiehler R, Lasky N, Ginsburg D, Broze GJ Jr. Prothrombotic phenotype of protein Z deficiency. Proc Natl Acad Sci USA 2000;97:6734–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Tu Y, Lu L, Lasky N, Broze GJ Jr. Protein Z-dependent protease inhibitor deficiency produces a more severe phenotype than protein Z deficiency. Blood 2008;111:4973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X, Yan Y, Tu Y, Gatti J, Broze GJ Jr, Zhou A, Olson ST. Structural basis for catalytic activation of protein Z-dependent protease inhibitor (ZPI) by protein Z. Blood 2012;120:1726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemker HC, Kremers R. Data Management in Thrombin Generation. Thromb Res 2013;131:3–11. [DOI] [PubMed] [Google Scholar]
- 31.Schiviz A, Magirr D, Leidenmuhler P, Schuster M, Muchitsch E-M, Hollriegl W for the Subcommittee on Animal Models. Influence of genetic background on bleeding phenotype in the tail-tip bleeding model and recommendations for standardization: communication from the SSC of the ISTH. J Thromb Haemost 2014;12:1940–2. [DOI] [PubMed] [Google Scholar]
- 32.Broze GJ Jr, Yin Z-F, Lasky N. A tail vein bleeding time model and delayed bleeding in hemophiliac mice. Thromb Haemost 2001;85:747–8. [PubMed] [Google Scholar]
- 33.Ampofo E, Spater T, Muller I, Eichler H, Menger MD Laschke MW. The marine-derived kinase inhibitor fascaplysin exerts anti-thrombotic activity. Mar Drugs 2015;13:6774–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou W, Schwarting S, Illanes S, Liesz A, Middelhoff M, Zorn M, Bendszus M, Heiland S, van Ryn J, Veltkamp R. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke 2011;42:3594–9. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, and Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood 2009;113:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumont JA, Liu T, Low SC, Zhang X, Kamphaus G, Sakorafas P, Fraley C, Drager D, Reidy T, McCue J, Franck HWG, Merricks EP, Nichols TC, Bitonti AJ, Pierce GF, Jiang H. Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood 2012;119:3024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan J, Liu T, Kim J-Y, Zhu D, Patel C, Cui Z-H, Zhang X, Newgren JO, Reames A, Canivel D, Jesmok G, Pierce GF, Sommer JM, Jiang H. Enhanced efficacy of recombinant FVIII in noncovalent complex with PEGylated liposome in hemophilia A mice. Blood 2009;114:2802–11. [DOI] [PubMed] [Google Scholar]
- 38.Buyue Y, Liu T, Kulman JD, Toby GG, Kamphaus GD, Patarroyo-White S, Lu Q, Reidy TJ, Mei B, Jiang H, Pierce GF, Sommer JM, Peters RT. A single chain variant of factor VIII-Fc fusion protein retains normal in vivo efficacy but exhibits altered in vitro activity. PLoS One 2014;9:e113600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansen PB, Tranholm M, Haaning J, Knudsen T. Development of a tail vein transection bleeding model in fully anaesthetized haemophilia A mice—characterization of two novel FVIII molecules. Haemophilia 2016;22:625–31. [DOI] [PubMed] [Google Scholar]
- 40.Buyue Y, Whinna HC, Sheehan JP. The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood 2008;112:3234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pastoft AE, Lykkesfeldt J, Ezban M, Tranholm M, Whinna HC, Lauritzen B. A sensitive venous bleeding model in haemophilia A mice: effects of two recombinant FVIII products (N8 and Advate). Haemophilia 2012;18:782–8. [DOI] [PubMed] [Google Scholar]
- 42.Dargaud Y, Beguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, Hemker HC, Negrier C. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost 2005;93:475–80. [DOI] [PubMed] [Google Scholar]
- 43.Beltran-Miranda CP, Khan A, Jaloma-Cruz AR, Laffan MA. Thrombin generation and phenotype correlation in haemophilia A. Haemophilia 2005;11:326–34. [DOI] [PubMed] [Google Scholar]
- 44.McDonald JF, Shah AM, Schwalbe RA, Kisiel W, Dahlback B, Nelsesteun GL. Comparison of naturally occurring vitamin K-dependent proteins: Correlation of amino acid sequences and membrane binding properties suggests a membrane contact site. Biochemistry 1997;36:5120–7. [DOI] [PubMed] [Google Scholar]