Abstract
Background:
Intraoperative plasma transfusion is common, yet little is known regarding its effects on perioperative coagulation tests or clinical outcomes.
Study Design and Methods:
This is a retrospective cohort study of adults receiving intraoperative plasma transfusion at a single center from 2011–2015. Relationships between plasma transfusion volume, changes in coagulation test values, and clinical outcomes, including a primary outcome of early postoperative RBC transfusion, were assessed with multivariable regression analyses. Secondary outcomes included hospital mortality, ICU and hospital free days, intraoperative RBC transfusions, and estimated blood loss.
Results:
3,393 unique patients were included, with median (IQR) transfusion of 2 (2, 4) units. In multivariable analyses, higher plasma volumes were associated with worse outcomes, with each 1 ml/kg increase associated with increased odds for postoperative [1.02 (1.01, 1.03); p<.001] and intraoperative RBCs [1.17 (1.16, 1.19); p<.001], and fewer ICU and hospital free days [mean difference (95% CI) −0.08 (−0.12, −0.05); p < 0.001 and −0.09 (−0.13, −0.06); p < 0.001, respectively]. Greater decreases in INR following plasma transfusion were associated with decreased odds of postoperative RBCs [0.35 (0.25, 0.47); p<.001], decreased mortality [0.50 (0.31, 0.83); p=0.007], and increased mean ICU [1.31 (0.41, 2.21); p=0.004] and hospital free days [1.15 (0.19, 2.10); p=0.018].
Conclusion:
In patients receiving intraoperative plasma transfusion, higher transfusion volumes were associated with inferior clinical outcomes; however, greater improvements in INR were associated with improved outcomes. Future prospective studies are necessary to better define these relationships and to explore plasma transfusion triggers beyond the limitations of INR.
Keywords: Plasma, INR, surgery, coagulation, bleeding, transfusion
Introduction:
Plasma transfusion is commonly performed in surgical patients, with a previously reported intraoperative plasma transfusion rate of 2.5% for all surgical procedures at a large tertiary care hospital and rates in excess of 20% when restricted to those undergoing cardiac, transplant, or spine surgery.1 Commonly accepted triggers for intraoperative plasma transfusion include massive acute surgical hemorrhage, correction of coagulation factor deficiencies when specific concentrates are unavailable, and microvascular bleeding with derangements in coagulation test results.2 Regarding the latter, the American Society of Anesthesiologists (ASA) Practice Guidelines for Perioperative Blood Management from 2015 endorse the use of plasma for microvascular bleeding when the International Normalized Ratio (INR) is greater than 2.0.3 While the INR has long been recognized for its inadequacies in predicting bleeding risk, it remains a commonly utilized laboratory modality for assessing perioperative coagulation status as well as patient-specific responses to plasma transfusion. Additionally, more liberal plasma transfusion strategies (e.g. triggered by INR > 1.5) are frequently utilized in the perioperative period.4 Given the inadequacies in INR, many centers routinely employ point-of-care viscoelastic testing such as thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to better assess coagulation status perioperatively in high risk patients. In these assays, reaction (R) time or clotting time (CT) values, respectively, are commonly utilized as triggers for plasma transfusion, as they measure the latency period between the start of the test and initial fibrin formation.
Notably, there is limited evidence to guide intraoperative plasma transfusion practices. While the cessation of microvascular bleeding and prevention of dilutional coagulopathy may seem ideal endpoints, these outcomes are often difficult to detect in complex surgical cases requiring time-sensitive transfusion decisions. When plasma is transfused intraoperatively, its impact on coagulation test results (i.e. INR, R-values) and downstream clinical outcomes are unknown.
Given substantial equipoise surrounding the clinical effects of intraoperative plasma transfusion practices, the goals of this investigation were: 1) to assess the relationships between intraoperative plasma transfusion volume (dose) and clinical outcomes in a diverse cohort of surgical patients; and 2) to assess the relationships between plasma-associated changes in coagulation test results and clinical outcomes. We hypothesized that after adjustment for confounding, 1) higher volumes of intraoperative plasma would be associated with worse clinical outcomes; and 2) greater plasma-mediated decreases in INR and R values would be associated with superior clinical outcomes.
Materials & Methods
This is a retrospective, single-center, cohort study conducted with approval from the Mayo Clinic (Rochester, Minnesota) Institutional Review Board with waived written informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used in the design and conduct of this study, as well as in the reporting of results.5
Inclusion criteria were: 1) age > 18 years; 2) intraoperative plasma transfusion (defined as occurring in the time frame between surgical incision and closure) between January 1st 2011 and December 31st 2015; 3) a pre-transfusion INR value measured in the 24 hours prior to the first qualifying intraoperative plasma transfusion and; 4) a post-transfusion INR value measured in the 24 hour interval following the last qualifying intraoperative plasma transfusion. The post-transfusion INR value was defined as the first INR value occurring after the last unit of intraoperative plasma transfusion. Exclusion criteria included: 1) plasma utilized as part of therapeutic plasma exchange or apheresis; 2) a normal pre-transfusion INR (i.e. < 1.1); 3) lack of research authorization and; 4) prior inclusion in the study. For patients receiving intraoperative plasma transfusion during multiple surgical encounters during the study period, only the first intraoperative encounter was included.
Screening for potential study participants was performed using an institutional data warehouse called the OR DataMart, which captures data for all transfused patients at the study institution.6 In addition, this resource contains clinical and procedural data for all patients admitted to an acute care environment. Additional pertinent baseline characteristics were obtained from a second validated database, the Mayo Clinic Life Sciences System (MCLSS).7 Both databases have undergone extensive validation with accuracy superior to manual data collection alone.8
The primary exposure variable of interest was the volume of plasma transfused (aim 1), while changes in INR and R-time values (pre-transfusion – post-transfusion) were analyzed as intermediary variables associated with the plasma transfusion episode (aim 2). Additional potentially confounding variables of interest included demographic features, surgical characteristics (e.g. procedure type, procedure length, emergency surgery, estimated blood loss), clinical features [e.g. comorbid medical conditions, Charlson comorbidity scores, Sequential Organ Failure Assessment (SOFA) scores, ASA physical status], perioperative transfusions, and perioperative laboratory tests. SOFA scores were calculated for each patient by taking the worst organ system score in the 24 hour window before surgery, with missing values assumed to represent no deficits in that domain. TEG-derived R-time values were included if the pre-transfusion TEG was obtained within 1 hour of the first intraoperative plasma transfusion and the post-transfusion TEG was obtained within 6 hours following the last unit of intraoperative plasma. These timeframes were chosen rather than 24 hours because 1) TEG values at the study institution are typically drawn intraoperatively for the immediate guidance of transfusion decisions, while INR values may be drawn as baseline laboratory parameters or for the verification of anticoagulation status prior to a surgical insult; and 2) anesthesiologists at the study institution typically refrain from using point-of-care viscoelastic results for the guidance of transfusion decisions extending beyond 1 hour, and repeat results are generally obtained within the first several hours of transfusion. At the study institution, TEG values are used routinely for all patients undergoing liver transplant surgery and in patients with anticipated or perceived coagulopathy following separation from cardiopulmonary bypass. R-time values obtained after the first intraoperative plasma unit transfused but preceding additional units of intraoperative plasma were not included. Similarly, R-time values obtained after the last intraoperative plasma unit but without corresponding pre-transfusion values were not included.
The primary outcome of interest was the administration of one or more units of allogeneic RBCs in the first 24 postoperative hours, as RBC transfusion in this early postoperative period is typically reflective of ongoing surgical blood loss. Secondary outcomes included ICU admission rates, hospital mortality, ICU free days, and hospital free days. Free days were defined by subtracting the ICU or hospital length of stay in days from 28, with patients dying during the ICU or hospital stay receiving a score of zero. Patients with ICU or hospital lengths of stay greater than 28 days also received a score of zero. Of note, intraoperative RBC transfusions and estimated blood loss were utilized as covariates to account for the severity of the surgical insult rather than outcome variables in primary analyses. However, recognizing that these variables may represent clinically important outcomes for intraoperative plasma transfusion, sensitivity analyses were performed utilizing 1) intraoperative RBC transfusions occurring after the first plasma unit, and 2) estimated blood loss as outcome variables. In these instances, only pre-transfusion characteristics were utilized as covariates with explicit exclusion of intraoperative features to avoid cause-effect reversal (i.e. surgery length, intraoperative transfusions of platelets and cell-salvaged blood, intraoperative RBC transfusions given prior to plasma, intraoperative factor concentrate use).
Regarding the indications for intraoperative plasma transfusion at the study institution, plasma is administered at the discretion of the covering anesthesiologist (often with consultation of the surgical team), and no documented indication is required prior to administration. While there are no mandatory triggers or targets for plasma transfusion, providers are encouraged to refrain from plasma transfusion in the absence of ongoing microvascular blood loss when the INR is less than 2. For cardiac surgery, anesthesiologists follow a previously published algorithm utilizing an INR > 1.6 as a trigger for plasma transfusion in the presence of microvascular bleeding.9 Of note, if heparin resistance is suspected, antithrombin-III concentrate is used rather than plasma. Hence, the overwhelming majority of intraoperative plasma transfusions are provided for the prevention or treatment of active bleeding. Regarding RBC transfusion practices, no documented indication is required for intraoperative RBC transfusion, though anesthesiologists are encouraged to avoid transfusion for patients with hemoglobin values greater than 8 g/dL unless in the presence of marked ongoing blood loss and cardiovascular instability. Postoperatively, institutional transfusion guidelines state that RBC transfusion is indicated for hemoglobin < 8 g/dL in the presence of coronary artery disease, end-organ ischemia, acute brain injury, or cardiovascular instability related to anemia; hemoglobin < 7 g/dL in hemodynamically stable patients without bleeding; and at any hemoglobin level in the presence of hemorrhagic shock.
Statistical Analysis
The sample size was estimated for the primary outcome of aim 1 (i.e. postoperative RBC transfusion) by assuming a postoperative RBC transfusion rate of 25% in plasma-transfused patients (historical data from institution) and an odds ratio (OR) of 1.25 for this outcome in those transfused with less than 10 ml/kg of plasma (estimated to comprise 50% of the cohort) compared to those receiving greater than 10 ml/kg. Under these assumptions, an approximate sample size of 3,200 patients was required (80% power; 5% two-sided alpha).
Baseline demographics and intraoperative characteristics were summarized using frequencies and percentages for categorical variables and median and interquartile ranges (IQR) for continuous variables. Associations between the volume of plasma administered (ml/kg), pre-transfusion INR and R-values, and plasma-mediated changes in INR and R-values were examined using multivariable linear regression adjusted for the effects of variables selected a priori. These included 1) preoperative demographics (age, gender, body mass index); 2) preoperative laboratory values potentially indicative of severity of illness (creatinine, sodium, glucose, leukocyte count) or risk for bleeding (hemoglobin, platelet count, INR); 3) preoperative medications associated with alterations in hemostasis (aspirin or non-steroidal anti-inflammatory drugs within 7 days of the procedure, warfarin within 5 days of the procedure, direct oral anticoagulants within 5 days of the procedure, therapeutic heparin or low-molecular weight heparin within 24 hours of the procedure) or severity of illness (vasopressors or inotropes within 24 hours of the procedure, insulin infusion within 24 hours of the procedure); 4) preoperative platelet, plasma, and RBC transfusions within 24 hours of the procedure, which may indicate pre-existing coagulation abnormalities, anemia, or bleeding; 5) intraoperative transfusion volumes of platelets, allogeneic RBCs, and cell-salvaged blood, and total crystalloid and colloid volumes, which may indicate the magnitude of the surgical insult; 6) intraoperative factor concentrate administration (including prothrombin complex concentrates and single factor replacements), which may indicate rapid or marked surgical bleeding; 7) surgical features [surgery type, surgery length, anesthesia type (general, monitored anesthesia care, primary regional), emergency surgery, estimated blood loss]; and 8) patient comorbidities (preoperative Charlson and SOFA scores).
We used a multiple imputation approach with 10 independent imputed data sets to fill in missing values (sex 0.1%, age 0.4%, surgery time and sodium 1.3%, hemoglobin and ASA PS 1.4%, WBC 1.8%, creatinine and glucose 2.2%, anesthesia type 3.1%, plasma volume 3.4%, BMI 5.5%, and estimated blood loss 8.8%) prior to analysis.10,11 Associations between the predictors of interest and clinical outcomes were analyzed using multivariable logistic or linear regression as appropriate (adjusted for the same effects as noted above).
Multiple sensitivity analyses were planned a priori, including: 1) restriction to patients undergoing cardiac surgery; 2) restriction to those on preoperative warfarin therapy; and 3) restriction to those with INR values less than or equal to 1.5, greater than 1.5 and less than 2, and greater than or equal to 2. Additionally, we also performed pre-defined analyses stratified by high or low intraoperative RBC requirements, separated at a threshold of 3 units of allogeneic RBCs during the surgical encounter (> 3 units for high, < 3 units for low). All statistical analyses were performed using SAS version 9.4. All tests were 2-sided, and P < 0.05 was determined to be significant.
Results:
A total of 3,393 unique patients met inclusion criteria and received intraoperative plasma transfusion (Figure 1). Baseline demographic, clinical, and laboratory features for the cohort are displayed in Table 1. The majority (74%) of patients were transfused with INR values less than 2. The median (IQR) pre-transfusion and post-transfusion INR values were 1.7 (1.5, 2.0) and 1.4 (1.3, 1.5), respectively. The median time from pre-transfusion INR measurement to plasma transfusion was 0.9 (0.6, 2.2) hours, and the median time to last intraoperative plasma transfusion end to post-transfusion INR measurement was 1.2 (0.6, 1.8) hours. TEG values were available prior to plasma transfusion for 795 patients (23%) with 70% obtained during cardiac surgery, 18% obtained during transplant surgery, and 5% obtained during trauma surgery. The median pre-transfusion R-value was 7.3 (5.9, 10.0) minutes (ref: 4 – 8 minutes) with a median post-transfusion R-value of 6.7 (5.4, 8.3) minutes. The median number of plasma units transfused was 2 (2, 4), which differed by the extent of derangement in the pre-transfusion INR such that 45% of patients with an INR > 3 received 4 or more units of plasma, compared to 25% and 34% for patients with INR between 1.5 – 3 and < 1.5, respectively (p<0.001). Cardiac surgery accounted for the majority of patients receiving intraoperative plasma transfusion (63%), followed by transplant surgery (10%), general/trauma surgery (9%), and vascular surgery (6%). Approximately 68% of patients received RBCs (median 996 ml), 60% received platelets (median 335 ml), and 18% received cryoprecipitate (median 206 ml) intraoperatively. Regarding postoperative outcome event rates, 1,201 patients (35.4%) received an allogeneic RBC transfusion in the first 24 postoperative hours, with a median RBC transfusion volume of 2 (2, 4) units. 1,590 patients (46.9%) received an intraoperative RBC transfusion after the administration of at least one unit of plasma. A total of 203 patients (6.0%) died in the hospital.
Figure 1.
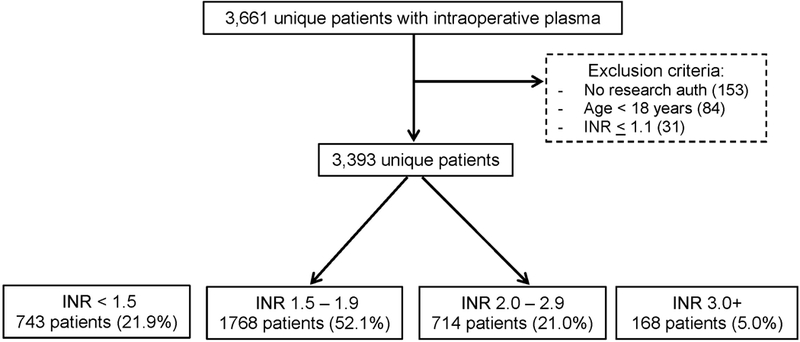
Study population flow diagram.
Table 1.
Demographic and clinical characteristics of patients receiving intraoperative plasma transfusion
| Characteristic | <10 ml/kg N=2013 |
10+ ml/kg N=1380 |
Total N=3393 |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 69 (59, 79) | 67 (56, 76) | 68 (58, 78) | <.001† |
| Male sex | 1360 (67.7%) | 841 (61.0%) | 2201 (65.0%) | <.001‡ |
| BMI (kg/m2) | 28.9 (25.3, 33.4) | 26.6 (23.3, 30.4) | 27.9 (24.4, 32.2) | <.001† |
| Laboratory values | ||||
| Pre-transfusion INR | 1.7 (1.5, 1.9) | 1.7 (1.4, 2.1) | 1.7 (1.5, 2.0) | <.001† |
| Pre-transfusion INR category | <.001‡ | |||
| <1.5 | 390 (19.4%) | 353 (25.6%) | 743 (21.9%) | |
| 1.5–3 | 1537 (76.4%) | 945 (68.5%) | 2482 (73.2%) | |
| 3+ | 86 (4.3%) | 82 (5.9%) | 168 (5.0%) | |
| Post-transfusion INR | 1.3 (1.2, 1.5) | 1.4 (1.3, 1.5) | 1.4 (1.3, 1.5) | 0.008† |
| INR Decrease (Pre – Post) | 0.3 (0.1, 0.5) | 0.4 (0.1, 0.7) | 0.3 (0.1, 0.5) | <.001† |
| Pre-transfusion R time (minutes; ref 4–8) | 7.2 (5.8, 9.5) | 7.8 (6.2, 10.3) | 7.4 (5.9, 10.0) | 0.003† |
| Post-transfusion R time (minutes; ref 4–8) | 6.4 (5.4, 7.9) | 6.9 (5.4, 8.9) | 6.7 (5.4, 8.3) | 0.05† |
| R time decrease (Pre – Post) | 0.5 (−0.9, 2.9) | 1.3 (−1.3, 3.9) | 0.8 (−1.0, 3.3) | 0.19† |
| Preoperative hemoglobin (g/dL) | 12.6 (10.6, 14.1) | 11.7 (9.7, 13.4) | 12.2 (10.2, 13.9) | <.001† |
| Preoperative creatinine (mg/dL) | 1.1 (0.9, 1.3) | 1.1 (0.8, 1.4) | 1.1 (0.9, 1.4) | 0.85† |
| Preoperative glucose (mg/dL) | 150 (126, 180) | 154 (122, 188) | 152 (124, 183) | 0.21† |
| Preoperative sodium (mEq/L) | 138 (136, 140) | 138 (135, 141) | 138 (135, 140) | 0.39† |
| Preoperative leukocyte count (x 109/L) | 7.0 (5.5, 9.4) | 6.6 (5.2, 8.9) | 6.9 (5.4, 9.2) | <.001† |
| Preoperative platelet count (x 109/L) | 187 (151, 238) | 181.0 (124.5, 235.0) | 185 (141, 237) | <.001† |
| Preoperative medications | ||||
| Clopidogrel within 14 days | 231 (11.5%) | 105 (7.6%) | 336 (9.9%) | <.001‡ |
| Aspirin within 7 days | 1054 (52.4%) | 713 (51.7%) | 1767 (52.1%) | 0.69‡ |
| NSAIDs within 7 days | 198 (9.8%) | 91 (6.6%) | 289 (8.5%) | <.001‡ |
| Insulin within 5 days | 968 (48.1%) | 723 (52.4%) | 1691 (49.8%) | 0.01‡ |
| Warfarin within 5 days | 547 (27.2%) | 399 (28.9%) | 946 (27.9%) | 0.27‡ |
| Factor Xa inhibitor within 5 days | 30 (1.5%) | 10 (0.7%) | 40 (1.2%) | 0.04‡ |
| Direct thrombin inhibitor within 5 days | 28 (1.4%) | 16 (1.2%) | 44 (1.3%) | 0.56‡ |
| Therapeutic unfractionated heparin within 1 day | 10 (0.5%) | 11 (0.8%) | 21 (0.6%) | 0.27‡ |
| Therapeutic LMW heparin within 1 day | 74 (3.7%) | 48 (3.5%) | 122 (3.6%) | 0.76‡ |
| Vitamin K within 1 day | 83 (4.1%) | 71 (5.1%) | 154 (4.5%) | 0.16‡ |
| Vasopressors or inotropes within 24 hours | 100 (5.0%) | 126 (9.1%) | 226 (6.7%) | <.001‡ |
| Intraoperative therapies | ||||
| Antibrinolytics | 1287 (63.9%) | 860 (62.3%) | 2147 (63.3%) | 0.34‡ |
| Vitamin K | 16 (0.8%) | 20 (1.4%) | 36 (1.1%) | 0.07‡ |
| Prothrombin complex concentrates | 7 (0.3%) | 49 (3.6%) | 56 (1.7%) | <.001‡ |
| Surgical characteristics | ||||
| Surgery Length (min) | 266 (189, 349) | 345 (251, 465) | 294 (213, 397) | <.001† |
| Surgery Type | <.001‡ | |||
| Cardiac | 1324 (65.8%) | 818 (59.3%) | 2142 (63.1%) | |
| General/Trauma | 207 (10.3%) | 83 (6.0%) | 290 (8.5%) | |
| Neuro/ENT | 22 (1.1%) | 13 (0.9%) | 35 (1.0%) | |
| OB/GYN/Urology | 44 (2.2%) | 29 (2.1%) | 73 (2.2%) | |
| Ortho | 49 (2.4%) | 30 (2.2%) | 79 (2.3%) | |
| Outfield | 105 (5.2%) | 22 (1.6%) | 127 (3.7%) | |
| Spine | 46 (2.3%) | 34 (2.5%) | 80 (2.4%) | |
| Thoracic | 22 (1.1%) | 19 (1.4%) | 41 (1.2%) | |
| Transplant | 98 (4.9%) | 228 (16.5%) | 326 (9.6%) | |
| Vascular | 96 (4.8%) | 104 (7.5%) | 200 (5.9%) | |
| Anesthesia Type* | <.001§ | |||
| General Anesthesia | 1917 (98.6%) | 1340 (99.8%) | 3257 (99.1%) | |
| Monitored Anesthesia Care | 28 (1.4%) | 3 (0.2%) | 31 (0.9%) | |
| ASA PS | 3 (3, 4) | 3 (3, 4) | 3 (3, 4) | <.001† |
| Emergency Procedure | 287 (14.3%) | 310 (22.5%) | 597 (17.6%) | <.001‡ |
| Intraoperative EBL (ml) | 903.5 (100.0, 1600.0) | 1584 (600, 3244) | 1092.0 (125.5, 2000.0) | <.001† |
| Intraoperative transfusion characteristics | ||||
| Number of plasma units given | 2 (2, 2) | 4 (2, 4) | 2 (2, 4) | <.001† |
| Number of plasma units given | <.001‡ | |||
| 1 | 298 (14.8%) | 0 (0.0%) | 298 (8.8%) | |
| 2 | 1335 (66.3%) | 338 (24.5%) | 1673 (49.3%) | |
| 3 | 240 (11.9%) | 223 (16.2%) | 463 (13.6%) | |
| 4+ | 140 (7.0%) | 819 (59.3%) | 959 (28.3%) | |
| Plasma volume (ml) | 550.0 (411.7, 589.0) | 1276.1 (1060.0, 2026.3) | 618 (541, 1129) | <.001† |
| Plasma dose (ml/kg) | 6.2 (4.7, 7.7) | 16.6 (12.6, 25.7) | 8.6 (5.9, 14.7) | <.001† |
| RBCs | 1113 (55.3%) | 1204 (87.2%) | 2317 (68.3%) | <.001‡ |
| RBC Volume (ml) | 663.0 (363.6, 1320.0) | 1574.9 (663.0, 2674.0) | 996 (660, 1980) | <.001† |
| Platelets | 948 (47.1%) | 1074 (77.8%) | 2022 (59.6%) | <.001‡ |
| Platelet volume (ml) | 289.0 (224.0, 408.5) | 524 (291, 819) | 335 (266, 590) | <.001† |
| Cryoprecipitate | 145 (7.2%) | 472 (34.2%) | 617 (18.2%) | <.001‡ |
| Cryoprecipitate volume (ml) | 198 (110, 212) | 208.0 (188.5, 390.5) | 206 (182, 290) | <.001† |
| Cell Saver | 1473 (73.2%) | 1117 (80.9%) | 2590 (76.3%) | <.001‡ |
| Cell saver volume (ml) | 610 (402, 856) | 953.4 (574.0, 1672.0) | 702.6 (458.3, 1134.9) | <.001† |
| Crystalloid volume (ml) | 5201 (3884, 6982) | 8905 (6097, 13658) | 6194 (4438, 9600) | <.001† |
| Colloid volume (ml) | 2246 (1412, 3320) | 4933 (3161, 7849) | 2994 (1795, 4915) | <.001† |
| Patient comorbidities | ||||
| Myocardial infarction | 161 (8.0%) | 117 (8.5%) | 278 (8.2%) | 0.62‡ |
| Congestive heart failure | 277 (13.8%) | 203 (14.7%) | 480 (14.1%) | 0.44‡ |
| Peripheral vascular disease | 69 (3.4%) | 48 (3.5%) | 117 (3.4%) | 0.94‡ |
| Dementia | 3 (0.1%) | 2 (0.1%) | 5 (0.1%) | 1.00§ |
| Cerebrovascular disease | 170 (8.4%) | 92 (6.7%) | 262 (7.7%) | 0.06‡ |
| Chronic obstructive lung disease | 139 (6.9%) | 92 (6.7%) | 231 (6.8%) | 0.79‡ |
| Asthma | 108 (5.4%) | 65 (4.7%) | 173 (5.1%) | 0.39‡ |
| Interstitial lung disease | 26 (1.3%) | 22 (1.6%) | 48 (1.4%) | 0.46‡ |
| Chronic pulmonary disease | 252 (12.5%) | 163 (11.8%) | 415 (12.2%) | 0.54‡ |
| Connective tissue disease | 56 (2.8%) | 34 (2.5%) | 90 (2.7%) | 0.57‡ |
| Diabetes mellitus | 81 (4.0%) | 35 (2.5%) | 116 (3.4%) | 0.05‡ |
| Peptic ulcer disease | 54 (2.7%) | 44 (3.2%) | 98 (2.9%) | 0.39‡ |
| Hemiplegia/paraplegia | 5 (0.2%) | 5 (0.4%) | 10 (0.3%) | 0.55‡ |
| Moderate to severe kidney disease | 257 (12.8%) | 204 (14.8%) | 461 (13.6%) | 0.09‡ |
| Solid organ malignancy | 430 (21.4%) | 278 (20.1%) | 708 (20.9%) | 0.39‡ |
| Solid organ malignancy with metastases | 65 (3.2%) | 48 (3.5%) | 113 (3.3%) | 0.69‡ |
| Leukemia | 4 (0.2%) | 6 (0.4%) | 10 (0.3%) | 0.33§ |
| Lymphoma | 46 (2.3%) | 28 (2.0%) | 74 (2.2%) | 0.62‡ |
| Moderate to severe liver disease | 60 (3.0%) | 99 (7.2%) | 159 (4.7%) | <.001‡ |
| AIDS | 0 (0.0%) | 1 (0.1%) | 1 (0.0%) | 0.41§ |
| Preoperative Charlson score | 5 (3, 7) | 5 (3, 7) | 5 (3, 7) | 0.45† |
| Preoperative SOFA score | 4 (2, 5) | 5 (3, 7) | 4 (2, 6) | <.001† |
Numbers indicate N (%) and median (Q1, Q3).
AIDS – acquired immunodeficiency syndrome; ASA PS – American Society of Anesthesiologists Physical Status; BMI – body mass index (kg/m2); EBL – estimated blood loss (ml); ENT – otorhinolaryngology; INR – International Normalized Ratio; LMW – low molecular weight; NSAIDs – non-steroidal anti-inflammatory drugs; OB/GYN – obstetric and gynecology; RBC – red blood cell; SOFA – sequential organ failure assessment.
Wilcoxon
Chi-square
Fisher exact
Missing anesthesia type for 105 patients.
There were significant differences in the magnitude of INR correction based upon the severity of pre-transfusion INR abnormality, such that patients with higher pre-transfusion INR values had more substantial reductions in the INR following plasma administration in multivariable analyses, even with adjustment for the volume of plasma transfused. Every 100 ml increase in plasma resulted in a decrease in INR by 0.26 (95% CI 0.17–0.35; p<0.001). Changes in INR values based upon the severity of pre-transfusion INR are displayed graphically in Figure 2. Of note, patients with pre-transfusion INR values less than 1.5 had no change in median post-transfusion INR values following transfusion. Plasma volume (per 100 ml) was not associated with changes in R-values [mean change (95% CI) 0.31 minutes (−4.45, 5.08); p=0.889].
Figure 2.
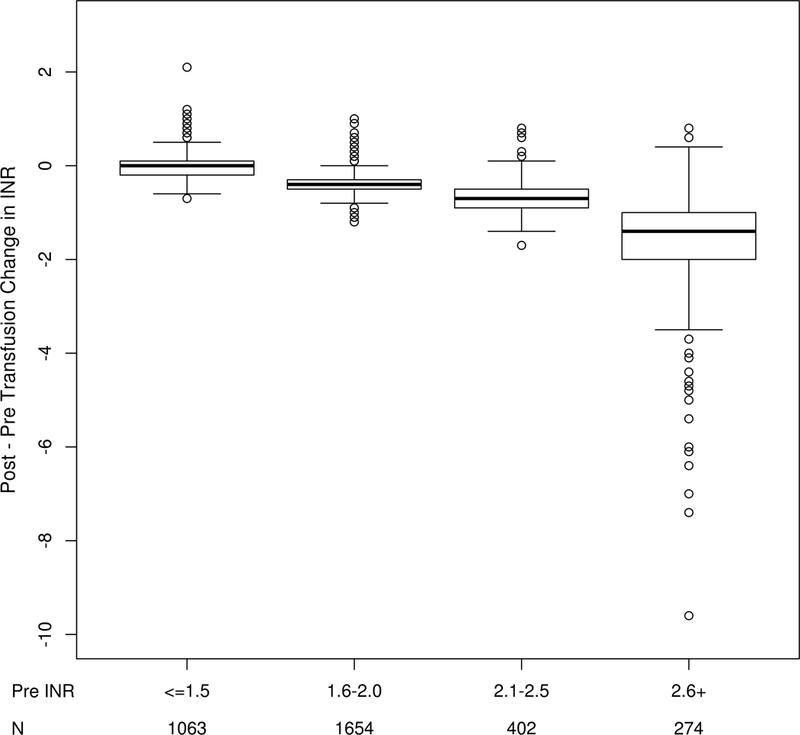
Changes in INR by severity of pre-transfusion INR.
Aim 1. Volume of Plasma Administered & Clinical Outcomes
After adjusting for potentially confounding variables, each ml/kg increase in the volume of plasma administered was associated with an increased odds for RBC transfusion in the 24 hours after surgery [OR (95% CI) 1.02 (1.01, 1.04); p<.001; Table 2]. Increased plasma volumes were also significantly associated with fewer ICU and hospital free days [mean difference (95% CI) −0.08 (−0.12, −0.05); p < 0.001 and −0.09 (−0.13, −0.06); p < 0.001, respectively], greater incidence of intraoperative allogeneic RBC transfusion following the first dose of plasma [OR 1.17 (1.16, 1.19); p<.001], higher intraoperative RBC volumes after the first dose of plasma [mean difference 0.20 (0.20, 0.21) units; p<.001], and greater estimated blood loss [73 (69, 78) ml; p<.001]. Plasma dose (per ml/kg) was not associated with hospital mortality or postoperative ICU admission, as 94.9% of all patients were admitted to the ICU postoperatively. Minimally-adjusted models for the outcomes of interest are shown in supplemental table 1.
Table 2.
Relationships between plasma transfusion characteristics, coagulation features, and clinical outcomes in multivariable analyses
| Characteristic | Postoperative RBCs OR (95% CI)† |
Hospital Mortality OR (95% CI)† |
ICU Free Days Mean (95% CI)‡ |
Hospital Free Days Mean (95% CI)‡ |
Intraoperative RBCs§ OR (95% CI)† |
Intraop RBC Units§ Mean (95% CI)‡ |
EBL (ml)§ Mean (95% CI)‡ |
|
|---|---|---|---|---|---|---|---|---|
| Plasma dose†† | 1.02 (1.01, 1.03)*** | 1.01 (1.00, 1.03) | −0.08 (−0.12, −0.05)*** | −0.09 (−0.13, −0.06)*** | 1.17 (1.16, 1.19)*** | 0.20 (0.20, 0.21)*** | 73 (69, 78)*** | |
| Pre-transfusion INR†† | 2.46 (1.86, 3.25)*** | 1.98 (1.25, 3.13) ** | −0.90 (−1.72, −0.08) * | −0.32 (−1.19, 0.55) | 0.79 (0.58, 1.07) | −0.11 (−0.39, 0.17) | 54 (−152, 259) | |
| Pre-Post INR†† | 0.35 (0.25, 0.47)*** | 0.50 (0.31, 0.83) ** | 1.31 (0.41, 2.21) ** | 1.15 (0.19, 2.10) * | 0.76 (0.54, 1.07) | −0.41 (−0.71, −0.10) ** | −177 (−396, 41) | |
| Pre-transfusion R‡‡ | 1.01 (0.97, 1.05) | 1.05 (0.98, 1.12)^ | −0.18 (−0.31, −0.06)* | −0.16 (−0.28, −0.03) * | 0.99 (0.94, 1.03) | −0.03 (−0.08, 0.01) | −20 (−48, 9) | |
| Pre-Post R‡‡ | 0.99 (0.96, 1.02) | 0.99 (0.93, 1.05)^ | 0.05 (−0.05, 0.15) | 0.05 (−0.05, 0.14) | 1.00 (0.97, 1.04) | 0.03 (−0.01, 0.07) | 20 (−2, 43) |
Plasma dose is per 1 ml/kg.
Regression models include row covariate plus preoperative demographics (age, gender, body mass index), pre-operative laboratory values (creatinine, sodium, glucose, leukocyte count, hemoglobin, platelet count), preoperative medications (aspirin, clopidogrel, warfarin, NSAIDS, heparin or low-molecular weight heparin, vasopressors, inotropes within 24 hours of the procedure, insulin), preoperative platelet, plasma, and RBC transfusions, intraoperative transfusion volumes (plasma, platelets, allogeneic RBCs, cell-salvaged blood), total intraoperative crystalloid and colloid volumes, intraoperative factor concentrate administration (including prothrombin complex concentrates and single factor replacements), surgical features [surgery type, surgery length, anesthesia type (general, monitored anesthesia care, primary regional), emergency surgery, estimated blood loss], and patient comorbidities (preoperative Charlson score, preoperative SOFA scores).
P < 0.05
P < 0.01
P < 0.001
Analyzed using multivariable logistic regression.
Analyzed using multivariable linear regression.
Only preoperative characteristics were included when analyzing the outcomes of intraoperative RBCs, intraoperative RBC units, and estimated blood loss.
Regression models additionally include plasma dose (per ml/kg), pre-transfusion INR, and decrease in INR.
Regression models additionally include plasma dose (per ml/kg), pre-transfusion R, and decrease in R.
Minimally-adjusted models utilized for hospital mortality data for R-time analyses (rather than fully-adjusted models) given only 25 mortality events for 627 eligible patients. Models included pre-transfusion R-value, change in R value after transfusion, and plasma dose.
Aim 2. Perioperative Coagulation Test Values & Clinical Outcomes
Pre-transfusion INR elevations were associated with increased odds of receiving postoperative allogeneic RBCs [2.46 (1.68, 3.25); p<.001; Table 2], increased hospital mortality [1.98 (1.25, 3.13); p=0.003], and fewer ICU [−0.90 (−1.72, −0.08); p=0.032] free days. There were no associations between pre-transfusion INR values and hospital free days, intraoperative RBC transfusion rates, RBC transfusion volumes, or estimated blood loss. Greater decreases in INR after plasma transfusion were associated with superior outcomes, including decreased odds of postoperative RBCs [OR=0.35 (0.25, 0.47); p<.001], decreased hospital mortality [OR=0.50 (0.31, 0.83); p=0.007], increased mean ICU [1.31 (0.41, 2.21); p=0.004] and hospital free days [1.15 (0.19, 2.10); p=0.018] and decreased mean intraoperative RBC units [−0.41 (−0.71, −0.10); p=0.009]. Pre-transfusion R-values and changes following plasma transfusion are also presented in Table 2. There were no significant associations between either measure and clinical outcomes except for a reduction in ICU free days with higher pre-transfusion R-times.
Sensitivity analyses
Analyses limited to cardiac surgery patients are displayed in Supplemental Table 2. Of the 2,142 patients, 711(33.2%) received postoperative RBCs, 896 (41.8%) received intraoperative RBCs following the first unit of plasma, and 114 (5.3%) patients died. Briefly, higher volumes of plasma administered intraoperatively were associated with higher postoperative RBC transfusion rates, fewer ICU and hospital free days, increased intraoperative RBC transfusion rates and higher RBC volumes following plasma transfusion, and greater estimated blood loss. Higher pre-transfusion INR values were associated with greater propensity for postoperative RBC transfusion and mortality, but not with intraoperative RBC transfusions or estimated blood loss. Greater decreases in INR following plasma transfusion were associated with decreased odds for intraoperative and postoperative RBC transfusion, decreased mortality, and more ICU and hospital free days.
A total of 946 patients (27.9%) received warfarin within 5 days of surgery. Of these, 316 (33.4%) received postoperative RBCs, 409 (43.2%) received intraoperative RBCs following plasma transfusion, and 40 (4.2%) patients died. Similar to the entire cohort, plasma transfusion volume was associated with fewer ICU and hospital free days, increased incidence and volume of intraoperative RBCs, and greater estimated blood loss (Supplemental Table 3). Higher preoperative INR values in patients on warfarin were not associated with the incidence of intraoperative or postoperative RBCs, though greater decreases in INR following plasma transfusion were associated a modest reduction in intraoperative RBC volume in this group [mean difference −0.56; 95% CI (−1.08, −0.05) units; p=0.032].
Outcomes by severity of pre-transfusion INR values (grouped as < 1.5, 1.5–2, and > 2) are displayed in Table 3. Briefly, the associations observed with plasma volume and the primary and secondary outcomes were largely consistent across preoperative INR groups, such that higher plasma volumes were associated with worse clinical outcomes including increased odds for intraoperative RBCs and higher estimated blood loss. Higher plasma volumes were associated with increased odds for postoperative RBCs in those with pre-transfusion INR values less than 2 but not in those with higher pre-transfusion INR values.
Table 3.
Associations with primary and secondary outcomes using multivariable regression stratified by preoperative INR
| Characteristic | Postoperative RBCs Odds Ratio (95% CI)† |
Hospital Mortality Odds Ratio (95% CI)† |
ICU Free Days Mean (95% CI)‡ |
Hospital Free Days Mean (95% CI)‡ |
Intraoperative RBCs§ Odds Ratio (95% CI)† |
Intraop RBC Units§ Mean (95% CI)‡ |
EBL (ml)§ Mean (95% CI)‡ |
|---|---|---|---|---|---|---|---|
| INR < 1.5 (n=743) | |||||||
| Plasma dose†† | 1.03 (1.01, 1.05) * | 1.03 (0.99, 1.07) | −0.10 (−0.17, −0.03)** | −0.17 (−0.24, −0.09)*** | 1.14 (1.11, 1.17)*** | 0.27 (0.25, 0.28)*** | 74 (65, 82)*** |
| Pre-transfusion INR†† | 5.30 (1.47, 19.08)* | 1.09 (0.09, 13.84) | −1.32 (−5.38, 2.73) | −1.43 (−5.73, 2.87) | 0.31 (0.08, 1.23) | 0.35 (−1.47, 2.17) | 152 (−798, 1102) |
| Pre-Post INR†† | 0.24 (0.11, 0.53)*** | 0.25 (0.07, 0.89)* | 5.33 (2.92, 7.75)*** | 5.65 (3.09, 8.21)*** | 0.35 (0.15, 0.82) * | −1.49 (−2.55, −0.43) ** | −758 (−1343, −173) * |
| Pre-transfusion R‡‡ | - | - | −0.22 (−0.45, 0.00) | −0.23 (−0.48, 0.02) | 1.11 (0.98, 1.25) | −0.02 (−0.14, 0.11) | −15 (−67, 37) |
| Pre-Post R‡‡ | - | - | −0.02 (−0.16, 0.11) | −0.02 (−0.17, 0.13) | 0.98 (0.92, 1.04) | 0.03 (−0.05, 0.11) | −7 (−38, 24) |
| 1.5 ≤ INR < 2 (n=1768) | |||||||
| Plasma dose†† | 1.03 (1.01, 1.05)*** | 1.01 (0.98, 1.04) | −0.08 (−0.13, −0.04)*** | −0.09 (−0.14, −0.04)*** | 1.18 (1.15, 1.21)*** | 0.18 (0.17, 0.19)*** | 69 (62, 76)*** |
| Pre-transfusion INR†† | 7.48 (2.79, 20.06)*** | 10.00 (1.41, 70.76)* | −1.58 (−4.00, 0.85) | −2.44 (−5.05, 0.18) | 1.02 (0.38, 2.72) | −0.05 (−0.73, 0.64) | 235 (−291, 760) |
| Pre-Post INR†† | 0.06 (0.03, 0.13)*** | 0.14 (0.04, 0.44)*** | 3.25 (1.51, 4.99)*** | 3.62 (1.74, 5.50)*** | 0.50 (0.25, 1.00) | −0.62 (−1.09, −0.15) * | −739 (−1103, −376)*** |
| Pre-transfusion R‡‡ | 1.01 (0.93, 1.10) | 0.91 (0.60, 1.38) | −0.08 (−0.30, 0.15) | −0.12 (−0.36, 0.13) | 0.96 (0.88, 1.05) | −0.06 (−0.12, −0.00) * | −37 (−86, 12) |
| Pre-Post INR‡‡ | 0.99 (0.93, 1.04) | 1.22 (0.82, 1.82) | −0.02 (−0.17, 0.13) | 0.06 (−0.10, 0.22) | 1.02 (0.96, 1.08) | 0.04 (−0.00, 0.08) | 47 (13, 80) ** |
| INR ≥ 2 (n=882) | |||||||
| Plasma dose†† | 1.02 (1.00, 1.04) | 1.01 (0.98, 1.04) | −0.08 (−0.14, −0.01) * | −0.04 (−0.10, 0.03) | 1.18 (1.15, 1.21)*** | 0.16 (0.15, 0.17)*** | 70 (61, 78)*** |
| Pre-transfusion INR†† | 1.78 (1.14, 2.80) * | 1.09 (0.49, 2.45) | 0.22 (−1.17, 1.60) | 1.22 (−0.20, 2.64) | 0.79 (0.48, 1.30) | −0.20 (−0.59, 0.19) | −214 (−550, 123) |
| Pre-Post INR†† | 0.51 (0.32, 0.81) ** | 0.87 (0.38, 1.99) | −0.27 (−1.67, 1.12) | −0.86 (−2.29, 0.56) | 1.12 (0.67, 1.88) | 0.02 (−0.38, 0.42) | 178 (−159, 514) |
| Pre-transfusion R‡‡ | 1.01 (0.89, 1.15) | - | −0.29 (−0.67, 0.09) | −0.27 (−0.61, 0.07) | 0.91 (0.78, 1.06) | −0.10 (−0.26, 0.06) | −59 (−167, 49) |
| Pre-Post R‡‡ | 1.00 (0.88, 1.13) | - | 0.21 (−0.16, 0.58) | 0.19 (−0.13, 0.51) | 1.08 (0.93, 1.26) | 0.11 (−0.04, 0.26) | 58 (−45, 162) |
Plasma dose is per 1 ml/kg. Change in INR and change in R-values were calculated as pre-transfusion – post-transfusion values.
Regression models include row covariate plus preoperative demographics (age, gender, body mass index), pre-operative laboratory values (creatinine, sodium, glucose, leukocyte count, hemoglobin, platelet count), preoperative medications (aspirin, clopidogrel, warfarin, NSAIDs, heparin or low-molecular weight heparin, vasopressors, inotropes within 24 hours of the procedure, insulin), preoperative platelet, plasma, and RBC transfusions, intraoperative transfusion volumes (plasma, platelets, allogeneic RBCs, cell-salvaged blood), total intraoperative crystalloid and colloid volumes, intraoperative factor concentrate administration (including prothrombin complex concentrates and single factor replacements), surgical features [surgery type, surgery length, anesthesia type (general, monitored anesthesia care, primary regional), emergency surgery, estimated blood loss], patient comorbidities (preoperative Charlson score, preoperative SOFA scores).
P < 0.05
P < 0.01
P < 0.001
Unable to analyze due to small event counts.
Analyzed using multivariable logistic regression.
Analyzed using multivariable linear regression.
Only preoperative characteristics were included when analyzing the outcomes of intraoperative RBCs, intraoperative RBC units, and estimated blood loss.
Regressions models additionally include plasma dose (per ml/kg), preoperative INR, and decrease in INR.
Regressions models additionally include plasma dose (per ml/kg), preoperative R, and decrease in R.
Analyses based upon the quantity of intraoperative allogeneic RBCs, divided at a quantity of 3 units, are shown in Supplemental Table 4. A total of 1,373 patients received 3 or more units of intraoperative RBCs. In this group, 627 (45.7%) received postoperative RBCs, 1048 (76.3%) received intraoperative RBCs after the first unit of plasma, and 21 (7.3%) patients died in the hospital. A total of 2,020 patients received less than 3 units of intraoperative RBCs. Among these, 574 (28.4%) received postoperative RBCs, 542 (26.8%) received intraoperative RBCs following plasma transfusion, and 4 (1.2%) patients died. In both groups, higher plasma volumes were associated with increased incidence of postoperative RBC transfusion, fewer ICU and hospital free days, greater incidence and volume of intraoperative RBCs, and higher estimated blood loss. In those receiving less than 3 units of intraoperative RBCs, higher plasma volumes were also associated with increased mortality, but this was not observed in those receiving 3 or more units of RBCs. Greater decreases in INR were associated with decreased risk for postoperative RBCs in both groups.
Discussion
In this cohort of more than 3,000 patients receiving intraoperative plasma transfusion, increased doses of plasma were associated with greater decrements in post-transfusion INR but not with improved clinical outcomes. On the contrary, higher plasma volumes were associated with increased odds for intraoperative and postoperative RBC transfusion, higher mortality, and fewer hospital and ICU free days, even with careful adjustment for the severity of coagulation abnormalities, patient comorbidities, and the severity of the surgical insult.
While it has long been recognized that higher doses of plasma result in greater changes in INR,12–14 there has been no objective evidence that this results in decreased bleeding or improved clinical outcomes in surgical patients. In this analysis, patients receiving higher doses of plasma indeed achieved greater improvement in the INR. However, higher doses of plasma did not translate to improvement in postoperative outcomes even with careful adjustment for important patient and procedure-related factors. This finding is quite distinct from what has been observed in patients with acute trauma, in which early plasma transfusion as part of a damage control resuscitation strategy has been associated with improve patient outcomes.15,16 While the precise circumstances surrounding each intraoperative transfusion episode were not available, there are plausible explanations for the lack of perceived benefit in this cohort. First, distinct from the patient with acute traumatic hemorrhage, some surgical patients likely receive plasma in reaction to abnormal coagulation test values in the absence of microvascular bleeding. As has been shown previously, the INR by itself is a poor marker of bleeding risk in non-hemorrhaging patients.17–19 In this study, higher pre-transfusion INR values were associated with increased rates of postoperative RBC transfusion but not with intraoperative RBC transfusion or estimated blood loss. In warfarin treated patients specifically, the pre-transfusion INR was not associated with clinical outcomes. Additionally, clotting factors may remain at sufficient levels until the INR is greater than 2.5,20 which falls beyond typical transfusion thresholds encountered in this study. Hence, rather than correcting any perceived abnormality in hemostatic balance, higher doses of plasma may have inadvertently been associated with hemodilution, potentially pushing hemoglobin values beyond RBC transfusion thresholds. Moreover, when plasma was given in response to ongoing surgical blood loss, it is unclear if the timing of plasma administration (early vs. late in response to hemorrhage) was associated with clinical outcomes. This may represent another explanation for the observed discrepancy between these findings and those from previous studies in acute trauma. Regarding plasma given in response to active bleeding, there is evidence to suggest that increased intravascular pressures (as may be experienced with the rapid administration of a high volume product) may paradoxically result in increased blood loss.21–23 For this reason, many anesthesiologists at the study institution cautiously allow permissive hypotension in times of acute exsanguination, in order to prevent worsening hemorrhage. Hence, higher doses of plasma may have theoretically perpetuated surgical bleeding. Third, while one would assume that plasma given for the purpose of combating microvascular bleeding would have the desired clinical effect, there is limited evidence to either support or refute this notion. Microvascular bleeding is generally the result of multiple complex and often interacting mechanisms.24 Hence, higher volume plasma administration by itself may not result in improved outcomes, especially at mild-to-moderate elevations in INR. Indeed, recent interventions for the prevention and treatment of microvascular bleeding have looked beyond traditional replacement of clotting factors (i.e. fibrinogen and factor concentrates, anti-fibrinolytic therapy, early platelet transfusion).25–28
Plasma administration has been associated with a variety of complications, including transfusion-related circulatory overload (TACO), transfusion-related acute lung injury (TRALI), allergic and febrile reactions, nosocomial infection, and multiorgan failure.29–32 TRALI and TACO occur more frequently than clinically recognized, are associated with higher plasma doses, and have been linked to increased lengths of stay and mortality.33–35 Fortunately, with improvements in transfusion practices and blood bank strategies (e.g. male-predominant plasma), clinically meaningful rates of TRALI remain low.36 Despite this, higher plasma volumes have also been associated with increased rates of infection and multiorgan failure.30 While this study was not specifically designed to assess the incidence of plasma-related complications, it is possible that their occurrence may have been associated with worse outcomes in our cohort. In addition to the potential effects of transfusion therapies, it should also be noted that high crystalloid volumes have recently been associated with an increased incidence of acute respiratory distress syndrome (ARDS) in patients with acute trauma.37 As our cohort received median intraoperative crystalloid volumes greater than 6L, with higher volumes observed in those with increased plasma volumes, it is important to note this potential modifier of postoperative outcomes.
It must also be noted that the achievement of greater decreases in INR following plasma transfusion was associated with improved clinical outcomes. This suggests that patients that received a greater decrement in INR following any given dose of plasma were phenotypically unique from those with less abrupt responses (e.g. greater severity of acute hemorrhage, ongoing hemorrhage without adequate control, worsening coagulopathy independent of the effects of plasma). Interestingly, this was true at all levels of pre-transfusion INR, although effects were greatest with INR values between 1.5 and 2.0. When limiting analyses to those receiving warfarin, these relationships were no longer apparent. This supports the role of unmeasured patient or surgical characteristics rather than the degree of therapeutic anticoagulation as the driver of outcomes. However, it must also be emphasized that when controlling for the severity of pre-transfusion INR and the magnitude of INR change after transfusion, patients receiving higher plasma volumes had inferior outcomes to those receiving lower volumes. This suggests that if two patients with equivalent derangements in coagulation test results, similar clinical features, and similar intraoperative characteristics receive an equal volume of plasma, the patient that achieves a greater decrease in INR is likely to experience better clinical outcomes. By the same token, if a patient receives an increased volume of plasma to achieve an equivalent drop in the INR, then that patient is likely to experience a worse outcome. Unfortunately, it is not clinically possible to monitor the temporal profile of in vivo changes in coagulation in real-time during plasma administration, and hence the optimal transfusion stopping point based on laboratory testing remains unclear. Cessation of microvascular bleeding would seem an obvious surrogate end-point for plasma transfusion, but that is a subjective marker based upon direct observation of the surgical field by experienced physicians. It is possible that administration of plasma beyond a critical end-point may be a driver of worse patient outcomes.
With regards to TEG values, there were no significant relationships observed between R-time derangements, plasma-mediated changes in R-values, and clinical outcomes. While surprising, caution must be taken before drawing conclusions. Only a minority of patients had TEG values obtained (23%), with the majority of these patients undergoing cardiac or liver transplant surgery. Many may have experienced refractory microvascular bleeding or ongoing massive hemorrhage. Hence, changes in R-values in this subset of patients may not reflect true clinical outcome relationships in those without massive or refractory bleeding. As an alternative explanation, it is possible that R-values are truly poor predictors of perioperative outcomes for this cohort of plasma-transfused patients. Regardless, further research is clearly warranted to better define these relationships.
Despite notable strengths of this investigation include a large sample size with robust adjustment of patient comorbidities and surgical features, there are limitations. First, this is a retrospective analysis, and we were unable to account for the precise circumstances surrounding each transfusion episode. Hence, it is unclear how many transfusions were prophylactic (e.g. in response to abnormal coagulation parameters) versus therapeutic (e.g. in response to bleeding). Furthermore, this study was not designed to compare patients receiving any plasma versus no plasma for the management of intraoperative laboratory coagulation derangements, as all patients received intraoperative plasma. As in all observational research, these results do not imply causality. Moreover, there exists the potential for unmeasured confounding despite careful protocol development and statistical adjustment. Specifically, those receiving higher plasma volumes may have been representative of a sicker patient population, though careful adjustment was performed based upon preoperative severity of illness (i.e. Charlson score, SOFA score) and laboratory and medication profiles. Results may also have been influenced by the extent of the surgical insult or the severity of intraoperative bleeding. To account for this, all analyses were adjusted by surgery type, duration, estimated blood loss, and intraoperative RBC volume. We also performed pre-defined sensitivity analyses by the magnitude of intraoperative allogeneic RBC transfusion (delineated at a threshold of 3 units) with consistent findings. Additionally, the use of postoperative RBC transfusion as an outcome measure for bleeding may have inadvertently included some patients being treated for non-hemorrhagic conditions (e.g. anemia of chronic disease). While we did not have a more reliable and universal marker of hemostasis available as an outcome measure, the presence of a hemostatic marker would strengthen future investigations. Despite this, we attempted to control for procedural characteristics including the severity of intraoperative blood loss. Recognizing that the magnitude of intraoperative blood loss itself may be a relevant outcome measure, we also performed analyses utilizing estimated blood loss and intraoperative RBC volume (occurring after the first unit of plasma) as outcome measures. To this end, increases in intraoperative plasma volume were associated with increased intraoperative RBC volumes and greater estimated blood loss despite careful statistical adjustment of patient illness, laboratory derangements, medications, and procedural factors.
Utilization of the INR for the guidance of plasma transfusion decisions is another limitation of the study. While this reflects real-world clinical practice (i.e. the INR is a widely utilized marker for the guidance of plasma transfusion decisions), this approach is inherently flawed. The INR was developed to standardize prothrombin time values across laboratories with the primary goal of optimizing warfarin dosing and monitoring. Previous studies have consistently noted a lack of correlation between mild-to-moderate derangements in the INR and bleeding complications.14,17–19 This may be explained, in part, by individual clotting factor levels that remain sufficient for thrombus formation (i.e. greater than 30 IU/dL) despite mild elevations in the INR.12,32 Moreover, an elevated INR does not implicitly reflect derangements in clinical hemostasis even in the presence of decreased clotting factor levels. In a previous investigation of non-anticoagulated critically ill patients with moderate elevations in INR (i.e. INR 1.5 – 3.0), viscoelastic test results, as measured by ROTEM, were largely unaffected despite reductions in individual factor levels, and plasma transfusion did not result in a more procoagulant state.38 Similarly, multiple investigations have observed higher rates of bleeding complications in patients that receive prophylactic plasma transfusion for the management of elevated INR values in the absence of clinical bleeding.39–41
As another study limitation, plasma transfusion volumes were modest (median 8.9 ml/kg), and hence “suboptimal” plasma dosing may have affected study results. Furthermore, most transfusions were administered with INR values less than 2 and hence within the range of potentially “preserved” hemostasis. This may have influenced the lack of observed benefit with higher doses of plasma transfusion; however, this provides a true snapshot of clinical practice at the study institution. Given that 95% of plasma transfusions occurred with INR values less than 3, we are unable to comment on the utility of plasma transfusion at greater levels of INR derangement. Theoretically, a critical threshold may exist beyond which higher plasma volumes are associated with improved outcomes. It should also be noted that with only 203 mortality events, multivariable analyses for this outcome are at risk for over-fitting. However, we opted to include the same adjustment covariates for this outcome for the sake of consistency in adjustment terms, and there were no glaring issues with model fit. Minimally adjusted analyses are provided as supplemental material (Supplemental Table 1). Additionally, we were underpowered for many of the sensitivity analyses. However, it was important to maintain the same adjustment terms in these exploratory analyses in order to be confident that any observed differences were truly representative of effects in the unique patient subgroup rather than a reflection of changes in adjustment variables. Finally, this study population is derived from a single academic medical center and is compromised predominantly of cardiac surgery patients with modest abnormalities in pre-transfusion INR, which limits external validity and generalizability.
In conclusion, in this single center investigation of intraoperative plasma transfusion practices consisting predominantly of patients with mild-to-moderate laboratory coagulation abnormalities, greater intraoperative plasma transfusion volumes were associated with greater decreases in INR values but not with improvements in clinical outcomes. Future prospective multicenter studies with pre-defined transfusion triggers, plasma transfusion volumes, and outcomes will be greatly beneficial in further assessing the impact of intraoperative plasma transfusion practices on hemostasis and clinical outcomes.
Supplementary Material
Acknowledgments
Financial Support:
This study was made possible by funding from the Mayo Clinic Department of Anesthesiology and Perioperative Medicine and the Critical Care Integrated Multidisciplinary Practice, Rochester, Minnesota. In addition, this study was supported by an NIH R01 grant (HL121232) to Dr. Kor and by CTSA Grant Number KL2 TR002379 to Dr. Warner from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The manuscript has been read and approved by all authors. There are no actual or potential conflicts of interest capable of influencing the judgement on the part of any author
Footnotes
Conflicts of Interests: The authors declare no competing interests.
Disclaimers/Disclosures: None
References
- 1.Frank SM, Savage WJ, Rothschild JA, et al. Variability in blood and blood component utilization as assessed by an anesthesia information management system. Anesthesiology 2012;117:99–106. [DOI] [PubMed] [Google Scholar]
- 2.Kor DJ, Gajic O. Blood product transfusion in the critical care setting. Current opinion in critical care 2010;16:309–16. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Anesthesiologists Task Force on Perioperative Blood M. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology 2015;122:241–75. [DOI] [PubMed] [Google Scholar]
- 4.Dzik W, Rao A. Why do physicians request fresh frozen plasma? Transfusion 2004;44:1393–4. [DOI] [PubMed] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 6.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform 2011;28:42, 4–5. [PubMed] [Google Scholar]
- 7.Chute CG, Beck SA, Fisk TB, Mohr DN. The Enterprise Data Trust at Mayo Clinic: a semantically integrated warehouse of biomedical data. J Am Med Inform Assoc 2010;17:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc 2012;87:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuttall GA, Oliver WC, Santrach PJ, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology 2001;94:773–81; discussion 5A-6A. [DOI] [PubMed] [Google Scholar]
- 10.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585–98. [DOI] [PubMed] [Google Scholar]
- 12.Chowdary P, Saayman AG, Paulus U, Findlay GP, Collins PW. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004;125:69–73. [DOI] [PubMed] [Google Scholar]
- 13.Dara SI, Rana R, Afessa B, Moore SB, Gajic O. Fresh frozen plasma transfusion in critically ill medical patients with coagulopathy. Crit Care Med 2005;33:2667–71. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006;46:1279–85. [DOI] [PubMed] [Google Scholar]
- 15.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. Jama 2015;313:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperry JL, Guyette FX, Brown JB, et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med 2018;379:315–26. [DOI] [PubMed] [Google Scholar]
- 17.Dzik WH. Predicting hemorrhage using preoperative coagulation screening assays. Curr Hematol Rep 2004;3:324–30. [PubMed] [Google Scholar]
- 18.Darcy MD, Kanterman RY, Kleinhoffer MA, et al. Evaluation of coagulation tests as predictors of angiographic bleeding complications. Radiology 1996;198:741–4. [DOI] [PubMed] [Google Scholar]
- 19.Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005;45:1413–25. [DOI] [PubMed] [Google Scholar]
- 20.Gulati G, Hevelow M, George M, Behling E, Siegel J. International normalized ratio versus plasma levels of coagulation factors in patients on vitamin K antagonist therapy. Arch Pathol Lab Med 2011;135:490–4. [DOI] [PubMed] [Google Scholar]
- 21.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21. [DOI] [PubMed] [Google Scholar]
- 22.Morrison CA, Carrick MM, Norman MA, et al. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma 2011;70:652–63. [DOI] [PubMed] [Google Scholar]
- 23.Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol 2014;109:81–8. [DOI] [PubMed] [Google Scholar]
- 24.Grottke O, Fries D, Nascimento B. Perioperatively acquired disorders of coagulation. Curr Opin Anaesthesiol 2015;28:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2013;30:270–382. [DOI] [PubMed] [Google Scholar]
- 26.Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care 2016;20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons J, Sikorski RA, Pittet JF. Tranexamic acid: from trauma to routine perioperative use. Curr Opin Anaesthesiol 2015;28:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohlauer MV, Moore EE, Thomas S, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg 2012;214:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma 2009;67:221–7; discussion 8–30. [DOI] [PubMed] [Google Scholar]
- 30.Inaba K, Branco BC, Rhee P, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg 2010;210:957–65. [DOI] [PubMed] [Google Scholar]
- 31.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest 2007;131:1308–14. [DOI] [PubMed] [Google Scholar]
- 32.Kor DJ, Stubbs JR, Gajic O. Perioperative coagulation management--fresh frozen plasma. Best Pract Res Clin Anaesthesiol 2010;24:51–64. [DOI] [PubMed] [Google Scholar]
- 33.Clifford L, Jia Q, Yadav H, et al. Characterizing the epidemiology of perioperative transfusion-associated circulatory overload. Anesthesiology 2015;122:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clifford L, Jia Q, Subramanian A, et al. Characterizing the epidemiology of postoperative transfusion-related acute lung injury. Anesthesiology 2015;122:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood 2012;119:1757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer DE, Reynolds JW, Hobbs R, et al. The Incidence of Transfusion-Related Acute Lung Injury at a Large, Urban Tertiary Medical Center: A Decade’s Experience. Anesth Analg 2018;127:444–9. [DOI] [PubMed] [Google Scholar]
- 37.Robinson BRH, Cohen MJ, Holcomb JB, et al. Risk Factors for the Development of Acute Respiratory Distress Syndrome Following Hemorrhage. Shock 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller MC, Straat M, Meijers JC, et al. Fresh frozen plasma transfusion fails to influence the hemostatic balance in critically ill patients with a coagulopathy. J Thromb Haemost 2015;13:989–97. [DOI] [PubMed] [Google Scholar]
- 39.Jia Q, Brown MJ, Clifford L, et al. Prophylactic plasma transfusion for surgical patients with abnormal preoperative coagulation tests: a single-institution propensity-adjusted cohort study. Lancet Haematol 2016;3:e139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warner MA, Woodrum DA, Hanson AC, Schroeder DR, Wilson GA, Kor DJ. Prophylactic Plasma Transfusion Before Interventional Radiology Procedures Is Not Associated With Reduced Bleeding Complications. Mayo Clin Proc 2016;91:1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warner MA, Chandran A, Jenkins G, Kor DJ. Prophylactic Plasma Transfusion Is Not Associated With Decreased Red Blood Cell Requirements in Critically Ill Patients. Anesth Analg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


