Abstract
Objective:
Examine the relationship between perioperative renal regional oximetry (rSO2), urinary biomarkers, and acute kidney injury (AKI) in infants after congenital cardiac surgery with cardiopulmonary bypass.
Design:
Prospective, observational.
Setting:
Cardiac operating room and intensive care unit (CICU)
Patients:
Neonates and infants without history of kidney injury or anatomic renal abnormality.
Interventions:
None.
Measurements and Main Results:
Renal rSO2 was measured intraoperatively and for 48 hours postoperatively. Urinary levels of neutrophil gelatinase-associated lipocalin (NGAL) and tissue inhibitor of metalloproteinases 2 (TIMP-2) together with insulin-like growth factor-binding protein 7 (IGFBP7) were measured preoperatively, 2, 12, and 24 hours postoperatively. Patients were categorized as no AKI, Stage 1, or Stage 2–3 AKI using KDIGO criteria with 43/70 (61%) meeting criteria for any stage AKI. Stage 2–3 AKI patients had higher [TIMP-2]•[IGFBP7] at 2 hours (0.3 vs. 0.14 for Stage 1 AKI and 0.05 for no AKI, P=0.052) and 24 hours postop (1.71 vs. 0.27 for Stage 1 AKI and 0.19 for no AKI, P=0.027) and higher NGAL levels at 24 hours postop (10.3 vs. 3.4 for Stage 1 AKI and 6.2 for no AKI, P=0.019). Stage 2–3 AKI patients had lower mean CICU renal rSO2 (66% vs. 79% for Stage 1 AKI and 84% for no AKI, P=0.038). Regression analyses showed that [TIMP-2]•[IGFBP7] at 2 hours postop and nadir intraoperative renal rSO2 to be independent predictors of postoperative kidney damage as measured by urinary NGAL.
Conclusions:
We observed modest differences in perioperative renal rSO2 and urinary biomarker levels compared between AKI groups classified by creatinine-dependent KDIGO criteria, but there were significant correlations between renal rSO2, [TIMP-2]•[IGFBP7], and postoperative NGAL levels. Kidney injury after infant cardiac surgery may be undetectable by functional assessment (creatinine) alone and continuous monitoring of renal rSO2 may be more sensitive to important subclinical AKI.
Keywords: acute kidney injury, near-infrared spectroscopy, biomarker, infant, congenital heart surgery, cardiac intensive care unit
INTRODUCTION
Pediatric cardiac surgery with cardiopulmonary bypass (CPB) has an incidence of acute kidney injury (AKI) ranging from 20–86% depending on which diagnostic classification method is used (1). The Kidney Disease: Improving Global Outcomes (KDIGO) criteria, relying on changes in serum creatinine and urine output (UOP), has been established as the preferred method to identify AKI in children and adults (1). However, creatinine and UOP may not be reliable for neonates and infants due to the presence of maternal creatinine, varying degrees of creatinine reabsorption in the proximal tubules, muscle mass, a lower percentage of the cardiac output going to the kidneys, and glomerular filtration rates that do not reach adults levels until two years of age (2, 3).
Because higher morbidity and mortality have been associated with pediatric AKI (4, 5), much effort has been made to develop novel biomarkers of renal injury to provide more precise identification of those at risk and allow for earlier detection and intervention. Neutrophil gelatinase-associated lipocalin (NGAL) has been described as one of the most promising biomarkers for early detection of AKI and even obviates the need for blood sampling because of urinary NGAL’s strong diagnostic capability (1, 6). More recently, the cell-cycle arrest biomarkers, tissue inhibitor of metalloproteinases 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7), have been validated in children after congenital cardiac surgery (7). Like NGAL, these promising biomarkers of kidney injury are measured from the urine. The product of the urinary concentrations of TIMP-2 and IGFBP7 divided by 1,000 yields an AKIRisk® Score, which was found to produce AUCs >0.7 for AKI after infant cardiac surgery (7). Several other studies suggest [TIMP-2]•[IGFBP7] may be superior to NGAL for AKI detection in various patient populations and disease states (8).
One disadvantage that both creatinine and biomarker (serum and/or urine) measurements share is that none provide continuous monitoring. Near-infrared spectroscopy allows for the continuous assessment of regional tissue oxygenation (rSO2), and is believed to reflect renal rSO2 when placed on the flank overlying the kidney. While some studies have observed an association between prolonged periods low perioperative renal rSO2 and AKI after congenital cardiac surgery (9–11), other studies have failed to show an association (12, 13). Interestingly, although the study by Hazle, et al did not find an association between prolonged low renal rSO2 and creatinine-based AKI after infant cardiac surgery, they did observe an association between renal rSO2 and higher postoperative NGAL and interleukin-18 levels, suggesting an association between renal rSO2 and kidney injury by more novel indicators (13).
Hence, in this study, we aimed to examine whether perioperative renal rSO2 and/or [TIMP-2]•[IGFBP7] would significantly correlate with postoperative renal injury as identified by urinary NGAL. First, we investigated perioperative AKI as defined by KDIGO criteria in infants after congenital cardiac surgery with CPB and the associations of renal rSO2 and the urinary biomarkers NGAL and [TIMP-2]•[IGFBP7]. Next, we examined renal rSO2 and [TIMP-2]•[IGFBP7] for their association with kidney damage as identified by NGAL as opposed to “clinical” AKI by creatinine/UOP-based definitions.
MATERIALS AND METHODS
Study Design
This was a prospective observational study of neonates and infants with congenital heart disease (CHD) undergoing congenital heart surgery. The primary outcome investigated was postoperative AKI defined by KDIGO criteria and its association with perioperative renal rSO2 and urinary biomarkers. Secondarily, we investigated associations between perioperative renal rSO2 values, biomarkers and postoperative kidney damage as identified by NGAL.
Setting and Participants
This study was conducted at a single academic pediatric medical center from 10/9/15 – 4/9/17. Data was collected during a single perioperative period without any further follow-up. Children with CHD requiring surgery with CPB prior to one year of age were recruited. Exclusion criteria were pre-existing renal dysfunction as defined by meeting KDIGO criteria for AKI preoperatively or preoperative dialysis requirement, radiographic evidence of any anatomic renal abnormality (e.g. polycystic kidney(s), solitary kidney, horseshoe kidney), and emergent procedures in children weighing <2.5 kg. Written parental informed consent was obtained prior to any sampling and all aspects of the study were conducted in accordance with our Institutional Review Board protocol (PRO15020326). Patient demographic information and perioperative variables were all abstracted from the electronic medical record.
Regional Oxygen Saturation
Renal rSO2 was continuously measured (Medtronic®, Minneapolis, MN, USA) from the time of entering the operating room through 48 postoperative hours. Sensors were placed just inferior to the left 12th rib and just lateral to the spinous process per manufacturer recommendations. We chose the left side to avoid any confounding by changes in liver size/position that could potentially alter right sided kidney position (14). Baseline renal rSO2 levels and both average and nadir levels for the intraoperative (INTRAOP) and postoperative (CICU) periods were recorded. No clinical interventions were made based on the renal rSO2 values.
Biomarkers
Preoperative, 2-, 12-, and 24-hour postoperative urine specimens were obtained from an indwelling urinary catheter. Infants in the CICU with an existing urinary catheter had urine sent just prior to departing for the operating room. Those without pre-existing catheters had urine sent immediately after induction of anesthesia and insertion of the urinary catheter. Urinary concentrations of NGAL were derived from NGAL plates (R&D Systems, Inc., Minneapolis, MN, USA) that were read at 450 nm using a Uniread 800 ELISA plate reader (GeneMate, Kaysville, UT, USA). [TIMP-2]•[IGFBP7] levels (the product of TIMP-2 and IGFBP7 in ng/ml divided by 1000) were derived from the NephroCheck® Test (Astute Medical, San Diego, CA, USA). In addition to [TIMP-2]•[IGFBP7] levels, patients were categorized based on [TIMP-2]•[IGFBP7] >0.3 per manufacturer recommendations and also by [TIMP-2]•[IGFBP7] >0.7 as demonstrated by Meersch, et al (7).
Statistical Analysis
Data are presented as the count with percentage, mean with standard deviation, or the median with interquartile range (IQR). Shapiro-Wilk test was used to determine normality of continuous data. Pearson Chi-squared testing was used to determine significance of categorical comparisons with Fisher exact test used when appropriate. One way analysis of variance (ANOVA) was used for normally distributed comparisons between AKI groups. Wilcoxon rank-sum test and Kruskal-Wallis testing with Dunn’s pairwise comparison was utilized for non-normally distributed intergroup comparisons. Bonferroni adjustment of the P-value was used for all post-hoc pairwise comparisons. Given increases in creatinine are heavily relied upon for determining AKI, Pearson product-moment correlation was utilized to investigate the association between patient characteristics (age, weight, body surface area) and preoperative baseline creatinine levels. Additionally, because serum creatinine has been described as an inaccurate metric for AKI in children (2, 6, 15, 16), and evidence has shown NGAL to be a highly specific marker of nephron damage (1, 6, 17), we performed an exploratory analysis investigating the relationship between renal rSO2, [TIMP-2]•[IGFBP7], and perioperative NGAL levels. Pearson product-moment correlation was used to examine perioperative renal rSO2 and [TIMP-2]•[IGFBP7] levels with NGAL levels. Also, we performed a backward stepwise regression incorporating variables into the model with P-values <0.2 for estimating 2-, 12-, and 24-hour postoperative NGAL levels. Predictor variables included perioperative renal rSO2, [TIMP-2]•[IGFBP7] levels, and clinical variables described to be risk factors for AKI after infant cardiac surgery (age, weight, single-ventricle status, Risk Adjustment in Congenital Heart Surgery [RACHS-1] score, CPB duration, and blood pressure for the first 24 postoperative hours) (1, 18). Inferential statistics were exploratory/hypothesis generating and thus were interpreted as such. Statistical analysis was completed using STATA 14.1 (StataCorp, College Station, TX, USA) and GraphPad Prism 7.0a (GraphPad Software, La Jolla, CA, USA).
RESULTS
Perioperative AKI
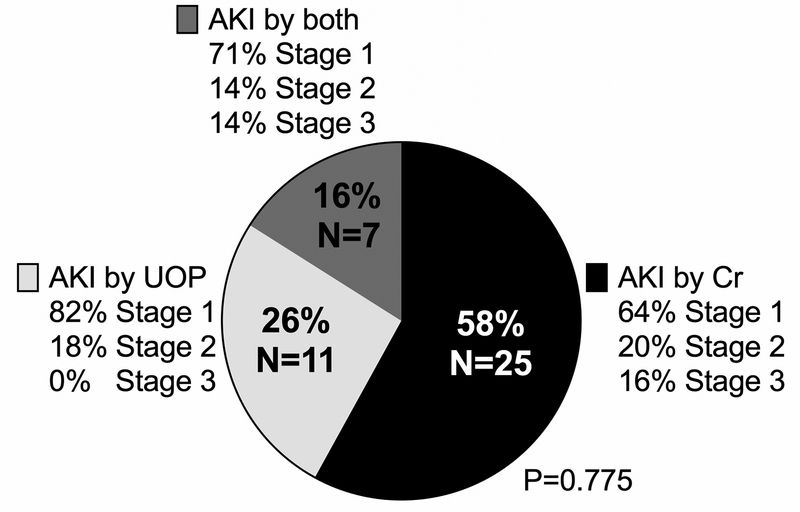
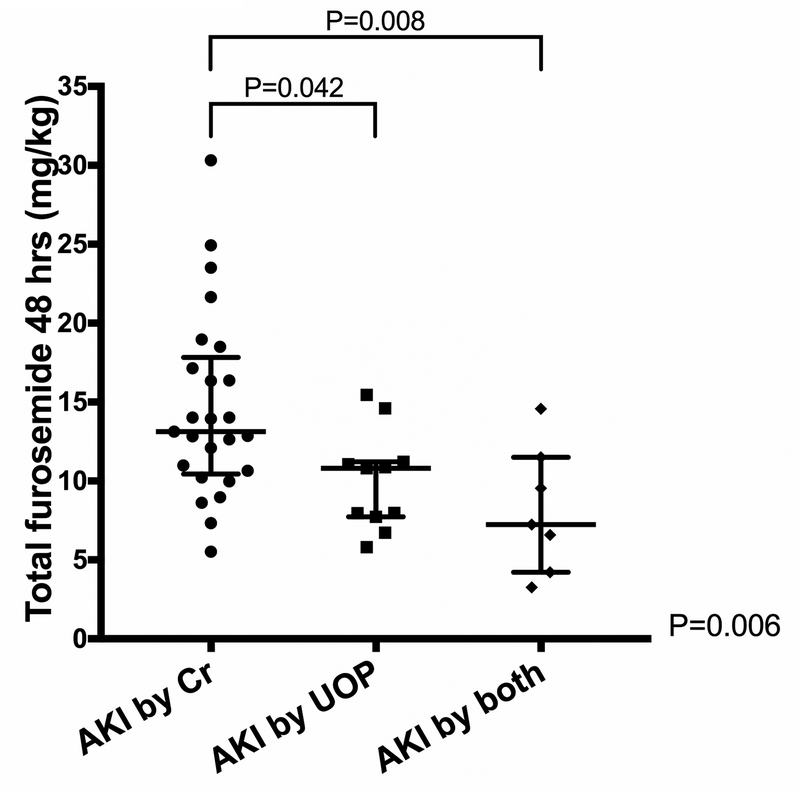
KDIGO criteria for postoperative AKI was met by 43/70 (61%). The median age at surgery was 58 days (7–123 days IQR), 31/70 (44%) were neonates (ranging 3–30 days old) with AKI (any stage) developing in 16/31 (52%) compared to 27/39 (69%) infants (ranging 40–360 days old) (P=0.133). The majority of patients met criteria for KDIGO Stage 1 AKI (30/43, 70%) and met criteria for AKI by creatinine alone (25/43, 58%) (Figure 1). Of those who met KDIGO criteria for AKI by creatinine (either alone or creatinine+UOP), 11/32 (34%) patients had a 0.1 mg/dL increase from their baseline creatinine level. Patients who met KDIGO criteria for AKI by low UOP alone and by both UOP+creatinine received significantly less cumulative furosemide than patients who met KDIGO criteria for AKI by creatinine increase alone (UOP alone 10.8 mg/kg [7.7–11.2 mg/kg], for UOP+creatinine 7.23 mg/kg [4.2–11.5 mg/kg], for creatinine alone 13.1 mg.kg [10.7–17.2 mg/kg], P=0.006) (Figure 2). Patients were categorized as no AKI (N=27, 39%), Stage 1 AKI (N=30, 43%), or Stage 2–3 AKI (N=13, 19%). Additional analyses categorizing patients as no AKI vs. Stage 1–3 AKI and no AKI/Stage 1 AKI vs. Stage 2–3 AKI are presented in supplemental results (Supplemental Tables 1–4).
Figure 1.
Pie chart showing distribution of acute kidney injury (AKI) diagnoses based on Kidney Disease: Improving Global Outcomes (KDIGO) criteria.
Figure 2.
Graphs showing cumulative amount of furosemide received by patients meeting various criteria for acute kidney injury (AKI) by Kidney Disease: Improving Global Outcomes (KDIGO) criteria.
Significant inverse correlations existed between preoperative baseline creatinine levels and patient age (r = −0.405, P<0.001), weight (r = −0.366, P=0.002), and body surface area (r = −0.386, P=0.005). Baseline creatinine levels were significantly higher in neonates (0.42 mg/dL [0.4–0.5]) compared to infants (0.22 mg/dL [0.2–0.3 mg/dL]) (P<0.001). There were significantly different preoperative creatinine levels between the three AKI groups with those developing Stage 2–3 AKI having the lowest preoperative creatinine levels (Table 1). Otherwise, there were no statistically significant differences in perioperative clinical variable comparisons between the three AKI groups (Table 1).
Table 1.
Perioperative variable comparisons between the three AKI groups
| Variables | No AKI | Stage 1 AKI | Stage 2–3 AKI | P-value |
|---|---|---|---|---|
| N = 27 | N = 30 | N = 13 | ||
| PREOPERATIVE | ||||
| Male, N (%) | 17 (63) | 19 (63) | 8 (62) | >0.999 |
| Surgery age (days) | 25 (5–150) | 84 (9–150) | 49 (10–60) | 0.521 |
| Neonate (≤30 days), N (%) | 15 (56) | 12 (40) | 4 (31) | 0.276 |
| Surgery weight (kg) | 3.7 (3–6) | 4.6 (3.2–7.1) | 3.5 (3.1–4.4) | 0.193 |
| Surgery BSA (m2) | 0.27 (0.2–0.33) | 0.28 (0.22–0.35) | 0.21 (0.19–0.23) | 0.083 |
| Single ventricle, N (%) | 4 (15) | 5 (17) | 4 (31) | 0.479 |
| Hemoglobin (g/dL) | 12.9 (11.7–15) | 13.4 (11.8–15.3) | 13.1 (11.2–13.7) | 0.254 |
| SpO2 (%) | 91 (84–98) | 93 (87–98) | 91 (87–98) | 0.754 |
| RACHS-1, N (%) | ||||
| 1 | 1 (4) | 0 (0) | 0 (0) | 0.721 |
| 2 | 7 (26) | 12 (40) | 6 (46) | |
| 3 | 6 (22) | 8 (27) | 2 (15) | |
| 4 | 11 (41) | 8 (27) | 3 (23) | |
| 6 | 2 (7) | 2 (7) | 2 (15) | |
| PRISM Score | 12 (6) | 10 (6) | 11 (7) | 0.425 |
| Creatinine (mg/dL) | 0.4 (0.3–0.5) | 0.3 (0.2–0.41) | 0.2 (0.16–0.3) | <0.001* |
| Blood urea nitrogen (mg/dL) | 14 (11–17) | 10 (9–18) | 12 (9–17) | 0.582 |
| INTRAOPERATIVE | ||||
| CPB (min) | 97 (55–113) | 100 (73–128) | 104 (76–127) | 0.548 |
| DHCA, N (%) | 9 (36) | 10 (33) | 3 (33) | >0.999 |
| Platelets (ml) | 205 (171–239) | 174 (145–228) | 169 (124–190) | 0.068 |
| Total products (ml) | 432 (168) | 357 (159) | 371 (207) | 0.247 |
| UOP (ml) | 36 (20–80) | 33 (15–70) | 19 (8–58) | 0.537 |
| Average OR ABP (mm Hg) | 47 (40–51) | 48 (38–53) | 47 (43–51) | 0.723 |
| Average OR CVP (mm Hg) | 10 (8–11) | 10 (8–13) | 9 (7–11) | 0.379 |
| POSTOPERATIVE | ||||
| Average CICU ABP (mm Hg) | 58 (53–66) | 60 (55–64) | 55 (51–60) | 0.613 |
| Average CICU CVP (mm Hg) | 9 (3) | 9 (3) | 11 (3) | 0.341 |
| †Peritoneal drain, N (%) | 13 (48) | 12 (40) | 10 (77) | 0.082 |
| †Peritoneal drain output (ml) | 214 (113) | 205 (138) | 321 (229) | 0.196 |
| Open sternum, N (%) | 13 (52) | 17 (57) | 7 (54) | 0.941 |
| Duration open sternum, (hr) | 44 (22–92) | 68 (42–114) | 68 (40–118) | 0.443 |
| UOP (ml/kg/hr) | ||||
| 12-hour postop | 3.4 (2.2–5.4) | 3.5 (2.3–4.8) | 2.8 (2–3.8) | 0.529 |
| 24-hour postop | 3.4 (2.1–4.5) | 3.2 (2.2–3.9) | 2.9 (1.8–3.4) | 0.37 |
| 48-hour postop | 3.7 (2.8–4.6) | 3.3 (2.7–4.1) | 3.7 (1.8–4.7) | 0.573 |
| Fluid Balance (ml) | ||||
| 12-hour postop | 381 (76–528) | 346 (73–572) | 213 (6–481) | 0.718 |
| 24-hour postop | 88 (226) | 52 (234) | −81 (255) | 0.124 |
| 48-hour postop | −111 (193) | −97 (150) | −173 (187) | 0.437 |
| SvO2 (%) | ||||
| 2-hour postop | 68 (14) | 63 (13) | 67 (18) | 0.394 |
| 12-hour postop | 63 (12) | 60 (11) | 59 (16) | 0.637 |
| 24-hour postop | 68 (10) | 63 (11) | 62 (16) | 0.169 |
| 48-hour postop | 67 (9) | 63 (11) | 64 (12) | 0.431 |
| Lactate (mMol/L) | ||||
| 2-hour postop | 3.4 (2.4–4.7) | 2.9 (2.4–4.3) | 2.6 (2.2–4) | 0.459 |
| 12-hour postop | 2.1 (1.2–3.1) | 1.7 (1.1–2.2) | 2 (1.6–2.7) | 0.272 |
| 24-hour postop | 1.4 (1–1.9) | 1.2 (1–1.4) | 1.3 (1–1.7) | 0.753 |
| 48-hour postop | 1.1 (0.9–1.4) | 1.2 (0.9–1.6) | 1.3 (1–1.5) | 0.581 |
| Creatinine (mg/dL) | ||||
| 2-hour postop | 0.4 (0.3–0.5) | 0.39 (0.3–0.5) | 0.44 (0.37–0.5) | 0.419 |
| 12-hour postop | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.705 |
| 24-hour postop | 0.35 (0.3–0.4) | 0.3 (0.26–0.4) | 0.4 (0.2–0.5) | 0.896 |
| 48-hour postop | 0.3 (0.2–0.4) | 0.34 (0.2–0.4) | 0.35 (0.24–0.45) | 0.736 |
| CICU LOS (days) | 11 (6–17) | 10 (3–22) | 10 (6–23) | 0.86 |
| Hospital LOS (days) | 16 (10–31) | 15 (8–30) | 29 (9–61) | 0.403 |
| Mechanical ventilation (days) | 3 (2–7) | 3 (0–6) | 4 (3–14) | 0.531 |
Data presented as count (%), median (IQR), or mean (SD)
Stage 2–3 AKI had lower preoperative Cr than both Stage 1 AKI (P=0.039) and no AKI (P<0.001), and Stage 1 AKI had lower preoperative Cr than no AKI (P=0.034)
Passive peritoneal drains, no patient received peritoneal dialysis
AKI - acute kidney injury; BSA - body surface area; RACHS - Risk Adjustment in Congenital Heart Surgery score; PRISM - Pediatric Risk of Mortality score; CPB - cardiopulmonary bypass; DHCA - deep hypothermic circulatory arrest; UOP - urine output; OR - operating room; ABP - mean arterial blood pressure; CVP central venous pressure; CICU - cardiac intensive care unit; LOS - length of stay
Perioperative Renal Oximetry
Intraoperative renal rSO2 data was collected for 65 (93%) patients and postoperative renal rSO2 data for 60 (86%). Baseline renal rSO2 was not significantly different between the three AKI groups (Table 2). Average and nadir INTRAOP renal rSO2 was not statistically significantly different between the three AKI groups, however there was a trend toward lower average renal rSO2 for the Stage 2–3 AKI group (no AKI 74% ± 11, Stage 1 AKI 78% ± 10, Stage 2–3 AKI 69% ± 12, P=0.075) (Table 2). Postoperative average CICU renal rSO2 significantly differed between the three groups (no AKI 84% [75–90%], Stage 1 AKI 79% [72–88%], Stage 2–3 AKI 66% [47–87%], P=0.038) with Stage 2–3 AKI having lower values than the no AKI group (P=0.016) (Table 2).
Table 2.
Regional tissue oximetry comparisons between the three AKI groups
| Variables | No AKI | Stage 1 AKI | Stage 2–3 AKI | P-value |
|---|---|---|---|---|
| N = 27 | N = 30 | N = 13 | ||
| BASELINE | ||||
| Renal rSO2 (%) | 67 (18) | 72 (14) | 66 (16) | 0.415 |
| Left cerebral rSO2 (%) | 59 (12) | 57 (10) | 62 (9) | 0.54 |
| Right cerebral rSO2 (%) | 61 (11) | 58 (11) | 63 (9) | 0.356 |
| INTRAOPERATIVE | ||||
| Average renal rSO2 (%) | 74 (11) | 78 (10) | 69 (12) | 0.075 |
| Nadir renal rSO2 (%) | 39 (28–65) | 49 (27–68) | 48 (19–58) | 0.499 |
| rSO2 <20% baseline, N (%) | 17 (65) | 17 (61) | 9 (82) | 0.476 |
| Average left cerebral rSO2 (%) | 62 (10) | 60 (10) | 62 (8) | 0.651 |
| Nadir left cerebral rSO2 (%) | 37 (11) | 37 (8) | 41 (9) | 0.435 |
| Average right cerebral rSO2 (%) | 63 (10) | 60 (9) | 63 (11) | 0.633 |
| Nadir right cerebral rSO2 (%) | 41 (13) | 40 (13) | 44 (11) | 0.668 |
| POSTOPERATIVE | ||||
| Average CICU renal rSO2 (%) | 84 (75–90) | 79 (72–88) | 66 (47–87) | 0.038* |
| Nadir CICU renal rSO2 (%) | 52 (16) | 46 (21) | 40 (19) | 0.22 |
Data presented as count (%), median (IQR), or mean (SD)
Stage 2–3 AKI had lower average CICU renal rSO2 than no AKI (P=0.016)
AKI - acute kidney injury; rSO2 – regional tissue oxygenation; CICU - cardiac intensive care unit
Perioperative Biomarkers
[TIMP-2]•[IGFBP7] measurements were obtained for 70 (100%) patients. Baseline [TIMP-2]•[IGFBP7] levels were not significantly different (Table 3). There was a trend toward higher 2-hour postoperative [TIMP-2]•[IGFBP7] levels in the AKI groups (no AKI 0.05 [0.04–0.13], Stage 1 AKI 0.14 [0.05–0.59], Stage 2–3 AKI 0.3 [0.06–2], P=0.052) with Stage 2–3 AKI patients having higher values than no AKI (P=0.033) (Table 3). At 24 hours postop, [TIMP-2]•[IGFBP7] levels were significantly different between the groups (no AKI 0.19 [0.07–0.49], Stage 1 AKI 0.27 [0.1–0.9], Stage 2–3 AKI 1.71 [0.41–2.59], P=0.027) with Stage 2–3 AKI having significantly higher values than no AKI (P=0.011) (Table 3). Furthermore, significantly more patients with Stage 2–3 AKI had 24-hour [TIMP-2]•[IGFBP7] levels >0.3 (no AKI 37%, Stage 1 AKI 47%, Stage 2–3 AKI 85%, P=0.017) (Table 3). The same was true for [TIMP-2]•[IGFBP7] levels >0.7 (no AKI 15%, Stage 1 AKI 37%, Stage 2–3 AKI 69%, P=0.003) (Table 3). The odds of meeting KDIGO criteria for Stage 2–3 AKI vs. Stage 1/no AKI were over seven times as high for those with 24-hour [TIMP-2]•[IGFBP7] levels >0.3 (11/35 [31%] vs. 2/35 [6%], respectively; OR 7.6, CI 1.4–74.4, P=0.006) and over six times as high for those with 24-hour [TIMP-2]•[IGFBP7] levels >0.7 (9/24 [38%] vs. 4/46 [9%], respectively; OR 6.3, CI 1.4–31.3, P=0.003).
Table 3.
Urinary biomarker comparisons between the three AKI groups
| Variables | No AKI | Stage 1 AKI | Stage 2–3 AKI | P-value |
|---|---|---|---|---|
| N = 27 | N = 30 | N = 13 | ||
| BASELINE | ||||
| [TIMP-2]•[IGFBP7] | 0.2 (0.1–0.9) | 0.4 (0.2–1.5) | 0.6 (0.1–2) | 0.146 |
| [TIMP-2]•[IGFBP7] >0.3, N (%) | 11 (41) | 17 (59) | 8 (73) | 0.157 |
| [TIMP-2]•[IGFBP7] >0.7, N (%) | 7 (26) | 13 (45) | 5 (45) | 0.261 |
| NGAL (ng/ml) | 5 (1.8–9.1) | 5.3 (2.1–13.6) | 3.9 (1.9–11.6) | 0.757 |
| POSTOPERATIVE | ||||
| [TIMP-2]•[IGFBP7] | ||||
| 2-hour postop | 0.05 (0.04–0.13) | 0.14 (0.05–0.59) | 0.3 (0.06–2) | 0.052* |
| 2-hour postop >0.3, N (%) | 5 (19) | 11 (37) | 6 (46) | 0.143 |
| 2-hour postop >0.7, N (%) | 4 (15) | 6 (20) | 5 (38) | 0.238 |
| 12-hour postop | 0.52 (0.18–1.63) | 0.57 (0.08–0.96) | 1.49 (0.28–3.25) | 0.225 |
| 12-hour postop >0.3, N (%) | 18 (67) | 17 (57) | 9 (69) | 0.685 |
| 12-hour postop >0.7, N (%) | 11 (41) | 12 (40) | 8 (62) | 0.381 |
| 24-hour postop | 0.19 (0.07–0.49) | 0.27 (0.1–0.9) | 1.71 (0.41–2.59) | 0.027* |
| 24-hour postop >0.3, N (%) | 10 (37) | 14 (47) | 11 (85) | 0.017 |
| 24-hour postop >0.7, N (%) | 4 (15) | 11 (37) | 9 (69) | 0.003 |
| NGAL (ng/ml) | ||||
| 2-hour postop | 2.5 (0.8–4.9) | 4.9 (1.4–6.9) | 2.2 (1.2–31.3) | 0.697 |
| 12-hour postop | 4.3 (3.5–9.8) | 4.1 (2.1–7.2) | 9 (3.7–18.9) | 0.269 |
| 24-hour postop | 6.2 (1.8–8.6) | 3.4 (2–8.6) | 10.3 (4.7–30.1) | 0.019** |
Data presented as count (%) or median (IQR)
Stage 2–3 AKI had higher 2-hr postop [TIMP-2]•[IGFBP7] than no AKI (P=0.033), and higher 24-hr postop [TIMP-2]•[IGFBP7] than no AKI (P=0.011)
Stage 2–3 AKI had higher 24-hr postop NGAL than both Stage 1 AKI (P=0.012) and no AKI (P=0.021)
AKI - acute kidney injury; [TIMP-2]•[IGFBP7] - tissue inhibitor of metalloproteinases 2, insulin-like growth factor-binding protein 7; NGAL - neutrophil gelatinase-associated lipocalin
NGAL measurements were available for 59 (84%) patients. Baseline NGAL levels did not significantly differ (Table 3). There were no statistically significant differences between NGAL levels at 2 and 12 hours postop. The 24-hour postoperative NGAL levels were significantly different between the three AKI groups (no AKI 6.2 ng/ml [1.8–8.6], Stage 1 AKI 3.4 ng/ml [2–8.6], Stage 2–3 AKI 10.3 ng/ml [4.7–30.1], P=0.019), with Stage 2–3 AKI having significantly higher values then no AKI (P=0.012) and Stage 1 AKI (P=0.021) (Table 3).
AKI, Renal Oximetry, and Biomarkers
Significant inverse correlations existed between nadir INTRAOP renal rSO2 levels and NGAL at baseline (r = −0.356, P=0.014), 2 hours (r = −0.332), P=0.034), 12 hours (r = −0.33, P=0.017), and 24 hours postop (r = −0.343, P=0.01). [TIMP-2]•[IGFBP7] levels at 2 hours postop significantly correlated with NGAL levels at 2 (r = 0.494, P<0.001) and 12 hours postop (r = 0.403, P=0.002), and [TIMP-2]•[IGFBP7] levels at 12 and 24 hours postop significantly correlated NGAL levels at 24 hours postop (r = 0.41, P=0.001 and r = 0.267, P=0.041, respectively). There were significant differences in postoperative urinary NGAL levels at various time-points based on varying percent decreases of INTRAOP renal rSO2 from baseline with a drop in INTRAOP renal rSO2 ≥30% showing higher NGAL levels at all postoperative time-points (Table 4). In addition, patients with [TIMP-2]•[IGFBP7] levels >0.7 at 2, 12, and 24 hours postop had significantly higher NGAL levels at those corresponding time-points (Table 4).
Table 4.
Urinary NGAL level differences for patients with various renal oximetry and [TIMP-2]•[IGFBP7] cutoff values
| Variables | 2-hour NGAL | P-value | 12-hour NGAL | P-value | 24-hour NGAL | P-value |
|---|---|---|---|---|---|---|
| INTRAOP renal rSO2 decline from baseline | ||||||
| ≥20% | 4.9 (1.5–13.9) | 0.085 | 6.5 (3.6–14.2) | 0.044 | 7.6 (3–10.7) | 0.004 |
| <20% | 1.7 (0.7–4.9) | 3.5 (2.1–6.2) | 2.7 (0.7–7.6) | |||
| ≥30% | 5 (2.5–25.1) | 0.019 | 7.4 (4.1–14.2) | 0.003 | 9.2 (3.4–14) | 0.002 |
| <30% | 1.7 (0.6–6) | 3.5 (2.1–6.2) | 3.5 (1.1–7.3) | |||
| ≥40% | 5.3 (1.5–26.9) | 0.017 | 6.9 (3.8–13.6) | 0.052 | 8.4 (3.1–14.7) | 0.014 |
| <40% | 2.2 (0.7–4.9) | 3.6 (2.1–8.6) | 3.7 (1.4–8.3) | |||
| ≥50% | 5.3 (1.5–26.9) | 0.042 | 6.6 (3.8–14.9) | 0.113 | 8.4 (3.4–14.3) | 0.014 |
| <50% | 2.5 (0.7–6) | 3.8 (2.1–9) | 3.7 (1.5–8.3) | |||
| [TIMP-2]•[IGFBP7] | ||||||
| Baseline >0.3 | 1.9 (1.1–6.8) | 0.398 | 3.6 (1.8–7.4) | 0.005 | 3.7 (1.5–8.6) | 0.12 |
| Baseline ≤0.3 | 4.9 (1.4–11.4) | 7.5 (3.8–19) | 8 (2.9–12.3) | |||
| 2-hour >0.3 | 11.6 (2.8–26.9) | 0.018 | 7.4 (2.1–15.7) | 0.213 | 6.5 (2.7–14.5) | 0.379 |
| 2-hour ≤0.3 | 2.5 (0.9–5.2) | 4 (2.6–8.1) | 4.9 (2–9.5) | |||
| 12-hour >0.3 | 4.5 (1.1–7) | 0.918 | 5.4 (2.9–10.4) | 0.563 | 4.9 (2.8–10) | 0.367 |
| 12-hour ≤0.3 | 2.8 (1.2–6.6) | 4.3 (2.1–8.2) | 7.3 (1.4–9.8) | |||
| 24-hour >0.3 | 4.9 (1.4–7.7) | 0.617 | 6.1 (2.1–14.2) | 0.357 | 8.1 (4.3–14.7) | <0.001 |
| 24-hour ≤0.3 | 3.2 (0.8–6.6) | 4.2 (2.9–7.2) | 2.8 (0.8–8.2) | |||
| Baseline >0.7 | 3.7 (1.1–6.8) | 0.866 | 3.6 (1.8–7) | 0.035 | 3.5 (1.8–7.2) | 0.075 |
| Baseline ≤0.7 | 4.1 (0.7–11.4) | 6 (3.5–16.6) | 8 (2.8–13.9) | |||
| 2-hour >0.7 | 26.9 (25.1–56.1) | 0.003 | 8.2 (6.2–26.9) | 0.044 | 4.9 (2.8–10.3) | 0.648 |
| 2-hour ≤0.7 | 2.6 (1–5.6) | 3.9 (2.4–8.3) | 5.4 (2–9.8) | |||
| 12-hour >0.7 | 4.9 (1.1–6) | 0.991 | 7.8 (3.5–14.9) | 0.033 | 6.5 (2.8–14) | 0.154 |
| 12-hour ≤0.7 | 2.7 (1.1–6.9) | 3.8 (2.1–6.1) | 4.5 (2–8.6) | |||
| 24-hour >0.7 | 3.8 (1.1–9.5) | 0.731 | 6.1 (2.1–14.2) | 0.637 | 9.3 (4.2–21.2) | <0.001 |
| 24-hour ≤0.7 | 3.7 (1.1–6) | 4.4 (2.9–8.2) | 3.4 (1.1–8.2) | |||
Data presented as median (IQR)
NGAL - neutrophil gelatinase-associated lipocalin; [TIMP-2]•[IGFBP7] - tissue inhibitor of metalloproteinases 2, insulin-like growth factor-binding protein 7
Ordinary least squares regression was observed to be inappropriate due to heteroscedasticity and unequal variance of fitted residuals. Thus, we utilized quantile regression for estimating the conditional median of postoperative NGAL levels. After backward stepwise regression, both the 2- and 12-hour postop NGAL models each yielded a single predictor variable, [TIMP-2]•[IGFBP7] levels at 2 hours postop (P<0.001 and P=0.048, respectively) (Table 5). Nadir INTRAOP renal rSO2 levels (P=0.016) and [TIMP-2]•[IGFBP7] levels at 2 (P=0.02) and 12 hours postop (P=0.013) were found to be significant predictors of 24-hour postop NGAL (Table 5).
Table 5.
Backward stepwise quantile regression for estimating the conditional median of the response variable NGAL at 2, 12, and 24 hours postop
| Variables | Coefficient | Standard Error | Confidence Interval | P-value |
|---|---|---|---|---|
| NGAL 2-hour postop | ||||
| 2-hour postop [TIMP-2]•[IGFBP7] | 10.696 | 2.134 | 6.331–15.06 | <0.001 |
| NGAL 12-hour postop | ||||
| 2-hour postop [TIMP-2]•[IGFBP7] | 1.467 | 0.72 | 0.013–2.921 | 0.048 |
| NGAL 24-hour postop | ||||
| Average INTRAOP renal rSO2 (%) | 0.187 | 0.111 | −0.037–0.41 | 0.099 |
| Nadir INTRAOP renal rSO2 (%) | −0.146 | 0.058 | −0.264 - (–)0.028 | 0.016 |
| Nadir CICU renal rSO2 (%) | 0.092 | 0.049 | −0.008–0.191 | 0.069 |
| 2-hour postop [TIMP-2]•[IGFBP7] | 1.411 | 0.582 | 0.233–2.588 | 0.02 |
| 12-hour postop [TIMP-2]•[IGFBP7] | 1.322 | 0.508 | 0.296–2.349 | 0.013 |
Full models for each time-point started with the variables: surgical age, weight, single-ventricle status, RACHS-1 score, cardiopulmonary bypass duration, blood pressure for the first 24-postoperative hours, nadir and average INTRAOP renal rSO2 levels, nadir and average CICU renal rSO2 levels, and [TIMP-2]•[IGFBP7] levels at baseline, 2, 12, and 24 hours postop, with stepwise removal of variables with P≥0.2
DISCUSSION
Post-cardiac surgery AKI (any stage) occurred in 61% of our patients based on KDIGO criteria, which is similar to other infant studies. This study adds the additional evaluation of renal rSO2 with NGAL, strengthening the analysis by adding a continuous measurement (renal rSO2) and newly identified sensitive measure of nephron injury (NGAL), whereas previous studies lack these comparisons. We also critically assessed how our infants were meeting the specific KDIGO criteria. In addition, we chose to subdivide our patients into three AKI groups based on KDIGO criteria: no AKI, Stage 1 AKI, and Stage 2–3 AKI in order to evaluate whether trends existed as the severity of AKI worsened. While we did not observe any statistically significant differences in INTRAOP renal rSO2 levels between the three AKI groups, the average CICU renal rSO2 levels were significantly lower in Stage 2–3 AKI compared to the no AKI group. We also observed significantly higher 2- and 24-hour [TIMP-2]•[IGFBP7] levels and 24-hour NGAL levels in the Stage 2–3 AKI group.
Interestingly, the majority of our patients met KDIGO criteria for AKI by sustaining a creatinine increase of only 0.1 mg/dL. While this small increase technically meets KDIGO criteria for AKI, its clinical utility has been questioned (2). We observed the no AKI group had the highest baseline creatinine levels and also the highest proportion of neonates, with the Stage 2–3 AKI group having the lowest of both. We also note the significant inverse correlations between baseline creatinine and age, weight, and body surface area. Again, this is described in the literature that up until the umbilical cord is clamped, fetal creatinine is equilibrated with maternal creatinine and thus early newborn creatinine levels more reflect maternal renal function than the neonate’s (3). Thus, a neonate with higher baseline creatinine who experiences a perioperative increase of 0.1 mg/dL, say from 0.3 to 0.4, would not meet KDIGO criteria for AKI whereas an increase from 0.2 to 0.3 does.
Furthermore, 11/43 (26%) of our patients met KDIGO criteria for AKI by low UOP alone. These patients received significantly less furosemide postoperatively than those with AKI by creatinine alone. It is possible that some of these 11 patients may not have met UOP criteria for AKI if they had received more furosemide. This may explain why the use of diuretics to prevent AKI has resulted in increased mortality (19), possibly due to the fact that giving diuretics may mask, and therefore possibly delay the recognition, diagnosis of, and intervention for AKI.
Given higher neonatal creatinine levels, renal physiologic maturational changes, confounding clinical interventions, and the fact that creatinine, as a marker of renal function and not renal injury, does not begin to rise until 48–72 hours after injury (2, 6), perhaps changes in serum creatinine after infant cardiac surgery hold very little, if any clinical value at all. The idea of serum creatinine as the gold standard for both diagnosing AKI and also the comparator to which novel biomarkers of renal injury are compared against has been challenged (16, 20).
Renal Oximetry and Biomarkers
Similar to other studies, we show an association with perioperative renal rSO2 levels, urinary biomarkers and AKI based on creatinine/UOP criteria. However, as previously suggested we may be searching for correlations with an imperfect gold standard marker of disease (16). Thus, in addition to investigating renal rSO2 and [TIMP-2]•[IGFBP7] trends in AKI defined by creatinine/UOP, we examined the correlations between renal rSO2, [TIMP-2]•[IGFBP7], and postoperative urinary NGAL because urinary NGAL has shown to be an early, sensitive, and readily detectable marker of renal damage (1, 6, 21–23).
We observed significant inverse correlations between nadir intraoperative renal rSO2 levels and postoperative NGAL at all time-points as well as significantly higher postop NGAL levels at various time-points based on the percent decline in renal rSO2 from baseline, suggesting that neonates and infants able to maintain higher renal oxygenation intraoperatively may suffer less perioperative kidney damage. In particular, an intraoperative renal rSO2 decrease of ≥30% from baseline was associated with significantly higher NGAL at all postoperative time-points and provided the largest effect sizes compared to other percentage decreases. These observations are similar to those made by Hazle et al, where they did not observe renal rSO2 to be correlated with AKI but rather there were significant correlations with NGAL (13).
Additional interesting trends were observed when examining the relationship between [TIMP-2]•[IGFBP7] and NGAL. Most striking were the observations of significant correlations between NGAL levels and the [TIMP-2]•[IGFBP7] levels from the preceding time-point. TIMP-2 and IGFBP7 are released from renal tubular cells in response to cellular stress (24) and elevated [TIMP-2]•[IGFBP7] levels can predict impending AKI within 12 hours of measurement (25), which is supported by our data. Also, similar to that described by Meersch et al (7), our sample’s postoperative [TIMP-2]•[IGFBP7] levels >0.7 appeared to have significantly higher NGAL levels at corresponding time-points. Consistent with the recently described kinetics of [TIMP-2]•[IGFBP7] in response to various insults (26), we also observed that [TIMP-2]•[IGFBP7] levels progressively climbed postoperatively for Stage 2–3 AKI whereas those with no AKI or Stage 1 AKI peaked at 12 hours and had a decline in [TIMP-2]•[IGFBP7] levels at 24 hours.
In summary, we have shown that decreased renal rSO2 may contribute to kidney cell stress, which is detectable by [TIMP-2]•[IGFBP7], and subsequently leads to kidney injury as indicated by NGAL. Finally, this injury leads to decreased function detectable by serum creatinine. The combination of a non-invasive, continuous oximetry monitor plus sensitive urinary biomarkers that obviate the need for repeated blood draws has great potential for altering the approach to identifying and trending the degree of AKI.
Limitations
This is a relatively small sample of patients from a single center. Intraoperative renal rSO2 missing for 5, postoperative renal rSO2 missing for 10, and NGAL missing for 11 patients may have affected the results of the study. Also, there was a heterogeneous mix of CHD phenotypes. We acknowledge that similar to all of the age-related changes that affect serum creatinine levels in neonates and infants, similar maturational changes may occur with levels of the novel biomarkers as well. For instance, baseline NGAL levels were found to be significantly higher in pre-term neonates, appear to nadir in the young-child age range, and then begin to increase in adolescent and adult age groups (6). We also acknowledge that our analysis is meant to be hypothesis generating and therefore some results may overestimate the true significance of the differences.
Future Directions
Our observations from this study, in addition to those presented in recent infant AKI literature, suggest that although the use of standardized criteria for classifying AKI in infants after cardiac surgery have helped to advance our knowledge of perioperative renal injury, we are likely striving to investigate and improve upon an imperfect, poor sensitivity, poor specificity standard. The results of this investigation suggest that kidney stress, as detected by [TIMP-2]•[IGFBP7] could be both a specific target as well as a trigger for various actions designed to prevent kidney damage. This approach has been successful in adults undergoing cardiac (27) and non-cardiac surgery (28) where applications of care bundles based on practice guidelines dramatically reduced the rates of AKI. Importantly, these interventions were triggered by increases in urinary [TIMP-2]•[IGFBP7]. In the future, we see the capacity to use [TIMP-2]•[IGFBP7] as a non-specific indicator of kidney stress and rSO2 to specifically monitor renal oxygen delivery. Then, interventions could be tailored to these indicators.
For example, during events that could potentially result in AKI, such as surgery, sepsis, and/or trauma, renal rSO2 can be used in real-time to tailor treatment regimens that optimize oxygen delivery to the kidneys as well as other interventions that may help to prevent AKI. Furthermore, if a renal insult is suspected based on a clinical event, [TIMP-2]•[IGFBP7] measurements can forecast impending AKI prompting earlier interventions that can mitigate the magnitude and/or duration of AKI.
Supplementary Material
ACKNOWLEDGEMENTS
This project was supported by a seed grant from the University of Pittsburgh School of Medicine, Department of Anesthesiology. PSA was supported in part by a training grant from the NIH (T32GM075770). JAK receives consulting fees and grant support from Astute Medical. Covidien provided a portion of the somatic rSO2 sensors. We thank the Heart Institute at UPMC Children’s Hospital of Pittsburgh and the children and families we are so fortunate to take care for and who selflessly participate in clinical studies.
Copyright form disclosure: Dr. Adams disclosed that Covidien provided a portion of the somatic rSO2 sensors, and he disclosed off-label product use of NEPHROCHECK® Test System (intended to be used in patients 21 years or older). Dr. Adams received support for article research from the National Institutes of Health and the University of Pittsburgh Department of Anesthesiology. Dr. Kellum and his institution received funding from Astute Medical. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Toda Y, Sugimoto K. AKI after pediatric cardiac surgery for congenital heart diseases-recent developments in diagnostic criteria and early diagnosis by biomarkers. J Intensive Care 2017;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selewski DT, Charlton JR, Jetton JG, et al. Neonatal Acute Kidney Injury. Pediatrics 2015;136(2):e463–473. [DOI] [PubMed] [Google Scholar]
- 3.Guignard JP, Drukker A. Why do newborn infants have a high plasma creatinine? Pediatrics 1999;103(4):e49. [DOI] [PubMed] [Google Scholar]
- 4.Watkins SC, Williamson K, Davidson M, et al. Long-term mortality associated with acute kidney injury in children following congenital cardiac surgery. Paediatr Anaesth 2014;24(9):919–926. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland SM, Byrnes JJ, Kothari M, et al. KI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 2015;10(4):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg JH, Parikh CR. Biomarkers for Diagnosis and Prognosis of AKI in Children: One Size Does Not Fit All. Clin J Am Soc Nephrol 2017;12(9):1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meersch M, Schmidt C, Van Aken H, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One 2014;9(10):e110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gist KM, Goldstein SL, Wrona J, et al. Kinetics of the cell cycle arrest biomarkers (TIMP-2*IGFBP-7) for prediction of acute kidney injury in infants after cardiac surgery. Pediatr Nephrol 2017;32(9):1611–1619. [DOI] [PubMed] [Google Scholar]
- 9.Ruf B, Bonelli V, Balling G, et al. Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: a case-control study. Crit Care 2015;19:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owens GE, King K, Gurney JG, et al. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol 2011;32(2):183–188. [DOI] [PubMed] [Google Scholar]
- 11.Neunhoeffer F, Wiest M, Sandner K, et al. Non-invasive measurement of renal perfusion and oxygen metabolism to predict postoperative acute kidney injury in neonates and infants after cardiopulmonary bypass surgery. Br J Anaesth 2016;117(5):623–634. [DOI] [PubMed] [Google Scholar]
- 12.Gist KM, Kaufman J, da Cruz EM, et al. A Decline in Intraoperative Renal Near-Infrared Spectroscopy Is Associated With Adverse Outcomes in Children Following Cardiac Surgery. Pediatr Crit Care Med 2016;17(4):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazle MA, Gajarski RJ, Aiyagari R, et al. Urinary biomarkers and renal near-infrared spectroscopy predict intensive care unit outcomes after cardiac surgery in infants younger than 6 months of age. J Thorac Cardiovasc Surg 2013;146(4):861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan H, Zerbino V. Rotation and forward displacement of the right kidney in hepatomegaly. Anat Clin 1985;7(2):137–141. [DOI] [PubMed] [Google Scholar]
- 15.Filler G, Guerrero-Kanan R, Alvarez-Elías AC. Assessment of glomerular filtration rate in the neonate: is creatinine the best tool? Curr Opin Pediatr 2016;28(2):173–179. [DOI] [PubMed] [Google Scholar]
- 16.Waikar SS, Betensky RA, Emerson SC, et al. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol 2012;23(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer E, Markó L, Paragas N, et al. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf) 2013;207(4):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh SP. Acute kidney injury after pediatric cardiac surgery. Ann Card Anaesth 2016;19(2):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endre ZH. Using biomarkers for acute kidney injury: barriers and solutions. Nephron Clin Pract 2014;127(1–4):180–184. [DOI] [PubMed] [Google Scholar]
- 21.Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int 2009;75(3):285–294. [DOI] [PubMed] [Google Scholar]
- 22.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 2003;14(10):2534–2543. [DOI] [PubMed] [Google Scholar]
- 23.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54(6):1012–1024. [DOI] [PubMed] [Google Scholar]
- 24.Emlet DR, Pastor-Soler N, Marciszyn A, et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol 2017;312(2):F284–F296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013;17(1):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostermann M, McCullough PA, Forni LG, et al. Kinetics of Urinary Cell Cycle Arrest Markers for Acute Kidney Injury Following Exposure to Potential Renal Insults. Crit Care Med 2018;46(3):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 2017;43(11):1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Göcze I, Jauch D, Götz M, et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann Surg 2018;267(6):1013–1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.