Abstract
Background:
Patients undergoing allogeneic hematopoietic stem cell transplant (HCT) require variable, often extensive transfusion support. Identification of factors that predict urgent, intensive, or special needs should improve management of these patients.
Study Design and Methods:
This is a retrospective study of red blood cell (RBC) and platelet transfusion support provided for sequential matched sibling donor (MSD) allogeneic transplants conducted at the Clinical Center, NIH, from 1993-2010. Factors potentially important for predicting quantity of RBC and platelet transfusions, and time to transfusion independence through Day 200 post-HCT were evaluated.
Results:
Subjects (n=800) received 10,591 RBC and 10,199 platelet transfusions. Multivariable analysis demonstrated that need for RBC pretransplant, CD34+ dose, transplant year, diagnostic category, and ABO match were significantly independently associated with quantity of RBC transfusions Days 0-30. Only pretransplant need for RBC, CD34+ dose and transplant year had significance during Days 0-100. Similar analyses for quantity of platelet transfusions demonstrated that for both Days 0-30 and 0-100, significant factors were need for platelet support pretransplant, CD34+ dose, transplant year, and transplant regimen. Of note, long term, during Days 101-200, only CD34+ dose remained significant for quantity of RBC and of platelet transfusions. Analysis of time to transfusion independence demonstrated that patients with ABO major mismatches required longer to achieve freedom from RBC transfusion support compared to identical matches or those with minor mismatches.
Conclusion:
Patient-specific factors including CD34+ dose and ABO match of the graft should be given particular consideration by transfusion services when planning support of patients receiving allogeneic HCT.
Keywords: Transfusion, allogeneic hematopoietic cell transplantation, CD34+ stem cell
Introduction
Hematopoietic stem cell transplant programs impose substantial and specialized challenges in clinical care1-6 and inventory management7-8 on blood banks and transfusion services. Patients undergoing hematopoietic stem cell transplantation (HCT) experience differing periods of prolonged cytopenia, necessitating close monitoring and often requiring intensive, urgent, or specialized transfusion support. Further, individual patients may present unexpected and complex hematologic complications arising from their primary disease, their transplant regimen, and/or graft-host interactions. For transfusion services to provide optimal care, prior knowledge of patient factors that might predict special needs or the requirement for prolonged component support post-transplant is desirable, to facilitate individualized care planning and acquisition of dedicated blood component inventories in advance of need.
We quantified red cell and platelet transfusion support, and the time course of these transfusions post-HCT, for a large group of human leukocyte antigen (HLA)-matched sibling donor (MSD) transplants performed at a single center. The relative importance of a few key baseline factors readily known to the transfusion medicine service, such as need for transfusions pre-transplant, the ABO match of the donor and recipient, the type of transplant regimen (myeloablative or nonmyeloablative), and the CD34+ stem cell dose, were analyzed. Patient-specific demographic and clinical factors including diagnosis, blood type, and disease stage were also studied.
Materials and Methods
Protocol:
Study protocol 13-CC-N026 was reviewed and approved by the Institutional Review Board (IRB) of the National Heart, Lung and Blood Institute (NHLBI), Clinical Center, NIH. A waiver of written informed consent was approved for this retrospective natural history study.
Demographic and baseline data:
Databases of the Department of Transfusion Medicine (DTM) and the Medical Record Department, NIH Clinical Center were researched to identify a total of 800 sequential patients who received HLA MSD HCT on prospective clinical protocols at the Clinical Center, from 1993 through 2010 (Figure S1).9-22 Subject baseline demographics are provided in Table 1A for all patients, and by transplant regimen in Table S1. The primary treatment protocols are listed by Institute in Appendix S1. Demographic and baseline data for each subject including age, gender, diagnosis, weight, subject ABO type, donor ABO type, category of transplant preparative regimen, date of graft infusion and CD34+ hematopoietic stem cell dose were obtained. For subject diagnoses and the categories used for this analysis, see Table S3. Transplant preparative regimen was specified by the subject’s primary transplant protocol (Table S4). Need, if any, for transfusion of RBC or platelets during Days −10 through −1 immediately preceding graft infusion was assessed as “none” (no transfusions) vs. “any” (1 or more transfusions). Patient-donor ABO match was recorded as: identical, major mismatched (recipient isohemagglutinins vs. ABO type of the donor), bidirectional mismatched, or minor mismatched (donor isohemagglutinins vs. ABO type of the recipient). Three CD34+ dose categories were designated, for use in the investigation of importance of CD34+ dose for red cell or platelet usage: “Small Dose” (≤2.0 ×106/kg), “Medium Dose” (>2.0 – 6.0 × 106/kg), and “Large Dose” (>6.0 × 106/kg). Categories designated for transplant year consisted of: 1993–2000, 2000–2005, and 2006–2010.
TABLE 1.
BASELINE AND TIME-DEPENDENT DATA
| A) BASELINE DEMOGRAPHIC, CLINICAL AND LABORATORY DATA | |
| Subjects enrolled* | |
| Number | n = 800 |
| Age, years | |
| Mean +/− SD | 41.6 +/− 14.4 |
| Range | 4 yr - 72 yr |
| Gender, n (% of enrolled) | |
| Female | 330 (41) |
| Male | 470 (59) |
| Weight, kg | |
| Mean +/− SD | 71.6 +/− 16.7 |
| Stem cell dose, CD34+ × 106/kg (Day 0)* | |
| Mean +/− SD | 6.8 +/− 3.7 |
| Missing, frequency | n = 20 |
| Stem cell dose category, CD34+ × 106/kg (Day 0), n (% of enrolled)* | |
| ≤ 2.00 | 53 (7) |
| > 2.00 - 6.00 | 322 (41) |
| > 6.00 | 405 (52) |
| Missing, frequency | 20 (3) |
| Received transfusion pre-HCT, n (% of enrolled) | |
| Any RBC, Days −10 through −1 | 355 (44) |
| Any platelets, Days −10 through −1 | 176 (22) |
| Any RBC and/or platelets, Days −10 through −1 | 371 (46) |
| ABO match, n (% of enrolled) | |
| Identical | 553 (69) |
| Major mismatch | 114 (14) |
| Bidirectional mismatch | 29 (4) |
| Minor mismatch | 104 (13) |
| Diagnostic category, n (% of enrolled) | |
| Hematologic malignancy, standard risk | 212 (27) |
| Hematologic malignancy, intermediate risk | 241 (30) |
| Hematologic malignancy, high risk | 80 (10) |
| Benign hematologic disorder | 98 (12) |
| Solid tumor | 169 (21) |
| Transplant regimen category, n (% of enrolled) | |
| Bone marrow myeloablative | 62 (8) |
| PBSC myeloablative | 220 (28) |
| PBSC nonmyeloablative | 292 (37) |
| TLD + PBSC nonmyeloablative | 226 (28) |
| Time period, years, n (% of enrolled) | |
| 1993 - 2000 | 270 (34) |
| 2001 - 2005 | 309 (39) |
| 2006 - 2010 | 221 (28) |
| B) TIME-DEPENDENT CLINICAL AND LABORATORY DATA | |
| Received transfusion post-HCT, n (% of enrolled) | |
| Any RBC, within 200 days | 513 (64) |
| Any platelets, within 200 days | 596 (75) |
| Any RBC or platelets, within 200 days | 706 (88) |
| Death post-HCT, n (% of enrolled) | |
| Within 30 days | 15 (2) |
| Within 60 days | 45 (6) |
| Within 100 days | 87 (11) |
| Within 200 days | 162 (20) |
| Survival & availability for transfusion post-HCT, n (% of enrolled)** | |
| At Day 0 | 800 (100) |
| At Day 101 | 705 (88) |
| At Day 200 | 623 (78) |
n=780 subjects had complete data, n=20 subjects lacked CD34 stem cell dose.
Patients became unavailable upon 1) death, or 2) censored due to second transplant.
Transfusion practices:
Standard transfusion practices were followed, to maintain target hemoglobin / hematocrit values and platelet counts.1-6 We note that transfusion medicine guidelines for patient hemoglobin (Hb) and platelet counts evolved during 1993 – 2010, the period of this study. For example, NIH Clinical Center studies written during 1993 and 1994 (Protocols 93-H-0212, 94-H-0092, and 94-H-0182 in Appendix S1) recommended maintaining Hb > 9.0 g/dl, while a later study from 2000 (Protocol 00-C-0119) specified Hb > 8.0 g/dl. The protocol for sickle cell disease (SCD) (Protocol 03-DK-170) recommended a Hb level of 9-10 g/dl. Most studies deferred to standard practice of the DTM, NIH Clinical Center, which since the early 2000’s has been that while allogeneic HCT patients with Hb > 9.0 g/dl do not require transfusion, those with Hb < 7.0 g/dl may benefit.6 In the case of ABO mismatched transplants, blood components were selected with the goal of reducing preventable complications.23-27 Since patients with minor ABO mismatches are at risk for immune mediated hemolysis, Hb > 9.0 g/dl was maintained for these individuals during and immediately following the period of engraftment and recovery of blood cell counts.6,26 RBC were transfused in quantities of 1 or 2 units, or more if clinically indicated. Platelet count targets of 10,000/μl were maintained for subjects who were stable, and >40,000/μl for those with overt hemorrhage.6 Platelets were transfused as single apheresis donor collections.
Time dependent post-transplant data:
Red cell and platelet component transfusion data was acquired for each subject from databases of the DTM. The DTM records included transfusions provided for each subject while inpatient at the Clinical Center, NIH, and while seen for day care as an outpatient of the Clinical Center. For each individual component, calculation of day of infusion with respect to the day of graft infusion, designated as Day 0, was made. Patients became unavailable for analysis upon: 1) death; or 2) censored due to a second transplant (Figure 1A and Table 1B). Patient medical records at the Clinical Center, NIH, were searched for occurrences of any second transplant events and/or death, for each subject.
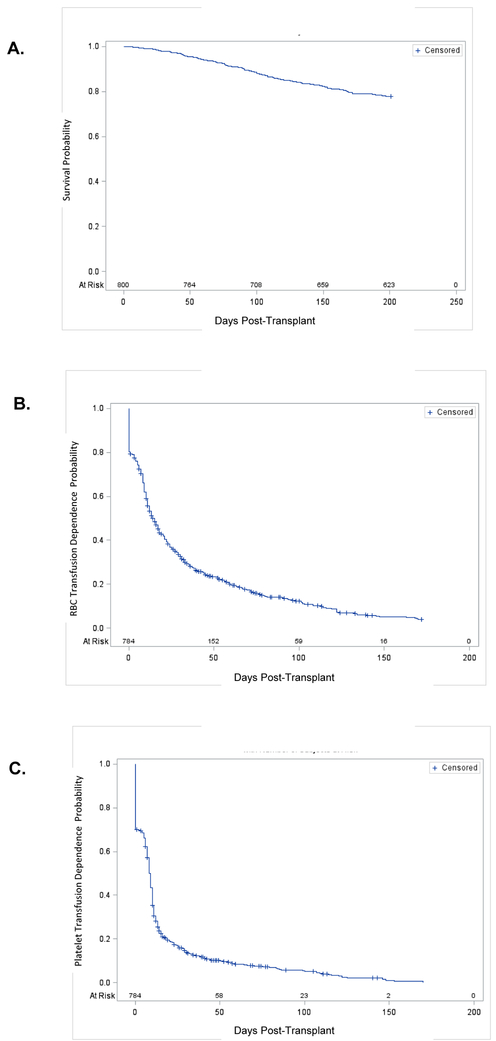
Figure 1. RBC and Platelet Transfusion Independence, All Patients.
Panel A: Survival and Availability for Transfusion. By Day 101, 705 (88%) of n=800 subjects remained available for transfusion, and by Day 200, 623 (78%) remained available. Patients became unavailable for analysis upon: 1) death; or 2) censored due to a second transplant.
Panel B: RBC Transfusion Independence. About 25% of subjects attained RBC transfusion independence at Day 5, 50% at Day 15 and 75% at Day 44.
Panel C: Platelet Transfusion Independence. About 25% of subjects were platelet transfusion independent at Day 0, 50% at Day 9 and 75% at Day 14.
Data for Panels B and C are displayed on a modified Kaplan-Meier plot in which transfusion independence, defined as the day of last transfusion preceding 30 days during which no transfusion is needed, is the criterion for removing an individual from the graph. Earlier removal of a patient represents more rapid attainment of independence, and is thus more favorable. For patients who attained independence, if subsequent transfusions were needed, the interval until the next transfusion was >30 days.
Quantity of transfusion support:
RBC and platelets transfused were summed for each patient for each of the intervals 0-30, 0-100, and 101-200 days post-transplant (Table 2). RBC were counted as units; platelets were counted as single apheresis collections (equivalent to a pool of 6 units of random donor platelets). The time intervals were selected for clinical relevance; by convention “immediate” complications due to the transplant regimen are generally seen by Day 30, and most complications attributable to the transplant are seen by Day 100. The interval 101-200 days post-HCT includes long-term follow-up.
TABLE 2.
CUMULATIVE RBC AND PLATELET TRANSFUSION EVENTS, BY INTERVAL, WITH UNIVARIATE ANALYSES OF IMPORTANCE OF BASELINE VARIABLES*
|
RBC TRANSFUSED PER SUBJECT, mean +/− SD |
PLATELETS TRANSFUSED PER SUBJECT, mean +/− SD |
|||||
|---|---|---|---|---|---|---|
| BASELINE VARIABLE | INTERVAL, DAYS POST-TRANSPLANT | INTERVAL, DAYS POST-TRANSPLANT | ||||
| Day 0-30 (n = 800) |
Day 0-100 (n = 800) |
Day 101-200 (n = 705) |
Day 0-30 (n = 800) |
Day 0-100 (n = 800) |
Day 101-200 (n = 705) |
|
| Stem cell dose category, CD34+ × 106/kg | ||||||
| ≤ 2.00 | 12.9 +/− 6.5 | 24.7 +/− 12.9 | 6.8 +/− 9.5 | 19.2 +/− 12.1 | 33.6 +/− 26.5 | 7.5 +/− 13.8 |
| > 2.00 - 6.00 | 5.5 +/− 6.0 | 10.8 +/− 13.8 | 3.6 +/− 7.9 | 6.6 +/− 9.9 | 11.0 +/− 20.0 | 3.5 +/− 9.9 |
| > 6.00 | 4.6 +/− 4.6 | 8.6 +/− 10.6 | 2.7 +/− 7.1 | 4.3 +/− 6.1 | 6.8 +/− 12.6 | 2.3 +/− 8.5 |
| Significance, CD34+ dose: ANOVA p value | < .0001 | <.0001 | 0.008 | < .0001 | <.0001 | 0.005 |
| RBC pre-HCT transfusion, Days −10 through −1 | ||||||
| No RBC | 3.6 +/− 4.2 | 7.1 +/− 10.0 | 2.7 +/− 7.6 | NA | NA | NA |
| Any RBC | 7.8 +/− 6.4 | 14.6 +/− 14.3 | 3.9 +/− 7.2 | NA | NA | NA |
| Significance, RBC pre-HCT: ANOVA p value | < .0001 | <.0001 | 0.04 | NA | NA | NA |
| Platelets pre-HCT transfusion, Days −10 through −1 | ||||||
| No platelets | NA | NA | NA | 4.5 +/− 7.2 | 7.8 +/− 16.2 | 2.8 +/− 9.1 |
| Any platelets | NA | NA | NA | 11.9 +/− 11.9 | 18.7 +/− 21.9 | 3.5 +/− 10.4 |
| Significance, platelets pre-HCT: ANOVA p value | NA | NA | NA | < .0001 | < .0001 | 0.04 |
| ABO match | ||||||
| Identical | 5.0 +/− 5.3 | 9.6 +/−12.0 | 3.2 +/− 7.9 | 5.8 +/− 8.7 | 9.5 +/− 16.5 | 3.1 +/− 9.8 |
| Major mismatch | 6.2 +/− 6.0 | 12.9 +/− 13.2 | 3.4 +/− 5.8 | 7.3 +/− 9.3 | 11.2 +/− 18.9 | 2.7 +/− 7.0 |
| Bidirectional mismatch | 6.0 +/−7.7 | 9.7 +/−14.1 | 2.9 +/−7.1 | 5.3 +/−10.1 | 8.0 +/−19.2 | 1.4 +/−5.7 |
| Minor mismatch | 6.9 +/−6.3 | 12.5 +/−14.9 | 3.0 +/−7.2 | 7.3 +/−10.0 | 13.5 +/−24.6 | 2.7 +/−10.2 |
| Significance, ABO match: ANOVA p value | 0.009 | 0.024 | 0.98 | 0.22 | 0.16 | 0.77 |
| Diagnostic category | ||||||
| Hematologic malignancy, standard risk | 5.4 +/− 5.6 | 10.4 +/− 12.9 | 3.4 +/− 8.1 | 7.2 +/− 8.2 | 11.9 +/− 19.4 | 3.8 +/− 10.7 |
| Hematologic malignancy, intermediate risk | 6.5 +/− 6.5 | 10.9 +/− 13.8 | 3.1 +/− 7.0 | 6.3 +/− 9.2 | 10.6 +/− 19.4 | 2.8 +/− 9.1 |
| Hematologic malignancy, high risk | 8.4 +/− 6.3 | 16.0 +/− 14.4 | 5.3 +/− 10.1 | 11.6 +/− 12.4 | 19.0 +/− 23.4 | 4.3 +/− 10.8 |
| Benign hematologic disorder | 3.8 +/− 4.0 | 7.3 +/− 8.6 | 1.9 +/− 5.6 | 5.4 +/− 9.9 | 7.7 +/− 14.9 | 1.9 +/− 9.3 |
| Solid tumor | 3.7 +/− 4.0 | 9.0 +/− 11.0 | 3.0 +/− 7.1 | 2.6 +/− 4.6 | 4.7 +/− 9.9 | 2.1 +/− 7.0 |
| Significance, diagnostic category: ANOVA p value | < .0001 | < .0001 | 0.1 | < .0001 | < .0001 | 0.28 |
| Transplant regimen category | ||||||
| Bone marrow myeloablative | 12.6 +/− 6.5 | 23.8 +/− 13.6 | 5.8 +/− 8.9 | 9.0 +/− 13.3 | 32.4 +/− 27.0 | 6.5 +/− 13.0 |
| PBSC myeloablative | 5.0 +/− 4.4 | 9.0 +/− 11.6 | 3.4 +/− 8.0 | 6.9 +/− 7.0 | 10.6 +/− 17.4 | 3.4 +/− 9.5 |
| PBSC nonmyeloablative | 4.1 +/− 4.8 | 8.9 +/− 11.5 | 2.7 +/− 6.5 | 3.3 +/− 6.3 | 5.8 +/− 13.1 | 2.0 +/− 7.7 |
| TLD + PBSC nonmyeloablative | 5.7 +/− 6.0 | 10.1 +/− 12.8 | 3.1 +/− 7.8 | 5.7 +/− 9.2 | 9.3 +/− 17.2 | 3.0 +/− 10.3 |
| Significance, regimen category: ANOVA p value | < .0001 | < .0001 | 0.11 | < .0001 | < .0001 | 0.03 |
| Time period, years | ||||||
| 1993 - 2000 | 7.6 +/− 6.6 | 14.1 +/− 13.9 | 4.2 +/− 8.3 | 8.7 +/− 10.7 | 14.3 +/− 21.2 | 3.3 +/− 9.0 |
| 2001 - 2005 | 5.1 +/− 5.4 | 10.0 +/− 13.3 | 3.0 +/− 7.5 | 6.2 +/− 8.7 | 10.4 +/− 18.6 | 3.5 +/− 10.8 |
| 2006 - 2010 | 3.4 +/− 3.7 | 6.5 +/− 8.2 | 2.3 +/− 6.4 | 3.1 +/− 5.6 | 4.8 +/− 10.5 | 1.8 +/− 7.4 |
| Significance, year of transplant: ANOVA p value | < .0001 | < .0001 | 0.03 | < .0001 | < .0001 | 0.1 |
Notes:
All n=800 subjects were considered available for the intervals 0-30 days, and 0-100 days. For the interval 101-200 days, the subset included those who completed at least one day in the interval. Patients became unavailable due to death, or censoring because of a second transplant.
Analysis of variance for patient age, or gender, or weight revealed no significance for RBC or platelets transfused.
Abbreviations: NA: not applicable / not analyzed
Time to transfusion independence:
Time to “red cell transfusion independence”, defined as the day of last transfusion preceding ≥ 30 days during which no transfusion of red cells was needed, was determined for each subject. The same interval was designated for platelets, for convenience. The definition of ≥ 30 days was used, as an adult patient unable to produce red cells and totally dependent on transfusion will require about one unit of red cells per week.
Statistical analyses:
Descriptive analyses of baseline data and quantity of transfusions post-HCT included mean and standard deviation for the number of RBC and of platelet transfusions per patient, for groups of interest. Univariate statistical significance of the association between each baseline variable and transfusion quantity was assessed by ANOVA (Table 2). Linear regression models were built stepwise to determine which baseline predictors were jointly significant in their association with RBC transfusion support as well as with platelet transfusion support for each time interval (Table 3). Analysis of every estimated effect was in comparison to a reference group we defined as: having standard risk hematologic malignancy, needing no transfusions pre-HCT, receiving a PBSC myeloablative stem cell transplant regimen and a large CD34+ dose, ABO identical with respect to recipient-donor, for a transplant procedure performed during 2006-2010.
TABLE 3.
FINAL MODELS FROM STEPWISE SELECTION OF MULTIVARIABLE LINEAR REGRESSION ANALYSIS
| Interval | Variable | Reference Group |
Effect Estimate* |
Standard Error |
F Value* | p* |
|---|---|---|---|---|---|---|
| A) RBC TRANSFUSION SUPPORT MODEL | ||||||
| 0-30 Days | Intercept* | NA | 1.28 | 0.50 | 6 | 0.0111 |
| RBC in 10 Days Prior | None | 4.04 | 0.37 | 117 | < .0001 | |
| CD34+ Dose Category | 29 | < .0001 | ||||
| Medium | Large | 0.84 | 0.36 | 5 | 0.02 | |
| Small | Large | 5.83 | 0.77 | 57 | < .0001 | |
| Diagnostic Category | 9 | < .0001 | ||||
| Intermediate risk heme | Standard risk heme | 0.50 | 0.48 | 1 | 0.3 | |
| High risk heme | Standard risk heme | 0.23 | 0.65 | 0 | 0.72 | |
| Benign | Standard risk heme | −2.52 | 0.65 | 15 | 0.0001 | |
| Solid tumor | Standard risk heme | −1.35 | 0.51 | 7 | 0.008 | |
| ABO Match | 4 | 0.014 | ||||
| Minor mismatch | Identical | 1.30 | 0.52 | 6 | 0.012 | |
| Bidirectional mismatch | Identical | 0.36 | 0.94 | 0 | 0.7 | |
| Major mismatch | Identical | 1.22 | 0.50 | 6 | 0.015 | |
| Time Period | 22 | < .0001 | ||||
| 2001-2005 | 2006-2010 | 1.91 | 0.44 | 19 | < .0001 | |
| 1993-2000 | 2006-2010 | 3.04 | 0.47 | 42 | < .0001 | |
| 0-100 Days | Intercept* | NA | 1.93 | 0.97 | 4 | 0.05 |
| RBC in 10 Days Prior | None | 7.16 | 0.83 | 74 | < .0001 | |
| CD34+ Dose Category | 27 | < .0001 | ||||
| Medium | Large | 2.71 | 0.86 | 10 | 0.002 | |
| Small | Large | 13.01 | 1.80 | 52 | < .0001 | |
| Time Period | 14 | < .0001 | ||||
| 2001-2005 | 2006-2010 | 4.16 | 1.03 | 16 | < .0001 | |
| 1993-2000 | 2006-2010 | 5.58 | 1.10 | 26 | < .0001 | |
| B) PLATELET TRANSFUSION SUPPORT MODEL | ||||||
| 0-30 Days | Intercept* | NA | 2.52 | 0.79 | 10.3 | 0.0014 |
| Platelets in 10 Days Prior | None | 6.73 | 0.66 | 104 | < .0001 | |
| CD34+ Dose Category | 5.4 | 0.0048 | ||||
| Medium | Large | 1.29 | 0.58 | 5 | 0.026 | |
| Small | Large | 6.60 | 2.33 | 8 | 0.0048 | |
| Regimen Category | 13.6 | < .0001 | ||||
| Bone marrow myeloablative | PBSC myeloablative | 5.39 | 2.21 | 6 | 0.0149 | |
| PBSC nonmyeloablative | PBSC myeloablative | −3.50 | 0.70 | 25.1 | < .0001 | |
| TLD + PBSC nonmyeloablative | PBSC myeloablative | −0.54 | 0.75 | 0.5 | 0.4679 | |
| Time Period | 13.1 | < .0001 | ||||
| 2001-2005 | 2006-2010 | 3.27 | 0.68 | 23.3 | < .0001 | |
| 1993-2000 | 2006-2010 | 3.06 | 0.76 | 16.1 | < .0001 | |
| 0-100 Days | Intercept* | NA | 3.12 | 1.71 | 3.4 | 0.07 |
| Platelets in 10 Days Prior | None | 9.81 | 1.44 | 46.8 | < .0001 | |
| CD34+ Dose Category | 6.4 | 0.002 | ||||
| Medium | Large | 2.99 | 1.26 | 5.6 | 0.018 | |
| Small | Large | 15.79 | 5.06 | 9.7 | 0.002 | |
| Regimen Category | 5.1 | 0.002 | ||||
| Bone marrow myeloablative | PBSC myeloablative | 7.54 | 4.80 | 2.5 | 0.12 | |
| PBSC nonmyeloablative | PBSC myeloablative | −4.45 | 1.52 | 8.6 | 0.004 | |
| TLD + PBSC nonmyeloablative | PBSC myeloablative | −0.30 | 1.62 | 0 | 0.85 | |
| Time Period | 8.6 | 0.0002 | ||||
| 2001-2005 | 2006-2010 | 6.01 | 1.48 | 16.6 | < .0001 | |
| 1993-2000 | 2006-2010 | 4.76 | 1.66 | 8.2 | 0.0042 | |
Panel A: For the RBC model, R-square = 0.329 for 0-30 days; R-square = 0.198 for 0-100 days.
Panel B: For the platelet model, R-square = 0.324 for 0-30 days; R-square = 0.217 for 0-100 days.
Notes and Definitions:
Categories listed in the “Variable” column are compared to the category listed in the “Reference Group” column.
R-square: The percentage of the total variance among responses (i.e. RBC usage or platelet usage) that is explained by the model.
Intercept: The mean value of the outcome among subjects for whom all variables in the model have the reference value.
Effect Estimate: The difference in the estimated mean associated with that variable or category, vs the reference group, while simultaneously accounting for effects of other factors in the model.
F Value: The F value measures the degree of evidence that the independent variable improves model fit. F is larger for more improvement of fit. p: The p-value for overall significance of each variable in the model is shown in bold. Non-bolded p-values are interpreted differently; they only help assess relative effects among different categories of the same variable (smaller suggests a stronger difference).
Time to transfusion independence was analyzed by Kaplan-Meier methods. Cox proportional hazards regression analysis was used to study the relative importance of baseline variables and covariates related to independence of transfusion support (Table 4).
TABLE 4.
FINAL MODELS FROM MULTIVARIABLE PROPORTIONAL HAZARDS REGRESSION ANALYSIS
| Variable* | Reference Group* | Parameter Estimate |
Standard Error |
Chi Square |
P* | Hazard Ratio* |
95% CI |
|---|---|---|---|---|---|---|---|
| A) RBC TRANSFUSION INDEPENDENCE MODEL | |||||||
| RBC in 10 Days Prior | None | −0.62 | 0.08 | 58.70 | < .0001 | 0.54 | 0.46, 0.63 |
| CD34+ Dose Category | 79.50 | < .0001 | |||||
| Medium | Large | −0.22 | 0.09 | 6.50 | 0.01 | 0.80 | 0.68, 0.95 |
| Small | Large | −1.15 | 0.13 | 79.40 | < .0001 | 0.32 | 0.25, 0.41 |
| ABO Match | 16.30 | 0.001 | |||||
| Major mismatch | Identical | −0.46 | 0.12 | 15.60 | < .0001 | 0.63 | 0.50, 0.79 |
| Bidirectional mismatch | Identical | 0.02 | 0.15 | 0.00 | 0.91 | 1.02 | 0.76, 1.37 |
| Minor mismatch | Identical | −0.14 | 0.14 | 1.00 | 0.31 | 0.87 | 0.67, 1.14 |
| B) PLATELET TRANSFUSION INDEPENDENCE MODEL | |||||||
| Platelets in 10 Days Prior | None | −0.63 | 0.10 | 40.00 | <.0001 | 0.53 | 0.44, 0.65 |
| Diagnostic Category | 18.40 | <.0001 | |||||
| Intermediate risk heme | Standard risk heme | −0.10 | 0.13 | 0.60 | 0.44 | 0.90 | 0.70, 1.17 |
| High risk heme | Standard risk heme | −0.11 | 0.15 | 0.50 | 0.48 | 0.90 | 0.66, 1.21 |
| Benign | Standard risk heme | −0.34 | 0.20 | 2.80 | 0.09 | 0.71 | 0.48, 1.06 |
| Solid tumor | Standard risk heme | 0.27 | 0.17 | 2.50 | 0.11 | 1.31 | 0.94, 1.83 |
| Regimen Category | 56.30 | < .0001 | |||||
| Bone marrow myeloablative | PBSC myeloablative | −0.88 | 0.14 | 37.70 | < .0001 | 0.41 | 0.31, 0.55 |
| PBSC nonmyeloablative | PBSC myeloablative | 0.54 | 0.15 | 12.60 | 0.0004 | 1.72 | 1.28, 2.32 |
| TLD + PBSC nonmyeloablative | PBSC myeloablative | 0.27 | 0.13 | 4.40 | 0.04 | 1.32 | 1.02, 1.70 |
| Time Period | 23.60 | < .0001 | |||||
| 2001-2005 | 2006-2010 | −0.43 | 0.10 | 20.60 | < .0001 | 0.65 | 0.54, 0.78 |
| 1993-2000 | 2006-2010 | −0.40 | 0.10 | 14.80 | 0.0001 | 0.67 | 0.55, 0.82 |
Notes and Definitions:
Categories listed in the “Variable” column are compared to the category listed in the “Reference Group” column.
P: The P value for overall significance of each variable in the model is shown in bold. Non-bolded P values are interpreted differently; they only help assess relative effects among different categories of the same variable (smaller suggests a stronger difference).
Hazard Ratio: The hazard ratio characterizes the ratio in instantaneous chance of becoming independent relative to those in the reference group; values greater than 1 reflect faster attainment of transfusion independence.
Since subgroup analyses on the many baseline variables of interest could lead to spurious findings, an alpha = 0.01 instead of 0.05 was used as a cutoff for statistical significance.
All analyses were performed using SAS E-Guide Version 5.1 for Windows or original SAS programs developed for this study, using SAS Version 9.3 (SAS Institute, Cary, NC).
Results
MSD Allogeneic HCT at the Clinical Center, NIH (1993-2010)
From 1993-2010, 800 matched sibling transplants were performed continuously, with more cases per year for the years 1999-2005 than before or since (Figure S1A). For 2006 through 2010, the most recent years examined, about 40 matched sibling allogeneic hematopoietic stem cell transplants were performed per year.
The initial transplant preparative regimens were fully myeloablative (Figure S1C and Table S4). Procedures are indicated as “Bone Marrow Myeloablative” in Figure S1C when grafts were collected by aspiration of donor bone marrow,9-11 and “PBSC Myeloablative” for those collected by apheresis of donor peripheral blood CD34+ stem cells after mobilization using growth factors.12-14 Regimens were similar for these two groups, with the difference being in the method of graft collection. Myeloablative transplants, either “Bone Marrow Myeloablative” or “PBSC Myeloablative” in Figure S1C, were performed continuously at the Clinical Center from 1993 through 2010.
More recent nonmyeloablative procedures (Figure S1C and Table S4) utilized lower doses of chemotherapy and/or radiotherapy, and some included components that were lymphoablative preferentially, to facilitate donor engraftment with fewer regimen-related toxicities. Grafts were collected by apheresis of donor peripheral blood CD34+ stem cells, after mobilization using growth factors.15-19 Nonmyeloablative regimens are indicated as “PBSC Nonmyeloablative” procedures in Figure S1C. While most of the group of 292 nonmyeloablative transplants (see Table 1) received regimens containing fludarabine and cyclophosphamide, we note that a cohort of 22 transplants performed for SCD (Protocol 03-DK-0170, see Appendix S1) received conditioning that included alemtuzumab and TBI (see Table S4). During 1999, there were more nonmyeloablative than myeloablative transplants performed at the Clinical Center (Figure S1C). By 2000, nonmyeloablative transplants were about half of all procedures, and from 2001 through 2010, they were about one-third to somewhat less than one-third of all procedures.
The regimen labeled “TLD + PBSC Nonmyeloablative” in Figure S1C was a modified nonmyeloablative approach, in which patients with malignancies were pre-treated with chemotherapy and T cell-depleting agents to achieve targeted lymphocyte depletion (TLD)20 prior to receiving a reduced intensity preparative regimen (Table S4); grafts were collected by apheresis of donor peripheral blood, after mobilization of CD34+ stem cells.20-22 The numbers and relative proportions of “TLD + PBSC Nonmyeloablative” procedures performed at the Clinical Center were reasonably consistent, during 2000 - 2010.
Demographic and Baseline Clinical and Laboratory Data (Table 1A)
Age, Sex and Weight:
The distributions of subject age (mean 41.6 years) and weight (mean 71.6 kg) were distributed normally (data not shown); female subjects were 41% (n=330) while males were 59% (n=470).
Diagnostic Category:
The categories (see Table S3) of “Standard Risk Hematologic Malignancy” (n=212), “Intermediate Risk Hematologic Malignancy” (n=241) and “Solid Tumor” (n=169) each had about 150 or more subjects enrolled, whereas the categories “High Risk Hematologic Malignancy” (n=80) and “Benign Heme / Non-Malignant Hematologic Disorder” (n=98) were less well represented.
The distribution of diseases studied at the Clinical Center in any given year was necessarily dependent upon the clinical trials open and recruiting subjects. Thus, while we classified the indications for HCT by risk, we note the distribution of cases at the levels of risk was not necessarily consistent from year to year. For example, while transplants done 1998 and earlier were predominantly for leukemia, indications during subsequent years included lymphomas and solid tumors, as well as non-malignant indications.
Preparative Regimen:
Subject enrollment was reasonably evenly distributed among the regimens (see Table S4) “PBSC Myeloablative” (220 subjects), “PBSC Nonmyeloablative” (292 subjects) and “TLD + PBSC Nonmyeloablative” (226 subjects) preparative regimens, with relatively fewer enrolled on the early “Bone Marrow Myeloablative” protocols (62 subjects).
Pre-transplant transfusions:
A substantial proportion of the subjects required pre-transplant transfusions on Days −10 through −1; among the study population 44.4% received RBC, 22.0% received platelets, and 46.4% received one or both components. This is consistent with these patients having a variety of diagnoses, in more than one diagnostic category (see Table S3). We note that only 12.3% of all diagnoses were a “Non-Malignant Hematologic Disorder” (see Table S3) including, for example, aplastic anemia, in which patients are RBC transfusion dependent, severe congenital anemias such as SCD, and primary immune deficiency diseases. Patients with SCD constituted 2.8% of all diagnoses. SCD patients with SS hemoglobin received exchange transfusion prior to Day −7 when their preparative regimen was started, if needed to reduce hemoglobin S levels to 30% or less.19
CD34+ Dose:
Mean CD34+ dose, for n = 780 subjects with known dose, was 6.79 +/− 3.69 × 106/kg (95% confidence interval 6.53 – 7.05 × 106/kg according to the t-distribution). The distribution of subjects with known CD34+ dose into the CD34+ dose categories was similar for “LargeDose” and “MediumDose”, with 405 subjects and 322 subjects respectively; fewer individuals (53 subjects) received the “SmallDose”.
ABO for Recipient and Donor:
Among the 800 recipient-donor pairs, most were ABO identical (553; 69.1%), while smaller numbers of patients with major (114; 14.3%), bidirectional (29; 3.6%) and minor (104; 13%) mismatches were transplanted.
Year of Transplant:
Transplants were reasonably equally distributed over the three time periods, 1993-2000, 2001-2005, and 2006-2010.
RBC and Platelet Transfusion Events Throughout 200 Days Follow-Up
Survival and Availability for Transfusion:
By Day 101, 705 (88%) of 800 subjects remained available for transfusion, and by Day 200, 623 (78%) remained available (Figure 1A and Table 1B). Patients became unavailable for further transfusions upon 1) death, or 2) inclusion of any further transfusion history was censored at the date of a second transplant.
Sum of RBC and Platelet Transfusion Events:
Of 800 subjects, 706 received RBC or platelets during follow-up through 200 days post-HCT (Table 1B). For all subjects, RBC transfusion support included 8,345 units during days 0-100, and 2,246 units during days 101-200, for a total of 10,590 RBC transfusion events (Table S2). Similarly, there were 10,199 platelet transfusion events.
Quantity of RBC Transfusions During Intervals:
Univariate analyses indicated that CD34+ stem cell dose, need for RBC transfusion pre-transplant (during the interval Day −10 to Day −1), ABO match of the donor vs. recipient, diagnostic category, transplant regimen, and transplant year were all significantly associated with quantity of RBC transfusions for Day 0-30 (p≤0.01). For Day 0-100, all of these factors, except ABO match, remained significant. Regarding the importance of transplant year, we acknowledge that evolution of transfusion practice guidelines may be contributory, as described above. Importantly, for the interval Day 101-200, CD34+ dose was the only factor that remained significant (p = 0.008) (Table 2).
Multivariable analyses (Table 3A) identified factors that were independently associated with quantity of RBC transfusions during 0-30 days post-HCT, namely, need for RBC pretransplant, CD34+ dose, year of transplant, diagnostic category, and ABO match. During 0-100 days post-HCT only need for RBC pretransplant, CD34+ dose and year of transplant were significant.
Quantity of Platelet Transfusions During Intervals:
Univariate analyses indicated that CD34+ stem cell dose, need for platelet transfusion pre-transplant (during the interval Day −10 to Day −1), diagnostic category, transplant regimen, and year of transplant were significantly associated with quantity of platelet transfusion support for both Day 0-30 and Day 0-100 at the level of p = .01 or less. As noted, regarding the importance of transplant year, we acknowledge that evolution of transfusion practice guidelines may be contributory. Again, importantly, for the interval Day 101-200, CD34+ dose was the only factor that remained significant (p = 0.005) (Table 2).
Multivariable analyses (Table 3B) for quantity of platelet transfusions demonstrated that during both the 0-30 days and 0-100 days post-HCT intervals, significant factors were need for platelet support pretransplant, CD34+ dose, year of transplant, and transplant regimen. Since need for platelet transfusion support declined rapidly during the first 10 days post-HCT (Figure 1C), with very few transfusion events during Days 31-100, it is reasonable that factors identified as important for platelet transfusion during 0-30 days were also important for the longer time interval 0-100 days.
Time to RBC Transfusion Independence:
Fully 25% of patients became RBC transfusion independent by Day 5, 50% by Day 15 and 75% by Day 44 (Figure 1B). Time course for RBC transfusion independence was compared for factors including need for RBC transfusion prior to Day 0 (Figure 2A), CD34+ dose (Figure 2B), and year of transplant (Figure 2C). The ABO type of the recipient as compared to the donor was associated with time to RBC transfusion independence (Figure 3A), with major mismatches requiring a significantly longer time to achieve freedom from RBC transfusion support, as compared to identical or minor mismatches. For patients transplanted using a nonmyeloablative regimen, those with major mismatches required 1-2 months longer to achieve RBC transfusion independence (Figure 3B).
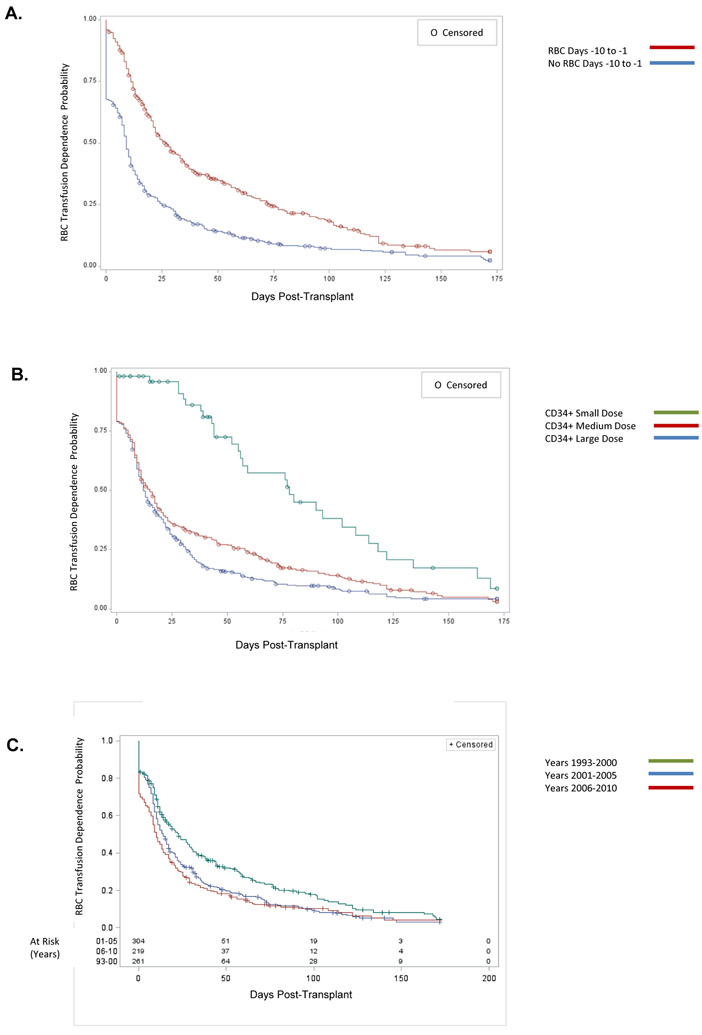
Figure 2. RBC Transfusion Independence, Importance of Factors.
Panel A: RBC Pre-Transplant. Compare those who received ≥ 1 RBC transfusion during days −10 through −1, vs. those who did not require transfusion. Those who received RBC pretransplant required more time before attaining RBC transfusion independence. (Logrank p < .0001).
Panel B: CD34 Dose. Compare Small Dose < 2 × 106/kg, vs. Medium Dose 2-6 × 106/kg, vs. Large Dose > 6 × 106/kg. Patients who received higher CD34+ doses became RBC transfusion independent sooner. (Logrank test for trend: p < .0001).
Panel C: Time Period of Transplant, Years. Compare transplants with Day 0 during 1993-2000 vs. 2001-2005 vs. 2006-2010. Patients who received the more recent transplants became RBC transfusion independent sooner. (Logrank test for trend: p < .003).
Data are displayed on a modified Kaplan-Meier plot in which RBC transfusion independence is defined as in Figure 1.
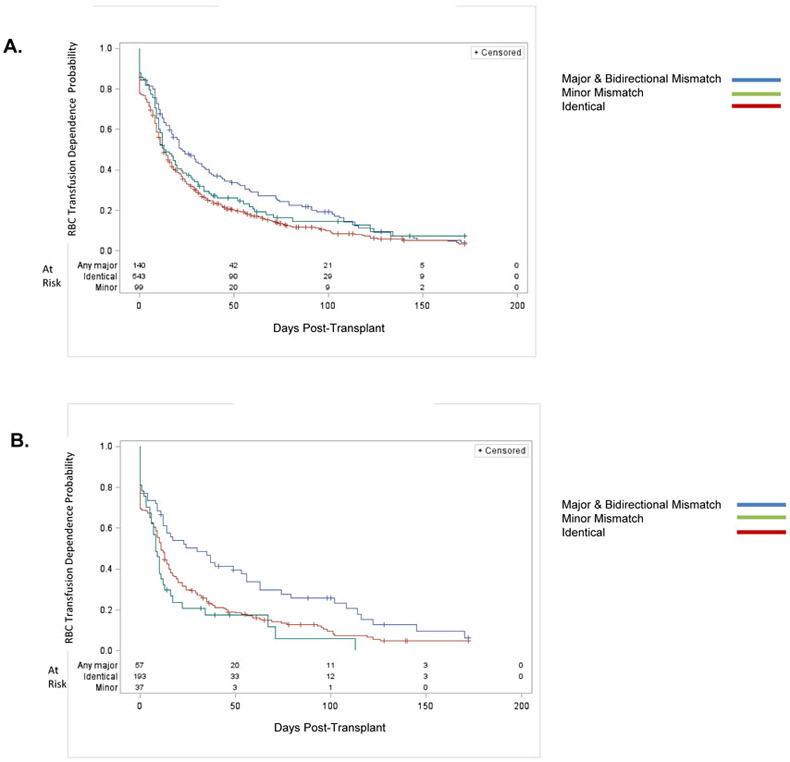
Figure 3. RBC Transfusion Independence, ABO Recipient—Donor Match.
Panel A: All Patients, All Regimens. Subjects who were an ABO major or bidirectional mismatch with their donor required longer to achieve independence of red cell transfusion, on average, as compared to ABO identical or minor mismatched transplants. (Logrank p = 0.005).
Panel B: PBSC Nonmyeloablative Regimen. For patients who received a PBSC nonmyeloablative transplant regimen, those who were an ABO major or bidirectional mismatch with their donor required 1 – 2 months longer to achieve independence of red cell transfusion, on average, as compared to ABO identical or minor mismatched transplants. (Logrank p = 0.004).
Data are displayed on a modified Kaplan-Meier plot in which RBC transfusion independence is defined as in Figure 1.
Multivariable analysis (Table 4A) demonstrated that RBC transfusions prior to Day 0, CD34+ dose category, and ABO mismatch were significant for time to RBC transfusion independence.
Time to Platelet Transfusion Independence:
For platelet support, 25% of subjects were transfusion independent at Day 0, 50% become independent by Day 9 and 75% at Day 14 (Figure 1C). The time course to achieve independence was very short for most subjects. Overall, this was a more rapid time course than described above for RBC. Multivariable analysis (Table 4B) identified the factors that were independently significant for time to platelet transfusion independence: need for platelet transfusions pre-transplant, diagnostic category, transplant regimen, and the year of the transplant.
Discussion
This report describes the substantial and sustained RBC and platelet transfusion support provided for MSD allogeneic HCT transplants for Phase I/II clinical trials conducted 1993 – 2010 at a single center. While several reports28-39 (Table S5) have examined one or more of the factors that we studied, we have been able to undertake a series of analyses to determine the relative importance of these variables. We include transplants performed continuously over many years, a variety of transplant practices, a substantial number of subjects, and extended follow-up. We have employed two independent and complementary approaches to analysis: quantitation of the number of transfusions needed during intervals of interest post-HCT, and determination of time to transfusion independence.
This study confirms the widely-accepted experience that ABO incompatibility is a potential complication in allogeneic HCT.40-42 We provide evidence that ABO mismatch is a relatively important variable, even after controlling for the other factors among those examined.
Multivariable analysis of factors important for quantity of RBC transfusions during Days 0-30 demonstrates the potential importance of major or minor mismatches as compared to identical grafts, with its p-value of 0.014 being near our 0.01 cut-off for significance. Further, for all subjects, multivariable analysis of time to RBC transfusion independence clearly demonstrates the importance of major mismatches compared to ABO minor mismatches or identical transplants (p = 0.001). RBC major mismatches required about 10 days longer to reach independence overall (Logrank p = 0.005); we also note that the effect appears exaggerated in the setting of nonmyeloablative conditioning, where ABO mismatches required 1-2 months longer to achieve transfusion independence (Logrank p = 0.004). This and other studies emphasize the need for the transfusion medicine service to carefully assess pre-HCT and monitor closely all such patients post-HCT.23-27
We provide evidence for the importance of CD34+ dose on the quantity and time course of transfusion support post-HCT. A broad range of CD34+ doses were available for analysis. Although the more historic cases in our dataset were bone marrow transplants, and laboratory methods for determination of CD34+ stem cell content were in development in the early 1990’s,43,44 CD34+ stem cell doses were determined for grafts continuously using contemporary methods at our center. As described here, it is especially noteworthy that beyond 100 days post-HCT, the only factor of importance for quantity of RBC and platelets transfused, among those we examined, was the CD34+ dose. Multiple factors were important for quantity of RBC and platelet transfusion support during the first 30 days post-HCT; those that retained importance through 100 days post-HCT included the CD34+ stem cell dose of the graft received, the need for transfusions during the 10 days preceding the day of graft infusion, and the transplant year.
These data confirm that “small” CD34+ doses (<2 × 106/kg) were independently associated with seriously compromised marrow recovery, as judged by need for increased RBC and platelet transfusion, and prolonged time to RBC transfusion independence.
Somewhat unexpectedly, we demonstrate that pre-HCT transfusion is an important predictor of both quantity of components needed post-HCT, and time to independence of transfusion support. This group included some diagnoses such as aplastic anemia with transfusion dependence due to inability to produce autologous red blood cells. Additionally, many patients in this study received extensive prior chemotherapy or radiation treatment for malignancy, which may have compromised the stem cell “niche” (see Figure 2.2, Barrett et al).45 The stem cell “niche” may be important for engraftment and recovery of hematopoietic activity after HCT.46-49 We observed that independence of platelet transfusion support was attained more rapidly, as compared to independence of RBC transfusions. Perhaps this is related to differences in the growth conditions permissive for lineage committed megakaryocyte progenitors and production of mature platelets, as compared to erythroid progenitors and development of fully differentiated RBC.
This cohort was comprised solely of HLA-matched sibling donors and we have focused our analysis on the relative importance of key baseline variables readily available to the transfusion medicine service. We have not examined the impact of acute GVHD incidence, its prophylaxis or treatment as a factor for transfusion support. For a series of NHLBI myeloablative transplants (excluding chronic leukemias) that included the cases evaluated in this study of transfusion support, acute GVHD grade II-IV was reported to be 42%, while grade III-IV was 14%.14
Contemporary HCT practice utilizes hematopoietic stem cells from HLA matched and mismatched donors and cord blood units as well as partially matched related donors (e.g. haploidentical). These sources may have greater major or minor histoincompatibility and greater potential for GVHD as compared to MSD. Acute GVHD occurring 100 or more days post-HCT may increase the need for transfusion for those individuals with involvement of the mucosa of the gastrointestinal tract. Other post-HCT complications such has autoimmune hemolytic anemia or immune thrombocytopenia may also contribute to the need for transfusion support. These are subjects for future analyses.
In conclusion, in this retrospective study, we describe several clinical factors associated with quantity of RBC and platelet transfusion support, and / or time to transfusion independence after MSD allogeneic HCT. Low doses of CD34+ stem cells and ABO mismatch of the graft, and a requirement for transfusion support pre-HCT due to marrow damage caused by disease or by chemotherapy used for treatment of the primary disease, are associated with impaired recovery of hematopoiesis post-transplant. This information should help to identify patients likely to have unusual or potentially urgent transfusion needs post-HCT, and plan prospectively for best patient care and maintenance of component inventory. We hope this report will assist with the management of such patients.
Supplementary Material
Acknowledgements
We thank the medical technologists and nursing staff of the Department of Transfusion Medicine for their professional expertise, dedication and team work which have contributed to the success of the hematopoietic stem cell transplantation programs of the Clinical Center, National Institutes of Health; and the patients who have been our partners in these studies for more than 25 years.
This work was supported by: the Intramural Research Programs of the Department of Transfusion Medicine, Clinical Center; the National Institute of Allergy and Infectious Diseases; the National Heart, Lung and Blood Institute; and the National Cancer Institute; and by the Division of Allergy, Immunology and Transplantation, and Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
This work was submitted in partial fulfillment of requirements for the degree of Master of Health Sciences in Clinical Research, NIH-Duke Training Program in Clinical Research, Department of Biostatistics and Bioinformatics, Duke University School of Medicine, Durham, NC.
The opinions expressed are those of the authors and do not represent the position of the National Institute of Allergy and Infectious Diseases; the National Heart, Lung and Blood Institute; the National Cancer Institute; the Department of Transfusion Medicine, Clinical Center; the National Institutes of Health, or the U.S. Government.
Footnotes
Authorship Statement: Dr. VanRaden has retired from NIAID, NIH.
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
References
- 1.McCullough J Principles of transfusion support before and after hematopoietic cell transplantation In: Forman SJ, Negrin RS, Antin JH, Appelbaum FR, editors. Thomas ’ Hematopoietic Cell Transplantation, Fifth Edition Oxford (UK) Wiley-Blackwell; 2016; p. 961–975. [Google Scholar]
- 2.Liu C, Grossman BJ. Red blood cell transfusion for hematologic disorders. Hematology American Society of Hematology Education Program. 2015; p. 454–461. [DOI] [PubMed] [Google Scholar]
- 3.Christou G, Iyengar A, Shorr R, et al. Optimal transfusion practices after allogeneic hematopoietic cell transplantation: a systematic scoping review of evidence from randomized controlled trials. Transfusion. 2016; 56: 2607–2614. [DOI] [PubMed] [Google Scholar]
- 4.Szczepiorkowski ZM, Dunbar NM. Transfusion guidelines: when to transfuse. Hematology American Society of Hematology Education Program; 2013: p. 638–644. [DOI] [PubMed] [Google Scholar]
- 5.Griffith LM, Klein HG. Immunohematology. In: Rich RR, Fleisher TA, Shearer WT, Kotzin BL, Schroeder HW, editors. Clinical Immunology: Principles and Practice, Second Edition London (UK): Mosby International Ltd; 2001; p. 127.1–127.10. [Google Scholar]
- 6.Vasu S, Bolan C. Transfusion medicine support for stem cell transplantation In: Treleaven J, Barrett AJ, editors. Hematopoietic Stem Cell Transplantation in Clinical Practice. Edinburgh (UK): Churchill Livingstone Elsevier; 2019; p. 315–329. [Google Scholar]
- 7.Dunbar NM. Hospital storage, monitoring, pretransfusion processing, distribution, and inventory management of blood components In: Fung MK, Grossman BJ, Hilly er C, Westhoff CM, editors. Technical Manual, 18th Edition Bethesda (MD): American Association of Blood Banks; 2014; p. 213–230. [Google Scholar]
- 8.Chapman JF, Hyam C, Hick R. Blood inventory management. Vox Sang 2004; 87 (Suppl. 2): S143–S145. [DOI] [PubMed] [Google Scholar]
- 9.Mavroudis DA, Read EJ, Cottier-Fox M, et al. CD34+ dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood. 1996; 88: 3223–3229. [PubMed] [Google Scholar]
- 10.Mavroudis DA, Read EJ, Molldrem J, et al. T cell-depleted granulocyte colony-stimulating factor (G-CSF) modified allogeneic bone marrow transplantation for hematological malignancy improves graft CD34+ cell content but is associated with delayed pancytopenia. Bone Marrow Transplant. 1998; 21: 431–440. [DOI] [PubMed] [Google Scholar]
- 11.Barrett AJ, Mavroudis DA, Tisdale J, et al. T Cell-depleted bone marrow transplantation and delayed T cell add-back to control acute GVHD and conserve a graft-versus-leukemia effect. Bone Marrow Transplant 1998; 21: 543–551. [DOI] [PubMed] [Google Scholar]
- 12.Bahceci E, Read EJ, Leitman S, et al. CD34+ cell dose predicts relapse and survival after T-cell-depleted HLA-identical haematopoietic stem cell transplantation (HSCT) for haematological malignancies. Br J Haematol. 2000; 108: 408–414. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura R, Bahceci E, Read EJ, et al. Transplant dose of CD34+ and CD3+ cells predicts outcome in patients with haematological malignancies undergoing T cell-depleted peripheral blood stem cell transplants with delayed donor lymphocyte add-back. Br J Haematol. 2001; 115: 95–104. [DOI] [PubMed] [Google Scholar]
- 14.Anandi P, Tian X, Ito S, et al. Ex vivo T-cell-depleted allogeneic stem cell transplantation for hematologic malignancies: the search for an optimum transplant T-cell dose and T-cell add-back strategy. Cytotherapy. 2017; 19: 735–743. [DOI] [PubMed] [Google Scholar]
- 15.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999; 94: 3234–3241. [PubMed] [Google Scholar]
- 16.Childs R, Chemoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. New Engl J Med. 2000; 343:750–758. [DOI] [PubMed] [Google Scholar]
- 17.Sloand E, Childs RW, Solomon S, et al. The graft-versus-leukemia effect of nonmyeloablative stem cell allografts may not be sufficient to cure chronic myelogenous leukemia. Bone Marrow Transplant. 2003; 32: 897–901. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz ME, Barrett AJ, Brown MR, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. New Engl J Med. 2001; 344: 881–888. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. New Engl J Med. 2009; 361: 2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salit RB, Fowler DH, Dean RM, et al. Host lymphocyte depletion as a strategy to facilitate early full donor chimerism after reduced-intensity allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013; 19: 1509–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salit RB, Fowler DH, Wilson WH, et al. Dose-adjusted EPOCH-rituximab combined with fludarabine provides an effective bridge to reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with lymphoid malignancies. J Clin Oncol. 2012; 30: 830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowler DH, Bishop MR, Gress RE. Immunoablative reduced intensity stem cell transplantation: potential role of donor Th2 and Tc2 cells. Semin Oncol. 2004; 31: 56–67. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell MR. Blood group incompatibilities and hemolytic complications of hematopoietic cell transplantation In: Forman SJ, Negrin RS, Antin JH, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation, Fifth Edition) Wiley-Blackwell; (Oxford, UK: ); 2016; p 955–960. [Google Scholar]
- 24.Cohn CS. Transfusion support issues in hematopoietic stem cell transplantation. Cancer Control. 2015; 22: 52–59. [DOI] [PubMed] [Google Scholar]
- 25.Staley EM, Schwartz J, Pham HP. An update on ABO incompatible hematopoietic progenitor cell transplantation. Transfus Apher Sci. 2016; 54: 337–344. [DOI] [PubMed] [Google Scholar]
- 26.Booth GS, Gehrie EA, Bolan CD, et al. Clinical guide to ABO-incompatible allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013; 19: 1152–1158. [DOI] [PubMed] [Google Scholar]
- 27.O’Donghaile D, Kelley W, Klein HG, et al. Recommendations for transfusion in ABO-incompatible hematopoietic stem cell transplantation. Transfusion 2012; 52: 456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hefazi M, Litzow M, Hogan W, et al. ABO blood group incompatibility as an adverse risk factor for outcomes in patients with myelodysplastic syndromes and acute myeloid leukemia undergoing HLA-matched peripheral blood hematopoietic cell transplantation after reduced-intensity conditioning. Transfusion 2016; 56: 518–527. [DOI] [PubMed] [Google Scholar]
- 29.Brierley CK, Littlewood TJ, Peniket AJ, et al. Impact of ABO blood group mismatch in alemtuzumab-based reduced-intensity conditioned haematopoietic SCT. Bone Marrow Transplant. 2015; 50: 931–938. [DOI] [PubMed] [Google Scholar]
- 30.Le Viellez A, P’Ng S, Buffery S, et al. Red cell and platelet transfusion burden following myeloablative allogeneic haemopoietic stem cell transplantation. Intern Med J. 2015; 45: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 31.Datta SS, Basu S, Chandy M. An analysis of transfusion support in haematopoietic stem cell transplantation - report from a centre in India. Transfus Apher Sci. 2015; 53: 373–377. [DOI] [PubMed] [Google Scholar]
- 32.Marenchino D, Maddalena L, Balbo R, et al. Consequences of ABO incompatibility in multiple myeloma patients undergoing peripheral blood stem cell transplantation after reduced intensity conditioning. Blood Transfus 2011; 9: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Sorror ML, Leisenring W, et al. The impact of donor type and ABO incompatibility on transfusion requirements after nonmyeloablative haematopoietic cell transplantation. Br J Haematol. 2010; 149: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erker CG, Steins MB, Fischer RJ, et al. The influence of blood group differences in allogeneic hematopoietic peripheral blood progenitor cell transplantation. Transfusion. 2005; 45: 1382–1390. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov V, Faucher C, Mohty M, et al. Decreased RBCTs after reduced intensity conditioning allogeneic stem cell transplantation: predictive value of prior Hb level. Transfusion. 2004; 44: 501–508. [DOI] [PubMed] [Google Scholar]
- 36.Xenocostas A, Yee A, Wong CJ, et al. RBC transfusion requirements after allogeneic marrow transplantation: impact of the before-transplant Hb level on transfusion and early survival. Transfusion. 2003; 43: 373–382. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz-Arguelles GJ, Lopez-Martinez B, Gomez-Rangel D, et al. Decreased transfusion requirements in patients given stem cell allografts using a non-myeloablative conditioning regimen: a single institution experience. Hematology. 2003; 8: 151–154. [DOI] [PubMed] [Google Scholar]
- 38.Weissinger F, Sandmaier BM, Maloney DG, et al. Decreased transfusion requirements for patients receiving nonmyeloablative compared with conventional peripheral blood stem cell transplants from HLA-identical siblings. Blood. 2001; 98: 3584–3588. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein SH, Nademanee AP, Vose JM, et al. for the Epidemiology of Platelet Recovery Study Group. A multicenter study of platelet recovery and utilization in patients after myeloablative therapy and hematopoietic stem cell transplantation. Blood. 1998; 91: 3509–3517. [PubMed] [Google Scholar]
- 40.Bolan CD, Childs RW, Procter JL, et al. Massive immune hemolysis after allogeneic peripheral blood stem cell transplantation with minor ABO incompatibility. Br J Haematol. 2001; 112: 787–795. [DOI] [PubMed] [Google Scholar]
- 41.Bolan CD, Leitman SF, Griffith LM, et al. Delayed donor red cell chimerism and pure red cell aplasia following major ABO-incompatible nonmyeloablative hematopoietic stem cell transplantation. Blood. 2001; 98: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 42. Griffith LM, McCoy JP Jr, Bolan CD, et al. Persistence of recipient plasma cells and antidonor isohemagglutinins in patients with delayed donor erythropoiesis after major ABO incompatible non-myeloablative allogeneic haematopoietic cell transplantation. Br J Haematol. 2005; 128: 668–675. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland DR, Anderson L, Keeney M, et al. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematotherapy. 1996; 5: 213–226. [DOI] [PubMed] [Google Scholar]
- 44.Dzik W, Sniecinski I, Fischer J. Toward standardization of CD34+ cell enumeration: an international study. Biomedical Excellence for Safer Transfusion Working Party. Transfusion. 1999; 39: 856–863. [DOI] [PubMed] [Google Scholar]
- 45.Barrett AJ. Essential biology of stem cell transplantation In: Treleaven J, Barrett AJ, editors. Hematopoietic Stem Cell Transplantation in Clinical Practice. Edinburgh (UK); Churchill Livingstone Elsevier; 2009; pages 9–21. [Google Scholar]
- 46.Weissman I Hematopoietic stem cells, regenerative medicine, and leukemogenesis In: Forman SJ, Negrin RS, Antin JH, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation, Fifth Edition Oxford (UK), Wiley-Blackwell; 2016; p. 25–49. [Google Scholar]
- 47.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu VWC, Scadden DT. Hematopoietic stem cell and its bone marrow niche. Curr Topics Dev Biol. 2016; 118: 21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoggatt J, Kfoury Y, Scadden DT. Hematopoietic stem cell niche in health and disease. Anna Rev Pathol. 2016; 11: 555–581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.