Abstract
Despite clear evidence that colorectal cancer (CRC) screening reduces mortality, screening, including fecal immunochemical tests (FIT), is underutilized. We conducted a systematic review to determine the evidence of efficacy of interventions to improve FIT completion that could be scaled and utilized in population health management. We systematically searched publication databases for studies evaluating provider- or system-level interventions to improve CRC screening by FIT between 1 January 1996 and 13 December 2017 without language restrictions. Twenty articles describing 25 studies were included, 23 were randomized controlled trials with 1 quasi-experimental and 1 observational study. Ten studies discussed mailed FIT outreach, 4 pre-FIT patient reminders, 3 tailored patient messages, 2 post-FIT reminders, 2 paired FIT with influenza vaccinations, 2 provider alerts and 1 study each described the use of high-quality small media and patient financial incentives. Mailed FIT outreach was consistently effective with median improvement in CRC screening of 21.5% (interquartile range (IQR) 13.6%−29.0%). FIT paired with vaccinations led to a median 15.9% (IQR 15.6%−16.3%) improvement, while pre-FIT and post-FIT reminders demonstrated modest efficacy with median 4.1% (IQR 3.6%−6.7%) and 3.1% (IQR 2.9%−3.3%) improvement in CRC screening, respectively. More than half the studies were at high or unclear risk of bias; heterogenous study designs and characteristics precluded meta-analysis. FIT-based CRC screening programs utilizing multilevel interventions (e.g. mailed FIT outreach, FIT paired with other preventative services, and provider alerts) have the potential to significantly increase screening participation. However, such programs must also follow-up patients with abnormal FIT results.
Introduction
Colorectal cancer (CRC) remains the 2nd leading cause of cancer deaths in the United States (U.S.) despite an over 30% decrease in incidence and mortality since 19851. There is clear evidence that screening by colonoscopy and stool-based tests is cost-effective2 and saves lives3,4; however screening remains underutilized5. While colonoscopy remains the primary modality for CRC screening in the U.S.6, there is increased uptake of stool-based CRC screening in large integrated health systems7 and in resource constrained settings such as safety-net populations, who also may have a preference8 for non-invasive screening modalities.
Fecal immunochemical test (FIT), when compared to the traditional 3-sample fecal occult blood test (FOBT), has superior test performance characteristics9 and adherence10. As a result, FIT is the preferred modality for fecal testing and has been adopted in programmatic CRC screening of average-risk adults around the world7,11–13. To accelerate progress in improving CRC screening rates in the U.S.,14 health systems will require programmatic screening efforts including increased use of FIT. Identifying low-cost, scalable interventions that improve FIT completion across large patient populations will be important in this effort. Scalable health interventions are interventions shown to be efficacious on a small scale/and or under controlled conditions that can be expanded under real world conditions to reach a greater proportion of the eligible population, while retaining effectiveness15.
The Center for Disease Control’s (CDC) Guide to Community Preventive Services recommends interventions to increase CRC screening completion from three broad strategies to (1) increase community demand (e.g. one-on-one education, client reminders, etc.), (2) increase community access (e.g. reducing structural barriers though mailed fecal-based tests, reducing client out-of-pocket costs, etc.), and (3) increase provider delivery of screening services (e.g. provider incentives, provider reminders, etc.)16. Using this CDC guide as a starting point, we sought to summarize the existing literature on interventions that could improve adherence to FIT-based CRC screening. Specifically, we focused on those interventions that did not require one-on-one patient and physician interactions and therefore have the potential to be scaled to improve CRC screening across healthcare systems or geographically designated regions. Given the emerging field of population health management, such interventions could theoretically lead to more rapid improvements in CRC screening rates and potentially address long-standing racial, ethnic, and socioeconomic inequities in CRC prevention. In sum, this paper aims to (1) systematically review the literature on potentially scalable healthcare system or provider-level interventions to improve FIT completion, (2) report the proportion screened by intervention types compared to controls and (3) recommend research and population health management strategies based on this evidence.
Methods
Data Sources and Literature Searches
We developed our search strategy with a medical librarian (EW) using keywords for fecal immunochemical tests and recommended interventions from the CDC Guide (Supplemental Table 1). We searched PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Web of Science databases from January 1st, 1996 through January 20th, 2017 and updated our search on December 13th, 2017. Additionally, related reviews17,18, bibliographies, reference lists of eligible papers, and registered clinical trials were also reviewed. This systematic review was conducted according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions19 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards20. A review protocol was registered a priori through PROSPERO, an international database of registered prospective systematic reviews (CRD42017054643, https://www.crd.york.ac.uk/PROSPERO/).
Study Eligibility and Selection
As we sought to report about interventions that could be potentially scaled and utilized in population-wide efforts to improve screening by FIT, studies that required one-on-one provider and patient interactions (e.g. one-on-one classes, patient navigators, etc.) were not included. Studies using the 3-sample FOBT, fecal deoxyribonucleic acid, studies on high-risk CRC populations, descriptive studies, methodology papers, out-of-scope review articles, population surveys, and conference abstracts without accompanying full citations were also not included. Additionally, due to the lack of validated criteria to assess the quality of feasibility studies, pilot studies were also excluded. Two reviewers (RBI and PA) independently appraised the pertinent studies to determine eligibility and studies were included if they: (1) evaluated asymptomatic adults aged 50–75 being screened for CRC by FIT, (2) examined a potentially scalable intervention to increase CRC screening completion by FIT compared to controls; and (3) reported the quantitative effect of this intervention on CRC screening completion. We included randomized controlled trials and non-randomized controlled trials. Studies that evaluated the effect of an intervention on multiple screening modalities were also included if the intervention effect on screening by FIT was provided.
Data Extraction
The reviewers (RBI and PA) independently extracted data from the included studies into a Microsoft Excel Spreadsheet. Information was abstracted on study design, setting, patient demographics, intervention attributes, usual care procedures, and study results. Whenever available, the type of FIT kit used, modality of FIT kit distribution, and colonoscopy rates for those with abnormal FIT results was also abstracted. Each intervention was then classified into the following discrete categories: (1) mailed FIT outreach, (2) pre-FIT reminders through letters or calls, (3) post-FIT reminders, (4) tailored patient messages, (5) high-quality small media (e.g. brochures, DVD’s, etc.), (6) patient financial incentives, (7) FIT screening paired with influenza vaccinations, and (8) provider alerts utilizing electronic health records.
Risk of Bias/Quality Assessment
Two authors (RBI and PA) independently assessed the methodologic quality of each included study using the tool developed by the Cochrane Collaboration for randomized and nonrandomized controlled trials19. This tool allows for the risk of bias to be assessed within each of the specified domains (e.g. selection bias, performance bias, reporting bias etc.). Rated studies were given an overall summary assessment of “low” risk of bias if the majority of key domains were rated as low, “high” risk of bias if one or more domain was rated as high, or “unclear” risk of bias if several domain ratings were rated as unclear due to lack of available information in the published manuscript. Any disagreement in abstraction or risk of bias assessments were resolved through discussion.
Data Synthesis and Analysis
For each study, we calculated the percent of CRC screening completed in the intervention arm compared to the control/usual care (hereinafter referred to as control) arm and reported the median difference in CRC screening completion and interquartile ranges (IQR) across treatment arms in the intervention categories. Due to heterogeneity in methods, settings and patient populations, we did not combine these studies in a quantitative meta-analysis.
Results
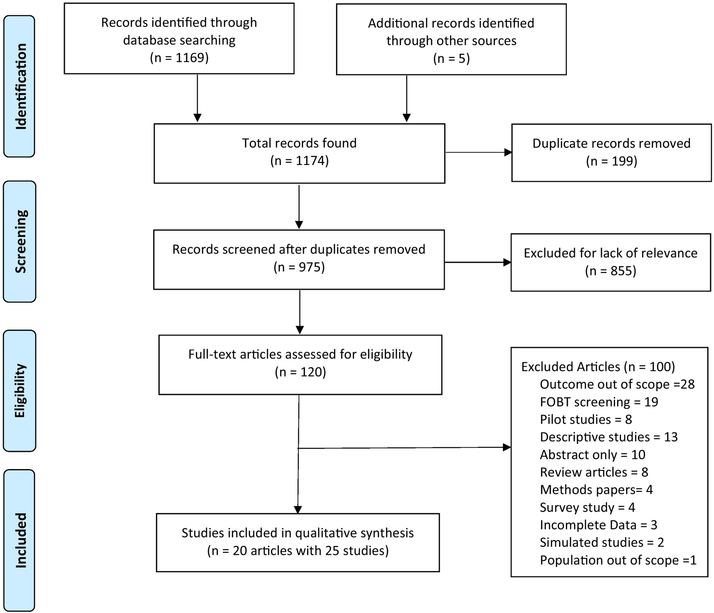
After removing duplicates, we identified 975 unique manuscripts; 120 full-text articles were assessed and 20 met inclusion criteria (Figure 1). Myers et al.21, Cole et al.22, and Giorgi Rossi et al.23 each evaluated multiple interventions across different study arms resulting in 25 unique studies (Table 1). Two studies described interventions at the provider-level24,25, the remaining 23 studies evaluated interventions at a clinic, healthcare system, or population-level. Interventions reported in this review include: (1) mailed FIT outreach21,23,26–32; (2) pre-FIT reminders through letters or calls22,33–35; (3) post-FIT reminders21,36; (4) tailored patient messages21,22; (5) high-quality small media37; (6) patient financial incentives38; (7) FIT screening paired with influenza vaccinations39,40; and (8) provider alerts24,25. Sixteen studies were conducted in the U.S., 3 in Australia, 3 in Italy, and 1 each in the Netherlands, France and Spain. Study sample sizes ranged from 330 to 49,815 and mean patient age varied from 55.7 to 63.1 years. The descriptive characteristics and findings of the 25 included studies are outlined in Tables 1 and 2. Due to insufficient details in study methods, lack of blinding or potential for selection bias, many of the included studies were rated as high or unclear risk of bias (Table 3).
Figure 1:
Flow Diagram of Included and Excluded Studies (1996–2017)
Table 1:
Summary of Included Studies
| Study, Year | Country | Study Design | Setting | Intervention Type | Follow-up Time | Intervention Screened/Total (%) | Control Screened/Total (%) | Change in screening (%) |
|---|---|---|---|---|---|---|---|---|
| Singal et al,32 2016 | USA | RCT | Safety-Net | Mailed FIT | 12 months | 1410/2400 (58.8) | 355/1199 (29.6) | 29.1 |
| Goldman et al,28 2015 | USA | RCT | FQHC | Mailed FIT | 6 months | 77/210 (36.7) | 31/210 (14.8) | 21.9 |
| Baker et al,26 2014 | USA | RCT | FQHC | Mailed FIT | 6 months | 185/225 (82.2) | 84/225 (37.3) | 44.9 |
| Hendren et al,30 2014 | USA | RCT | Safety-Net | Mailed FIT | 12 months | 43/114 (37.8) | 21/126 (16.7) | 21.1 |
| Charlton et al,27 2014 | USA | RCT | VA | Mailed FIT | 6 months | 71/500 (14.2) | 0/500 (0) | 14.2 |
| Gupta et al,29 2013 | USA | RCT | Safety-Net | Mailed FIT | 12 months | 648/1593 (40.7) | 471/3898 (12.1) | 28.6 |
| Levy et al,31 2013 | USA | RCT | PCCs | Mailed FIT | 15 months | 105/186 (56.4) | 33/185 (17.8) | 38.6 |
| Giorgi Rossi et al,23 2011 | Italy | RCT | National | Mailed FIT | 3 months | 1006/1569 (64.1) | 908/1600 (56.8) | 7.4 |
| Giorgi Rossi et al,23 2011 | Italy | RCT | National | Mailed FIT | 3 months | 307/2107 (14.6) | 226/ 2112 (10.7) | 3.9 |
| Myers et al,21 2007 | USA | RCT | Safety-Net | Mailed FIT | 24 months | 177/387 (45.7) | 126/387 (32.6) | 13.2 |
| Senore et al,34 2015 | Italy | RCT | National | Pre-FIT Reminder | 9 months | 3888/10257 (37.9) | 3580/10444 (34.3) | 3.6 |
| Kempe et al,33 2012 | USA | Quasi-Exp | KPCO | Pre-FIT Reminder | 12 months | 3497/8583 (40.7) | 14952/41232 (36.3) | 4.5 |
| Van Roon et al,35 2011 | Netherlands | RCT | National | Pre-FIT Reminder | N/A | 1539/2390 (64.4) | 1462/2394 (61.1) | 3.3 |
| Cole et al,22 2007 | Australia | RCT | CHC | Pre-FIT Reminder | 3 months | 290/600 (48.3) | 237/600 (39.5) | 8.8 |
| Levy BT et al,36 2012 | USA | RCT | PCCs | Post-FIT Reminder | 15 months | 91/187 (48.7) | 84/186 (45.2) | 3.5 |
| Meyers et al,21 2007 | USA | RCT | Safety-Net | Post-FIT Reminder | 24 months | 187/386 (48.4) | 177/387 (45.7) | 2.7 |
| Myers et al,21 2007 | USA | RCT | Safety-Net | Tailored Messaging | 24 months | 168/386 (43.5) | 177/387 (45.7) | −2.2 |
| Cole et al,22 2007 | Australia | RCT | CHC | Tailored Messaging | 3 months | 242/600 (40.3) | 237/600 (39.5) | 0.8 |
| Cole et al,22 2007 | Australia | RCT | CHC | Tailored Messaging | 3 months | 216/600 (36.0) | 237/600 (39.5) | −3.5 |
| Davis et al,37 2016 | USA | RCT | FQHC & CHC | Small Media | 6 months | 164/210 (78.1) | 172/206 (90.3) | −5.4 |
| Gupta et al,38 2016 | USA | RCT | Safety-Net | Financial Incentives | 12 months | 738/2000 (36.9) | 2379/6565 (36.2) | 0.7 |
| Potter et al,39 2013 | USA | RCT | KPNC | FIT w/vaccination | 3 months | 900/3351 (26.9) | 336/2884 (11.7) | 15.2 |
| Potter et al,40 2011 | USA | Obs. | KPNC | FIT w/vaccination | 3 months | 853/2812 (30.3) | 635/4653 (13.7) | 16.6 |
| Rat et al,25 2017 | France | Cluster RCT | National | Provider Alerts | 12 months | 123/496* (24.8) | 94/455* (20.6) | 4.2 |
| Guiriguet et al,24 2016 | Spain | Cluster RCT | National | Provider Alerts | 12 months | 9536/21619 (44.1) | 8196/19423 (42.2) | 1.9 |
Abbreviations: RCT (Randomized Controlled Trial); FQHC (Federally Qualified Heath Center); VA (Veterans Administration Hospital); PCCs (Primary Care Clinics); KPCO (Kaiser Permanente Colorado); CHC (Community Health Centers); KPNC (Kaiser Permanente Northern California); Obs. (Observational Study)
Authors did not provide numbers of patients in intervention and control arms but did provide percentage screened.
Table 2:
Detailed Characteristics of Included Studies
| Study, Year | Informed Consent Waiver | Prior screening rate (%) | Type of FIT | FIT follow-up rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean age (Years) | Women (%) | NHC (%) | AA (%) | Hispanic (%) | English speakers (%) | |||||
| Singal,32 2016 | 56.1 | 61.6 | 21.5 | 24.1 | 48.5 | 61.3 | Yes | N/A | Polymed co OC-Auto FIT CHEK | 49.3 |
| Goldman,28 2015 | 57.3 | 66.0 | 15. 7 | 16. 4 | 62.1 | 51.5 | Yes | 0 | Polymed co OC-Light | 30.0 |
| Baker,26 2014 | 59.5 | 71.6 | N/A | N/A | 89.3 | N/A | Yes | 100 | Polymed co OC-Light | 58.6 |
| Hendren,30 2014 | 53.9 | N/A | 53.3 | 39.6 | N/A | N/A | Yes | N/A | N/A | N/A |
| Charlton,27 2014 | 59.3 | 0.1 | N/A | N/A | N/A | N/A | No | N/A | N/A | 81.2 |
| Gupta,29 2013 | 59.0 | 63.9 | 41.0 | 23.3 | 28.9 | 83.3 | Yes | N/A | Polymed co OC-Auto FIT CHEK | 81.7 |
| Levy,31 2013 | N/A | 52.8 | 98.1 | 0.01 | N/A | N/A | No | N/A | Clearview Ultra FIT | N/A |
| Giorgi Rossi,23 2011 | N/A | N/A | N/A | N/A | N/A | N/A | No | 100 | OC-Hemodia | N/A |
| Giorgi Rossi,23 2011 | N/A | N/A | N/A | N/A | N/A | N/A | No | Previously unresponsivea | OC-Hemodia | N/A |
| Myers,21 2007b | N/A | N/A | N/A | N/A | N/A | N/A | No | N/A | InSure FIT | N/A |
| Senore,34 2015 | N/A | 53.1 | N/A | N/A | N/A | N/A | Yes | N/A | N/A | N/A |
| Kempe,33 2012b | N/A | N/A | N/A | N/A | N/A | N/A | Yes | N/A | OC-Sensor Micro | 79.0 |
| Van Roon,35 2011 | 60.3 | 51.0 | N/A | N/A | N/A | N/A | No | N/A | OC-Sensor Micro | N/A |
| Cole,22 2007 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | InSure FIT | N/A |
| Levy BT,36 2012 | 61.2 | 51.7 | 99.2 | 0.5 | N/A | N/A | No | N/A | Clearview Ultra FIT | N/A |
| Meyers,21 2007b | N/A | N/A | N/A | N/A | N/A | N/A | No | N/A | InSure FIT | N/A |
| Myers,21 2007b | N/A | N/A | N/A | N/A | N/A | N/A | No | N/A | InSure FIT | N/A |
| Cole,22 2007 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Insure FIT | N/A |
| Cole,22 2007 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Insure FIT | N/A |
| Davis,37 2016 | 55.7 | 53.6 | 66 | 28.2 | N/A | N/A | No | 32.0 | N/A | 76.2 |
| Gupta,38 2016 | 56.0 | 61.8 | 37.1 | 24.4 | 29.2 | 83.3 | Yes | N/A | Polymed co OC-Sensor | 57.0–63.0 |
| Potter,39 2013 | 61.5 | 57.4 | 46.6 | 5.7 | N/A | 87.5 | Yes | N/A | Polymed co OC-Auto FIT CHEK | N/A |
| Potter,40 2011 | 63.1 | 55.4 | 51.0 | 1.7 | 11.3 | 88.0 | Yes | N/A | Polymed co OC-Micron | N/A |
| Rat,25 2017 | 60.9 | 38.9 | N/A | N/A | N/A | N/A | Yes | Previously unresponsivea | N/A | N/A |
| Guiriguet,24 2016 | 58.7 | 53.6 | N/A | N/A | N/A | N/A | Yes | 0 | N/A | N/A |
Abbreviations: N/A- not available
Authors stated that participants had been previously unresponsive but did not specify proportion that had never completed screening.
Demographics published on the sub-groups combined but was not available by study sub-groups.
Table 3:
Quality Assessment for Randomized Controlled Trials or Controlled Trialsa
| Author, Year | Cochrane Collaboration’s tool for assessing risk of bias (ROB) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | ||||
| Study Design | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias | Summary assessment High/Low/Unclear ROB | |
| Singal,32 2016 | RCET | Yes | Yes | Yes | Yes | No | No | No | Low |
| Goldman,28 2015 | RCT | Yes | Yes | Unclear | Yes | No | No | Unclear | Low |
| Baker,26 2014 | RCT | Yes | Yes | Unclear | Yes | No | No | No | Low |
| Hendren,30 2014 | RCT | Yes | Yes | Unclear | Yes | Unclear (outcomes reported values vary from patients randomized). | No | Unclear | Unclear |
| Charlton,27 2014 | RCT | Yes | Unclear | Unclear | Yes | No | No | Potential for volunteer bias | High |
| Gupta,29 2013 | RCET | Yes | Yes | Yes | Yes | Yes (small and similar across study arms) | No | No | Low |
| Levy,31 2013 | RCT | Yes | Unclear | Unclear | Yes | Unclear | No | Unclear, overall low participation | Unclear |
| Giorgi Rossi,23 2011 | RCT | Yes | Unclear | Unclear | Unclear | No | No | No | Unclear |
| Myers,21 2007 | RCT | Unclear | No | Unclear | Yes | Unclear | No | No | Unclear |
| Senore34, 2015 | RCT | Yes | Yes | Unclear | Unclear | Yes (one site completed 2/3 study arms) | Unclear (outcomes not explicitly stated) | No | High |
| Kempe,33 2012 | Quasi-Experimental | No | Unclear | Unclear | Unclear | No | No | No | Unclear |
| Van Roon,35 2011 | RCT | Yes | Unclear | Unclear | Unclear | Yes (not intention-to-treat analysis) | No | No | High |
| Cole,22 2007 | RCT | Unclear | Unclear | Unclear | Unclear | No | No | Unclear | Unclear |
| Levy,36 2012 | RCT | Unclear | Unclear | Unclear | Unclear | No | No | Unclear | Unclear |
| Davis,37 2016 | RCT | Unclear | Unclear | Unclear | Unclear | No | No | Potential for volunteer bias | High |
| Gupta,38 2016 | RCET | Yes | Unclear | Unclear | Unclear | No | No | No | Unclear |
| Potter,39 2013 | RCT | Yes | Unclear | Unclear | Unclear | No | No | Unclear, overall low participation | Unclear |
| Rat,25 2017 | Clust er RCT | Yes | Unclear | Unclear | Yes | No | No | No | Low |
| Guiriguet,24 2016 | Cluster RCT | Yes | Yes | Yes | Yes | No | No | No | Low |
Clinic, Healthcare System and Population-Level Interventions
Mailed FIT Outreach
Ten studies from 9 articles investigated direct mailing of FIT kits to improve CRC screening (Table 1)21,23,26–32. Among included studies, the median efficacy of mailed FIT outreach to improve CRC screening compared to controls was 21.5% (IQR 13.6%−29.0%). All were randomized controlled trials (RCT’s), over half (6/10, 60%) were conducted in safety-net systems or federally qualified health centers (FQHC’s) and follow-up time varied from 3 to 24 months.
Although the studies by Goldman et al.28 and Baker et al.26 were conducted in the same health care setting, all the patients in Goldman et al. had never been previously screened while all the patients in Baker et al. had a prior negative FOBT. As a result, Baker et al. reported higher participation in both intervention and control arms (82.7% and 37.3%, respectively) compared to Goldman et al. (36.7% and 14.8%). Notably, the community health centers where the Goldman et al. and Baker et al. studies were conducted, implemented strategies to improve overall CRC screening prior to mailed FIT outreach including provider performance feedback and financial incentives.
A similar pattern of differential screening by prior participation was also observed in the studies by Giorgi Rossi et al23. Among those that had previously completed stool-based screening, CRC screening rates were higher in both the intervention and control arms (64.1% and 56.8% respectively) compared to the previously non-responsive cohort (14.6% and 10.7%). Myers et al.21 reported that 41% of the entire study population had previously completed CRC screening, while 59% had never previously been screened, but the authors did not report the effect of mailed FIT outreach by prior screening behavior. Other mailed FIT outreach studies did not describe the intervention effect across treatment arms by prior screening behavior (Table 2).
The lowest efficacy of mailed FIT outreach was noted in Charlton et al.27, where 14.2% of the intervention arm completed CRC screening by FIT. Charlton et al. targeted a primarily rural veteran population which was primarily male and under age 65, factors associated with lower screening participation1,41. Additionally, the authors relied upon a pre-participation survey to screen for eligibility and did not obtain a waiver of informed consent. Ultimately, 21% (107/500) of patients agreed to participate in the intervention and 14% (71/500) completed screening after mailed FIT outreach.
Among the studies included, CRC screening completion rates in the control populations were also highly variable, median 17.3% (IQR 12.8%−31.8%). Studies provided limited information on usual care, but when available varied from opportunistic clinic-based discussions29,32 to scheduled appointments to discuss CRC screening communicated via a mailed letter23.
Pre-FIT patient reminders
Four studies described the use of a letter containing background information on CRC and the potential benefits of screening prior to FIT distribution22,33–35. The benefit of pre-FIT reminders compared to control was small but consistently effective with a median improvement of 4.1% (IQR 3.6%−6.7%) in CRC screening. Settings included a large integrated health care system and population-based screening programs with follow-up times of 3 to 12 months.
Only 1 study discussed the impact of a pre-FIT reminder by prior screening behavior. In Senore et al.34, FIT completion rates were similar in the pre-FIT reminder arm compared to controls for initial screeners (52.0% vs. 52.8%), regular screeners (23.5% vs. 22.8%), and never screeners (24.5% vs. 24.4%). When the authors extended follow-up, overall CRC screening completion in the pre-FIT reminder group increased from 37.9% at 9 months to 50.7% at 18 months, whereas the control arm increased from 34.3% to 48.8%. This study also attempted to report the effect of a pre-FIT reminder letter in combination with an offer for in-person CRC counseling from the participants primary care provider (PCP), these results were incompletely published as some PCPs chose not to participate.
Post-FIT patient reminders
Two studies described the use of phone calls or letters to improve the return of FIT kits after distribution. In Levy et al.36, patients randomized to a mailed FIT kit and educational materials alone were compared to patients who received a mailed FIT kit, educational materials, and a reminder telephone call 2–4 weeks after mailed materials. On follow-up, there was a 3.5% (48.7% vs. 45.2%, P=0.50) improvement in FIT completion among patients randomized to a post-FIT telephone reminder compared to those who did not receive this telephone call.
In Myers et al.21, patients receiving an intervention which included a mailed FIT kit, a letter with tailored messages, and a reminder letter were randomized to also receive a reminder telephone call or no telephone call. On follow-up, there was a non-significant increase in FIT completion among patients who received a telephone call compared to those who didn’t receive a telephone call (48.5% vs. 43.8%, P=0.19).
Tailored patient messaging
Three studies from 2 articles evaluated the impact of tailored messaging on CRC screening by FIT. In Myers et al.21, patients randomized to receive a mailed FIT kit, reminder letter, and tailored messaging pages addressing screening barriers were compared to (1) patients receiving mailed FIT kits and a reminder letter only and (2) patients not receiving any interventions (controls). On follow-up, patients who received the tailored messages were more likely to complete FIT compared to controls (43.8% vs. 32.6%, P=0.002); however, the tailored messages did not improve FIT completion when compared to those who received a mailed FIT kit and reminder letter alone (43.8% vs. 45.7%, P=0.68).
In Cole et al.22, patients receiving a mailed FIT kit were randomized to (1) standard insert alone, (2) standard insert and a pre-FIT reminder, (3) standard insert and a letter with brief messages about the risk of CRC, or (4) standard insert and a letter with messages from other patients advocating for CRC screening. There was no significant difference in FIT completion between patients who received the CRC risk messages compared to mailed FIT kit alone (40.3% vs. 39.5%, P=0.77). There was also no significant difference in FIT completion between patients who received messages from other patients compared to FIT kit alone (36% vs. 39.5%, P=0.21).
High-quality small media
In Davis et al.,37 patients receiving mailed FIT outreach through FQHCs and community health centers were randomized to either a low-literacy picture-booklet and DVD containing storylines of local citizens modeling screening using a FIT kit or a trifold CRC screening brochure by the CDC. At the 6-month follow-up, the overall FIT completion rate was 81% with a non-significant increase in FIT completion among those randomized to the low-literacy materials compared to the CDC brochure (83.5% vs. 78.1%, P=0.17). The majority of patients in the intervention and control arms, 68% and 69% respectively, had never previously completed CRC screening. The cohort was educationally and economically diverse: 24% reported having less than a high school education, 63% reported a household income of less than $10,000, and 61% had health insurance primarily through the county.
Patient financial incentives
Gupta et al38. randomized patients to mailed FIT outreach with or without a $5 or $10 incentive after the receipt of FIT kits to determine the effect of a patient financial incentive on screening completion. The mailed FIT outreach consisted of a 1-sample FIT kit, two automated telephone reminders in English and Spanish 1 week after mailed FIT kit, and up to two live telephone reminders within 4 weeks of the originally mailed FIT kit. Compared to no incentive, the additional financial incentive did not significantly improve FIT completion at $5 (39.2% vs. 36.2%, P=0.07) or at $10 (34.6% vs. 36.2%, P=0.32). In subgroup analysis, the effect of a $5 or $10 financial incentive on CRC screening did not differ substantially by age, gender, race, ethnicity or neighborhood poverty rate.
FIT paired with vaccination
Two studies completed by the same lead author evaluated the impact of pairing FIT with influenza (FLU) vaccinations. In an initial observational study, Potter et al40. designated FLU vaccination clinics at a single Kaiser Permanente Northern California clinic as FLU vaccination clinics only or FLU-FIT clinics. FIT kits for CRC screening were distributed to patients who received care in designated FLU-FIT clinics. At 90-days, the authors reported a statistically significant improvement in CRC screening by FIT among those seen in the FLU-FIT clinics compared to FLU only clinics (14% vs. 4.8%, P<0.001).
In a follow-up RCT39, patients in 5 Kaiser Permanente influenza vaccination clinics were randomized to either vaccination alone or vaccination with a FIT kit at point-of-care. CRC screening by FIT significantly improved in the intervention group compared to controls (26.9% vs. 11.7%, P<0.01). In an adjusted multivariable logistic regression, the odds of completing FIT for CRC screening was 2.75 for those randomized to the intervention compared to controls. Older age (66–74), being of Asian American race, and having at least 1 but no more than 10 primary care visits in the previous year were all positively associated with completing the FIT within 90 days of vaccination.
Provider-Level Interventions
Provider Alerts
Two studies reported on the impact of provider alerts in CRC screening by FIT. Guiriguet et al.24 cluster randomized physicians assigned to a population of 1st time screeners as part of a national FIT-based CRC screening program to either receive an electronic alert reminding them to discuss CRC screening (N=67) or to not receive a reminder (N=63). On follow-up, electronic alerts only minimally increased FIT completion (44.1% vs. 42.2%, OR 1.08, P=0.15). Ultimately, 25.6% of all patients did not visit their PCP during the study period while 44.3% visited ≥ 5 times, however the authors did not provide details of clinic attendance by treatment arms.
In the study by Rat et al.25, PCPs in France were cluster randomized to either (1) receive a list of their patients aged 50–74 who had not yet completed FIT screening (N=496 providers), (2) receive general CRC screening rates in their local administrative district (N=495 providers), or (3) receive no intervention with their patients receiving usual CRC screening care (N=455 providers). Usual care in this setting consisted of an invitation encouraging patients to pick-up a FIT kit from their PCPs. At 1-year follow-up, the mean patient participation per PCP was 24.8% (95% CI 23.4%−26.2%) among those randomized to receive a specific patient list, 21.7% (95% CI 20.5%−22.8%) in the generic CRC screening rate group, and 20.6% (95% CI 19.3%−21.8%) in the usual care group.
Discussion
This systematic review evaluated several interventions to increase population-wide FIT-based CRC screening participation. Mailed FIT outreach was the most commonly studied intervention and consistently improved CRC screening rates. FIT paired with influenza vaccination, pre- and post-FIT reminders and provider alerts, also modestly improved FIT completion. In contrast, tailored patient messages, high-quality small media and patient financial incentives did not improve CRC screening when compared to controls. This review synthesizes the evidence and expected impact when adopting various interventions aimed to improve FIT-based CRC screening. Consequently, healthcare systems focused on population-wide CRC screening will be better informed as they invest in interventions that improve the health of their patients.
While mailing FIT kits effectively increased screening participation, the rates varied widely across studies. Considering the factors that may enhance the effect of direct mailing, in the study with the highest participation, Baker et al.,26 all patients had previously participated in stool-based screening, 60% had 0–1 chronic conditions, 75% visited their PCP at least once during the 6-month follow-up, and there were few incorrect addresses. Additionally, this network of FQHCs implemented point-of-care strategies to improve CRC screening prior to the initiation of the mailed FIT outreach including performance feedback, provider financial incentives, and transitioned to FIT from FOBT. These strategies led to an improvement in baseline CRC screening rates from 17% to 43% prior to the initiation of the study.
One challenge noted across multiple studies was reaching an upper limit of patients willing to participate in CRC screening. For example, while Goldman et al.28 noted that mailed FIT outreach significantly increased the rate of CRC screening compared to controls, the absolute screening rate remained low. Despite the promise of mailed FIT outreach, many patients do not respond to mailings. In a study by Coronado et al.42, 35% of patients who failed to return a mailed FIT kit stated they never received the kit. In qualitative surveys, patients who did not complete FIT after multiple rounds of mailed outreach reported low self-efficacy, avoidance, and concerns about handling stool43, highlighting a few of the challenges when dealing with health beliefs and stool-based screening. To improve participation in CRC screening and reduce CRC-related mortality, the National Institutes of Health (NIH) has recently called for the evaluation of interventions that target change at two or more levels of care (i.e. multilevel interventions).44 Organized outreach programs that utilize multilevel interventions may overcome some of these challenges and lead to more durable and sustainable improvements in CRC screening participation.7
Prior research has demonstrated that FIT participation rates remain stable through multiple rounds of screening45, and as such prior FIT completion is predictive of future FIT completion. The studies by Baker26, Goldman28, and Giorgi Rossi23 provides further evidence to support this observation. Knowing this, it was our intention to examine intervention effects by prior screening status. Unfortunately, we were unable to do so given the lack of available information from several included studies. Other factors that might influence effectiveness and inform the implementation of mailed FIT outreach such as systems screening structure and implementation strategies were also not reported in several instances.
Ultimately, when screening programs rely upon one-on-one patient and provider interactions, these efforts may be challenging to sustain; in particular, the durability of the activities are susceptible to staff turnover46. The capacity of electronic health records to create patient registries, facilitate implementation of interventions and monitor the impact of interventions over time is an asset to population-wide outreach that could lead to rapid improvements in CRC screening participation and potentially reduce inequities in screening rates.
Inherent to FIT-based screening is that colonoscopy is performed for all patients with abnormal FIT results. Rates of diagnostic colonoscopy for patients with abnormal FIT results were available in only 32% (8/25) of included studies and varied from 30%−82%. While FIT follow-up is beyond the scope of this review, prior to implementing FIT-based CRC screening, programs should ensure the appropriate infrastructure is present to support maximal follow-up of abnormal FIT results. Several studies suggest that colonoscopy completion after abnormal FIT results are inadequate across many settings.47,48 A recent systematic review found moderate evidence supporting the use of patient navigators and provider reminders/performance data to improve colonoscopy rates of adults with positive fecal blood tests49.
Our results are consistent with a recent systematic review of RCTs aimed at increasing uptake of fecal tests for CRC screening by Rat et al.18 which found that advance notification letters and mailed outreach of fecal tests increased completion of fecal based screening tests. Our study, which focuses on interventions that could be scaled and utilized in population health, differs from the review by Rat et al. which included all fecal tests for CRC screening and interventions that required one-on-one provider and patient interactions.
The strengths of this review include a comprehensive evaluation of several interventions to improve CRC screening by FIT in diverse patient cohorts and health care settings. However, there are several limitations. First, although many health systems have adopted FIT, we were unable to evaluate intervention effects because these systems did not have a control arm7. Second, while pre- or post-FIT patient reminders are widely utilized, there were few published controlled trials likely due to its low-cost and ease of implementation. While high-cost personnel-intensive interventions (e.g. patient navigation, one-on-one classes, etc.) may be impractical in certain settings, a low-cost intervention such as a patient reminder using population notification systems may be feasible to implement. Third, due to heterogeneity in methods, settings and patient populations, we did not combine these studies in a quantitative meta-analysis as originally planned. Finally, several studies did not report about patient factors (e.g. prior screening status) or implementation strategies that might influence and inform the effectiveness of a given intervention.
Conclusions
In summary, to achieve population-wide health improvements in CRC screening, interventions that are effective in research settings should be implemented as widely as possible, considering infrastructural factors and implementation strategies that will promote high-quality screening programs. The impact of programmatic CRC screening can be further enhanced by multilevel interventions and this systematic review provides evidence that mailed FIT outreach, pre- and post-FIT reminders, pairing FIT with vaccinations, and provider alerts are potential strategies to improve CRC screening. In addition to these interventions, population-wide screening efforts should also consider tailored interventions based on prior screening behaviors and enhanced utilization of electronic health records to identify patients due for screening and track patients through the screening continuum. Altogether, these strategies have the potential to lead to rapid improvements in CRC screening participation and reduce downstream inequities in care outcomes beyond any singular intervention.
Supplementary Material
Highlights.
Population health approaches are important in colorectal cancer (CRC) screening.
Fecal immunochemical tests (FIT) use in CRC screening is rapidly increasingly.
Mailed FIT outreach consistently increases CRC screening rates, but magnitude varies.
Pre- and post-FIT reminders lead to small increases in screening completion.
Multilevel interventions may accelerate improvements in CRC screening rates.
Acknowledgements
Guarantor of the article: Rachel Issaka, MD, MAS
Grant Support: This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (T32DK007007; RI), the Jacobsohn Fund for Excellence (MS), and the Centers for Disease Control and Prevention (U48 DP004998; MS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- CRC
Colorectal Cancer
- FIT
Fecal Immunochemical Test
- RCT
Randomized Controlled Trial
- FQHCs
Federally Qualified Health Centers
Footnotes
Conflict of Interest: No conflicts of interest were reported by the authors of this paper
Financial Disclosures: No financial disclosures were reported by the authors of this paper
Writing Assistance: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Heitman SJ, Hilsden RJ, Au F, Dowden S, Manns BJ. Colorectal cancer screening for average-risk North Americans: an economic evaluation. PLoS Med. 2010;7(11):e1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. [DOI] [PubMed] [Google Scholar]
- 4.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8. [DOI] [PubMed] [Google Scholar]
- 5.Cyhaniuk A, Coombes ME. Longitudinal adherence to colorectal cancer screening guidelines. Am J Manag Care. 2016;22(2):105–111. [PubMed] [Google Scholar]
- 6.Centers for Disease Control. Vital Signs: Colorectal Cancer Screening Test Use — United States, 2012. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 7.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–110. [DOI] [PubMed] [Google Scholar]
- 8.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan C, Giorgi Rossi P, Camilloni L, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther. 2012;36(10):929–940. [DOI] [PubMed] [Google Scholar]
- 11.Australian Institute of H, Welfare, Australian Government Department of H. Analysis of colorectal cancer outcomes for the Australian National Bowel Cancer Screening Program. Asia Pac J Clin Oncol. 2016;12(1):22–32. [DOI] [PubMed] [Google Scholar]
- 12.Minozzi S, Armaroli P, Segnan N. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Principles of evidence assessment and methods for reaching recommendations. Endoscopy. 2012;44 Suppl 3:SE9–14. [DOI] [PubMed] [Google Scholar]
- 13.Telford J, Gentile L, Gondara L, McGahan C, Coldman A. Performance of a quantitative fecal immunochemical test in a colorectal cancer screening pilot program: a prospective cohort study. CMAJ Open. 2016;4(4):E668–E673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Colorectal Cancer Roundtable. 80% by 2018. 2016; http://nccrt.org/tools/80percent-by-2018/. Accessed May 1, 2017.
- 15.Milat AJ, King L, Bauman AE, Redman S. The concept of scalability: increasing the scale and potential adoption of health promotion interventions into policy and practice. Health Promot Int. 2013;28(3):285–298. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control. Guide to Community Preventive Services. 2016; https://www.thecommunityguide.org/findings/cancer-screening-multicomponentinterventions-colorectal-cancer. Accessed September 1, 2017.
- 17.Davis MM, Freeman M, Shannon J, et al. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States - How, what and when? BMC cancer. 2018;18(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rat C, Latour C, Rousseau R, et al. Interventions to increase uptake of faecal tests for colorectal cancer screening: a systematic review. Eur J Cancer Prev. 2018;27(3):227–236. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2011: http://handbook-5-1.cochrane.org/. Accessed March 5 2018. [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336341. [DOI] [PubMed] [Google Scholar]
- 21.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):20832091. [DOI] [PubMed] [Google Scholar]
- 22.Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J Med Screen. 2007;14(2):73–75. [DOI] [PubMed] [Google Scholar]
- 23.Giorgi Rossi P, Grazzini G, Anti M, et al. Direct mailing of faecal occult blood tests for colorectal cancer screening: a randomized population study from Central Italy. J Med Screen. 2011;18(3):121–127. [DOI] [PubMed] [Google Scholar]
- 24.Guiriguet C, Munoz-Ortiz L, Buron A, et al. Alerts in electronic medical records to promote a colorectal cancer screening programme: a cluster randomised controlled trial in primary care. Br J Gen Pract. 2016;66(648):e483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rat C, Pogu C, Le Donne D, et al. Effect of Physician Notification Regarding Nonadherence to Colorectal Cancer Screening on Patient Participation in Fecal Immunochemical Test Cancer Screening: A Randomized Clinical Trial. JAMA. 2017;318(9):816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–1241. [DOI] [PubMed] [Google Scholar]
- 27.Charlton ME, Mengeling MA, Halfdanarson TR, et al. Evaluation of a home-based colorectal cancer screening intervention in a rural state. J Rural Health. 2014;30(3):322332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman SN, Liss DT, Brown T, et al. Comparative Effectiveness of Multifaceted Outreach to Initiate Colorectal Cancer Screening in Community Health Centers: A Randomized Controlled Trial. J Gen Intern Med. 2015;30(8):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendren S, Winters P, Humiston S, et al. Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. J Gen Intern Med. 2014;29(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy BT, Xu Y, Daly JM, Ely JW. A randomized controlled trial to improve colon cancer screening in rural family medicine: an Iowa Research Network (IRENE) study. J Am Board Fam Med. 2013;26(5):486–497. [DOI] [PubMed] [Google Scholar]
- 32.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer. 2016;122(3):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kempe KL, Shetterly SM, France EK, Levin TR. Automated phone and mail population outreach to promote colorectal cancer screening. Am J Manag Care. 2012;18(7):370378. [PubMed] [Google Scholar]
- 34.Senore C, Ederle A, DePretis G, et al. Invitation strategies for colorectal cancer screening programmes: The impact of an advance notification letter. Prev Med. 2015;73:106–111. [DOI] [PubMed] [Google Scholar]
- 35.Van Roon AHC, Hol L, Wilschut JA, et al. Advance notification letters increase adherence in colorectal cancer screening: A population-based randomized trial. Preventive Medicine. 2011;52(6):448–451. [DOI] [PubMed] [Google Scholar]
- 36.Levy BT, Daly JM, Xu Y, Ely JW. Mailed fecal immunochemical tests plus educational materials to improve colon cancer screening rates in Iowa Research Network (IRENE) practices. J Am Board Fam Med. 2012;25(1):73–82. [DOI] [PubMed] [Google Scholar]
- 37.Davis SN, Christy SM, Chavarria EA, et al. A randomized controlled trial of a multicomponent, targeted, low-literacy educational intervention compared with a nontargeted intervention to boost colorectal cancer screening with fecal immunochemical testing in community clinics. Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta S, Miller S, Koch M, et al. Financial Incentives for Promoting Colorectal Cancer Screening: A Randomized, Comparative Effectiveness Trial. Am J Gastroenterol. 2016;111(11):1630–1636. [DOI] [PubMed] [Google Scholar]
- 39.Potter MB, Ackerson LM, Gomez V, et al. Effectiveness and reach of the FLU-FIT program in an integrated health care system: a multisite randomized trial. Am J Public Health. 2013;103(6):1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potter MB, Somkin CP, Ackerson LM, et al. The FLU-FIT program: an effective colorectal cancer screening program for high volume flu shot clinics. Am J Manag Care. 2011;17(8):577–583. [PubMed] [Google Scholar]
- 41.Liles EG, Perrin N, Rosales AG, et al. Change to FIT increased CRC screening rates: evaluation of a US screening outreach program. Am J Manag Care. 2012;18(10):588595. [PMC free article] [PubMed] [Google Scholar]
- 42.Coronado GD, Schneider JL, Sanchez JJ, Petrik AF, Green B. Reasons for non-response to a direct-mailed FIT kit program: lessons learned from a pragmatic colorectal-cancer screening study in a federally sponsored health center. Transl Behav Med. 2015;5(1):6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green BB, BlueSpruce J, Tuzzio L, Vernon SW, Aubree Shay L, Catz SL. Reasons for never and intermittent completion of colorectal cancer screening after receiving multiple rounds of mailed fecal tests. BMC Public Health. 2017;17(1):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institutes of Health. Accelerating Colorectal Cancer Screening and follow-up through Implementation Science (ACCSIS). 2017; https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-17-039.html. Accessed November 3, 2017.
- 45.Jensen CD, Corley DA, Quinn VP, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang PS, Wheat CL, Abhat A, et al. Adherence to Competing Strategies for Colorectal Cancer Screening Over 3 Years. Am J Gastroenterol. 2016;111(1):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Issaka RB, Singh MH, Oshima SM, et al. Inadequate Utilization of Diagnostic Colonoscopy Following Abnormal FIT Results in an Integrated Safety-Net System. Am J Gastroenterol. 2017;112(2):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems. Cancer Epidemiol Biomarkers Prev. 2016;25(2):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selby K, Baumgartner C, Levin TR, et al. Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests: A Systematic Review. Ann Intern Med. 2017;167(8):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.