Abstract
Perinatal smoking, including smoking during pregnancy and postpartum smoking relapse, is a persistent public health problem. While childhood trauma has been linked to perinatal smoking, less is known about the association with more proximal stressful life events (SLEs). The objective of this study was to examine the association between SLEs that occurred during the year prior to childbirth with perinatal smoking.
Using the Pregnancy Risk Assessment Monitoring System 2009–2011, perinatal smoking was assessed at three time points: (1) three months prior to pregnancy, (2) the last three months of pregnancy, and (3) two to six months postpartum. Survey respondents endorsed up to 13 SLEs (i.e., death of someone close). SLEs were analyzed individually, as well as using a cumulative score (range 0–13). Weighted analyses included unadjusted and adjusted logistic regression.
Among those who smoked prior to pregnancy (n=15,316), 48% (n=7,308) reported quitting smoking during pregnancy. Of those, 44% (n=3,126) reported postpartum smoking relapse. A total of 11 SLEs were associated with smoking during pregnancy and 2 SLEs were associated with postpartum smoking relapse. The odds of continued smoking during pregnancy was 12% higher for each SLE endorsed (adjusted odds ratio [aOR] =1.12, 95% confidence interval [CI]: 1.09, 1.15) and this association was attenuated in relation to the odds of postpartum smoking relapse (aOR=1.03, 95% CI: 0.99, 1.08).
SLEs are associated with perinatal smoking. Additional research is needed to elucidate the mechanisms of action and to develop interventions specific to the needs of women who experience SLEs.
Keywords: Cigarette Smoking, Postpartum, PRAMS, Pregnancy, Stress
1. Introduction
Among women in the United States (US) who smoked cigarettes prior to pregnancy, approximately half continue to smoke during pregnancy.1 Smoking during pregnancy causes serious health risks for both women and their unborn children. Women who smoke during pregnancy have an increased risk of placenta previa, placenta abruption, premature rupture of membranes and maternal death.2,3 Unborn children with prenatal exposure to cigarette smoke have an increased risk of premature birth, low birth weight, fetal and infant mortality, as well as problems later in life including obesity, type two diabetes and possibly academic, social and behavioral problems.4–6
Unfortunately, of the 20% of women who quit smoking cigarettes during pregnancy, about half relapse within six months of childbirth and 70% relapse within two years.7,8 This observation occurs against the background of grim statistics indicating that postpartum relapse rates have been stagnant since 2000.7 Smoking after pregnancy entails serious health risks for the mother and newborn child. Women are more likely to suffer smoking-related morbidity and mortality compared to men.2,9 Mothers are the primary source of secondhand smoke exposure for children, which increases the child’s risk of acute (e.g., ear infection) and chronic (e.g., asthma) illnesses, as well as death (e.g., sudden infant death syndrome).2 Further, postpartum relapse increases the risk of subsequent miscarriage, as well as health risks for additional pregnancies.2,10 Thus, identifying novel risk factors for perinatal smoking will help improve the health of both mother and child via the development of innovative and effective perinatal smoking cessation interventions.
Stress has a complex relationship with cigarette smoking.11 Psychological and social stress increases the risk of smoking initiation.12,13 Chronic nicotine exposure has been shown to be associated with the dysregulation of the stress response systems including the hypothalamic-pituitary-adrenal (HPA) axis.14,15 Tobacco use is associated with elevated baseline cortisol levels, as well as blunted cortisol response to acute stress.14,16,17 Experimental studies have shown that inducing stress decreases the time to the next cigarette.18 Finally, a sex-specific relationship between cortisol and smoking cessation has been documented indicating that lower cortisol levels are predictive of smoking relapse in men whereas higher cortisol levels are predictive in women.16 Despite these observations, the relationship between stress and perinatal smoking is not well characterized.
Stressful life events (SLEs), such as job loss or death of a loved one, are experiences or events that cause severe strain on the individual. While childhood trauma, a type of SLE, has been linked to smoking during pregnancy,19 less is known about the effect of more proximal life stress (i.e., within a year of pregnancy) on perinatal smoking in US women. SLEs are easily identifiable in a clinical setting and may serve as a marker of someone who is at high risk of perinatal smoking. Therefore, the purpose of this study was to explore the association between SLEs during the year prior to childbirth and perinatal smoking. We hypothesized that: (1) SLEs would be positively associated with the odds of smoking during pregnancy, and further that, (2) SLEs would be positively associated with odds of postpartum smoking relapse. Understanding the relationship between SLEs and perinatal smoking may inform clinical guidelines, as well as the development of novel perinatal smoking interventions.
2. Material and methods
2.1. Data source and study design
This cross-sectional study utilized data from Phase 6 (2009–2011) of Pregnancy Risk Assessment Monitoring System (PRAMS). PRAMS is an ongoing, mixed-mode, population-based surveillance system developed between specific state health departments and the Centers for Disease Control and Prevention (CDC). It evaluates certain maternal behaviors and experiences before and during pregnancy as well as during early postpartum.
Using state birth certificate files, the PRAMS population is sampled from women who have had a recent live birth (between 1,300 and 3,400 women per year per participating state).20 Women were contacted two to six months after delivery. This population is stratified to oversample certain subpopulations including mothers from racial and ethnic minority groups and mothers who delivered low birthweight babies.20 Data are collected via mailed questionnaires and later via telephone for repeated non-responders. All data collection protocols and instruments are standardized to allow comparisons between states. From 2009 through 2011, the Phase 6 PRAMS Questionnaire was administered to 38 participating sites. Data from the sites that met a 65% response threshold were included in the analysis. PRAMS data are weighted to account for the sampling design, nonresponse, and non-coverage. Detailed information about the PRAMS surveillance methodology and questionnaires have been described previously.20 The CDC Institutional Review Board approved the PRAMS protocol.
2.2. Eligible participants
The present analysis consisted of PRAMS participants who responded to the Phase 6 PRAMS Questionnaire administered from 2009 through 2011. Respondents were excluded if they had missing data on stress or smoking variables. We also excluded respondents who were defined as nonsmokers (i.e., those who reported not smoking any cigarettes in the three months prior to pregnancy). In the analyses examining postpartum relapse, we further restricted our analysis to respondents who quit smoking during pregnancy (defined as reporting any smoking in the three months before pregnancy and no smoking during the last three months of pregnancy; i.e., we excluded those who reported smoking both before and during pregnancy, as well as those who reported not smoking before and during pregnancy).
2.3. Study variables
Stressful life events (SLEs), the primary exposure of interest, was measured in PRAMS using a 13-item subset of the Modified Life Events Inventory.21 Participants endorsed each SLE that occurred during the 12 months prior to delivery. We assessed each stressful item as a binary event (yes/no) as well as a cumulative SLE score where the presence of each item contributed one point (0–13 points).
One of the study outcomes was smoking during pregnancy. Participants were asked, “In the last 3 months of your pregnancy, how many cigarettes did you smoke on an average day?” Participants who reported “I didn’t smoke then” were categorized as not smoking during pregnancy and participants with responses from “less than 1 cigarette” through “41 cigarettes or more” were categorized as smoking during pregnancy. The second study outcome was postpartum smoking relapse. Participants were asked at approximately two to six months postpartum, when the survey was completed, “How many cigarettes do you smoke on an average day now?” Mothers who sustained smoking abstinence had responses of “I don’t smoke now”, and mothers who relapsed had responses of “less than 1 cigarette” through “41 cigarettes or more.”
The following information was included as covariates and/or sample descriptors: maternal age (≤17, 18–19, 20–24, 25–29, 30–34 and ≥35 years), years of education (0–8, 9–11, 12,13–15, and 16+ years), marital status (married or other), race (white, black, American Indian/Alaskan Native, Asian/Pacific Islander, other), ethnicity (Hispanic, non-Hispanic), parity (primiparous, multiparous), type of insurance (Medicaid or private insurance/other), quality of prenatal care (measured and categorized using the Kessner Adequacy of Prenatal Care Index 22 as adequate, intermediate and inadequate), physical abuse during pregnancy (yes, no or not reported), mode of survey participation (mail or telephone), and number of days between delivery and survey completion.
2.4. Statistical methods
All analyses were weighted for the complex survey design. Descriptive statistics (percentages, means) were used to describe the study sample, with chi-square or t-tests used to compare study groups. Separate logistic regression were used to estimate unadjusted and adjusted associations between SLEs (cumulative score and each item separately) and smoking outcomes. To determine adjusted associations, covariates included in the models were prespecified and based on theoretical relevance. All reported adjusted odds ratios (aORs) between SLEs and smoking and 95% confidence intervals (CIs) were adjusted for all covariates in the model. The association between SLEs and smoking during pregnancy was adjusted for maternal age, race, ethnicity, level of education, marital status, parity, method of payment, days between delivery and survey completion, and mode of survey participation. The association between SLEs and smoking relapse after pregnancy was adjusted for the same variables as well as quality of prenatal care and reported physical abuse, as these additional variables were associated with postpartum smoking status.
Sensitivity analysis was performed to assess the influence of covariates with more than 10% missingness.23 Separate logistic regression models were re-run including missing responses combined with existing categories, and as a separate additional category. Results that were significantly different than the main results were reported. Linearity in the logit for continuous variables was tested by using restricted cubic splines.24 If linearity was not met for a continuous covariate, the covariate was categorized to meet the assumption for logistic regression. All statistical tests were conducted in 2017–2018, used a significance level of 0.05, and were performed using SAS version 9.4 (SAS Institute Inc).
3. Results
3.1. Study Sample
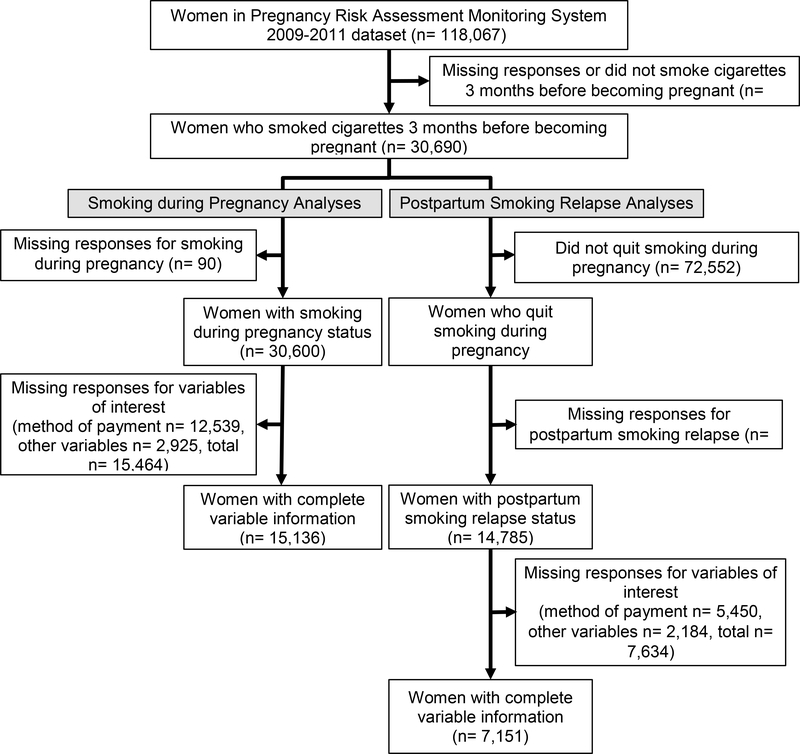
Among the 118,067 women in the PRAMS 2009–2011 dataset, 30,690 reported smoking cigarettes in the three months before becoming pregnant (Figure 1). Regarding smoking during pregnancy, a total of 15,136 women had complete data and were used to analyze the association between SLEs and smoking during pregnancy. There were 7,308 women (48%) who reported smoking during pregnancy and 7,828 (52%) who reported not smoking during pregnancy. Regarding postpartum smoking relapse, a total of 7,151 women had complete data and were used to analyze the association between SLEs and postpartum smoking relapse. There were 3,126 women (44%) who reported postpartum smoking relapse and 4,025 (56%) who reported sustained abstinence postpartum. Within our study sample, the highest rate of missingness (~40%) occurred within the variable method of payment for childbirth (e.g., private insurance, Medicaid), which was the only variable with more than 10% missingness. The results of the sensitivity analysis generally supported the results of the main analyses (see Supplemental Tables 1 and 2).
Figure 1. Study Sample Selection.
The study sample is described by smoking status in Table 1. In general, women who reported smoking during pregnancy or in the postpartum were more likely to be younger, less educated, unmarried, non-Hispanic, multiparous, receive less than adequate prenatal care, have Medicaid coverage, and report physical abuse compared to women who did not smoke during pregnancy or who sustained postpartum abstinence. However, women who did smoke during pregnancy were more likely to be White compared to those who did not smoke during pregnancy. In comparison, women who reported postpartum smoking relapse were more likely to be Black compared to those who reported postpartum sustained abstinence.
Table 1:
Study Sample Description, PRAMS, 2009 – 2011.
| Women who smoked before pregnancy n=15,136 | Women who quit smoking during pregnancy n=7,151 | |||||
|---|---|---|---|---|---|---|
| Did not smoke during pregnancy n=7,828 | Smoked during pregnancy n= 7,308 | p-value | Sustained abstinence postpartum n=4,025 | Postpartum smoking relapse n=3,126 | p-value | |
| Age (years) | <.0001 | <.0001 | ||||
| <=17 | 278 (3.6) | 185 (2.6) | 121 (3.0) | 137 (4.4) | ||
| 18–19 | 777 (9.9) | 665 (9.1) | 311 (7.7) | 390 (12.5) | ||
| 20–24 | 2,556 (32.7) | 2,541 (34.8) | 1,219 (30.3) | 1,110 (35.5) | ||
| 25–29 | 2,170 (27.7) | 2,083 (28.5) | 1,136 (28.2) | 847 (27.1) | ||
| 30–34 | 1,391 (17.8) | 1,160 (15.9) | 825 (20.5) | 459 (15.0) | ||
| 35–39 | 552 (7.0) | 518 (7.1) | 344 (8.6) | 158 (5.1) | ||
| >=40 | 104 (1.3) | 156 (2.1) | 69 (1.7) | 25 (0.8) | ||
| Education level (years) | <.0001 | <.0001 | ||||
| 0–8 | 82 (1.1) | 173 (2.4) | 40 (1.0) | 36 (1.2) | ||
| 9–11 | 1,262 (16.1) | 1,843 (25.2) | 503 (12.5) | 644 (20.6) | ||
| 12 | 2,440 (31.2) | 2,814 (38.5) | 1,136 (28.2) | 1,082 (34.6) | ||
| 13–15 | 2,858 (36.5) | 2,202 (30.1) | 1,588 (39.5) | 1,016 (32.5) | ||
| >=16 | 1,186 (15.2) | 276 (3.8) | 758 (18.8) | 348 (11.1) | ||
| Marital Status | <.0001 | <.0001 | ||||
| Married | 3,531 (45.1) | 2,511 (34.4) | 2,068 (51.4) | 1,194 (38.2) | ||
| Other | 4,297 (54.9) | 4,797 (65.6) | 1,957 (48.6) | 1,932 (61.8) | ||
| Race | <.0001 | <.0001 | ||||
| Other Asian | 110 (1.4) | 64 (0.9) | 61 (1.5) | 39 (1.3) | ||
| White | 5,178 (66.2) | 5,012 (68.6) | 2,810 (69.8) | 1,936 (61.9) | ||
| Black | 1,081 (13.8) | 1,087 (14.9) | 367 (9.1) | 577 (18.5) | ||
| American Indian | 600 (7.7) | 444 (6.1) | 308 (7.7) | 256 (8.2) | ||
| Chinese | 9 (0.1) | 1 (0.0) | 7 (0.2) | 2 (0.1) | ||
| Japanese | 11 (0.1) | 6 (0.1) | 9 (0.2) | 2 (0.1) | ||
| Filipino | 27 (0.3) | 17 (0.2) | 21 (0.5) | 4 (0.1) | ||
| Hawaiian | 4 (0.1) | 4 (0.1) | 2 (0.1) | 2 (0.1) | ||
| Other race | 251 (3.2) | 104 (1.4) | 140 (3.5) | 91 (2.9) | ||
| Alaskan Native | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Mixed race | 557 (7.1) | 5269 (7.8) | 300 (7.5) | 217 (6.9) | ||
| Hispanic ethnicity | <.0001 | 0.0055 | ||||
| No | 6,918 (88.4) | 6,817 (93.3) | 3,506 (87.1) | 2,790 (89.3) | ||
| Yes | 910 (11.6) | 491 (6.7) | 519 (12.9) | 336 (10.8) | ||
| Quality of prenatal care | <.0001 | 0.0037 | ||||
| Adequate | 4,931 (63.0) | 3,905 (53.4) | 2,835 (70.4) | 2,088 (66.8) | ||
| Intermediate | 1,743 (22.3) | 1,915 (26.2) | 933 (23.2) | 803 (25.7) | ||
| Inadequate | 493 (6.3) | 767 (10.5) | 257 (6.4) | 235 (7.5) | ||
| Unknown | 661 (8.4) | 721 (9.9) | ||||
| Parity | <.0001 | <.0001 | ||||
| Primiparous | 3,956 (50.5) | 5,658 (36.4) | 2,157 (53.6) | 1,480 (47.3) | ||
| Multiparous | 3,872 (49.5) | 4,650 (63.6) | 1,868 (46.4) | 1,646 (52.7) | ||
| Method of payment | <.0001 | <.0001 | ||||
| Medicaid | 4,143 (52.9) | 5,191 (71.0) | 1,892 (47.1) | 1,881 (60.2) | ||
| Other | 3,685 (47.1) | 2,117 (29.0) | 2,133 (53.0) | 1,245 (39.8) | ||
| Physical abuse during pregnancy | <.0001 | <.0008 | ||||
| Yes | 355 (4.5) | 578 (7.9) | 152 (3.8) | 170 (5.4) | ||
| Not reported | 7,473 (95.5) | 6,730 (92.1) | 3,873 (96.2) | 2,956 (94.6) | ||
| Days since delivery, mean (SD) | 119.33 (34.6) | 121.13 (37.0) | 0.0021 | 114.85 (31.5) | 123.88 (37.1) | <.0001 |
| Mode of participation | 0.0426 | <.0001 | ||||
| 6,095 (77.9) | 5,589 (76.5 ) | 3,319 (82.5) | 2,269 (72.6) | |||
| Phone | 1,733 (22.1) | 1,719 (23.5) | 706 (17.5) | 857 (27.4) | ||
PRAMS= Pregnancy Risk Assessment Monitoring System; SD= standard deviation; n= number; %= percentage Distribution of characteristics and smoking status was assessed using chi-square tests or t-tests. Percentages may not sum to 100 due to rounding.
3.2. Association between Stressful Life Events and Smoking during Pregnancy
Among women who reported smoking during the three months prior to pregnancy, women who quit smoking during pregnancy (n=7,828) had significantly lower cumulative SLEs compared to women who continued to smoke during pregnancy (n=7,308; 2.53±2.2 versus 3.29±2.4, respectively; difference between means and 95% CI of difference = −0.759 (−0.83, - 0.68); p<0.0001; Table 2). The women who continued to smoke during pregnancy were significantly more likely to endorse each individual SLE compared to the women who quit smoking (p-values<0.0001).
Table 2:
Stressful Life Events by Perinatal Smoking Status, PRAMS 2009 – 2011.
| Women who smoked before pregnancy n=15,136 | Women who quit smoking during pregnancy n= 7,151 | |||||
|---|---|---|---|---|---|---|
| Did not smoke during pregnancy n=7,828 | Smoked during pregnancy n= 7,308 | p-value | Sustained abstinence postpartum n=4,025 | Postpartum smoking relapse n=3,126 | p-value | |
| Cumulative stress score (0 –13), mean (SD) | 2.53 (2.2) | 3.29 (2.4) | <.0001 | 2.35 (2.1) | 2.71 (2.2) | <.0001 |
| Stressful life eventsa | ||||||
| Husband/partner lost job | <.0001 | 0.0198 | ||||
| Yes | 1,398 (17.9) | 1,742 (23.8) | 667 (16.6) | 584 (18.7) | ||
| No | 6,430 (82.1) | 5,566 (76.2) | 3,358 (83.4 | 2,542 (81.3) | ||
| Lost job despite wanting to continue working | 0.0214 | |||||
| Yes | 1,169 (14.9) | 1,463 (20.0) | <.0001 | 553 (13.7) | 490 (15.7) | |
| No | 6,659 (85.1) | 5,845 (80.0) | 3,472 (86.3) | 2,636 (84.3) | ||
| Had a lot of bills couldn’t pay | <.0001 | 0.0004 | ||||
| Yes | 2,332 (29.8) | 3,064 (41.9) | 1,120 (27.8) | 990 (31.7) | ||
| No | 5,496 (70.2) | 4,244 (58.1) | 2,905 (72.2) | 2,136 (68.3) | ||
| Moved to new address | <.0001 | 0.0005 | ||||
| Yes | 3,534 (45.2) | 3,620 (49.5) | 1,750 (43.5) | 1,489 (47.6) | ||
| No | 4,294 (54.9) | 3,688 (50.5) | 2,275 (56.5) | 1,637 (52.4) | ||
| Mother was homeless | <.0001 | 0.0011 | ||||
| Yes | 387 (4.9) | 719 (9.8) | 166 (4.1) | 181 (5.8) | ||
| No | 7,441 (95.1) | 6,589 (90.2) | 3,859 (95.9) | 2,945 (94.2) | ||
| Close family member was very sick and went to hospital | <.0001 | 0.0064 | ||||
| Yes | 2,053 (26.2) | 2,159 (29.5) | 994 (24.7) | 861 (27.5) | ||
| No | 5,775 (73.8) | 5,149 (70.5) | 3,031 (75.3) | 2,265 (72.5) | ||
| Someone close to mother died | <.0001 | 0.0024 | ||||
| Yes | 1,647 (21.0) | 1,856 (25.4) | 783 (19.5) | 700 (22.4) | ||
| No | 6,181 (79.0) | 5,452 (74.6) | 3,242 (80.6) | 2,426 (77.6) | ||
| Mother got separated/divorced | <.0001 | 0.0167 | ||||
| Yes | 1,003 (12.8) | 1,236 (16.9) | 476 (11.8) | 429 (13.7) | ||
| No | 6,825 (87.2) | 6,072 (83.1) | 3,549 (88.2) | 2,697 (86.3) | ||
| Argued with husband/partner more than usual | <.0001 | <.0001 | ||||
| Yes | 2,584 (33.1) | 2,979 (40.8) | 1,233 (30.6) | 1,104 (35.3) | ||
| No | 5,244 (67.0) | 4,329 (59.2) | 2,792 (69.4) | 2,022 (64.7) | ||
| Husband/partner or mother went to jail | <.0001 | <.0001 | ||||
| Yes | 615 (7.9) | 1,086 (14.9) | 263 (6.5) | 298 (9.5) | ||
| No | 7,213 (92.1) | 6,222 (85.1) | 3,726 (93.5) | 2,828 (90.5) | ||
| Was in a physical fight | <.0001 | <.0001 | ||||
| Yes | 513 (6.6) | 747 (10.2) | 222 (5.5) | 247 (7.9) | ||
| No | 7,315 (93.5) | 6,561 (89.8) | 3,802 (94.5) | 2,879 (92.1) | ||
| Husband/partner didn’t want mother to be pregnant | <.0001 | 0.0007 | ||||
| Yes | 888 (11.3) | 1,063 (14.6) | 408 (10.1) | 397 (12.7) | ||
| No | 6,940 (88.7) | 6,245 (85.5) | 3,617 (89.9) | 2,729 (87.3) | ||
| Someone close to mother had drug or alcohol problem | <.0001 | 0.0863 | ||||
| Yes | 1,683 (21.5) | 2,300 (31.5) | 830 (20.6) | 697 (22.3) | ||
| No | 6,145 (78.5) | 5,008 (68.5) | 3,195 (79.4) | 2,429 (77.7) | ||
PRAMS= Pregnancy Risk Assessment Monitoring System; SD= standard deviation; n= number; %= percentage Distribution of characteristics and smoking status was assessed using chi-square tests or t-tests. Percentages may not sum to 100 due to rounding
13-item stressful life events a subset of Modified Life Events Inventory.
Significant associations were seen in unadjusted and adjusted models, although adjusted associations were slightly attenuated (Table 3). Of the 13 individual SLEs, 11 were significantly associated with increased odds of smoking during pregnancy. This included three with at least 70% higher odds of smoking during pregnancy: being in a physical fight (aOR= 1.70; 95% CI= 1.35, 2.14), the husband/partner or mother going to jail (aOR=1.74; 95% CI= 1.41, 2.15), and being homeless (aOR= 2.03; 95% CI= 1.55, 2.66). The two that were not related to smoking during pregnancy were having a close family member who was sick and had to go to the hospital and the mother becoming separated or divorced.
Table 3:
Associations between maternal stressful life events and smoking outcomes,PRAMS 2009–2011
| Smoking during pregnancy among women who smoked before pregnancy (n=15,136) | Postpartum smoking relapse among women who quit during pregnancy (n=7,151) | |||
|---|---|---|---|---|
| UnadjustedaOR (95% CI) | Adjusteda,bOR (95% CI) | UnadjustedaOR (95% CI) | Adjusteda,cOR (95% CI) | |
| Cumulative stressful life events score (0–13) | 1.15 (1.12, 1.18) | 1.12 (1.09, 1.15) | 1.07 (1.03, 1.12) | 1.03 (0.99, 1.08) |
| Stressful life eventsd (yes vs. no) | ||||
| Husband/Partner lost job | 1.32 (1.15, 1.52) | 1.21 (1.04, 1.41) | 1.34 (1.07, 1.68) | 1.30 (1.03, 1.63) |
| Lost job despite wanting to continue working | 1.44 (1.24, 1.67) | 1.27 (1.08, 1.49) | 1.01 (0.80, 1.27) | 0.86 (0.68, 1.09) |
| Had a lot of bills couldn’t pay | 1.68 (1.49, 1.89) | 1.45 (1.28, 1.65) | 1.23 (1.02, 1.47) | 1.17 (0.97, 1.42) |
| Moved to new address | 1.32 (1.18, 1.47) | 1.24 (1.10, 1.40) | 1.10 (0.93, 1.29) | 1.01 (0.85, 1.20) |
| Was homeless | 2.31 (1.82, 2.93) | 2.03 (1.55, 2.66) | 1.18 (0.80, 1.72) | 0.91 (0.62, 1.35) |
| Close family member was very sick and had to go to the hospital | 1.08 (0.95, 1.22) | 1.07 (0.94, 1.21) | 1.19 (0.99, 1.43) | 1.19 (0.99, 1.43) |
| Someone close to mother died | 1.28 (1.12, 1.46) | 1.24 (1.07, 1.43) | 1.44 (1.18, 1.76) | 1.36 (1.11, 1.67) |
| Mother got separated/divorced | 1.26 (1.07, 1.49) | 1.11 (0.93, 1.32) | 1.16 (0.90, 1.49) | 0.96 (0.74, 1.25) |
| Argued with husband/partner more than usual | 1.43 (1.28, 1.61) | 1.39 (1.23, 1.58) | 1.12 (0.94, 1.34) | 0.99 (0.82, 1.20) |
| Husband/partner or mother went to jail | 2.01 (1.66, 2.44) | 1.74 (1.41, 2.15) | 1.37 (0.99, 1.91) | 1.04 (0.74, 1.46) |
| Was in a physical fight | 1.69 (1.35, 2.10) | 1.70 (1.35, 2.14) | 1.43 (0.99, 2.00) | 1.22 (0.81, 1.82) |
| Husband/partner didn’t want mother to be pregnant | 1.31 (1.11, 1.55) | 1.23 (1.03, 1.47) | 1.16 (0.90, 1.50) | 0.99 (0.77, 1.28) |
| Someone close to mother had drug or alcohol problem | 1.67 (1.46, 1.90) | 1.55 (1.35, 1.79) | 1.10 (0.90, 1.35) | 1.01 (0.82, 1.25) |
PRAMS= Pregnancy Risk Assessment Monitoring System
Odds ratios and 95% CIs have been weighted to account for the PRAMS survey design and the statistical weighting of the data.
Adjusted for maternal age, race, ethnicity, level of education, marital status, parity, methods of payment and days since delivery.
Adjusted for maternal age, race, ethnicity, level of education, marital status, parity, method of payment, quality of prenatal care, reported physical abuse, days since delivery and mode of participation.
13-item stressful life events, a subset of Modified Life Events Inventory.
3.3. Association between Stressful Life Events and Postpartum Smoking Relapse
Among women who smoked in the three months prior to pregnancy and reported smoking abstinence during the last three months of pregnancy, women who sustained abstinence into the postpartum period (n=4,025) reported significantly lower cumulative SLEs compared to women who relapsed to smoking during the postpartum (n=3,126; 2.35±2.1 versus 2.71±2.2, respectively; difference between means and 95% CI of difference = −0.357 (−0.46, 0.26); p<0.0001; Table 3). The women who relapsed to smoking during the postpartum were significantly more likely to endorse each individual SLE compared to the women who remained abstinent (p-values<0.03) except for the SLE of being close to someone who had a drug or alcohol problem (p=0.086).
The association between the cumulative SLE score and postpartum smoking relapse was significant in the unadjusted model (OR=1.07 per additional SLE; 95% CI=1.03, 1.12). However, the association was attenuated in the adjusted model (aOR=1.03 per additional SLE; 95% CI=0.99, 1.08). After adjustment, the individual SLEs that remained significantly associated with postpartum smoking relapse were having a husband/partner lose their job (aOR= 1.30; 95% CI= 1.03–1.63) and having someone close to the mother die (aOR=1.36; 95% CI= 1.11, 1.67).
4. Discussion
This study, which utilized data from a large, representative, population-based surveillance system, indicated that stressful life events (SLEs) were positively associated with smoking during pregnancy and, to a lesser degree, with postpartum smoking relapse. Specifically, of the 13 SLEs assessed, 11 were significantly associated with higher odds of smoking during pregnancy and two were significantly associated with higher odds of postpartum smoking relapse. The strength of the relationship with smoking during pregnancy ranged from 21% higher odds for those who endorsed husband/partner job loss to 74% higher odds for those who endorsed jail time for mother or husband/partner. The SLEs related to increased odds of postpartum smoking relapse including husband/partner lost job (30% higher odds) and the death of someone close to the mother (36% higher odds). Two SLEs were not significantly related with either prenatal smoking or postpartum smoking relapse – hospitalization of a family member and separation/divorce. While only the minority of events predicted relapse, the fact that they were particularly relevant to relapse and not to prenatal smoking suggests a unique association, direct or indirect, exerted by these particular events on relapse.
The increased odds of perinatal smoking could be driven by resource loss and the related emotional distress. Many SLEs measured in this study, such as becoming homeless or having a partner lose their job, involved a significant loss of financial and social resources. Resource loss has previously been identified as a significant source of emotional distress for women in general,25 and losing a significant amount of resources shortly before or during pregnancy may also influence women to engage in perinatal smoking, perhaps in an attempt to cope with resource loss, or the emotional distress from resource loss.26 This may be one of the reasons why contingency management interventions (i.e., financial incentives for smoking abstinence) are effective during the perinatal period. 27 However, resource loss is just one of the many possible mechanisms that may play a role in perinatal smoking, and additional research is needed to elucidate the specific mechanisms of action responsible for these observations. This knowledge will inform additional research that should develop, examine and implement novel smoking cessation interventions aimed at effectively managing stress to reduce the prevalence of prenatal smoking.
Further evidence for the relationship between stress and perinatal smoking is available from research utilizing other indicators of stress. For example, perceived stress, stress about money, and symptoms of abuse-related post-traumatic stress disorder (i.e., inability to cope with significant life event-related emotions and problems) have been implicated as predictors of smoking during pregnancy.28–30 However, the advantage to utilizing SLEs as compared to other measures of stress, is that SLEs may be particularly easy to identify in a clinical setting, as well as more applicable to a diverse patient population. Some, however, have strongly challenged the reliability of self-report of SLEs using paper and pencil measures like the one used in this study and they suggest that clinical interviews are a preferred approach.31,32 While such intensive interviewing is prohibitive in population surveys such as the PRAMS, within clinical practice stressful life event screening may be a useful tool for individualized care and referral to treatment programs where providers skilled in substance use and addiction evaluation and treatment can assess the potential impact of SLEs. Ease of identification may allow clinical providers to determine who may be at high risk for continued smoking during pregnancy and then, refer that patient to more intensive smoking cessation services. For example, Tragea and colleagues randomized 60 pregnant women to a stress management program or a placebo-control condition. The stress management program, which included twice a day relaxation breathing and progressive muscle relaxation, was found to significantly lower perceived stress compared to the placebo-control condition.33 However, the effect of this type of intervention has not yet been examined within the context of perinatal smoking.
This study, while strengthened by a large, diverse, and representative sample, relies on self-reported cross-sectional data, which limits our ability to assess the temporality of the observed relationships and limits the reliability of our observations. Most notably, perhaps, is that the item used to measure postpartum smoking status is a single item that asks participants how many cigarettes they are smoking per day “now.” It is possible that individuals who quit smoking during pregnancy, relapsed early in the postpartum period, then quit again at the time of the survey. These individuals did not “sustain postpartum abstinence.” However, we expect these instances to be few and would also expect this to bias our results towards the null. Further, our findings are consistent with previous observations made by Hauge and colleagues who followed over 71,000 women from pregnancy to six month postpartum in the Norwegian Mother and Child Cohort Study. Specifically, women who experienced SLEs were at significantly lower odds (OR=0.93, 95% CI: 0.90–0.96) of achieving smoking abstinence during pregnancy; however, SLEs were unrelated to postpartum smoking relapse (OR=0.97, 95% CI=0.93–1.01).34 In contrast, observations from a smaller sample of women (n=403) from the Netherlands indicated that SLEs were not related to smoking during pregnancy.35 These conflicting observations may be driven by societal differences. Similarly, the PRAMS survey did not assess smoking earlier in pregnancy, which made investigation into early pregnancy smoking cessation impossible. This is an area of future study. Lastly, all of these data rely on self-report, which contains error and bias.
5. Conclusions
Women who experience stressful life events are at higher odds of perinatal smoking. The urgency for novel smoking cessation interventions for pregnant women is particularly high given that women in general are more vulnerable to stress-related relapse, including life events stress, than men.36 Additional research is needed to elucidate the specific mechanisms of action responsible for these observations. This knowledge will inform additional research that should develop, examine and implement novel smoking cessation interventions aimed at effectively managing stress to reduce the prevalence of prenatal smoking.
Supplementary Material
Highlights.
The prevalence of perinatal cigarette smoking (PCS) remains high despite known risks.
Stressful life events (SLEs) may increase the risk of PCS, but this is not yet known.
Continued smoking during pregnancy was significantly associated with 11 of 13 SLEs.
Postpartum smoking relapse was significantly associated with 2 of 13 SLEs.
SLEs are associated with PCS, especially continued smoking during pregnancy.
Acknowledgements
We would like to extend our thanks to the PRAMS Working Group for granting us access to this data and for their review of the manuscript, as well as Evelyn Rens for her editorial assistance.
Role of Funding Sources
The sponsor did not play any role in study design; collection, analysis or interpretation of the data; writing of the report; or in the decision to submit the article for publication. Drs. al’Absi and Lemieux were supported in part by grants from the National Institute of Health (R01DA016351 and R01DA027232).
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alicia M. Allen, Department of Family & Community Medicine, College of Medicine, University of Arizona, 3950 South Country Club Road, Suite 330, Tucson, Arizona 85714-2238, Work: (520) 626-7864, Fax: (520) 626-1080, aliciaallen@email.arizona.edu.
Alesia M. Jung, Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health, University of Arizona, 1295 N Martin Ave, PO Box 245211, Tucson, Arizona 85724-5211.
Andrine M. Lemieux, Department of Family Medicine & Biobehavioral Health, Medical School, University of Minnesota, Duluth, 1035 University Drive, 291 SMed, Duluth, Minnesota, 55812-3031.
Adam C. Alexander, Department of Social and Behavioral Sciences, School of Public Health, University of Memphis 3825 DeSoto Avenue, Room 207, Memphis, Tennessee 38152.
Sharon S. Allen, Department of Family Medicine & Community Health, Medical School, University of Minnesota 420 Delaware Street SE, Room A682, Minneapolis, Minnesota 55455-0341.
Kenneth D. Ward, School of Public Health, University of Memphis, 201 Robison Hall, Memphis, TN 38152-3420.
Mustafa al’Absi, Department of Family Medicine & Biobehavioral Health, Medical School, University of Minnesota, Duluth, 1035 University Drive, Duluth, Minnesota, 55812-3031.
References
- 1.Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62(6):1–19. http://www.ncbi.nlm.nih.gov/pubmed/24196750. Accessed August 10, 2018. [PubMed] [Google Scholar]
- 2.CDC. Smoking and Tobacco Use; Surgeon General’s Reports; 2001.; 2001.
- 3.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83(11):713–720. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Ion R, Bernal AL. Smoking and Preterm Birth. Reprod Sci. 2015;22(8):918–926. doi: 10.1177/1933719114556486. [DOI] [PubMed] [Google Scholar]
- 5.Marufu TC, Ahankari A, Coleman T, Lewis S. Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health. 2015;15(1):239. doi: 10.1186/s12889-015-1552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polańska K, Jurewicz J, Hanke W. Smoking and alcohol drinking during pregnancy as the risk factors for poor child neurodevelopment - A review of epidemiological studies. Int J Occup Med Environ Health. 2015;28(3):419–443. doi: 10.13075/ijomeh.1896.00424. [DOI] [PubMed] [Google Scholar]
- 7.Rockhill KM, Tong VT, Farr SL, Robbins CL, D’Angelo D V., England LJ Postpartum Smoking Relapse After Quitting During Pregnancy: Pregnancy Risk Assessment Monitoring System, 2000–2011. J Women’s Heal. 2016;25(5):480–488. doi: 10.1089/jwh.2015.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtin SC, Mathews TJ. National Vital Statistics Reports Smoking Prevalence and Cessation Before and During Pregnancy: Data From the Birth Certificate, 2014. 2016. https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_01.pdf. Accessed October 10, 2017. [PubMed]
- 9.Pirie K, Peto R, Reeves GK, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381(9861):133–141. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andres RL, Day M-C. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5(3):231–241. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- 11.Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36(5):1418–1441. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyko EJ, Trone DW, Peterson A V., et al. Longitudinal Investigation of Smoking Initiation and Relapse Among Younger and Older US Military Personnel. Am J Public Health. 2015;105(6):1220–1229. doi: 10.2105/AJPH.2014.302538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holliday E, Gould TJ. Nicotine, adolescence, and stress: A review of how stress can modulate the negative consequences of adolescent nicotine abuse. Neurosci Biobehav Rev. 2016;65:173–184. doi: 10.1016/j.neubiorev.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74(2):401–410. http://www.ncbi.nlm.nih.gov/pubmed/12479961. Accessed November 28, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Ussher M, West R, Evans P, et al. Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosom Med. 2006;68(2):299–306. doi: 10.1097/01.psy.0000204926.27215.a1. [DOI] [PubMed] [Google Scholar]
- 16.al’Absi M Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59(3):218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Wong JA, Pickworth WB, Waters AJ, al’Absi M, Leventhal AM. Cortisol levels decrease after acute tobacco abstinence in regular smokers. Hum Psychopharmacol. 2014;29(2):152–162. doi: 10.1002/hup.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee SA, Sinha R, Weinberger AH, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25(4):490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blalock JA, Nayak N, Wetter DW, et al. The relationship of childhood trauma to nicotine dependence in pregnant smokers. Psychol Addict Behav. 2011;25(4):652–663. doi: 10.1037/a0025529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman HB, Gilbert BC, Msphbrenda CG, Lansky A. The Pregnancy Risk Assessment Monitoring System (PRAMS): current methods and evaluation of 2001 response rates. Public Health Rep. 2006;121(1):74–83. doi: 10.1177/003335490612100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton RW, Webster PA, Binu PS, Maskrey N, Phillips AB. Psychosocial stress in pregnancy and its relation to the onset of premature labour. Br Med J. 1979;2(6187):411–413. http://www.ncbi.nlm.nih.gov/pubmed/486966. Accessed October 26, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessner D, Kalk C, Schlesinger E. Infant Death: An Analysis by Maternal Risk and Health Care Infant Death: An Analysis by Maternal Risk and Health Care. In: Institute of Medicine and National Academy of Sciences; 1973. [Google Scholar]
- 23.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001;25(5):464–469. http://www.ncbi.nlm.nih.gov/pubmed/11688629. Accessed October 26, 2017. [PubMed] [Google Scholar]
- 24.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):n/a-n/a. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 25.Hobfoll SE, Johnson RJ, Ennis N, Jackson AP. Resource loss, resource gain, and emotional outcomes among inner city women. J Pers Soc Psychol. 2003;84(3):632–643. http://www.ncbi.nlm.nih.gov/pubmed/12635922. Accessed December 21, 2017. [PubMed] [Google Scholar]
- 26.Sperlich S, Maina MN. Are single mothers’ higher smoking rates mediated by dysfunctional coping styles? BMC Womens Health. 2014;14(1):124. doi: 10.1186/1472-6874-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson SM, Newins AR, Medenblik AM, et al. Contingency Management Versus Psychotherapy for Prenatal Smoking Cessation: A Meta-Analysis of Randomized, Controlled Trials. Women’s Heal Issues. July 2018. doi: 10.1016/j.whi.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crittenden KS, Manfredi C, Cho YI, Dolecek TA. Smoking cessation processes in low-SES women: The impact of time-varying pregnancy status, health care messages, stress, and health concerns. Addict Behav. 2007;32(7):1347–1366. doi: 10.1016/j.addbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez WD, Konrath SH, Seng JS. Abuse‐Related Post‐Traumatic Stress, Coping, and Tobacco Use in Pregnancy. J Obstet Gynecol Neonatal Nurs. 2011;40(4):422–431. doi: 10.1111/j.1552-6909.2011.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers JR, McDermott LJ, Loxton DJ, Chojenta CL. A Prospective Study of Prevalence and Predictors of Concurrent Alcohol and Tobacco Use During Pregnancy. Matern Child Health J. 2013;17(1):76–84. doi: 10.1007/s10995-012-0949-3. [DOI] [PubMed] [Google Scholar]
- 31.Harkness KL, Monroe SM. The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. J Abnorm Psychol. 2016;125(5):727–745. doi: 10.1037/abn0000178. [DOI] [PubMed] [Google Scholar]
- 32.Wagner MF, Skowronski JJ. Social influence and mental routes to the production of authentic false memories and inauthentic false memories. Conscious Cogn. 2017;51:34–52. doi: 10.1016/j.concog.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Tragea C, Chrousos GP, Alexopoulos EC, Darviri C. A randomized controlled trial of the effects of a stress management programme during pregnancy. Complement Ther Med. 2014;22(2):203–211. doi: 10.1016/j.ctim.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Hauge LJ, Torgersen L, Vollrath M. Associations between maternal stress and smoking: findings from a population-based prospective cohort study. Addiction. 2012;107(6):1168–1173. doi: 10.1111/j.1360-0443.2011.03775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beijers C, Ormel J, Meijer JL, Verbeek T, Bockting CLH, Burger H. Stressful Events and Continued Smoking and Continued Alcohol Consumption during Mid-Pregnancy. Franken IH, ed. PLoS One. 2014;9(1):e86359. doi: 10.1371/journal.pone.0086359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98(6):847–855. http://www.ncbi.nlm.nih.gov/pubmed/12780373. Accessed December 8, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.