Abstract
Outpatient cardiac rehabilitation (OCR) reinforces patients’ efforts to quit smoking, but the association between participation in OCR and long-term smoking status after acute myocardial infarction (AMI) is unknown. We studied hospitalized smokers with confirmed AMI from two multicenter prospective registries (PREMIER, from January 1, 2003, to June 28, 2004, and TRIUMPH, from April 11, 2005, to December 31, 2008) to describe the association of OCR participation with smoking cessation. Eligible patients smoked at least 1 cigarette per day on average in the 30 days prior to enrollment and completed 12-month follow-up (N=1,307). Structured interviews were completed on subjects at baseline and during follow-up. OCR participation and abstinence from smoking within the prior 30-days (30-day point prevalence abstinence, PPA) were self-reported. We constructed a propensity model of OCR participation based on 22 baseline sociodemographic and clinical characteristics, and constructed hierarchical modified Poisson regression models of 30-day PPA at 12 months after matching on the propensity for OCR participation (with clinical site treated as a random effect). Seventy-four percent of subjects were referred to OCR at hospital discharge, but only 36% participated during follow-up. At 12-month follow-up, 30-day PPA was 57% in OCR participants, compared to 41% in matched OCR non-participants. Participation in OCR was a significant predictor of 30-day PPA at 12 months (adjusted RR 1.38, 95% CI 1.20–1.57). In conclusion, smokers who participated in OCR were significantly more likely to abstain from smoking 12 months after AMI hospitalization.
Keywords: Cardiac rehabilitation, Smoking cessation, Acute myocardial infarction, Patient adherence
Cardiac rehabilitation is an evidence-based strategy to decrease morbidity and mortality after acute myocardial infarction (AMI) through exercise, counseling on cardiovascular risk factors, patient education to improve symptom recognition, and psychosocial support during treatment.1 A recent meta-analysis of randomized controlled trials showed that participation in exercise-based cardiac rehabilitation significantly reduces cardiovascular mortality (relative risk 0.74, 95% CI 0.64 to 0.86) and the risk of hospital admissions (relative risk 0.82, 95% CI 0.70 to 0.96) in patients with ischemic heart disease (IHD).2
Smoking cessation is a cornerstone of secondary prevention among smokers, as it reduces adverse cardiac events through several potential mechanisms: decreased platelet aggregation and thrombus formation, increased oxygen delivery, decreased catecholamine release and coronary vasoconstriction, reduced oxidative stress and vascular inflammation, and decreased myocardial workload.3 Approximately one-quarter of deaths prevented by outpatient cardiac rehabilitation (OCR) have been attributed to smoking cessation.4 Although OCR provides reinforcement for patients in their efforts to quit smoking, there are few empiric data on the association between OCR participation and long-term smoking cessation. Prior studies of smoking behavior in hospitalized smokers with acute coronary syndrome (ACS, which includes AMI and unstable angina) have focused on the effects of discharge recommendations for OCR,5 have been limited to short-term cessation outcomes (less than 6 months),6 or did not examine cardiac rehabilitation as a potential predictor of abstinence.7–11 Moreover, the relationship between participation in OCR and smoking cessation outcomes might be explained by selection bias (i.e., patients who participate in OCR may be more health conscious than those who do not participate).12 This study aims to extend prior research on the effects of OCR referral5 by examining the association between participation in OCR and smoking status in two large cohorts of patients with biochemically confirmed acute myocardial infarction, using propensity score matching to account for potential selection biases introduced by measured patient characteristics. These data have the potential to further illuminate the benefits of OCR and the need to better support patient participation in such programs.
METHODS
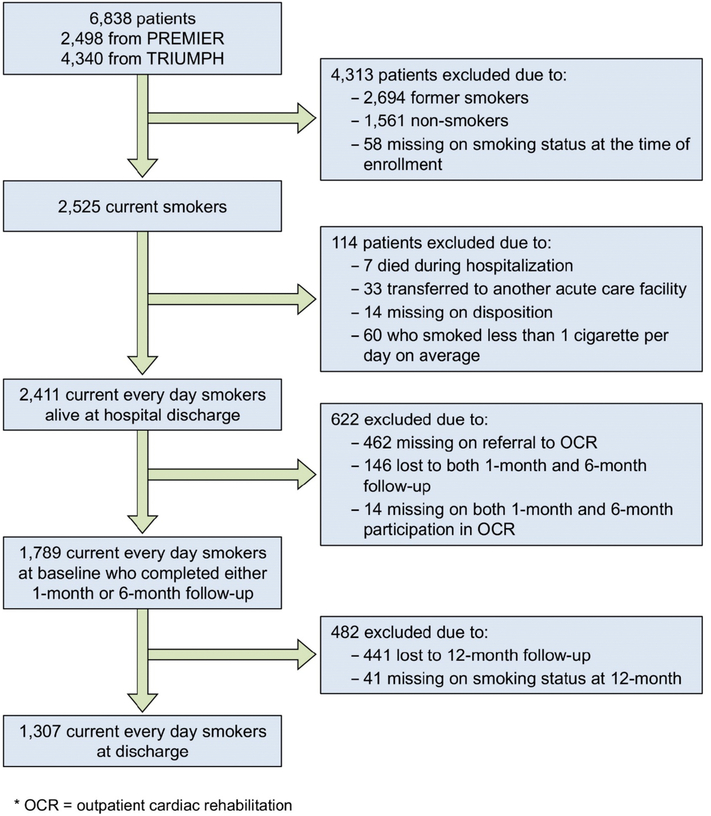
This study examined patients enrolled in two US multicenter, prospective, observational registries with similar study protocols. The Prospective Registry Evaluating Outcomes after Myocardial Infarction: Events and Recovery (PREMIER) Quality Improvement Registry enrolled patients (n=2,498) at 19 clinical centers from January 1, 2003 to June 28, 2004.13 Similarly, the Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Health Status (TRIUMPH) study enrolled patients (n=4,340) at 24 clinical centers from April 11, 2005, and December 31, 2008.14 Patients in both registries were at least 18 years of age, with elevated cardiac biomarkers (troponin or creatine kinase-MB fraction assessed within 24 hours of admission) and supporting evidence of AMI (electrocardiographic [EKG] ST-segment changes or prolonged ischemic symptoms/signs). Participants were required to either present to the enrolling institution or to have been transferred within 24 hours of original presentation, so that the primary clinical decision-making occurred at the enrolling center; patients with elevated cardiac biomarkers resulting from an elective coronary revascularization were excluded. The primary analysis was limited to 1307 patients who survived the initial hospitalization, who reported having smoked cigarettes at least once within 30 days prior to enrollment, and who smoked at least 1 cigarette per day on average (current every day smokers)(Figure 1). All patients signed an informed consent approved by the participating institution and institutional review board approval was obtained at each participating center.
Figure 1.
Study flow diagram
Data were collected on two groups of variables: 1) factors that may be associated with participation in OCR, and 2) factors associated with continued smoking following a cardiac event. Specifically, trained data collectors performed detailed baseline chart abstraction to document patients’ medical history and cardiovascular risk factors (e.g., hypertension, hyperlipidemia, diabetes, chronic heart failure, chronic lung disease), prior revascularization, MI severity (ST-segment elevation MI [STEMI] vs non–ST-segment elevation MI),13 inpatient care and treatments (in-hospital revascularization, prescription of aspirin, β-blocker, statin, angiotensin-converting enzyme inhibitor, or angiotensin receptor blocker), and laboratory results. We also collected data on body mass index (BMI, based on first height and weight in medical record), which was categorized as: <25 (normal), 25 to <30 (overweight), and ≥ 30 kg/m2 (obese). Prescription of first-line smoking cessation medications (nicotine patch, gum, lozenge, inhaler, or nasal spray; bupropion; varenicline) at discharge was also abstracted from the medical record.
During hospitalization, each patient underwent a standardized interview by research staff to document sociodemographic and economic variables (age, sex, self-identified race, marital status, education, insurance coverage, and financial status),5, 10, 11, 14, 15 smoking-related variables (cigarette intake, age at smoking initiation, counseling by staff on smoking cessation),7, 8, 10 psychosocial variables (depression, social support, perceived stress, current alcohol use),11, 16, 17 and self-reported health status.18 Follow-up interviews were conducted at one, six, and 12 months by a centralized, highly experienced, follow-up center.
Measures of health status at baseline and follow-up included the Seattle Angina Questionnaire (angina frequency, quality of life),19 and the Physical and Mental Health components of the Short-Form 12 (SF-12), version 2.20 We also asked participants if they avoided getting health care due to cost and if they had insurance coverage for prescription drugs. An additional measure of financial burden was based on monthly budget, with responses of “some money left over,” “just enough to make ends meet,” or “not enough to make ends meet.”21 Depressive symptoms were quantified with the 9-item Patient Health Questionnaire (PHQ-9); a PHQ-9 score ≥10 indicates a high probability of clinical depression.22 Social support was measured with the Enhancing Recovery in Coronary Heart Disease (ENRICHD) social support inventory.23 To assess psychological stress, we used a 4-item version of the Perceived Stress Scale (PSS); changes in the PSS are predictive of changes in smoking rate.24 Current alcohol use was defined as having at least 1 drink per month. During follow-up, participants were asked about total outpatient clinic visits since the prior interview (measure of health seeking behavior).
The primary independent variable of interest was participation in OCR, which was assessed at the 1- and 6-month follow-up interviews. Specifically, patients were asked if they had been referred to OCR and, if so, whether or not they had participated in a cardiac rehabilitation program since hospitalization for their “heart attack or heart problem.” In the analysis of 1- and 12-month cessation outcomes, those who confirmed that they had participated in at least 1 session within 1 month and within 6 months of hospital discharge were defined as OCR participants, respectively. Referral to OCR was determined by the presence of an order for cardiac rehabilitation at discharge in the medical record. Cardiac rehabilitation programs at all study sites were institution-based at the time of this study.
Smoking status was systematically collected at each follow-up interview. Patients who responded affirmatively to the following question were considered current smokers: “I have smoked (even a puff) in the past 30 days.” The primary outcome was 30-day point prevalence abstinence (PPA) at 12-month follow-up, which is associated more closely with lifelong cessation than assessments at 6-month follow-up;25 30-day PPA also allows comparison with other recent studies of hospitalized smokers.26 Smoking-related characteristics included cigarettes per day (<10, 11–20, >20), age of first regular cigarette smoking (in tertiles of < 15 years, 15 to 17 years, and 18 years or older), in-hospital receipt of smoking cessation counseling, and prescription of cessation medication at discharge.
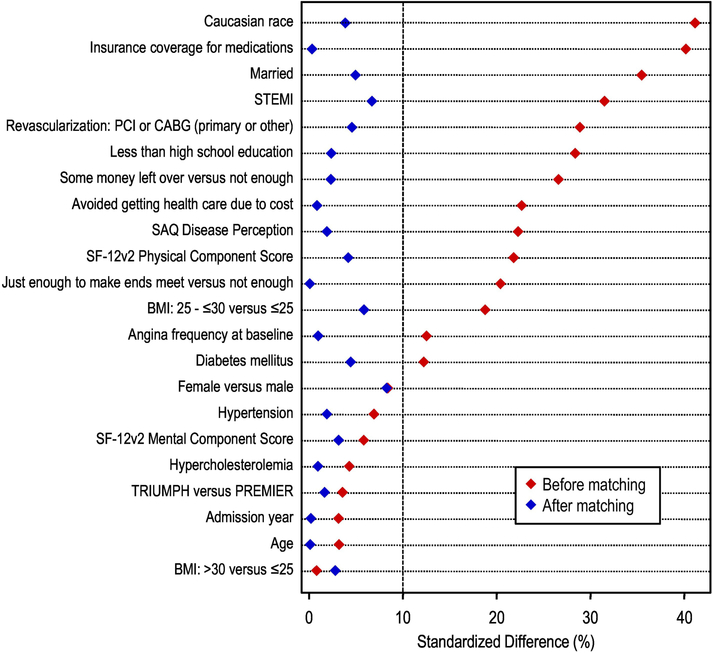
Baseline characteristics of OCR participants and nonparticipants were compared using the two-independent-sample t-test, Wilcoxon rank-sum, or chi-square tests as appropriate for the type of data. As the primary outcome of interest (30-day PPA) was anticipated to be a common occurrence, we used a modified Poisson regression model with robust variance estimates in the main analysis.27 As selection bias may confound the relationship between the exposure of interest and outcome, we developed a propensity model for OCR participation during the 6 months after hospital discharge, based on baseline data available at the time of initial hospital discharge (see Figure 2 for list of explanatory variables in both propensity models).28 We selected a set of variables common to both registries that could potentially influence the decision to refer to OCR (shown in Figure 2), based on prior literature and expert opinion.29-33 Model discrimination was assessed by the c-statistic (which is equivalent to the area under the receiver operating characteristic curve)34 and model calibration was evaluated with the Hosmer-Lemeshow goodness of fit test.35
Figure 2.
Standardized difference in covariate means or proportions between comparison groups at 12-month follow-up, before and after propensity score matching.
We then performed a matched analysis in which each OCR participant was matched by propensity score to an OCR non-participant. Only those matches that were within 0.2 SD of the logit of the propensity score were included;36 once a match was identified, we removed the matched pair from the sample and then repeated the process. We checked for balance of covariates by calculating the standardized difference in covariate means between patients within matched strata - small absolute values of the standardized difference (<10%) support the assumption of balance between comparison groups in measured characteristics. The relative risk (RR) for OCR participation on PPA was calculated at twelve months;37 clinical site was modeled as a random effect. Smoking-related factors, psychosocial factors (PHQ-9, social support, PSS, current alcohol use), and number of outpatient clinic visits were then added sequentially to these models to assess whether they attenuated the association between OCR participation and cessation outcomes. To evaluate the association between OCR participation and smoking status during the entire 12-month follow-up period, we also conducted a repeated-measures analysis of PPA (at 1, 6, and 12 months) using time-dependent propensity scores and generalized estimating equations (GEE)10 to calculate odds ratios and 95% confidence intervals.
We assessed the sensitivity of our results to assumptions regarding missing follow-up data. In the main analysis, patients who died or were lost to follow-up at 12 months were excluded (i.e., complete case analysis, N=1,307). We performed an analysis using inversely-weighted propensity scores, which represents the probability of each participant having available data at 12-month follow-up (N=1,789, including those who were lost to follow-up or were missing smoking status at 12-months); this approach more heavily weights the values of those subjects most likely to have missing follow-up data.38 We also repeated the analysis using penalized imputation (N=1,789), which assumes that subjects who are lost to follow-up are still smoking at 12 months (subjects who died during 12-month follow-up were excluded in this analysis).
RESULTS
An overview of the study sample is shown in Figure 1. Of 6,838 AMI patients in both registries, 2,525 (37%) were current smokers at the time of their AMI. Of 2,411 smokers who were alive at discharge, 1,307 completed follow-up (54%) and 1,104 subjects were excluded because they were missing data on OCR referral or participation (N=476), were lost to follow-up (N=587), or were missing data on smoking status at 12-month follow-up (N=41). The mean age of the analysis sample was 54.8 years, 68% were male, and 73% were White/Caucasian. At baseline, 34, 38, and 27% of subjects were light (≤10 cigarettes per day, cpd), medium (11–20 cpd), and heavy (> 20 cpd) smokers, respectively.
Seventy-four percent of subjects were referred to OCR at the time of discharge and 475 (36% of total) reported any participation during the 6 months following MI hospitalization. In bivariate analyses, OCR participants were significantly more likely to have had an ST-elevation MI and to have received revascularization during the index hospitalization (p<0.001 for both; Table 1). Despite having more severe ischemic heart disease at presentation, OCR participants had a better health outlook (based on the SAQ Quality of Life subscale), better physical function (SF-12), fewer depressive symptoms (PHQ-9), and greater social support (based on the ENRICHD score) at baseline. With regard to tobacco treatment, instructions for smoking cessation were more likely to have been documented in OCR participants, but prescription of smoking cessation medications at discharge was similar in both groups.
Table 1.
Baseline characteristics of participants and non-participants in outpatient cardiac rehabilitation (OCR).
| Participants (n = 475) | Non-participants (n = 832) | P–Value | |
|---|---|---|---|
| Sociodemographics | |||
| Age, mean (sd) | 54.6 (10.1) | 54.9 (9.9) | 0.58 |
| Sex, % male | 70.3 | 66.5 | 0.15 |
| Race Category, % | |||
| White/Caucasian | 84.0 | 66.7 | |
| Black/African American | 12.0 | 27.1 | |
| Other | 4.0 | 6.1 | < 0.001 |
| Less than high school education, % | 44.3 | 58.3 | < 0.001 |
| Marital status, % married | 64.2 | 46.9 | < 0.001 |
| Insurance coverage for medications, % | 81.4 | 63.8 | < 0.001 |
| Monthly financial situation, % | < 0.001 | ||
| Some money center over | 55.2 | 34.2 | |
| Just enough to make ends meet | 27.8 | 40.3 | |
| Not enough to make ends meet | 17.0 | 25.5 | < 0.001 |
| Avoided getting health care due to cost, % | 23.6 | 33.7 | < 0.001 |
| Study registry, % | 0.52 | ||
| PREMIER | 42.3 | 40.5 | |
| TRIUMPH | 57.7 | 59.5 | |
| MI characteristics and self-reported health | |||
| Final Study Diagnosis, % | < 0.001 | ||
| ST-Elevation Myocardial Infarction | 65.5 | 50.1 | |
| Non-ST-Elevation Myocardial Infarction | 34.5 | 49.9 | |
| Revascularization: PCI or CABG, % | 88.8 | 78.2 | < 0.001 |
| SAQ Quality of life, mean (sd) | 66.2 (22.7) | 61.0 (24.0) | < 0.001 |
| SF-12v2 Physical Component Score, mean (sd) | 45.3 (11.8) | 42.5 (12.1) | < 0.001 |
| SF-12v2 Mental Component Score, mean (sd) | 49.0 (11.9) | 48.2 (11.8) | 0.29 |
| Angina frequency at baseline, % | 0.03 | ||
| Good | 53.6 | 47.3 | |
| Mild/moderate/severe | 46.4 | 52.7 | 0.03 |
| Smoking-related factors | |||
| Cigarettes per day, % | 30.5 | 36.4 | 0.18 |
| 1 to 10 | 40.4 | 37.1 | |
| 11 to 20 | 29.0 | 26.4 | |
| Greater than 20 | |||
| Age started smoking regularly, % | 0.06 | ||
| Tertile 1 (0 to <15) | 26.1 | 30.7 | |
| Tertile 2 (15 to <18) | 27.8 | 29.8 | |
| Tertile 3 (18 to 75) | 46.0 | 39.5 | |
| Patient Instructions: Smoking Cessation, % | 83.8 | 73.6 | < 0.001 |
| Cessation medication prescribed at discharge, %* | 13.3 | 11.1 | 0.23 |
| Other cardiovascular risk factors | |||
| Diabetes mellitus, % | 16.4 | 21.3 | 0.03 |
| Hypertension, % | 52.8 | 56.4 | 0.22 |
| Hypercholesterolemia, % | 44.8 | 42.7 | 0.45 |
| Body Mass Index category, % | < 0.001 | ||
| Normal Weight (BMI < 25) | 18.8 | 27.4 | |
| Overweight (BMI 25 – <30) | 42.8 | 33.7 | |
| Obese (BMI ≥ 30) | 38.4 | 39.0 | |
| Psychosocial factors | |||
| ENRICHD Social Support score, mean (sd) | 21.9 (4.3) | 21.3 (4.8) | 0.02 |
| Alcohol use greater than monthly, % | 31.4 | 33.9 | 0.37 |
| PHQ-9 Depression score, mean (sd) | 5.3 (5.3) | 6.0 (5.5) | 0.03 |
| Treatment-related factors | |||
| Average number of outpatient clinic visits per month, median (IQR) | 0.50 (0.33, 0.67) | 0.42 (0.25, 0.67) | 0.02 |
Only FDA-approved medications for smoking cessation were included (nicotine patch, gum, lozenge, inhaler, or nasal spray; bupropion; varenicline).
Abbreviations: MI = Myocardial Infarction; PCI = Percutaneous coronary intervention; CABG = Coronary artery bypass graft; SF-12 = Short Form, 12 question, version 2; SAQ = Seattle Angina Questionnaire; SD = Standard deviation; IQR = Interquartile range
Propensity score models for OCR participation at 12-month follow-up (based on sociodemographic variables, cardiovascular risk factors, AMI characteristics, and self-reported health) showed good discrimination (C-statistic= 0.77). After optimal matching, comparison of OCR participants and non-participants showed standardized differences of less than 10% across all model covariates (Figure 2). These analyses (in which all candidate variables were forced into multivariable models) showed that low educational attainment, financial hardship, and lack of health insurance were associated with OCR non-participation, whereas patients with STEMI and those who were overweight (BMI 25–30 kg/m2) were more likely to participate.
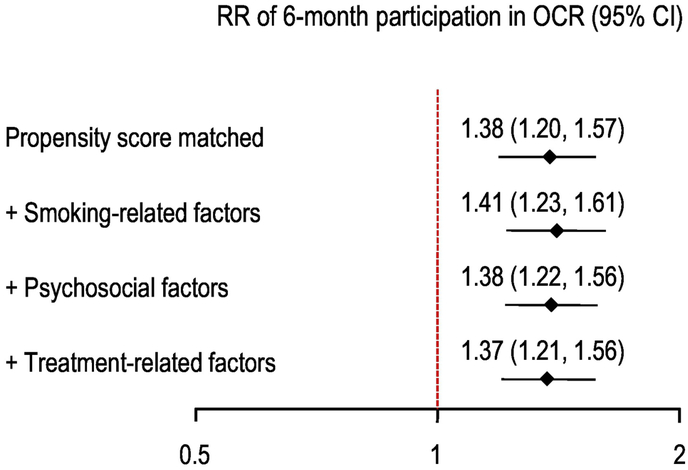
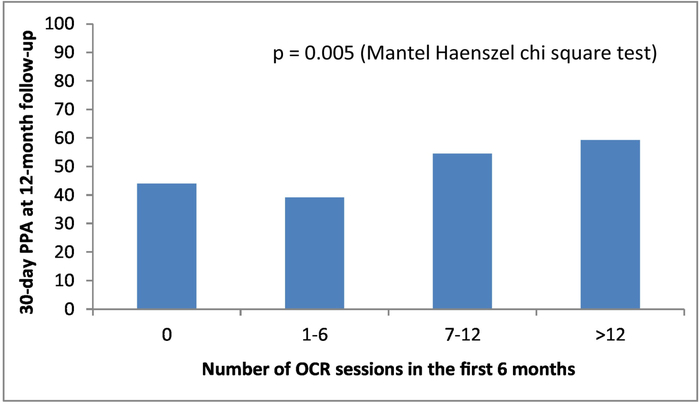
The unadjusted 30-day PPA for OCR participants and non-participants was 57% and 41% at 12-month follow-up, respectively (p<.001). After propensity score matching (N=1,255), the estimated relative risk (RR) of 30-day PPA for OCR participation was 1.38 (95% CI 1.20–1.57). This estimate changed minimally after adjustment for smoking-related variables, psychosocial factors, and outpatient visit rates: RR = 1.37, 95% CI = 1.21–1.56)(Figure 3). Repeated measures analysis with time-dependent covariates showed a somewhat attenuated, but still significant, treatment effect (RR = 1.22, 95% CI 1.11–1.33). In an unadjusted analysis of PREMIER subjects with complete data on OCR attendance during 6-month follow-up, there was a significant trend toward greater 30-day PPA as the number of OCR sessions increased (Figure 4).
Figure 3.
Association between participation in OCR and point-prevalence abstinence (PPA). OCR=outpatient cardiac rehabilitation; RR=relative risk, CI=confidence interval.
Figure 4.
Dose-response relationship between OCR attendance and point-prevalence abstinence (PPA) in the PREMIER registry. (TRIUMPH participants were not included, as the number of OCR sessions for these patients was only assessed through 1-month follow-up). OCR=outpatient cardiac rehabilitation.
Sensitivity analyses demonstrated that the original results were robust across different methods for handling missing data. Patients with missing smoking status at 12-month follow-up were significantly younger, were more likely to be non-white, have less than a high school education, to avoid getting healthcare due to cost, and to be treated without revascularization; they also had lower social support and worse SF-12 physical and mental health. In an analysis of 12-month survivors, using inversely weighted propensity scores (N=1,892), the fully adjusted relative risk was unchanged: RR = 1.37, 95% CI = 1.21–1.56). Using penalized imputation, the estimated RR for 30-day PPA was somewhat larger (fully adjusted RR = 1.66, 95% CI = 1.47–1.86).
DISCUSSION
Engaging AMI patients who smoke to quit tobacco is a critically important secondary prevention goal. In this large study of smokers recovering from an AMI, we found that participation in OCR was associated with an increased likelihood of smoking cessation during short- and long-term follow-up, after accounting for potential selection bias using propensity score matching. Although hospitalization is a teachable moment for smoking cessation,39, 40 40–69% of patients with ACS resume smoking, despite the known risks for recurrent coronary events,6, 9, 41, 42 and most patients who quit relapse within the first few weeks following hospital discharge.43 Through its focus on exercise and heart-healthy lifestyle, participation in OCR can reinforce patients’ efforts to maintain abstinence from smoking after hospital discharge. There remains a large gap between evidence and practice, however; although most of the patients (74%) in this study were referred to OCR, only 36% attended 1 or more sessions during follow-up.
This study extends the results of an earlier analysis of data from the PREMIER registry, which showed that the odds of smoking cessation were significantly greater among those receiving discharge recommendations for cardiac rehabilitation (OR=1.80, 95% confidence interval=1.17–2.75).5 We found that the absolute risk reduction associated with actual OCR participation in the current study was 17% in multivariable models, which is smaller than the effect observed for being merely referred to OCR.5 Our findings also confirm the results of Attebring et al., who found that non-participation in cardiac rehabilitation was an independent predictor of continued smoking at 3-month follow-up in patients hospitalized with ACS at a university hospital in Sweden.6 The authors suggested two possible interpretations for this finding: that more patients who had stopped smoking had chosen to participate in OCR or that OCR itself had a positive influence on smoking cessation.
Negative attitudes toward smoking cessation, nicotine dependence, low self-efficacy, and negative social influences reduce the likelihood of long-term abstinence from smoking in AMI patients.7, 8 Moreover, AMI patients frequently report symptoms of depression, and depressed smokers who attempt to quit are more likely to relapse,5, 44 in part because of strong nicotine withdrawal symptoms.45 OCR may improve cessation outcomes by strengthening motivation to quit, building perceptions of smoking self-efficacy, providing social support during treatment, and capitalizing on potential synergies with other cardiovascular risk behaviors.46, 47 An example of the latter is the finding that increasing physical activity during OCR may have positive impacts on both depressive symptoms and smoking cessation.48
The overall proportion of patients who reported any OCR participation in the current study (36%) is comparable to that reported in the 2005 Behavioral Risk Factor Surveillance System survey (34.7%).49 Compared with nonsmokers, smokers who are hospitalized for IHD-related events are less likely to enroll in OCR14, 29 and those who continue to smoke after hospital discharge are more likely to drop out of OCR.6 We identified several correlates of OCR participation that have been previously reported in other studies, including age,18, 30 low educational attainment,31 lack of insurance,31 financial constraints,32, 33 depression,17 and social support.32, 33 Reasons for not attending center-based OCR may also involve multiple logistical and contextual factors, such as competing work and family obligations, lack of transportation, and the accessibility of rehabilitation program sites and timing of sessions.50, 51
Our results should be interpreted in the context of the following limitations. First, the use of observational data is always subject to unmeasured confounding. Although we attempted to minimize confounding on account of selection bias through the use of propensity scores, there are potentially other unmeasured factors, such as personality types, social influences, and treatment adherence factors, that were not included in our model and may have been related both to OCR participation and smoking cessation. Such confounding would suggest that OCR participation is merely a marker of patients’ readiness to quit. Second, OCR participation was based on participant self-report; however, agreement between self-reported and program-reported rates of OCR participation has been shown to be quite high. In one prospective study of cardiac rehabilitation, participants self-reported attending a mean of 81.8% (sd=25.8%) of prescribed OCR sessions by self-report, with OCR sites reporting that participants completed 80.8% (sd=31.3%) of sessions (r = 0.66).52 Third, we did not capture the type, intensity, frequency, and length of OCR participation and cannot determine whether there is a dose-response association between OCR participation and smoking cessation.53 Similarly, we were unable to determine the specific components or attributes of OCR that were associated with improved cessation outcomes. Cardiac rehabilitation is a complex intervention that often incorporates several therapies, including exercise training, education for heart-healthy living and behavior change, stress management, psychological support, and strategies that target traditional risk factors for cardiovascular disease (including smoking cessation).2, 50 Fourth, we did not have data on level of nicotine dependence and confidence in quitting, which have been associated with long-term smoking cessation.8 Fifth, varenicline (which was approved by the FDA in May, 2006) was not available during the entire study period (2003–2008). Our findings should be confirmed in a contemporary cohort of patients with AMI. Finally, it was not feasible to obtain biochemical confirmation of smoking status during follow-up. Although a small minority of patients with ACS may misreport abstinence,6 we would not expect the rate of misreporting to differ based on OCR participation status. Moreover, self-reported abstinence is more likely to be accurate in observational studies without specific smoking cessation interventions and in the absence of strong incentives to deceive.54, 55
Despite these limitations, our findings have potential implications for the clinical care of smokers with AMI. In addition to referring these patients to OCR, clinicians should actively encourage current smokers to participate on an ongoing basis, which may substantially improve cessation outcomes.56 Patients who perceive that their physician considers OCR to be important are more likely to participate.57 The Center for Medicare & Medicaid Services (CMS) should also revisit policies and incentive payments that aim to reduce barriers to patient participation in OCR.58 Moreover, cardiac rehabilitation specialists need to be well versed in motivational interviewing, which has been shown to increase quit rates,59 and should look for natural synergies between smoking cessation and other cardiovascular risk behaviors (especially exercise and diet). Eligible AMI patients should also be routinely offered pharmacotherapy for smoking cessation60, 61 and all FDA-approved medications should be covered by health insurance plans, as recommended by the US Public Health Service guideline (and required by the 2010 Affordable Care Act).62, 63 More recently, varenicline has been shown to attenuate nicotine withdrawal symptoms during smoking abstinence and improve long-term cessation rates in patients with ACS.41
Conclusions.
In this observational study, cigarette smokers who participate in OCR after an AMI are more likely to self-report abstinence from smoking at 12-month follow-up compared to propensity-matched smokers who do not participate in CR. Strengths of this study are its prospective design, large effective sample size, and the use of propensity-score matching to account for selection bias (related to OCR participation). Limitations of this analysis include the possibility of residual confounding, the assessment of smoking status and OCR participation by self-report, and the lack of information on the quality of OCR-related cessation interventions. Future studies should better characterize the frequency, intensity, and duration of OCR and the specific elements that were delivered in order to determine possible causal mechanisms that could explain the association between OCR participation and smoking cessation. Novel strategies to improve OCR participation and to integrate state-of-the-art tobacco treatment into OCR programs should be evaluated in randomized clinical trials or controlled quasi-experimental trials.
Highlights.
Most smokers with acute myocardial infarction do not attend outpatient cardiac rehabilitation.
Older, less educated, poor, and uninsured smokers were less likely to participate.
Smokers who participated in outpatient cardiac rehabilitation were more likely to quit smoking.
Acknowledgements:
The authors thank Dominic Cirillo, MD, PhD for his intellectual contributions on an earlier version of this manuscript, Yan Li, PhD and Kensey Gosch, PhD for assistance with statistical analysis, and Charlotte Dean for assistance with manuscript preparation.
Funding: The Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) study was funded by grant P50 HL 077113 from the National Heart, Lung, and Blood Institute. Financial support for creation of the PREMIER registry was provided by CV Therapeutics, Palo Alto, California.
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interest.
The Prospective Registry Evaluating Outcomes After Myocardial Infarction: Events and Recovery (PREMIER) study was funded by CV Therapeutics, Palo Alto, California. The Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) study was funded by grant P50 HL 077113 from the National Heart, Lung, and Blood Institute.
Disclaimer: The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF and Yancy CW. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: A presidential advisory from the American Heart Association. Circulation. 2011;124:2951–60. [DOI] [PubMed] [Google Scholar]
- 2.Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N and Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016:Cd001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benowitz NL and Gourlay SG. Cardiovascular toxicity of nicotine: Implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29:1422–31. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RS, Unal B, Critchley JA and Capewell S. Mortality reductions in patients receiving exercise-based cardiac rehabilitation: How much can be attributed to cardiovascular risk factor improvements? Eur J Cardiovasc Prev Rehabil. 2006;13:369–74. [DOI] [PubMed] [Google Scholar]
- 5.Dawood N, Vaccarino V, Reid KJ, Spertus J, Hamid N, Parashar S, for the PREMIER Registry Investigators. Predictors of smoking cessation after a myocardial infarction: The role of institutional smoking cessation programs in improving success. Arch Intern Med. 2008;168:1961–7. [DOI] [PubMed] [Google Scholar]
- 6.Attebring MF, Hartford M, Hjalmarson A, Caidahl K, Karlsson T and Herlitz J. Smoking habits and predictors of continued smoking in patients with acute coronary syndromes. J Adv Nurs. 2004;46:614–23. [DOI] [PubMed] [Google Scholar]
- 7.van Berkel TF, van der Vlugt MJ and Boersma H. Characteristics of smokers and long-term changes in smoking behavior in consecutive patients with myocardial infarction. Prev Med. 2000;31:732–41. [DOI] [PubMed] [Google Scholar]
- 8.Quist-Paulsen P, Bakke PS and Gallefoss F. Predictors of smoking cessation in patients admitted for acute coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:472–7. [DOI] [PubMed] [Google Scholar]
- 9.Holtrop JS, Stommel M, Corser W and Holmes-Rovner M. Predictors of smoking cessation and relapse after hospitalization for acute coronary syndrome. J Hosp Med. 2009;4:E3–9. [DOI] [PubMed] [Google Scholar]
- 10.Gerber Y, Koren-Morag N, Myers V, Benyamini Y, Goldbourt U and Drory Y. Long-term predictors of smoking cessation in a cohort of myocardial infarction survivors: A longitudinal study. Eur J Cardiovasc Prev Rehabil. 2011;18:533–41. [DOI] [PubMed] [Google Scholar]
- 11.Berndt N, Bolman C, Mudde A, Verheugt F, de Vries H and Lechner L. Risk groups and predictors of short-term abstinence from smoking in patients with coronary heart disease. Heart Lung. 2012;41:332–43. [DOI] [PubMed] [Google Scholar]
- 12.Gaalema DE, Elliott RJ, Morford ZH, Higgins ST and Ades PA. Effect of socioeconomic status on propensity to change risk behaviors following myocardial infarction: Implications for healthy lifestyle medicine. Prog Cardiovasc Dis. 2017;60:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C and Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)-evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–97. [DOI] [PubMed] [Google Scholar]
- 14.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM and Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): Design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motivala AA, Cannon CP, Srinivas VS, Dai D, Hernandez AF, Peterson ED, Bhatt DL and Fonarow GC. Changes in myocardial infarction guideline adherence as a function of patient risk: An end to paradoxical care? J Am Coll Cardiol. 2011;58:1760–5. [DOI] [PubMed] [Google Scholar]
- 16.Tzou W, Vitcenda M and McBride P. Smoking status after cardiac events and participation in outpatient cardiac rehabilitation. J Cardiopulm Rehabil. 2004;24:94–9. [DOI] [PubMed] [Google Scholar]
- 17.Friedman DB, Williams AN and Levine BD. Compliance and efficacy of cardiac rehabilitation and risk factor modification in the medically indigent. Am J Cardiol. 1997;79:281–5. [DOI] [PubMed] [Google Scholar]
- 18.Perez GH, Nicolau JC, Romano BW and Laranjeira R. Depression: A predictor of smoking relapse in a 6-month follow-up after hospitalization for acute coronary syndrome. Eur J Cardiovasc Prev Rehabil. 2008;15:89–94. [DOI] [PubMed] [Google Scholar]
- 19.Pogosova N, Saner H, Pedersen SS, Cupples ME, McGee H, Hofer S, Doyle F, Schmid JP and von Kanel R. Psychosocial aspects in cardiac rehabilitation: From theory to practice. A position paper from the cardiac rehabilitation section of the European Association of Cardiovascular Prevention and Rehabilitation of the European Society of Cardiology. Eur J Prev Cardiol. 2015;22:1290–306. [DOI] [PubMed] [Google Scholar]
- 20.Cupples ME, Tully MA, Dempster M, Corrigan M, McCall DO and Downey B. Cardiac rehabilitation uptake following myocardial infarction: Cross-sectional study in primary care. Br J Gen Pract. 2010;60:431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spertus JA, Windar JA and Dewhurst TA. Development and evaluation of the Seattle Angina Questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B and Maruish ME. User’s manual for the SF-36v2 Health Survey: Quality Metric; 2008. [Google Scholar]
- 23.Rahimi AR, Spertus JA, Reid KJ, Bernheim SM and Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA. 2007;297:1063–72. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL and Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CR, Froelicher ES, Czajkowski S, Youngblood M, Huber M and Berkman LF. A short social support measure for patients recovering from myocardial infarction: The ENRICHD Social Support Inventory. J Cardiopulm Rehabil. 2003;23:398–403. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Kamarck T and Mermelstein R. A global measure of perceived stress. J Health Sci Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 27.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL and Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 28.Riley WT, Stevens VJ, Zhu SH, Morgan G and Grossman D. Overview of the Consortium of Hospitals Advancing Research on Tobacco (CHART). Trials. 2012;13:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 30.D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment ot a non-randomized control group. Stat Med. 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 31.Gaalema DE, Cutler AY, Higgins ST and Ades PA. Smoking and cardiac rehabilitation participation: Associations with referral, attendance and adherence. Prev Med. 2015;80:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, Victory J, Brown J, Taylor RS and Ebrahim S. Provision, uptake and cost of cardiac rehabilitation programmes: Improving services to under-represented groups. Health Technol Assess. 2004;8:iii-iv, ix-x, 1–152. [DOI] [PubMed] [Google Scholar]
- 33.Fang J, Ayala C, Luncheon C, Ritchey M and Loustalot F. Use of outpatient cardiac rehabilitation among heart attack survivors - 20 states and the District of Columbia, 2013 and four states, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grace SL, Gravely-Witte S, Brual J, Monette G, Suskin N, Higginson L, Alter DA and Stewart DE. Contribution of patient and physician factors to cardiac rehabilitation enrollment: A prospective multilevel study. Eur J Cardiovasc Prev Rehabil. 2008;15:548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark AM, King-Shier KM, Thompson DR, Spaling MA, Duncan AS, Stone JA, Jaglal SB and Angus JE. A qualitative systematic review of influences on attendance at cardiac rehabilitation programs after referral. Am Heart J. 2012;164:835–45. e2. [DOI] [PubMed] [Google Scholar]
- 36.Hanley JA and McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. [DOI] [PubMed] [Google Scholar]
- 37.Hosmer DW and Lemeshow S. Applied logistic regression. 2nd edition ed. New York: John Wiley & Sons; 2000. [Google Scholar]
- 38.Rosenbaum PR and Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 39.Ockene JK, Emmons KM, Mermelstein RJ, Perkins KA, Bonollo DS, Voorhees CC and Hollis JF. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19:17–31. [DOI] [PubMed] [Google Scholar]
- 40.Lunceford JK and Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: A comparative study. Stat Med. 2004;23:2937–60. [DOI] [PubMed] [Google Scholar]
- 41.McBride CM, Emmons KM and Lipkus IM. Understanding the potential of teachable moments: The case of smoking cessation. Health Educ Res. 2003;18:156–70. [DOI] [PubMed] [Google Scholar]
- 42.Boudreaux ED, Bock B and O’Hea E. When an event sparks behavior change: An introduction to the sentinel event method of dynamic model building and its application to emergency medicine. Acad Emerg Med. 2012;19:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, Iskander A, Lauzon C, Srivastava N, Clarke A, Cassavar D, Dion D, Haught H, Mehta SR, Baril JF, Lambert C, Madan M, Abramson BL and Dehghani P. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016;133:21–30. [DOI] [PubMed] [Google Scholar]
- 44.Minneboo M, Lachman S, Snaterse M, Jorstad HT, Ter Riet G, Boekholdt SM, Scholte Op Reimer WJM and Peters RJG. Community-based lifestyle intervention in patients with coronary artery disease: The RESPONSE-2 trial. J Am Coll Cardiol. 2017;70:318–327. [DOI] [PubMed] [Google Scholar]
- 45.Colivicchi F, Mocini D, Tubaro M, Aiello A, Clavario P and Santini M. Effect of smoking relapse on outcome after acute coronary syndromes. Am J Cardiol. 2011;108:804–8. [DOI] [PubMed] [Google Scholar]
- 46.McGee HM, Doyle F, Conroy RM, De La Harpe D and Shelley E. Impact of briefly-assessed depression on secondary prevention outcomes after acute coronary syndrome: A one-year longitudinal survey. BMC Health Serv Res. 2006;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres-Finnerty N and Rigotti NA. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Arch Intern Med. 2008;168:186–91. [DOI] [PubMed] [Google Scholar]
- 48.Gordon NF, Salmon RD, Mitchell BS, Faircloth GC, Levinrad LI, Salmon S, Saxon WE and Reid KS. Innovative approaches to comprehensive cardiovascular disease risk reduction in clinical and community-based settings. Curr Atheroscler Rep. 2001;3:498–506. [DOI] [PubMed] [Google Scholar]
- 49.Doherty SC, Steptoe A, Rink E, Kendrick T and Hilton S. Readiness to change health behaviors among patients at high risk of cardiovascular disease. J Cardiovasc Risk. 1998;5:147–53. [PubMed] [Google Scholar]
- 50.Ussher MH, Taylor A and Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2012;1:Cd002295. [DOI] [PubMed] [Google Scholar]
- 51.Receipt of outpatient cardiac rehabilitation among heart attack survivors--United States, 2005. MMWR Morb Mortal Wkly Rep. 2008;57:89–94. [PubMed] [Google Scholar]
- 52.Savage PD, Sanderson BK, Brown TM, Berra K and Ades PA. Clinical research in cardiac rehabilitation and secondary prevention: Looking back and moving forward. J Cardiopulm Rehabil Prev. 2011;31:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karmali KN, Davies P, Taylor F, Beswick A, Martin N and Ebrahim S. Promoting patient uptake and adherence in cardiac rehabilitation. Cochrane Database Syst Rev. 2014:Cd007131. [DOI] [PubMed] [Google Scholar]
- 54.Kayaniyil S, Leung YW, Suskin N, Stewart DE and Grace SL. Concordance of self- and program-reported rates of cardiac rehabilitation referral, enrollment and participation. Can J Cardiol. 2009;25:e96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kureshi F, Kennedy KF, Jones PG, Thomas RJ, Arnold SV, Sharma P, Fendler T, Buchanan DM, Qintar M, Ho PM, Nallamothu BK, Oldridge NB and Spertus JA. Association between cardiac rehabilitation participation and health status outcomes after acute myocardial infarction. JAMA Cardiol. 2016;1:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velicer WF, Prochaska JO, Rossi JS and Snow MG. Assessing outcome in smoking cessation studies. Psych Bull. 1992;111:23–41. [DOI] [PubMed] [Google Scholar]
- 57.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. [DOI] [PubMed] [Google Scholar]
- 58.Wallentin L, Kristensen SD, Anderson JL, Tubaro M, Sendon JL, Granger CB, Bode C, Huber K, Bates ER, Valgimigli M, Steg PG and Ohman EM. How can we optimize the processes of care for acute coronary syndromes to improve outcomes? Am Heart J. 2014;168:622–31. [DOI] [PubMed] [Google Scholar]
- 59.McCorry NK, Corrigan M, Tully MA, Dempster M, Downey B and Cupples ME. Perceptions of exercise among people who have not attended cardiac rehabilitation following myocardial infarction. J Health Psychol. 2009;14:924–32. [DOI] [PubMed] [Google Scholar]
- 60.Centers for Medicare & Medicaid Services. Cardiac Rehabilitation (CR) Incentive Payment Model. 2017.
- 61.Lindson-Hawley N, Thompson TP and Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015:Cd006936. [DOI] [PubMed] [Google Scholar]
- 62.Katz DA, Tang F, Faseru B, Horwitz PA, Jones P and Spertus J. Prevalence and correlates of smoking cessation pharmacotherapy in hospitalized smokers with acute myocardial infarction. Am Heart J. 2011;162:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rigotti NA, Clair C, Munafo MR and Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012:Cd001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Treating tobacco use and dependence: 2008 update. In: Clinical Practice Guideline. 2008. US Department of Health and Human Services, US Public Health Service, Rockville, MD: http://bphc.hrsa.gov/buckets/treatingtobacco.pdf. (accessed 12/10/17) [Google Scholar]
- 65.Centers for Disease Control and Prevention, Coverage for Tobacco Use Cessation Treatments. 2012. https://www.cdc.gov/tobacco/quit_smoking/cessation/pdfs/coverage.pdf (accessed 12/10/17).