Abstract
Objective:
Nonverbal learning disability (NVLD) is a putative neurodevelopmental disorder characterized by spatial processing deficits as well as social deficits similar to those characteristic of autism spectrum disorder (ASD). Nonetheless, NVLD may be a distinct disorder that is differentially associated with the functioning and connectivity of the salience (SN) and default mode (DMN) networks that support social processing. Thus, we sought to assess and compare connectivity across these networks in children with NVLD, ASD, and typically developing children.
Method:
Resting-state fMRI data were examined in 17 children with NVLD, 17 children with ASD selected from the Autism Brain Imaging Data Exchange (ABIDE), and 40 TD children (20 from ABIDE). Average DMN and SN functional connectivity and pairwise region-to-region connectivity were compared across groups. Associations with social impairment and IQ were assessed.
Results:
Children with NVLD showed reduced connectivity between SN regions (anterior insula to anterior cingulate and to rostral prefrontal cortex [rPFC]), whereas children with ASD showed greater connectivity between SN regions (supramarginal gyrus to rPFC) relative to the other groups. Both clinical groups showed higher levels of parent-reported social problems, which related to altered SN connectivity in the NVLD group. No differences were detected in overall average connectivity within or between networks.
Conclusions:
The social deficits common across children with NVLD and ASD may derive from distinct alterations in connectivity within the SN. Such findings represent the first step toward identifying a neurobiological signature of NVLD.
Keywords: resting state fMRI, nonverbal learning disability, autism spectrum disorder, salience network, default mode network
Nonverbal learning disability (NVLD) is a putative neurodevelopmental disorder first described by Johnson and Mykelbust (1967). Although the exact criteria for a diagnosis are debated, NVLD is most often characterized by deficits in visuospatial ability, or a discrepancy between visuospatial and verbal ability, that is accompanied by problems in math but not reading or verbal ability; in addition problems in visuospatial memory, attention, executive functions, as well as fine-motor and socioemotional skills are often present (Cornoldi, Mammarella, & Fine, 2016; Fine, Semrud-Clikeman, Bledsoe, & Musielak, 2013; Mammarella & Cornoldi, 2014). Autism spectrum disorder (ASD) is characterized by deficits in reciprocal communication and social function, as well as stereotyped, restricted interests and repetitive behaviors (Diagnostic and Statistical Manual of Mental Disorders, 5th ed., APA, 2013). NVLD and ASD share some characteristics, such as parent report of problems in social functioning, but these difficulties may derive from different underlying deficits. For example, both groups show deficits in understanding humor and processing pragmatic language (Cardillo, Garcia, Mammarella, & Cornoldi, 2017; Semrud-Clikeman, Fine, & Bledsoe, 2015; Semrud-Clikeman, Walkowiak, Wilkinson, & Minne, 2010; Semrud-Clikeman & Glass, 2010), but children with NVLD do not have the repetitive behaviors or restricted interests that contribute to the social deficits of children with ASD (Fine et al., 2013; Mammarella & Cornoldi, 2014). Given these differences and differences in spatial processing deficits, NVLD may be a distinct disorder (Klin, Volkmar, Sparrow, Cicchetti, & Rourke, 1995; Palombo, 2006; Semrud-Clikeman, Walkowiak, Wilkinson, & Christopher, 2010). The current study represents a first step in examining whether parent report of social problems in NVLD and ASD is differentially associated with brain function and connectivity.
Findings from resting state functional MRI (fMRI) studies of healthy individuals have identified canonical brain networks associated with broad aspects of psychological functioning (Power et al., 2011). Social processing functions are thought to be supported, in part, by two such networks: the default mode network (DMN) and the salience network (SN; Laird et al., 2011; Li, Mai, & Liu, 2014; Menon, 2015). In addition, task-based findings from healthy individuals suggest that DMN regions are activated during social cognition and mentalizing (Li et al., 2014; Mar, 2011; Schilbach, Eickhoff, Rotarska-Jagiela, Fink, & Vogeley, 2008), and that activation of SN regions, particularly the anterior insula (AI), supports the interpretation of social norms (Xiang, Lohrenz, & Montague, 2013) and emotional awareness (Gu, Hof, Friston, & Fan, 2013). Thus, both resting-state and task-based fMRI data implicate these networks in supporting social processing.
Altered function and connectivity of DMN and SN regions have been identified in children with ASD, consistent with their social processing deficits. Relative to typically developing (TD) children, those with ASD show reduced connectivity within the DMN in contrast to increased connectivity within and across other networks (Hull et al., 2017). Reduced DMN connectivity is associated with symptom severity in children with ASD and, specifically, social processing problems (Assaf et al., 2010). Children with ASD also show altered connectivity within the SN, with one study reporting decreased connectivity compared with TD children (Abbott et al., 2016), and others reporting increased connectivity (Uddin et al., 2013) associated with social problems (Elton, Di Martino, Hazlett, & Gao, 2015). Further, meta-analytic data suggest altered functioning of AI and anterior cingulate cortex (ACC) during social processing tasks in children with ASD (Di Martino et al., 2009). Although no previous studies of NVLD have used task-based or resting-state fMRI, structural MRI findings suggest reduced gray matter volume in bilateral ACC in both children with NVLD and those with ASD (Semrud-Clikeman, Fine, Bledsoe, & Zhu, 2013). Notably, the functioning and connectivity of the networks has yet to be studied and compared across these disorders.
The current study is the first to examine resting state functional connectivity (RSFC) within and between the SN and DMN in a sample of children with NVLD, children with ASD, and TD children. Given that parental reports of social dysfunction are common to both children with NVLD and ASD, we anticipated that both groups of children would show altered connectivity of the DMN and SN. However, children with NVLD and ASD have different clinical presentations and therefore the groups may show distinct patterns of alterations within or between these networks. Specifically, we hypothesized that compared with TD children, those with ASD would show decreased DMN and increased SN connectivity, consistent with prior RSFC findings (Uddin et al., 2013; von dem Hagen, Stoyanova, Baron-Cohen, & Calder, 2013). Prior structural findings implicate the ACC in NVLD (Semrud-Clikeman et al., 2013), but the absence of prior functional findings from this population precludes our making specific directional hypotheses regarding DMN and SN connectivity in children with NVLD. However, given recent work suggesting that the pathophysiology and behavioral presentation of NVLD is distinct from that of ASD (Fine, Musielak, & Semrud-Clikeman, 2014; Mammarella & Cornoldi, 2014; Semrud-Clikeman et al., 2013; Semrud-Clikeman et al., 2010), we hypothesized that children with NVLD would show altered RSFC within and between the SN and DMN compared with TD children and those with ASD.
Method
Participants
An age- and sex-matched sample of 20 typically developing children and 35 children suspected to exhibit NVLD were recruited through announcements posted at local schools, on social media, and in the newsletter of The NVLD Project, a nonprofit aimed at developing resources for families of children with NVLD. Of the 35 children evaluated for NVLD, 21 met criteria for NVLD (see description below and Table S1) and completed an MRI scan. Four children with NVLD and no TD children were excluded from imaging analyses due to excessive head motion, as described below, yielding 17 children with NVLD and 20 TD children in the final analyses (see Table 1). The Institutional Review Board (IRB) of the New York State Psychiatric Institute (NYSPI) approved the study; children and their guardians provided written informed assent and consent, respectively.
Table 1.
Demographic Characteristics
| Demographics | TD (n = 40) | NVLD (n = 17) | ASD (n = 17) | Group differences | Direction |
|---|---|---|---|---|---|
| Age | 10.534 (1.693) | 11.46 (2.084) | 11.539 (2.373) | 2.247 | — |
| 7.17–14.10 | 8.00–15.25 | 7.40–16.80 | |||
| Sex, N (%) female | 17 (43%) | 10 (59%) | 6 (35%) | .357 | — |
| VIQ | 114.125 (16.054) | 106.882 (11.027) | 96.176 (12.787) | 9.449*** | TD = NVLD > ASD |
| 73–144 | 87–127 | 75–116 | |||
| PIQ | 112.875 (15.310) | 86.000 (12.160) | 108.588 (16.492) | 19.693*** | TD = ASD > NVLD |
| 83–143 | 69–114 | 86–145 | |||
| VIQ-PIQ discrepancy | 1.975 (12.160) | 19.176 (11.775) | −12.411 (15.776) | 24.207*** | NVLD > TD > ASD |
| −27–24 | −1–40 | −43–14 | |||
| SRS total | 43.321 (6.066) | 71.411 (16.681) | 80.866 (8.025) | 82.862*** | ASD = NVLD > TD |
| 35–64 | 45–105 | 64–94 | |||
| Useable frames | 152.200 (25.076) | 121.941 (13.631) | 168.705 (15.490) | 22.043*** | ASD > TD > NVLD |
| 81–180 | 88–140 | 129–180 | |||
| Percent usable frames | 94.87% (7.8%) | 87.10% (9.7%) | 93.73% (8.9%) | 5.154* | TD = ASD > NVLD |
| 58%–100% | 63%–100% | 72%–100% | |||
| Mean motion | .180 (.189) | .347 (.197) | .223 (.023) | 6.616* | NVLD > TD = ASD |
| .030–1.162 | .068–.822 | .055–.775 |
Note.Table displays demographic characteristics for the typically developing (TD) children and children with nonverbal learning disability (NVLD) or with autism spectrum disorder (ASD). Means, standard deviations, and ranges are presented for all continuous variables. The group differences column indicates ANOVA F-statistics comparing across all three groups. The number and percent of female participants was presented in the sex row and group differences were tested using chi-squared. The direction column indicates significant post hoc tests (Fisher’s least significant difference or Dunnet’s T3 when inhomogeneity of variance was present) to parse any significant ANOVA results. VIQ = verbal IQ; PIQ = performance IQ; SRS = Social Responsiveness Scale. For all IQ scores, TD n = 40, NVLD n = 17, and ASD n = 17.
p < .05.
p < .001.
A sample of participants (N = 20 TD, N = 17 ASD) was selected from data made publicly available by the Autism Brain Imaging Data Exchange (ABIDE). Participants were selected from the ABIDE I (N = 8 TD, N = 2 ASD) and II (N = 12 TD, N = 15 ASD) releases to minimize age and sex differences between the groups. ABIDE data collected at one site, San Diego State University (SDSU), were utilized to best match the MRI scanner, acquisition parameters, and sample demographics at our site and to increase comparability across sites.
Diagnostic Criteria
A diagnosis of NVLD was established in accord with prior research criteria (e.g., Fine et al., 2014; Semrud-Clikeman et al., 2013). Children were included in the NVLD group if they had: (a) perceptual deficits (block design, matrix reasoning, or performance IQ [PIQ] at or below the 16th percentile) or a discrepancy between verbal IQ (VIQ) and PIQ greater than or equal to 15 points; (b) intact single word reading abilities (WJ-III Letter Word Identification at or above the 16th percentile); (c) poor performance (at or below the 16th percentile) on at least two measures of: fine motor skills (Perdue Pegboard), math calculation (WJ-III Calculation), visual executive functioning (Rey Osterrieth Complex Figure Test: Copy), and social skills (Vineland-II Socialization domain at or below the 16th percentile, or CBCL Social Problems at or above the 95th percentile); and (d) Autism Diagnostic Interview-Revised [ADI-R] Interests and Behaviors Module (Rutter, Le Couteur, & Lord, 2003) score less than or equal to 4. Table S2 presents data describing these children on these measures. Children were excluded for MRI contraindication or if they had any lifetime diagnosis of neurological or neurodevelopmental disorder other than specific learning disorder, ADHD, or social anxiety disorder, as determined by clinical interview and administration of the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS; Kaufman et al., 1997). All measures in the study were administered by a certified school psychologist (Ed.M.) who had formal ADI-R and KSADS clinical training.
A diagnosis of autism/autism spectrum disorder according to DSM–IV–TR criteria for participants in the ABIDE I release or DSM–5 criteria in the ABIDE II release was confirmed by an expert clinician and performance on the Autism Diagnostic Observation Schedule-2 (Lord et al., 2012) and the ADI-R (Rutter et al., 2003). Children were excluded for MRI contraindication, ASD-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis), and other neurological conditions (e.g., epilepsy, Tourette’s Syndrome). As children from the ABIDE dataset were not explicitly screened for NVLD, those who had a PIQ less than or equal to 16th percentile or a VIQ greater than PIQ discrepancy greater than or equal to 15 points were not selected for inclusion in our analyses because such a discrepancy could be indicative of undiagnosed NVLD.
Typically developing children had no diagnoses. In our dataset, the presence or absence of lifetime diagnoses was determined using the KSADS. In the ABIDE and ABIDE-II data sets, a phone screen followed by an in-person interview with the child and family determined the absence of personal or family history of ASD as well as any other neurological or psychiatric conditions. Because these children were considered typically developing, undiagnosed NVLD was not a concern and therefore neither PIQ deficits nor VIQ-PIQ discrepancies were used as selection criteria.
Neuropsychological and Psychosocial Outcome Measures
Children at both sites completed the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), a measure of intellectual functioning. Parents at both sites completed the Social Responsiveness Scale (SRS), a dimensional measure of autistic social impairment (Constantino & Gruber, 2005). Data from a number of measures used to diagnose NVLD (listed above) were only available for participants assessed at our site.
Demographic and Clinical Characteristics
Demographic and clinical characteristics (see Table 1) were compared across the three groups (TD vs. NVLD vs. ASD). Specifically, group differences in sex were tested by chi-squared test and group differences in continuous outcomes (age, IQ, SRS, head motion) were tested by ANOVA (Welch test when variances were not equal across groups). Independent samples t tests were used to examine differences in ADI-R scores, which were only available in the two patient groups.
Neuroimaging Acquisition
NYSPI site.
Functional and anatomical images were collected on a 3T GE 750 scanner. Structural T1 images were collected with an eight-channel head coil using a three-dimensional (3D) FSPGR sequences (flip angle = 11, TE = 2.588 ms, TR = 6.412 ms, 180 slices, 1 mm × 1 mm × 1 mm resolution). Two runs of resting state data were acquired with a 32-channel head coil using an echo planar imaging (EPI) sequence (flip angle = 77, TE = 30 ms, TR = 2,000 ms, 34 slices, 3.5 mm × 3.5 mm × 3.5 mm resolution, 140 acquisition frames, 4 min and 40 s long).
ABIDE SDSU site.
Functional and anatomical images were collected on a 3T GE MR750 scanner with an eight-channel head coil. Structural T1 images were collected with a 3D SPGR sequence (flip angle = 8, TE = 3.17 2ms, TR = 8.13 6ms, 172 slices, 1 mm × 1 mm × 1 mm resolution). One run of resting state data was acquired using an EPI sequence (flip angle = 90, TE = 30 ms, TR = 2,000 ms, 42 slices, 3.4375 mm × 3.4375 mm × 3.4 mm resolution, 180 acquisition frames, 6 min and 10 s long).
Resting State Functional Connectivity Preprocessing
For participants scanned at our site, one run of resting state data was selected (the first run, unless this run had too much head motion, as defined below) to better match the length of the ABIDE acquisition. Analysis was performed in the CONN toolbox v 17.f (www.nitrc.org/projects/conn; Whitfield-Gabrieli & Nieto-Castanon, 2012) for SPM 12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/ ). Preprocessing included realignment; unwarping; centering; slice timing correction; Artifact Detection Tools outlier detection; segmentation of cerebral spinal fluid, gray, and white matter; normalization to the Montreal Neurological Institute (MNI) template; and 8-mm full-width half-maximum smoothing for functional images. Structural images were centered, segmented, and normalized to the MNI template. BOLD signal was band-pass filtered (0.008 Hz–0.09 Hz). Anatomical component-based noise correction (aCompCor; Behzadi, Restom, Liau, & Liu, 2007) was used for further denoising, specifically regressing 10 white matter and 10 cerebral spinal fluid (CSF) components (detrended and despiked). Given significant debate regarding selection of confound regression methods (Ciric et al., 2017), supplemental analyses were conducted after denoising with global signal regression (GSR).
Motion Correction
To minimize effects of head motion, frames exceeding 0.9 mm frame-wise displacement or frame-to-frame changes in global signal above a z-value of 5, a more stringent correction than prior work with the ABIDE data (Nair et al., 2014; Carper, Solders, Treiber, Fishman, & Muller, 2015; Fishman, Keown, Lincoln, Pineda, & Muller, 2014) were regressed from the data in the first level models. In other words, frames with motion exceeding these thresholds (frame-wise displacement >.9 mm or frame-to-frame change z > 5) were assigned a value of 1 on individual regressors representing those frames, rather than removing the frame, in order to keep the total number of frames constant across participants. Next, participants with fewer than 80 usable frames were excluded from the analyses (N = 4/21 NVLD). In addition, 12 head motion parameters (motion + first-order derivatives) were included as regressors. Total number of usable frames and percent usable frames for each group were significantly correlated with mean motion, r(72) = −.694, p < .001; r(72) = −.947, p <.001, respectively (see Table 1).
Comparability of Data Across Sites
Recent findings suggest that seed-to-voxel connectivity findings are consistent across scanners and sites (Noble et al., 2017). To maximize comparability of data across sites, however, we selected data from ABIDE that was acquired at a single site using a scanner (3T GE 750) and pulse sequence that matched ours most closely: 1 mm × 1 mm × 1 mm resolution for the T1, and TE = 30, TR = 2,000 ms, and nearly identical spatial resolution for EPI sequences. In addition, we sought to determine whether site differences in head coils and MRI acquisition parameters could confound results. Specifically, an independent samples t test was used to compare the average temporal signal-to-noise ratio (tSNR) across the samples acquired at the two sites. Additionally, the number of frames acquired differed by site and thus contributed to differences in the number of usable frames between participants with NVLD and ASD (see Table 1). Because the ability to detect connectivity between regions is likely influenced by the number of available frames, differences in sequence length between sites could confound group differences in connectivity. Thus, the number of usable frames (after outlier regression) was included as a covariate to account for differences in site. The number of usable frames was highly correlated with mean motion, and therefore controlling for it also controlled for individual differences in head motion.
Connectivity Measures
Connectivity was derived from bivariate correlations between seven region of interest (ROI) seeds of the SN and four seeds of DMN (Table S3; Figure S1). Seeds were available through CONN and were previously defined using independent components analyses of data from the Human Connectome Project (Smith et al., 2013). First, average network connectivity, that is, mean of all pairwise correlations among SN seeds, among DMN seeds, and between SN and DMN seeds, was examined. Next, pairwise ROI-to-ROI connectivity strength values within and between the networks were examined.
Statistical Analyses
Average network connectivity and pairwise ROI-ROI connectivity strength values were compared across diagnostic groups (TD vs. NVLD vs. ASD) using second-level F tests in CONN. ANOVAs were performed on the Fisher r-to-Z transformed correlation values, the standard procedure in CONN. False discovery rate (FDR) was used in two steps, at the seed- and then connection-level, to control for multiple comparisons. First, multivariate tests were used to identify ROIs that showed omnibus group differences in connectivity with any other ROIs (FDR corrected p values: q<.05, controlling for 11 omnibus tests). Next, connectivity between each ROI significant in this first step and all 10 other ROIs was examined to parse significant omnibus effects (FDR corrected for 10 tests per ROI, q<.05). Only connections showing significant group differences after this two-step FDR correction (see Table 2) were extracted for post hoc analyses and visualization. Post hoc analyses included follow-up ANCOVAs performed in SPSS controlling for the number of usable frames to account for potential differences associated with head motion and scan site; these are reported in Table 2. Further, associations between RSFC and behavioral measures (SRS total, VIQ-PIQ discrepancy, VIQ, and PIQ) were explored in hierarchical linear regression models that included group, RSFC, and a group-by RSFC interaction term as predictors, controlling for number of usable frames. Nonsignificant interaction terms were dropped from the models.
Table 2.
Salience Network Connectivity
| Connection | F | FDR q | η2 | Direction | F-usable |
|---|---|---|---|---|---|
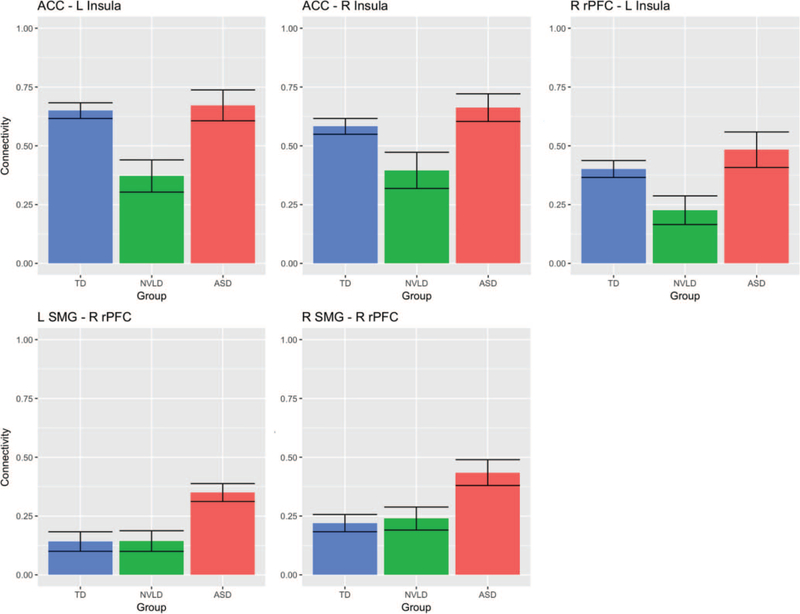
| ACC—L AI | 8.01*** | .007 | .184 | TD = ASD > NVLD | 3.761* |
| ACC—R AI | 5.29** | .036 | .130 | TD = ASD > NVLD | 1.875 |
| L SMG—R rPFC | 5.49** | .030 | .134 | ASD > TD = NVLD | 4.065* |
| R SMG—R rPFC | 5.84** | .030 | .141 | ASD > TD = NVLD | 3.590* |
| R rPFC—L AI | 4.54* | .046 | .113 | TD = ASD > NVLD | 2.312 |
Note. Table displays the connections between salience network seeds that showed significant group differences after false discovery rate (FDR) correction for multiple comparisons. The F column indicates F-statistics for group differences in each connection. The FDR q column indicates the FDR-corrected p-value from the second-step of the FDR correction (i.e. controlling for ten tests for each seed passing the first step of FDR correction). Effect size (partial eta-squared) of the group difference are presented in the η2 column. The direction column indicates the direction of effects among the three groups indicated by significant post hoc tests (Fisher’s least significant difference, p < .05). The F-useable column indicates the F-statistic for group differences from post hoc ANCOVAs including the number of usable frames as a covariate. ACC = anterior cingulate cortex; AI = anterior insula; SMG = supramarginal gyrus; rPFC = rostral prefrontal cortex; TD = typically developing; ASD = autism spectrum disorder; NVLD = nonverbal learning disability. For all SRS scores, TD n = 32, NVLD n = 17, and ASD n = 15.
p < .05.
p < .01.
p < .001.
Results
Demographic and Clinical Characteristics
Groups did not differ in age or sex (see Table 1). As expected, the two clinical groups differed in VIQ (NVLD > ASD), PIQ (ASD > NVLD), and VIQ-PIQ discrepancy (NVLD > ASD). Relative to TD children, children with NVLD and ASD had significantly higher total scores on the SRS (see Table 1) and children with NVLD showed more variance in SRS scores than did the other two groups (Levene statistic (2,61) = 8.86, p < .001). Average whole-brain tSNR did not differ across the samples acquired at the two sites, t(72) = −1.33, p = .19.
Resting State Functional Connectivity
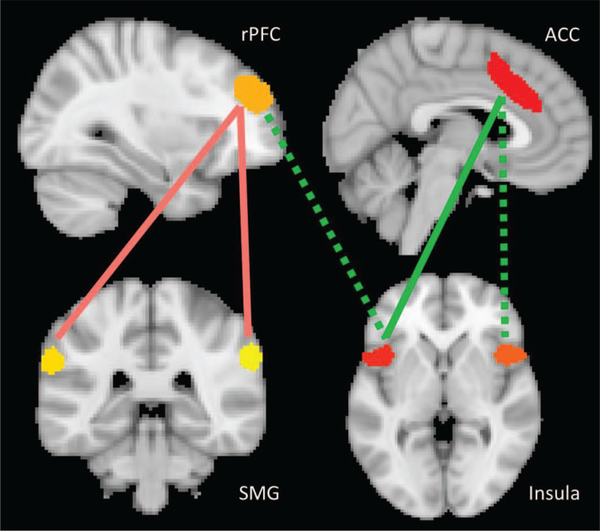
Group differences in RSFC between SN seeds were detected (Figures 1 and 2, Table 2), specifically from left and right AI to ACC and from left AI to right rostral prefrontal cortex (rPFC), deriving from reduced RSFC in children with NVLD relative to children with ASD and TD children. Group differences were also detected from left and right supramarginal gyrus (SMG) to right rPFC, deriving from greater RSFC in children with ASD relative to those with NVLD and TD children. Group differences in RSFC from right AI to ACC and from left AI to right rPFC were no longer significant when controlling for number of usable frames. No differences were detected in overall average connectivity within the DMN or SN or between the two networks. A supplemental analysis after denoising with GSR produced a similar pattern of results to those using aCompCor; children with NVLD showed reduced RSFC from left AI to ACC relative to children with ASD and TD children, and children with ASD showed greater RSFC from right SMG to right rPFC relative to children with NVLD and TD children (Table S4).
Figure 1.
Group differences in salience network connectivity. This figure displays differences in salience network connectivity across the typically developing (TD) children, children with nonverbal learning disability (NVLD), and children with autism spectrum disorder (ASD). Bars indicate mean connectivity values (Fisher r-to-Z transformed correlation values) and error bars indicate one standard error of the mean. ACC = anterior cingulate cortex; SMG = supramarginal gyrus; rPFC = rostral prefrontal cortex.
Figure 2.
Group differences in connectivity between SN seeds. This figure displays the seeds of the salience network and pairwise connections between them that showed significant group differences. Red lines between the rostral prefrontal cortex (rPFC) and bilateral supramarginal gyrus (SMG) indicate greater connectivity in children with autism spectrum disorder (ASD) compared with the other groups. Green lines between the anterior insula seeds and the anterior cingulate cortex (ACC) and rPFC indicate lower connectivity in the children with nonverbal learning disability (NVLD). The solid lines remained significant when controlling for the number of usable frames while the dotted lines indicate those connections which did not remain significant in this control analysis.
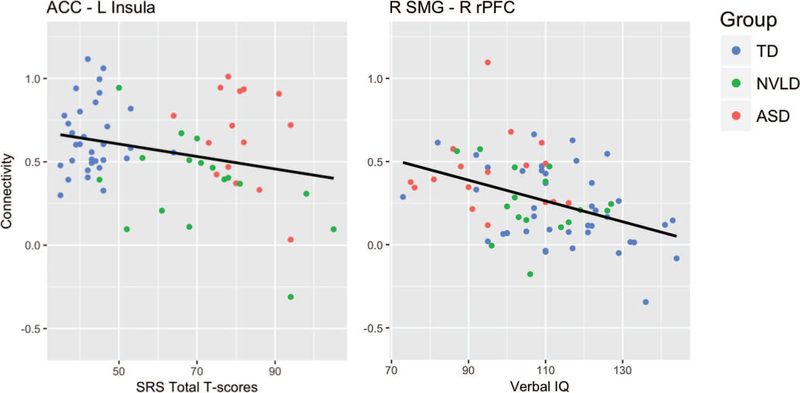
Exploratory analyses revealed a significant group-by-RSFC (ACC-left AI) interaction predicting SRS total scores (b = −26.323, t(63) = −2.33, p = .023; Table S5), deriving from a trend level inverse association between RSFC and SRS in the NVLD group (b = −24.890, t(15) = −1.946, p = .071) but nonsignificant associations in the other groups. No other significant group-by-RSFC interactions were detected, but main effects of right SMG to right rPFC connectivity on VIQ (b = −14.726, t(69) = −2.087, p = .041; Table S5) and of left SMG to right rPFC connectivity on PIQ (b = −15.793, t(69) = −2.122, p = .037; Table S5) were detected, controlling for group and number of usable frames (see Figure 3).
Figure 3.
Behavioral correlates of salience network connectivity. This figure displays scatter plots of behavioral measures that showed significant associations with connectivity values. The graph on the left shows the association between Social Responsiveness Scale (SRS) total t-scores and ACC—left AI connectivity values. A significant group-by-connectivity interaction on SRS total score (p = .023) was driven by a trend level inverse SRS-connectivity association in the NVLD children (p = .071). The graph on the right shows the association between verbal IQ scores and right SMG—right rPFC connectivity values. Inverse IQ-connectivity associations were significant across all participants while controlling for group (p < .04). Typically developing children (TD) are shown in blue, children with nonverbal learning disability (NVLD) are shown in green, and children with autism spectrum disorder (ASD) are shown in red. ACC = anterior cingulate cortex; SMG = supramarginal gyrus; rPFC = rostral prefrontal cortex.
Discussion
This is the first study to examine resting state functional connectivity in children with NVLD and specifically to compare connectivity within and across the SN and DMN in children with NVLD, children with ASD, and TD children. Children with NVLD and children with ASD had more parent-reported social problems than TD children. Whereas within-SN connectivity was reduced in children with NVLD, specifically between AI-ACC, SN connectivity was increased in children with ASD, specifically between rPFC-SMG. Weaker left AI-ACC connectivity was associated with more social problems in the NVLD group, while right SMG to right rPFC connectivity was increased in the ASD group and associated with reduced verbal abilities. These findings point to distinct alterations within a common network in NVLD and ASD that likely contribute to social or cognitive deficits in each disorder.
Children with ASD showed increased connectivity within the SN relative to TD children, specifically between left and right SMG and right rPFC. Our findings converge with findings from prior studies showing hyperconnectivity within the SN (Uddin et al., 2013) and no differences in AI-ACC connectivity (Abbott et al., 2016). Increased connectivity between right SMG and rPFC associated with lower VIQ scores in the ASD group, consistent with the role of SMG in language functions (Hartwigsen et al., 2010). Reduced interhemispheric connectivity between auditory cortical areas necessary for language functions is associated with reduced VIQ in children with ASD (Linke, Jao Keehn, Pueschel, Fishman, & Müller, 2018). Thus, aberrant connectivity across contralateral language processing areas and to PFC is associated with reduced verbal IQ in ASD, consistent with the language-related deficits commonly reported in these children (Rice, Warren, & Betz, 2005). Further, deficits in language-related processes associate with decreased social functioning in ASD (Linke et al., 2018), suggesting that social deficits in children with ASD may derive, in part, from language related deficits. Because adults with ASD do not show altered rSMG connectivity (Hoffmann, Koehne, Steinbeis, Dziobek, & Singer, 2016), future longitudinal studies should investigate whether an altered developmental trajectory of SN connectivity may contribute to the development of ASD.
Children with NVLD showed reduced connectivity within the SN relative to children with ASD or TD children, specifically between left and right AI and ACC as well as between left AI and right rPFC. Reduced connectivity from left AI to ACC was associated with greater social problems, suggesting that this pattern of altered functional connectivity may underlie the social difficulties detected in children with NVLD. Relative to healthy adults, healthy children typically show reduced connectivity within the SN (Menon, 2015), specifically between the AI and ACC, suggesting that further reduced SN connectivity in children with NVLD may reflect delayed network maturation, pointing to the need for longitudinal studies within this population. Consistent with clinical observations that children with NVLD have difficulty interpreting social cues (Semrud-Clikeman et al., 2015), AI-ACC functional activation and connectivity has been associated with interpreting social norms (Xiang et al., 2013), processing the experience of reward and pain in others (Lockwood, 2016), as well as the production of subjective feelings and coordinating responses to internal and external events (Medford & Critchley, 2010). Reduced AI-ACC functional connectivity in NVLD may underlie the specific difficulties that children with NVLD have in social interactions, which may derive from difficulty interpreting social cues. Such difficulty may thus disrupt their active engagement in social interactions.
That ROI-to-ROI connectivity between nodes, and not average connectivity within networks, showed group differences suggests that different methodological approaches to measuring network connectivity may identify different aspects of pathophysiology. This may be particularly relevant in studies of children as networks change and develop. ROI-to-ROI analyses may allow for more specific insight, revealing group differences that would be lost on a larger scale. Other studies have similarly found different connectivity patterns (hyper- and hypoconnectivity) between different nodes within the SN in individuals with obsessive compulsive disorder (Fan et al., 2017).
Our study has several limitations. The small sample size limits the generalizability of findings and requires replication in a larger independent sample. Further, it was not possible to compare performance of children with NVLD and ASD on direct tests of social function as this was not available in the ABIDE dataset. While leveraging publicly available data to compare children with NVLD and ASD was a strength of this study, the MRI acquisition parameters across sites were not matched identically. Although a limitation of our data, average whole-brain tSNR was comparable across the samples scanned at the two different sites, suggesting that our findings of group differences cannot be attributed to differences in MRI acquisition. Further, recent findings show that seed-to-voxel connectivity analyses are robust to scanner and site differences (Noble et al., 2017). Notably, the length of resting state MRI scans varied across sites; as longer scans could lead to better estimates of the correlations between brain regions, we covaried for number of usable frames to control for these differences. Because number of usable frames was highly correlated with mean motion, this also allowed us to control potential effects of motion on observed group differences. Additionally, although the criteria for NVLD is debated (Fine et al., 2013), the diagnostic criteria used herein build on those used in prior neuroimaging studies of NVLD (Fine et al., 2014; Semrud-Clikeman & Fine, 2011; Semrud-Clikeman et al., 2013). Our approach also excludes children with comorbid NVLD and ASD, which may have biased the results by increasing the likelihood of finding group differences. Future studies should compare functional connectivity across three groups of children: ASD only, NVLD only, ASD + NVLD. Last, correlations between behavioral variables and functional connectivity values were not corrected for multiple comparisons and therefore should be interpreted with caution and require replication.
In summary, this is the first study to provide evidence for distinct functional neural signatures in NVLD as compared with both TD children and those with ASD. Relative to TD children, children with ASD and children with NVLD showed parent-reported deficits in social functioning. Patterns of altered connectivity within the SN were associated with social problems in children with NVLD and language problems in those with ASD; importantly, these problems derived from alterations between distinct nodes of the same network. Children with ASD showed increased connectivity from rPFC to right SMG, a region implicated in language and social functions, whereas those with NVLD showed reduced AI–ACC connectivity, regions that support the processing of social cues, consistent with the phenomenology of these two disorders. Thus, our findings suggest that distinct alterations in RSFC within the SN may distinguish between children with NVLD and ASD.
Supplementary Material
General Scientific Summary.
Nonverbal learning disability (NVLD) is a putative neurodevelopmental disorder characterized by spatial and social deficits. This study suggests that the social deficits common across children with NVLD and autism spectrum disorder may derive from distinct alterations in connectivity within the salience network, which is believed to support social processing.
Acknowledgments
This work was supported by The NVLD Project and K23ES026239.
References
- Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, & Müller RA (2016). Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cerebral Cortex, 26, 4034–4045. 10.1093/cercor/bhv191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, … Pearlson GD (2010). Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage, 53, 247–256. 10.1016/j.neuroimage.2010.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37, 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo R, Garcia RB, Mammarella IC, & Cornoldi C (2017). Pragmatics of language and theory of mind in children with dyslexia with associated language difficulties or nonverbal learning disabilities. Appl Neuropsychol Child. Advance online publication. 10.1080/21622965.2017.1297946 [DOI] [PubMed] [Google Scholar]
- Carper RA, Solders S, Treiber JM, Fishman I, & Muller RA (2015). Corticospinal tract anatomy and functional connectivity of primary motor cortex in autism. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 859–867. 10.1016/j.jaac.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, … Satterthwaite TD (2017). Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage, 154, 174–187. 10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2005). The social responsiveness scale manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Cornoldi C, Mammarella IC, & Fine JG (2016). Nonverbal learning disabilities. New York, NY: Guilford Press. [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, & Milham MP (2009). Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biological Psychiatry, 65, 63–74. 10.1016/j.biopsych.2008.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Di Martino A, Hazlett HC, & Gao W (2015). Neural connectivity evidence for a categorical-dimensional hybrid model of autism spectrum disorder. Biological Psychiatry. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Zhong M, Zhu X, Gan J, Liu W, Niu C, … Tan C (2017). Resting-state functional connectivity between right anterior insula and right orbital frontal cortex correlate with insight level in obsessive-compulsive disorder. NeuroImage Clinical, 15, 1–7. 10.1016/j.nicl.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine JG, Musielak KA, & Semrud-Clikeman M (2014). Smaller splenium in children with nonverbal learning disability compared to controls, high-functioning autism and ADHD. Child Neuropsychology, 20, 641–661. 10.1080/09297049.2013.854763 [DOI] [PubMed] [Google Scholar]
- Fine JG, Semrud-Clikeman M, Bledsoe JC, & Musielak KA (2013). A critical review of the literature on NLD as a developmental disorder. Child Neuropsychology, 19, 190–223. 10.1080/09297049.2011.648923 [DOI] [PubMed] [Google Scholar]
- Fishman I, Keown CL, Lincoln AJ, Pineda JA, & Muller RA (2014). Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry, 71, 751–760. 10.1001/jamapsychiatry.2014.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, & Fan J (2013). Anterior insular cortex and emotional awareness. The Journal of Comparative Neurology, 521, 3371–3388. 10.1002/cne.23368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, & Siebner HR (2010). Phonological decisions require both the left and right supramarginal gyri. Proceedings of the National Academy of Sciences of the United States of America, 107, 16494–16499. 10.1073/pnas.1008121107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F, Koehne S, Steinbeis N, Dziobek I, & Singer T (2016). Preserved self-other distinction during empathy in autism is linked to network integrity of right supramarginal gyrus. Journal of Autism and Developmental Disorders, 46, 637–648. 10.1007/s10803-015-2609-0 [DOI] [PubMed] [Google Scholar]
- Hull JV, Dokovna LB, Jacokes ZJ, Torgerson CM, Irimia A, & Van Horn JD (2017). Resting-state functional connectivity in autism spectrum disorders: A review. Frontiers in Psychiatry, 7, 205 10.3389/fpsyt.2016.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DJ, & Mykelbust HR (1967). Learning disabilities: Educational principles and practices. New York: Grune & Stratton. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Klin A, Volkmar FR, Sparrow SS, Cicchetti DV, & Rourke BP (1995). Validity and neuropsychological characterization of Asperger syndrome: convergence with nonverbal learning disabilities syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines, 36, 1127–1140. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, … Fox PT (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23, 4022–4037. 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mai X, & Liu C (2014). The default mode network and social understanding of others: What do brain connectivity studies tell us. Frontiers in Human Neuroscience, 8, 74 10.3389/fnhum.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke AC, Jao Keehn RJ, Pueschel EB, Fishman I, & Müller RA (2018). Children with ASD show links between aberrant sound processing, social symptoms, and atypical auditory interhemispheric and thalamocortical functional connectivity. Developmental Cognitive Neuroscience, 29, 117–126. 10.1016/j.dcn.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL (2016). The anatomy of empathy: Vicarious experience and disorders of social cognition. Behavioural Brain Research, 311, 255–266. http:// [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule (2nd ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- Mammarella IC, & Cornoldi C (2014). An analysis of the criteria used to diagnose children with Nonverbal Learning Disability (NLD). Child Neuropsychology, 20, 255–280. 10.1080/09297049.2013.796920 [DOI] [PubMed] [Google Scholar]
- Mar RA (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–134. 10.1146/annurev-psych-120709-145406 [DOI] [PubMed] [Google Scholar]
- Medford N, & Critchley HD (2010). Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Structure & Function, 214, 535–549. 10.1007/s00429010-0265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2015). Salience network In Toga AW (Ed.), Brain mapping: An encyclopedic reference (pp. 597–611). Cambridge, MA: Academic Press; 10.1016/B978-0-12-397025-1.00052-X [DOI] [Google Scholar]
- Nair A, Keown CL, Datko M, Shih P, Keehn B, & Muller RA (2014). Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Human Brain Mapping, 35, 4035–4048. 10.1002/hbm.22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S, Scheinost D, Finn ES, Shen X, Papademetris X, McEwen SC, … Constable RT (2017). Multisite reliability of MR-based functional connectivity. NeuroImage, 146, 959–970. 10.1016/j.neuroimage.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo J (2006). Nonverbal Learning Disabilities: A clinical perspective: W. W. Norton & Company. [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, … Petersen SE (2011). Functional network organization of the human brain. Neuron, 72, 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ML, Warren SF, & Betz SK (2005). Language symptoms of developmental language disorders: An overview of autism, Down syndrome, Fragile X, specific languag e impairment, and Williams syndrome. Applied Psycholinguistics, 26, 7–27. 10.1017/S0142716405050034 [DOI] [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2003). Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, & Vogeley K (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17, 457–467. 10.1016/j.concog.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, & Glass K (2010). The relation of humor and child development: social, adaptive, and emotional aspects. Journal of Child Neurology, 25, 1248–1260. 10.1177/0883073810373144 [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, & Fine J (2011). Presence of cysts on magnetic resonance images (MRIs) in children with Asperger disorder and nonverbal learning disabilities. Journal of Child Neurology, 26, 471–475. 10.1177/0883073810384264 [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Fine JG, & Bledsoe J (2015). Social functioning using direct and indirect measures with children with high functioning autism, nonverbal learning disability, and typically developing children. Child Neuropsychology, 22, 318–335. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Fine JG, Bledsoe J, & Zhu DC (2013). Magnetic resonance imaging volumetric findings in children with Asperger syndrome, nonverbal learning disability, or healthy controls. Journal of Clinical and Experimental Neuropsychology, 35, 540–550. 10.1080/13803395.2013.795528 [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, & Christopher G (2010). Neuropsychological differences among children with Asperger syndrome, nonverbal learning disabilities, attention deficit disorder, and controls. Developmental Neuropsychology, 35, 582–600. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, & Minne EP (2010). Direct and indirect measures of social perception, behavior, and emotional functioning in children with Asperger’s disorder, nonverbal learning disability, or ADHD. Journal of Abnormal Child Psychology, 38, 509–519. 10.1007/s10802-009-9380-7 [DOI] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, … Glasser MF (2013). Resting-state fMRI in the Human Connectome Project. NeuroImage, 80, 144–168. 10.1016/j.neuroimage.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, … Menon V. (2013). Salience network-based classification and prediction of symptom severity in children with autism. Journal of the American Medical Association Psychiatry, 70, 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron-Cohen S, & Calder AJ (2013). Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Social Cognitive and Affective Neuroscience, 8, 694–701. 10.1093/scan/nss053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2, 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Xiang T, Lohrenz T, & Montague PR (2013). Computational substrates of norms and their violations during social exchange. The Journal of Neuroscience, 33, 1099–1108. 10.1523/JNEUROSCI.1642-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.